ABSTRACT
Clostridium perfringens can produce up to three different sialidases, including NanI, its major exosialidase. The current study first showed that human intestinal strains of C. perfringens can grow by utilizing either glucose or sialic acids, such as N-acetylneuraminic acid (Neu5Ac), which are the end products of sialidase activity. For the human enteropathogenic strain F4969, it was then determined that culture supernatant sialidase activity and expression of exosialidase genes, particularly nanI, are influenced by the presence of Neu5Ac or glucose. Low Neu5Ac concentrations increased culture supernatant sialidase activity, largely by stimulating nanI transcription. In contrast, low glucose concentrations did not affect exosialidase activity or nanI transcription. However, either high Neu5Ac or high glucose concentrations repressed F4969 culture supernatant sialidase activity and nanI transcription levels. Furthermore, high glucose levels repressed F4969 culture sialidase activity and nanI expression even in the presence of low Neu5AC concentrations. To begin to evaluate the mechanistic basis for nanI expression, a nanR null mutant was used to demonstrate that NanR, a member of the RpiR family of regulatory proteins, decreases exosialidase activity and nanI transcription in the absence of sialic acid. The ability of C. perfringens to regulate its exosialidase activity, largely by controlling nanI expression, may affect intestinal pathogenesis by affecting the production of NanI, which may affect C. perfringens growth, adhesion, and toxin binding in vivo.
KEYWORDS: Clostridium perfringens, sialidase, NanI, NanR, gene regulation, growth
INTRODUCTION
Clostridium perfringens is an important causative agent of human intestinal infections (1, 2). Type A strains producing C. perfringens enterotoxin (CPE) are responsible for C. perfringens type A food poisoning, which involves acute diarrhea and abdominal cramps that affect about 1 million Americans/year (3). CPE-producing type A strains also cause the chronic diarrhea of several nonfoodborne gastrointestinal diseases, most notably CPE-associated antibiotic-associated diarrhea (4). Type C strains, which produce beta toxin, are responsible for a human intestinal infection known as enteritis necroticans (EN). EN was first reported in post-World War II Germany (5–7) and then became a major cause of childhood death in the Papua New Guinea Highlands in the 1970s and -80s (5, 8, 9). EN outbreaks still occur sporadically in malnourished people in developing countries, and occasional cases of this disease are observed in developed countries (10).
Beyond its toxin armory, C. perfringens also produces a slew of extracellular enzymes that may assist growth and, possibly, virulence. Among those exoenzymes are two exosialidases: NanI (77 kDa) and NanJ (129 kDa) (11–16). C. perfringens also produces a third sialidase, NanH (43 kDa), but this enzyme has a cytoplasmic localization in log-phase cultures (11, 12). Many C. perfringens strains produce all three sialidases, and for those strains, NanI is responsible for most of the in vitro supernatant sialidase activity (14–16). Recent studies suggest that NanI may contribute to intestinal infections by promoting the adherence of NanI-producing C. perfringens strains to enterocyte-like cells and by increasing the production and binding to host cells (and therefore activity) of some C. perfringens toxins (11, 13, 17).
Sialidases are also known to assist in the growth of some pathogenic bacteria by liberating sialic acids, particularly N-acetylneuraminic acid (Neu5Ac), from complex host glycoconjugates, such as mucins, which are major components of gastrointestinal or respiratory tract mucus (18–20). Similarly, it has been proposed that sialidases contribute to the in vivo growth of C. perfringens (16). Supporting that possibility, C. perfringens was the first bacterium shown to be capable of using sialic acid for in vitro growth (21, 22), likely because it possesses a nan operon that encodes the complete Nan pathway for transporting and metabolizing sialic acid (16, 23).
The C. perfringens nan operon also encodes NanR, which shares sequence homology with the RpiR family of regulatory proteins (16). Since NanR can bind to nanE or nanI promoter sequences, it has been proposed (16) that NanR (i) regulates transcription of genes encoding exosialidases and other proteins involved in sialic acid transport and metabolism and (ii) responds, in a positive-feedback loop, to sialic acid. However, there is no direct evidence demonstrating NanR involvement in regulating exosialidase production or whether, if there is involvement, that regulation is responsive to sialic acid.
Though still poorly characterized, nutrient conditions can influence exosialidase production by at least some C. perfringens strains. Specifically, low concentrations of sialic acid were shown to induce in vitro exosialidase activity for type A gas gangrene-causing strain 13 (16). However, it remains unknown whether (i) this effect also occurs in C. perfringens human enteropathogenic strains, (ii) high sialic acid concentrations also affect exosialidase activity levels, and (iii) the presence of glucose affects exosialidase activity levels.
Similarly, the regulatory pathway by which low sialic acid concentrations regulate exosialidase activity levels is not understood. While several regulators have been identified that control transcription of nanH, nanI, and nanJ, none of those regulators are sialic acid specific (24–27). As mentioned above, NanR binds with high affinity and specificity to the nanI promoter, suggesting that it may regulate NanI production by C. perfringens, but this hypothesis remains untested.
Given the emerging interest in possible contributions of sialidases, particularly NanI, to C. perfringens intestinal growth and pathogenesis, the current study compared sialic acid and glucose effects on intestinal strain exosialidase production and growth. The involvement of NanR in regulating C. perfringens exosialidase gene expression was then directly evaluated.
RESULTS
C. perfringens human intestinal strains can use glucose or sialic acid for growth.
Using glucose or sialic acid for growth would have pathogenic relevance for C. perfringens intestinal strains, since glucose is present in the small intestinal lumen (28) and sialic acids, particularly Neu5Ac, are present on macromolecules located throughout the intestinal tract, e.g., on mucins (18). However, the ability of C. perfringens intestinal strains to use glucose or sialic acid for growth has not previously been evaluated carefully, so this study first characterized glucose and sialic acid utilization by a collection of strains (Table 1) that included human intestinal disease strains and a normal human intestinal microbiota strain of this bacterium. For this survey, the human intestinal strains were cultured for 24 h in glucose-free Dulbecco's modified Eagle medium (DMEM) or DMEM supplemented with (i) a low (1.6 mM) or high (16 mM) Neu5Ac concentration, creating DMEM-SL or DMEM-SH, respectively, or (ii) a low (1.6 mM) or high (16 mM) d-glucose concentration, creating DMEM-GL or DMEM-GH, respectively. Glucose-free DMEM was used as a defined carbohydrate-free medium that could readily be supplemented with desired concentrations of carbohydrates. Oxyrase was added to all DMEM-based cultures to facilitate anaerobic growth.
TABLE 1.
Human intestinal isolates used in this study
| Isolate (type) | Sialidase PCR typea | Description (disease, location, time of isolation) |
|---|---|---|
| SM101 (A) | H | Food poisoning, Europe, 1950s |
| C1841 (A) | J/H | Food poisoning, Vermont, 1980s |
| FD1041 (A) | H | Food poisoning, North America, 1980s |
| 01E809 (A) | J/H | Food poisoning, Oklahoma, 1999 |
| F4969 (A) | J/I/H | Sporadic diarrhea, Europe, 1990s |
| F5603 (A) | J/I/H | Sporadic diarrhea, Europe, 1990s |
| B40 (A) | J/I/H | Antibiotic-associated diarrhea, Europe, 1980s |
| F38660 (A) | J/I/H | Antibiotic-associated diarrhea, North America, 1990s |
| 8-6 (A) | J/I/H | Healthy North Americans |
| CN5383 (C) | J/I/H | Pigbel, Papua, New Guinea, 1970s |
| CN5388 (C) | J/I/H | Pigbel, Papua, New Guinea, 1970s |
| Bar 3 (C) | J/I/H | Pigbel, Papua, New Guinea,? |
| CN2076 (C) | J/H | Darmbrand (enteritis necroticans), Hamburg, Germany, 1955 |
| CN3758 (C) | J/H | Darmbrand (enteritis necroticans), Hamburg, Germany, 1955 |
| CN3763 (C) | J/H | Darmbrand (enteritis necroticans), Hamburg, Germany, 1955 |
From reference 13.
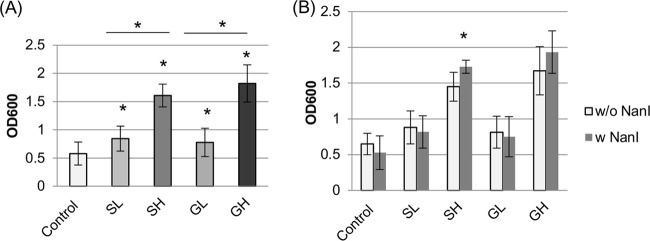
Compared to equivalent growth in control DMEM without any carbohydrate supplementation, the addition of even a low (1.6 mM) concentration of sialic acid or d-glucose significantly increased growth yields of all surveyed human intestinal strains, supporting the ability of these strains to use either carbohydrate for growth (Fig. 1A). Growth yields of these strains increased even further when they were cultured in DMEM supplemented with the higher (16 mM) concentration of either sialic acid or d-glucose (Fig. 1A).
FIG 1.
Comparison of growth yields for C. perfringens intestinal strains cultured at 37°C in DMEM with or without glucose or sialic acid supplementation. Fifteen intestinal strains (Table 1) were grown for 24 h in control DMEM, DMEM with 1.6 mM sialic acid (SL), DMEM with 1.6 mM glucose (GL), DMEM with 16 mM sialic acid (SH), or DMEM with 16 mM glucose (GH), as indicated. (A) Growth yields of the surveyed intestinal strains. Compared to that for equivalent growth in control DMEM, the OD600 of all carbohydrate-supplemented cultures increased significantly (*, P < 0.05). SH and GH cultures also had significantly higher growth yields than those of similar SL and GL cultures, respectively (* with bar, P < 0.05). (B) Growth yields of the eight intestinal strains in Table 1 that do and the seven intestinal strains in Table 1 that do not carry the nanI gene. Only when cultures were supplemented with the higher Neu5Ac concentration were growth yields slightly increased for strains with the nanI gene versus strains without the nanI gene (*, P < 0.05). All experiments were repeated three times, and mean values are shown. The error bars indicate standard deviations.
While most C. perfringens strains, including many human enteropathogenic strains, produce three sialidases (NanJ, NanI, and NanH), some C. perfringens type A food poisoning strains or type C human enteropathogenic strains lack the nanI gene (Table 1). Therefore, the effects of supplementing DMEM with glucose or free Neu5Ac on growth yields were compared between C. perfringens intestinal strains that can and cannot naturally produce NanI (Fig. 1B). Supplementation of DMEM with either carbohydrate significantly increased the 24-h growth yields of all intestinal strains, regardless of their ability to produce NanI. DMEM-SH supported a slight further increase in growth yields for the NanI-producing versus non-NanI-producing strains. Overall, these results indicated that intestinal strains can utilize free glucose or sialic acid for growth, regardless of whether they produce NanI.
Analysis of growth and sialidase production by F4969 cultured with sialic acid or glucose.
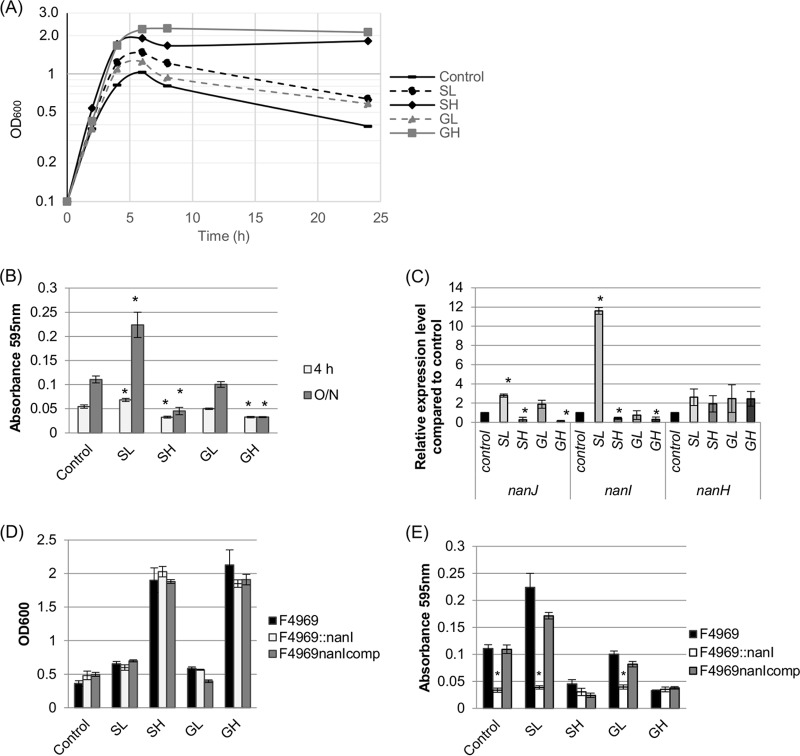
Detailed growth and sialidase activity experiments were then performed using CPE-positive type A strain F4969 as a representative human enteropathogenic strain of C. perfringens. Using control DMEM or DMEM supplemented with a low (1.6 mM) or high (16 mM) concentration of Neu5Ac or d-glucose showed that both sialic acid and d-glucose were able to promote the 8-h or 24-h growth of F4969 (Fig. 2A), with higher concentrations of the two carbohydrates supporting additional growth. For example, compared to those of equivalent cultures grown in control DMEM without carbohydrate supplementation, 24-h growth yields of F4969 cultures were significantly higher in DMEM supplemented with SL, SH, or GH. While the optical densities at 600 nm (OD600) were also higher for 24-h F4969 cultures grown in DMEM-GL, the differences relative to equivalent control DMEM cultures did not reach statistical significance. Viable plate counts mirrored these results (data not shown).
FIG 2.
Growth, sialidase activity, and sialidase gene expression by F4969, an isogenic nanI null mutant, and a complemented strain. (A) Cultures of F4969 were grown for 24 h at 37°C in control DMEM, DMEM with 1.6 mM sialic acid (SL), DMEM with 16 mM sialic acid (SH), DMEM with 1.6 mM glucose (GL), or DMEM with 16 mM glucose (GH), as indicated. At the designated time points, the OD600 of cultures were determined. Results representative of three repetitions are shown. (B) Supernatant sialidase activities in supernatants from F4969 grown at 37°C for 4 or 24 h (O/N) in control DMEM versus DMEM supplemented with Neu5Ac or d-glucose, as indicated. (C) qRT-PCR analyses of nanJ, nanI, and nanH expression levels in F4969 cultures grown in DMEM with or without carbohydrate supplementation. Transcript levels were determined with 20 ng of RNA isolated from 4-h cultures grown at 37°C in control DMEM or DMEM supplemented with GL, GH, SL, or SH. Average CT values were normalized to that of the housekeeping 16S rRNA gene, and fold differences were calculated using the comparative CT method (2−ΔΔCT). The value of each bar indicates the calculated fold change relative to the control. (D) Growth yields of 24-h cultures of wild-type, nanI null mutant, and complemented strains of F4969 grown at 37°C in control DMEM versus DMEM supplemented with Neu5Ac or d-glucose, as indicated. (E) Sialidase activities in supernatants from 24-h cultures of wild-type, nanI null mutant, and complemented strains of F4969 grown at 37°C in control DMEM versus DMEM supplemented with Neu5Ac or d-glucose, as indicated. For panels B to E, the data shown are mean values for three independent experiments. The error bars indicate standard deviations. *, P < 0.05 compared to the control.
Culture levels of glucose or end products, such as sialic acid, can influence enzyme production levels by bacterial cells. Therefore, sialidase activity was measured (Fig. 2B) in supernatants from cultures of F4969 grown in DMEM with or without sialic acid or glucose supplementation. Supernatants from both 4- and 24-h F4969 cultures were assessed because young (<6 h) C. perfringens culture supernatants contain only the NanI and NanJ exosialidases (when produced), while supernatants of >6-h cultures contain those two exosialidases plus some NanH released from bacterial cells (12). At both time points, sialidase assays detected significantly higher sialidase activity in DMEM-SL culture supernatants than in control DMEM supernatants. These results resemble those of a previous report (16) observing that the addition of a similarly low (3.2 mM) sialic acid concentration induced exosialidase activity for strain 13, which can cause gas gangrene.
No study has yet examined the effects of glucose or higher sialic acid concentrations on supernatant sialidase activity for any C. perfringens strains. When the effects of supplementing DMEM with those carbohydrates were evaluated (Fig. 2B), growth of F4969 for 4 or 24 h in DMEM-SH significantly reduced the supernatant sialidase activity compared to that with equivalent growth of this strain in control DMEM. Relative to 4- or 24-h DMEM control cultures of F4969, equivalent growth of F4969 in DMEM-GL did not significantly affect the supernatant sialidase activity. However, 4 or 24 h of growth of this strain in DMEM-GH significantly reduced the supernatant sialidase activity compared to that with equivalent growth of this strain in control DMEM. This is the first report indicating that supernatant sialidase activity in C. perfringens cultures is significantly decreased in the presence of high concentrations of either glucose or sialic acid.
Given the differences in exosialidase activity when F4969 was grown in media supplemented with different concentrations of glucose or sialic acid, transcript levels of sialidase genes in the 4-h cultures were compared by quantitative reverse transcription-PCR (qRT-PCR) (Fig. 2C). Relative to those with equivalent growth for 4 h in DMEM, expression levels of nanJ and, particularly, nanI increased significantly when F4969 was grown for 4 h in DMEM-SL. In contrast, 4 h of growth of F4969 in DMEM-SH significantly repressed nanJ and nanI expression. While 4 h of growth of F4969 in DMEM-GL did not significantly affect nanI or nanJ transcript levels, similar growth of this strain in DMEM-GH significantly repressed both nanJ and nanI expression. The presence of carbohydrates increased nanH transcript levels in 4-h cultures, but those increases did not reach statistical significance (Fig. 2C). Collectively, the results shown in Fig. 2 indicate that expression levels of the nanJ and nanI exosialidase genes can be strongly affected by sialic acid or glucose levels.
Since glucose and Neu5Ac affected both nanI and nanJ expression levels, a previously prepared F4969 nanI null mutant and complemented strain (13) were then used to specifically evaluate NanI's contributions to the variations in supernatant sialidase activity observed in Fig. 2B. When F4969 was cultured for 24 h in DMEM supplemented with sialic acid or d-glucose, growth yields of the parent strain, the nanI mutant, and the complemented strain were similar in each tested medium (Fig. 2D). Measuring sialidase activities in the corresponding culture supernatants then demonstrated (Fig. 2E) that changes in NanI activity were responsible for most of the increased supernatant sialidase activity induced by the addition of sialic acid at low concentrations. Supernatant sialidase activity was so strongly repressed that no significant differences could reliably be detected between supernatant sialidase activities of F4969, the nanI null mutant, and the complemented strain grown for 24 h in DMEM-SH or DMEM-GH.
Further analysis of Neu5AC and glucose concentration effects on supernatant sialidase activity.
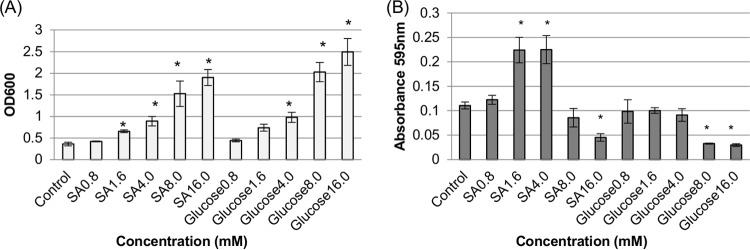
Supernatant sialidase activity differed for 16 mM versus 1.6 mM concentrations of either Neu5Ac or d-glucose (Fig. 2), so a dose-response experiment was performed using additional Neu5Ac or glucose concentrations to determine more precisely the concentrations of these carbohydrates that affect supernatant sialidase activity levels. The results (Fig. 3A) indicated that 24-h growth yields of F4969 increased steadily with higher Neu5Ac or glucose concentrations. Compared to 24-h growth yields when F4969 was grown in control DMEM, significantly increased 24-h growth yields were seen with the addition of Neu5Ac at 1.6 mM or higher or d-glucose at 4 mM or higher.
FIG 3.
Comparison of growth yields and supernatant sialidase activities for F4969 grown for 24 h at 37°C in control DMEM versus DMEM supplemented with different concentrations of Neu5Ac (SA) or d-glucose. (A) Growth yields for 24-h cultures in DMEM supplemented with specified concentrations of sialic acid (SA) or glucose. (B) Sialidase activities in supernatants from 24-h cultures. The data shown in each panel are mean values for three independent experiments. The error bars indicate standard deviations. *, P < 0.05 compared to the control.
Supernatant sialidase activity in those cultures varied with different carbohydrate concentrations (Fig. 3B). When F4969 was grown for 24 h in DMEM supplemented with 1.6 mM or 4 mM Neu5Ac, supernatant sialidase activity increased significantly relative to supernatant sialidase activity from equivalent DMEM cultures. However, at concentrations beyond 4 mM Neuc5Ac, sialidase activity dropped until it became significantly repressed by a 16 mM sialic acid concentration. In 24-h cultures of F4969, supernatant sialidase activity was significantly repressed when glucose levels of >4 mM were added to DMEM.
Glucose represses supernatant sialidase activity in the presence of low Neu5Ac concentrations.
The results in Fig. 2 and 3 show that a low Neu5Ac concentration induces supernatant sialidase activity, while a high glucose concentration represses supernatant sialidase activity. Therefore, an experiment was performed to evaluate which of those effects is dominant when F4969 is cultured in DMEM supplemented with both a low (1.6 mM) Neu5Ac concentration and a high (16 mM) glucose concentration. Growth curve results (Fig. 4A) indicated that when DMEM-SL was supplemented with low (1.6 mM) glucose (creating DMEM-SLGL) or, especially, high (16 mM) glucose (creating DMEM-SLGH), growth increased for both strains for up to 8 h. Similarly, after 24 h of culture in the same media, both DMEM-SLGL and DMEM-SLGH supported increased growth yields relative to those of equivalent DMEM cultures (Fig. 4A). However, despite that increased growth, supernatant sialidase activity (Fig. 4B) was significantly repressed, relative to that in equivalent DMEM cultures, by growth of F4969 for 24 h in DMEM containing the copresence of a high glucose concentration and a Neu5Ac concentration that, in the absence of glucose, boosts supernatant sialidase activity.
FIG 4.
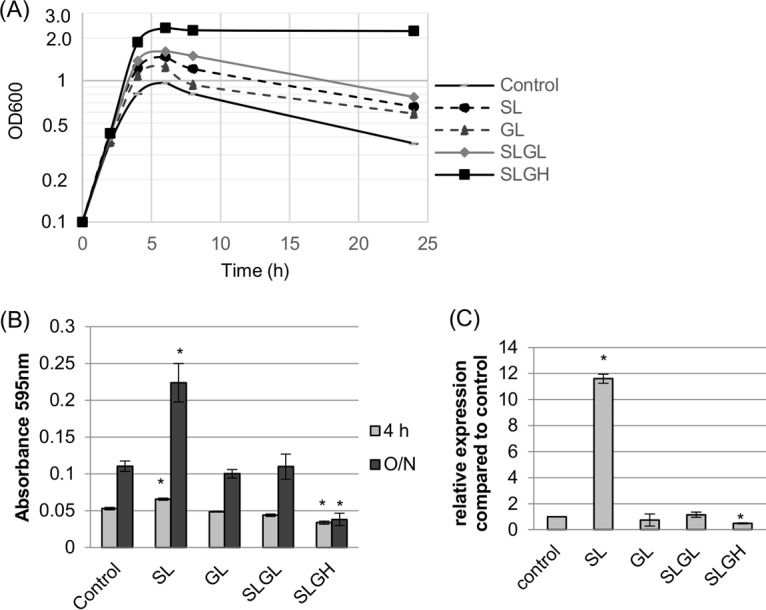
Growth yields and supernatant sialidase activities for F4969 grown in DMEM (control) or DMEM supplemented with sialic acid, glucose, or both sialic acid and glucose. (A) F4969 growth for 24 h at 37°C in DMEM (control), DMEM with 1.6 mM sialic acid (SL), DMEM with 1.6 mM glucose (GL), DMEM with 1.6 mM sialic acid plus 1.6 mM glucose (SLGL), or DMEM with 1.6 mM sialic acid plus 16 mM glucose (SLGH). Every 2 h for up to 8 h, the OD600 of the culture was measured. Results representative of three repetitions are shown. (B) Supernatant sialidase activities detected in 4-h or 24-h supernatants of the cultures used for panel A. (C) qRT-PCR results for nanI gene expression, using RNAs extracted from pelleted cells of the 4-h cultures used for panel A. Transcript levels were analyzed using 20 ng of RNA isolated from 4-h cultures. Average CT values were normalized to that of the housekeeping 16S rRNA gene, and the fold differences were calculated using the comparative CT method (2−ΔΔCT). The value of each bar indicates the calculated fold change relative to the control. The data shown in panels B and C are mean values for three independent experiments. The error bars indicate standard deviations. *, P < 0.05 compared to the control by ordinary one-way ANOVA.
To determine if repression of nanI gene transcription is involved in the decreased sialidase activity of supernatants collected from cultures grown in DMEM-SLGH, a nanI qRT-PCR was performed. Since pelleted cells from 4-h cultures were used to perform the nanI qRT-PCR, exosialidase activity in 4-h cultures was also determined. The same patterns of supernatant sialidase activity detected for the 24-h cultures were also observed for 4-h cultures (Fig. 4B), i.e., the copresence of a high glucose concentration and a low sialic acid concentration repressed exosialidase activity. The nanI qRT-PCR then determined (Fig. 4C) that although nanI gene transcription levels were significantly induced by the addition of a low concentration of Neu5Ac alone (no glucose) to DMEM, the copresence of a high glucose concentration attenuated this induction despite strong growth of the culture (Fig. 4A).
Construction and characterization of an F4969 nanR null mutant and reversed mutant.
To utilize Neu5AC, bacteria such as Escherichia coli, Vibrio vulnificus, Streptococcus pneumoniae, Staphylococcus aureus, and Corynebacterium glutamicum employ a Nan system that mediates the uptake and metabolism of sialic acids (29–32). Similarly, an operon encoding a Nan system is conserved among C. perfringens strains, where it is thought to mediate sialic acid uptake and metabolism (16). NanR, which is encoded within the nan operon, is a proposed sialic acid-induced regulator of nanI expression, as well as a regulator of transporters and enzymes involved in sialic acid metabolism (16).
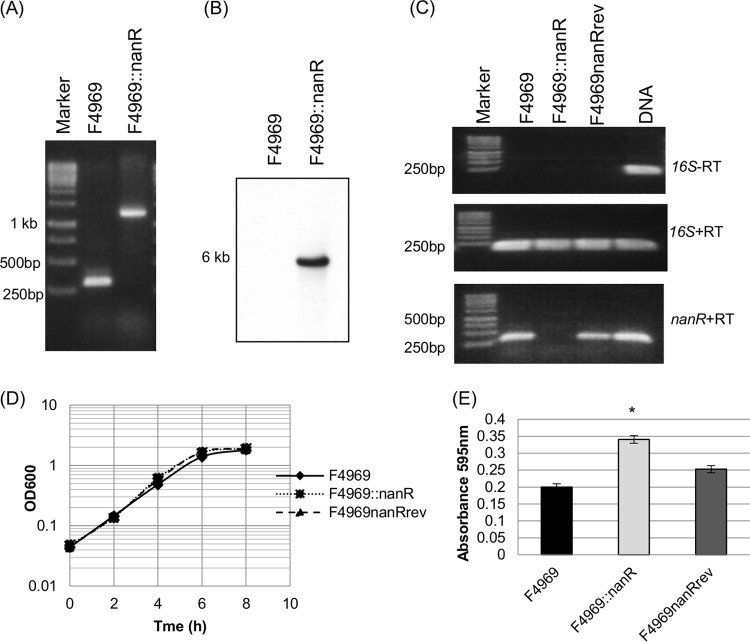
To evaluate whether NanR controls nanI expression, a nanR null mutant of F4969 was constructed using Clostridium-modified TargeTron technology (33). Verifying the null mutant, nanR PCR primers amplified an ∼300-bp product by using DNA from wild-type F4969 but amplified an ∼1,200-bp product by using DNA from the nanR null mutant, consistent with insertion of an ∼900-bp intron into the nanR open reading frame (ORF) of the mutant (Fig. 5A). To confirm that a single intron was inserted into the nanR null mutant genome, Southern blotting was performed with an intron-specific probe. This Southern blot detected no intron insertion in the wild-type strain but a single intron insertion in the nanR null mutant, named F4969::nanR (Fig. 5B).
FIG 5.
Construction and characterization of an F4969 nanR null mutant and a reversed mutant strain. (A) PCR confirmation of isogenic nanR null mutant strain construction. Using DNA from F4969, a PCR using internal nanR gene primers amplified the expected product of ∼300 bp. Using template DNA isolated from the F4969::nanR strain, which has a 900-bp intron insertion in the nanR gene, the same PCR assay amplified a larger product, of ∼1,200 bp. The left lane shows a 1-kb molecular ruler. (B) Intron-specific Southern blot hybridization with DNA from the wild-type or nanR null mutant strain. The size of the DNA fragment is indicated at left. (C) RT-PCR analysis of nanR gene transcription in wild-type, null mutant, and reversed strains grown for 4 h at 30°C. (Top) Transcription analysis of the housekeeping 16S rRNA gene without reverse transcriptase (−RT) indicated that all RNAs were free of DNA contamination. (Middle) transcription analysis of the housekeeping 16S rRNA gene with reverse transcriptase (+RT) indicated that the isolated RNA was of high quality. (Bottom) Transcription analysis of the nanR gene. The left lane shows the 1-kb molecular ruler, and the right lane shows the F4969 DNA (as a positive control). (D) Postinoculation changes in OD600 for cultures of wild-type, null mutant, and reversed mutant strains grown in TH medium at 30°C. The experiment was repeated three times, and representative results are shown. (E) Supernatant sialidase activity analysis of wild-type, null mutant, and reversed mutant strains grown overnight in TH medium at 30°C. The experiment for panel E was repeated three times, and mean values are shown. The error bars indicate standard deviations. *, P < 0.05 compared to the wild-type strain by ordinary one-way ANOVA.
In F4969::nanR, the group II intron was inserted into the nanR target gene in a sense orientation. Therefore, to help rule out effects from nonspecific secondary mutations, the pJIR750nanRi plasmid, which encodes LtrA, was transformed into the nanR null mutant to create a reversed mutant named F4969nanRrev. As demonstrated for other C. perfringens mutants with genes disrupted by sense-oriented introns (17, 34), growth of this reversed mutant at 30°C would allow the LtrA protein to remove the intron from some copies of nanR mRNA, thus partially restoring NanR production.
Therefore, F4969, F4969::nanR, and F4969nanRrev were compared based on their nanR expression levels at 30°C by nanR RT-PCR. As a control for RNA quality, Fig. 5C shows that all three strains expressed 16S rRNA. These RT-PCR analyses demonstrated that wild-type F4969 and the isogenic reversed mutant expressed wild-type nanR transcripts, although (as expected) the nanR expression level in the reversed mutant was lower (∼70%) than that in the wild-type strain. Notably, no nanR expression was detected for the nanR null mutant strain. It is also noteworthy that no products were amplified without the addition of reverse transcriptase, confirming that the RNA preparations were not contaminated with DNA.
Wild-type F4969, the nanR null mutant, and the reversed mutant all grew similarly in TH medium at 30°C (Fig. 5D). Measuring sialidase activity in the ∼20-h culture supernatants revealed that relative to that of wild-type F4969, sialidase activity in this rich medium (Fig. 5E) was significantly higher for the nanR null mutant but not for the reversed mutant. This result establishes that NanR can repress sialidase activity.
Supernatant sialidase activity of F4969::nanR grown in DMEM with or without d-glucose or sialic acid.
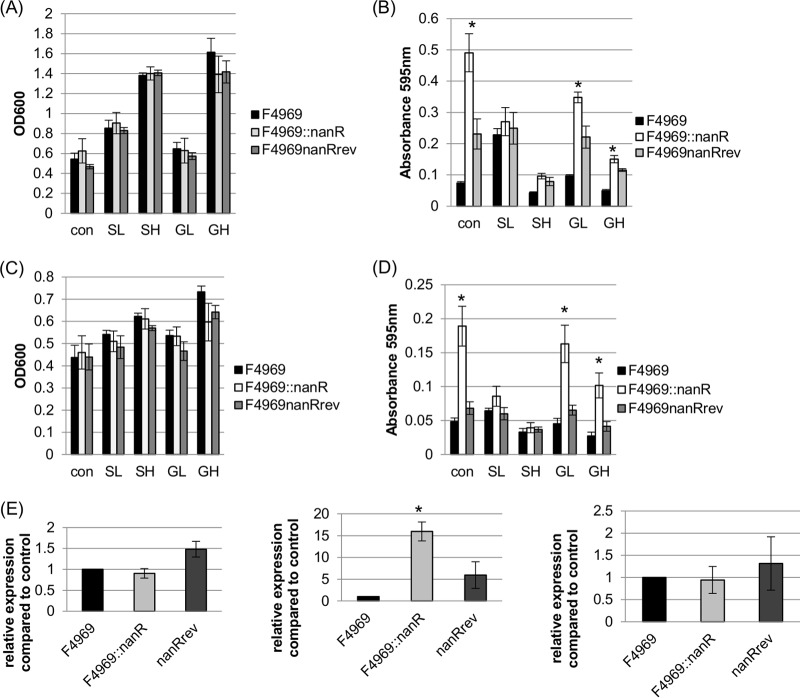
To understand if the presence of carbohydrates affects NanR regulation of nanI transcription, growth and supernatant sialidase activity were first compared for wild-type F4969 versus F4969::nanR or F4969nanRrev by using cultures grown at 30°C for 24 h in DMEM that was or was not supplemented with sialic acid or glucose. The 24-h growth yields of the three strains were similar in each medium (Fig. 6A). When supernatants from the 24-h cultures were analyzed for sialidase activity (Fig. 6B), supernatants from the nanR mutant cultured in DMEM or DMEM with glucose had more sialidase activity than that in supernatants from the F4969 culture; in contrast, supernatant sialidase activity was not significantly different between F4969 and the reversed mutant cultured in DMEM or DMEM with glucose. Significant supernatant sialidase activity differences were not detected between the three isogenic strains grown in DMEM with sialic acid.
FIG 6.
Supernatant sialidase activities and nanI transcription levels of wild-type F4969, an isogenic nanR null mutant, and the reversed mutant cultured at 30°C in DMEM with or without glucose or sialic acid. (A) Postinoculation OD600 changes for 24-h cultures of the wild-type, nanR null mutant, and reversed mutant strains of F4969 grown in control DMEM, DMEM with 1.6 mM sialic acid (SL), DMEM with 1.6 mM glucose (GL), DMEM with 16 mM sialic acid (SH), or DMEM-with 16 mM glucose (GH). (B) Supernatant sialidase activities in supernatants of the cultures used for panel A. (C) Postinoculation changes in OD600 for 4-h cultures of the wild-type, nanR null mutant, and reversed mutant strains of F4969 grown in control DMEM, DMEM-SL (SL), DMEM-GL (GL), DMEM-SH (SH), or DMEM-GH (GH). (D) Exosialidase activity detection in the supernatants of the cultures used for panel C. (E) qRT-PCR analyses of expression of the nanJ gene (left), the nanI gene (middle), and the nanH gene (right) in control DMEM cultures. All RNA samples were isolated from cells grown for 4 h at 30°C. Transcript levels were determined using 20 ng of RNA. Average CT values were normalized to that of the housekeeping 16S rRNA gene, and the fold differences were calculated using the comparative CT method (2−ΔΔCT). The value of each bar indicates the calculated fold change relative to the control. All panels show mean values for three independent experiments. The error bars indicate standard deviations. *, P < 0.05 compared to the control by ordinary one-way ANOVA.
To further evaluate the contributions of NanR to the regulation of exosialidase production, growth yield and exosialidase activity assays were repeated using young (4 h) cultures grown at 30°C in DMEM with or without sialic acid or glucose. Growth levels were similar among the wild-type, nanR null mutant, and reversed mutant strains cultured in all media (Fig. 6C). Again, the results indicated that supernatants from the nanR mutant cultured for 4 h in DMEM, DMEM-GL, or DMEM-GH had significantly more sialidase activity than that from equivalently grown cultures of F4969. However, after 4 h of growth, there was no significant difference between supernatant sialidase activities of F4969 and the reversed mutant. Importantly, there were no significant differences in exosialidase activity between cultures of F4969, the nanR null mutant, and the reversed mutant grown for 4 h in DMEM supplemented with Neu5Ac (Fig. 6D).
To determine which sialidase genes are regulated by NanR, qRT-PCR was performed (Fig. 6E) to evaluate nanJ, nanI, and nanH expression levels by the three isogenic strains grown in control DMEM, as a representative medium in which exosialidase activity is repressed by NanR in wild-type F4969 (Fig. 6B and D). The results of this assay indicated that only nanI gene transcription levels were significantly increased in the nanR null mutant strain compared to those in wild-type F4969. By comparison, under these growth conditions, no significant differences in nanI expression levels were detected between F4969 and the reversed mutant. Expression levels of the nanJ and nanH genes were not significantly different between wild-type F4969, F4969::nanR, and the reversed mutant cultured in control DMEM (Fig. 6E).
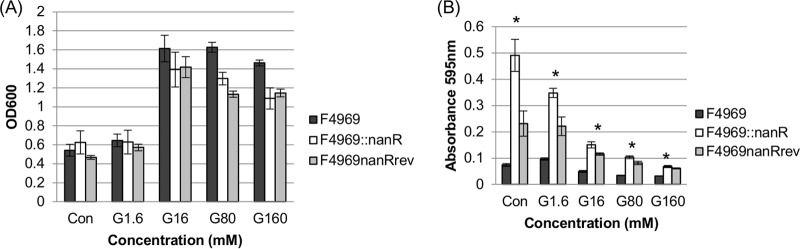
Since the nanR null mutant produced significantly higher supernatant sialidase activity than that of F4969 when grown in DMEM-GL versus DMEM-GH (Fig. 6), we performed an experiment to examine whether the glucose effects are dose dependent. Using even higher (up to 160 mM) concentrations of glucose added to DMEM, the 24-h growth yields at 30°C were similar for F4969, F4969::nanR, and the reversed mutant (Fig. 7A). However, supernatant sialidase activities in those cultures were increasingly repressed as glucose supplementation increased, even for the nanR null mutant (Fig. 7B). Using 160 mM glucose supplementation, little or no supernatant sialidase activity remained among the three strains. These results indicate that another repressor besides NanR is involved in control of sialidase expression in very glucose-rich nutrient conditions.
FIG 7.
Measurement of culture growth yields and supernatant sialidase activities of F4969, the isogenic nanR null mutant, and the reversed mutant grown for 24 h at 30°C in DMEM without (Con) or with d-glucose (1.6 mM, 16 mM, 80 mM, and 160 mM [G1.6, G16, G80, and G160, respectively]). (A) Growth yields in 24-h cultures grown in the specified media. (B) Sialidase activities in supernatants of the cultures used for panel A. The data shown in both panels are mean values for three independent experiments. The error bars indicate standard deviations. *, P < 0.05 compared to the control by ordinary one-way ANOVA.
DISCUSSION
C. perfringens intestinal diseases in humans are not intoxications but true infections involving in vivo growth (35), so the current results demonstrating that human enteropathogenic strains of this bacterium can use sialic acid as well as glucose for growth have potential pathogenic relevance. Glucose levels in the lumen of the mammalian small intestine vary greatly, ranging from 0.2 mM up to 48 mM (28), supporting the physiologic relevance of our findings. While free glucose is limited in the large intestine due to its absorption in the small intestine and utilization by the normal colonic microbiota, the intestines are rich in another potential nutrient, the sialic acids. An especially prevalent sialic acid is Neu5Ac, which accounts for 32 to 59% of all sialic acids in the human gut due to its presence on mucin proteins, which are abundant in intestinal mucus (18). By determining that most or all human enteropathogenic strains of C. perfringens can use free Neu5Ac for growth, the current results are in agreement with similar findings for the nonenteropathogenic C. perfringens strain 13 (16) and are consistent with the presence of the Nan system, which encodes sialic acid transporters and metabolic enzymes, in all genome-sequenced strains of C. perfringens, including F4969 (16, 36, 37).
Since intestinal sialic acid is bound to macromolecules, such as mucins, producing a sialidase may be helpful for intestinal growth because it can liberate free sialic acid for uptake and metabolism. However, some intestinal pathogens, e.g., Clostridium difficile and Salmonella enterica serovar Typhimurium, do not produce sialidases yet utilize sialic acids generated by the action of sialidases produced by other intestinal bacteria (38, 39). Similarly, NanI-negative strains of C. perfringens might utilize sialic acids generated by other normal intestinal microbiota. This would not exclude contributions by NanI, when produced, to C. perfringens intestinal growth and colonization, particularly during disease. While acute intestinal disease is associated with NanI-negative strains, chronic disease is typically caused by NanI-positive strains of this bacterium (13). These associations may suggest that, during chronic disease, NanI locally generates sialic acids for C. perfringens growth in an intestinal environment where chronic diarrhea has reduced the luminal levels of free glucose and sialidases made by normal microbiota organisms. Potential growth promotion during chronic disease may be another potential contribution of NanI to intestinal virulence, along with promoting adhesion and (at least sometimes) increased toxin binding (11, 13).
Despite the potential pathogenic importance of exosialidases, especially NanI, the regulation of exosialidase activity by C. perfringens has been poorly understood. End products often regulate enzyme expression, so the current study first examined whether sialic acid affects sialidase production levels. Confirming a previous report (16), the current study first determined that supernatant sialidase activity in C. perfringens cultures can be induced by the presence of a low sialic acid concentration (16). Importantly, this study then presented the first evidence that exosialidase activity in C. perfringens cultures is also strongly influenced by high sialic acid concentrations. Specifically, exosialidase activity is repressed by a high sialic acid concentration, mainly due to decreased nanI expression. This pattern of sialic acid regulation of exosialidase activity should be beneficial for C. perfringens intestinal strains. Generation of low sialic acid concentrations by basal levels of exosialidases may initially signal to these bacteria that sialic acid-containing glycoconjugates are nearby, making it beneficial to increase exosialidase (mainly NanI) production. However, as local sialic acid concentrations increase due to the action of those exosialidases, C. perfringens will stop producing the exosialidases that are no longer necessary.
Glucose levels can also affect enzyme production via processes such as catabolite repression. Therefore, the current study also evaluated the effects of glucose on exosialidase production. Unlike low sialic acid concentrations, low glucose concentrations were shown to have limited effects on exosialidase activity, revealing that C. perfringens can distinguish between the presence of sialic acids and glucose for regulating exosialidase production. In contrast, high glucose concentrations were found to repress exosialidase activity. This repression will allow C. perfringens to stop producing exosialidases when sufficient concentrations of a directly utilizable substrate, such as glucose, are available, i.e., exosialidase production is regulated by catabolite repression. Using qRT-PCR assays of nanI, nanJ, and nanH transcription, together with nanI null mutant strains, the current study then demonstrated that low sialic acid concentrations primarily increase, but high sialic acid or glucose concentrations primarily decrease, expression of the nanI gene, which encodes the major exosialidase of most C. perfringens strains, including F4969 (13). Since nanI expression and exosialidase activity are induced by low sialic acid concentrations but repressed by high glucose concentrations, the current study then examined the effects on exosialidase activity when F4969 is cultured in the presence of both low sialic acid and high glucose concentrations. The results show that C. perfringens supernatant sialidase activity, and nanI expression specifically, remain repressed even in the presence of a low sialic acid concentration. This dominance of glucose-induced repression further supports the hypothesis that nanI expression is subject to catabolite repression.
NanR is a member of the RpiR transcriptional regulator family, which includes regulators of sialic acid metabolism in S. aureus, S. pneumoniae, and V. vulnificus (30–32). It was recently suggested that, as in those other bacteria, NanR may repress NanI production and expression of proteins involved in sialic acid metabolism when C. perfringens is grown in the absence of sialic acid (16). Therefore, a major contribution of the current study was testing that proposal by inactivating the nanR gene in C. perfringens strain F4969. Compared to wild-type F4969, the isogenic nanR null mutant produced significantly more supernatant sialidase activity in rich TH medium, and this difference was attenuated when the mutation was reversed. These results provide the first direct evidence that NanR regulates C. perfringens supernatant sialidase activity. Comparison of supernatant sialidase activities of F4969 and the nanR null mutant cultured in control DMEM versus DMEM supplemented with glucose or sialic acid then revealed that NanR represses supernatant sialidase activity in the absence of sialic acid. However, the supernatant sialidase activity of the nanR null mutant remained low in the presence of high glucose concentrations, similar to that observed for wild-type F4969.
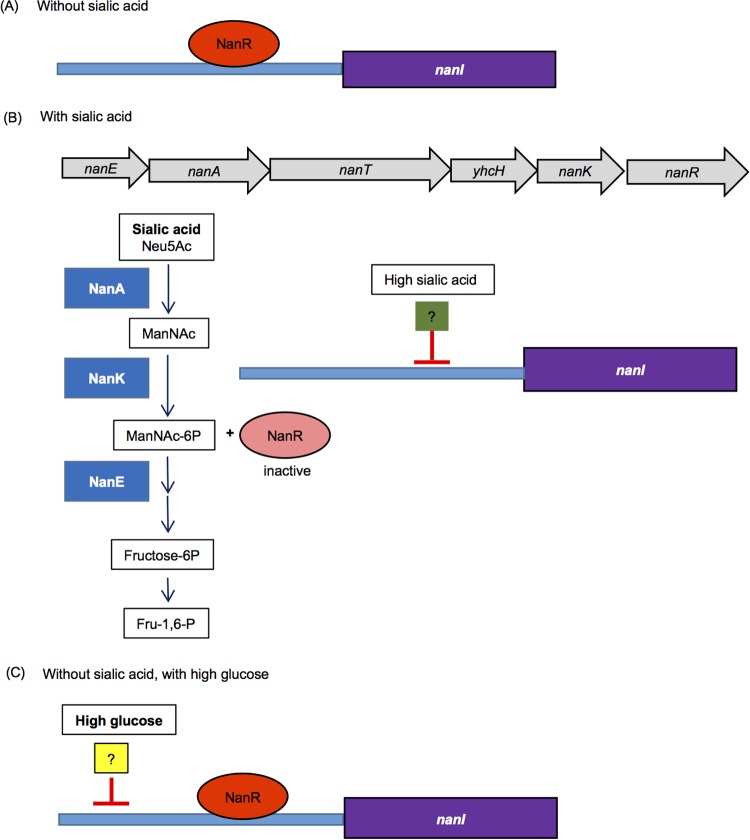
Based on these and previous (16) findings, an updated model can be proposed for C. perfringens nanI gene regulation. In the absence of sialic acid (Fig. 8A), NanR represses nanI expression. The nanI promoter region has six NanR binding sites (16), so this repression likely involves NanR binding to some or all of those sites in the nanI promoter. Similar NanR binding sites are also located near the nan operon (16), so NanR may also reduce expression of this operon in the absence of sialic acid, but this requires experimental verification.
FIG 8.
Updated model for regulation of expression of the C. perfringens nanI sialidase gene. (A) In the absence of sialic acid, NanR represses nanI expression. (B) In the presence of sialic acid, ManNAc-6P is generated from enzymes (blue boxes) encoded by the nan operon (gray arrows); ManNAc-6P then binds to and inactivates NanR to relieve NanR-mediated repression of nanI expression. This leads to strong nanI expression in the presence of low sialic acid levels. However, in the presence of high sialic acid levels, another, unidentified regulator (green box) also represses nanI expression. (C) In the absence of sialic acid but the presence of high glucose levels, both NanR and a second (unidentified) regulator (yellow box), possibly the same non-NanR regulator shown in panel B, repress nanI expression. The second repressor also functions in the presence of high glucose levels but low sialic acid levels.
However, when it is present (Fig. 8B), sialic acid is metabolized by C. perfringens to generate ManNAc-6P, which has been shown for NanR orthologs in other bacteria (40, 41) to displace NanR bound to promoters. This effect likely removes NanR repression of nanI (and possibly nan operon) expression. Our work reveals that another (still unidentified) regulator can also control nanI expression in the presence of sialic acid, i.e., this second (not NanR) repressor reduces nanI expression in the presence of high sialic acid concentrations.
The observed reduction in nanI expression in the presence of high glucose concentrations (Fig. 8C) also involves a repressor other than NanR, since (i) in a nanR null mutant, exosialidase activity is still reduced in the presence of high glucose levels, and (ii) exosialidase activity of wild-type F4969 is repressed by high glucose even when low concentrations of sialic acid are present. Whether this is the same non-NanR repressor that inhibits nanI expression in the presence of high sialic acid concentrations remains to be determined. Since NanI can affect ccpA and codY expression levels (17), the CcpA and CodY regulatory proteins are candidates for non-NanR regulators of nanI expression. In support of this possibility, putative CcpA and CodY binding boxes are located upstream of the nanI ORF. Future studies will investigate whether these two master regulatory proteins are involved in controlling nanI expression in order to more thoroughly understand how C. perfringens regulates NanI production.
MATERIALS AND METHODS
Chemicals, bacterial strains, and growth conditions.
d-Glucose and N-acetylneuraminic acid (Neu5Ac) (synthetic sialic acid; ≥95%) were purchased from Sigma-Aldrich. 5-Bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid sodium salt was purchased from Santa Cruz. Oxyrase for broth (OB; Oxyrase, Inc.) and all antibiotics used in this study were purchased from Fisher Scientific Company.
The previously characterized wild-type C. perfringens intestinal strains used in this study are listed in Table 1 (13). Of those 15 strains, 8 are nanI positive and 7 are nanI negative (Table 1). An F4969 nanI null mutant and complemented strain were prepared previously (13). All C. perfringens strains were stored as stock cultures in cooked meat medium (Oxford) at −20°C.
Rich media used for culture of C. perfringens included FTG medium (fluid thioglycolate; Difco Laboratories), TH medium (Bacto Todd-Hewitt broth [Becton-Dickinson] with 0.1% sodium thioglycolate [Sigma-Aldrich]), TGY medium (3% tryptic soy broth [Becton-Dickinson], 2% glucose [Fisher Scientific], 1% yeast extract [Becton-Dickinson], and 0.1% sodium thioglycolate [Sigma-Aldrich]), and brain heart infusion (BHI) agar plates (Becton-Dickinson).
To assess C. perfringens growth in carbohydrate-free medium, DMEM without glucose, l-glutamine, phenol red, sodium pyruvate, and sodium bicarbonate (Sigma-Aldrich) was used after the addition of glutamine and nonessential amino acids. DMEM-SL refers to DMEM supplemented with 1.6 mM sialic acid, while DMEM-SH refers to DMEM supplemented with 16 mM sialic acid. Similarly, DMEM-GL is DMEM supplemented with 1.6 mM d-glucose and DMEM-GH is DMEM supplemented with 16 mM d-glucose. Other sialic acid or d-glucose concentration supplements of DMEM are indicated for specific experiments. All DMEM types were supplemented with 10% Oxyrase; once Oxyrase was added, the media were preincubated for 30 min at 37°C to create anaerobic conditions.
Escherichia coli DH5α cells (New England BioLabs) were used as the cloning host and cultured in Luria-Bertani (LB) broth (1% tryptone [Becton-Dickinson], 0.5% yeast extract [Becton-Dickinson], 1% NaCl [Fisher Scientific]) and on LB agar (1.5% agar [Becton-Dickinson]).
Plasmids and primers.
A plasmid named pJIR750nanRi was constructed to prepare an F4969 nanR (AC5_0235) null mutant via the Clostridium-modified TargeTron gene knockout system (33). The primers used for intron targeting of the nanR gene were nanR-546∣547s-IBS primer (5′-AAAAAAGCTTATAATTATCCTTAGATGTCATAGTTGTGCGCCCAGATAGGGTG-3′), nanR-546∣547s-EBS1d primer (5′-CAGATTGTACAAATGTGGTGATAACAGATAAGTCATAGTTGCTAACTTACCTTTCTTTGT-3′), and nanR-546∣547s-EBS2 primer (5′-TGAACGCAAGTTTCTAATTTCGATTACATCTCGATAGAGGAAAGTGTCT-3′). The 350-bp intron PCR product was then inserted into pJIR750ai (33) between the HindIII and BsrGI enzyme sites to construct the pJIR750nanRi vector. The nanR null mutant screening primers were nanRKOF (5′-GGTATAGGTTATTCAGGAATAGCTG-3′) and nanRKOR (5′-TCAGCTGATGTATATGTCAATTCAT-3′).
The qRT-PCR primers used in this study were designed using the Integrated DNA Technologies (IDT) website. These primers were specific for the 16S rRNA gene (as a control housekeeping gene) (11), the nanJ gene (forward, 5′-TGACAGCTAGTGCAACTTCAG-3′; and reverse, 5′-CGCATCACTCCCATTCCAT-3′), the nanI gene (forward, 5′-TGATGCACCTCAATTAACTGAATG-3′; and reverse, 5′-CTTTCTCTACTGTCTCATCCCAAG), and the nanH gene (forward, 5′-TGCAGGCTCATGGAATACAA-3′; and reverse, 5′-TGGACAGACCAATCACTTCTTC-3′).
Construction of an F4969 nanR null mutant and reversed nanR mutant.
The nanR gene in F4969 was inactivated by insertion of a targeted group II intron. The intron insertion was targeted between nucleotides 546 and 547 of the nanR ORF. To construct this mutant, plasmid pJIR750nanRi was electroporated into wild-type F4969. The F4969 nanR null mutant (named F4969::nanR) was selected by using BHI agar plates containing 15 mg/liter of chloramphenicol. The PCR primers nanRKOF and nanRKOR were used to PCR screen the F4969 nanR null mutant (see Results). After reintroducing the pJIR750nanRi plasmid into F4969::nanR, the resultant transformant was used as a nanR reversed mutant, which was named F4969nanRrev. Growth at 30°C partially reversed the intron insertion in the nanR gene of the F4969nanRrev strain by causing Ltr-mediated splicing removal of the intron at the transcriptional level, as noted with other reversed C. perfringens mutants (17, 34). The F4969nanRrev strain was confirmed by PCR and RT-PCR as described below.
Measurement of C. perfringens growth and supernatant sialidase activity.
For analysis of C. perfringens growth in rich medium, a 0.2-ml aliquot of an overnight FTG culture was inoculated into 10 ml of TH medium at 37°C. On the next day, a 0.2-ml aliquot of that overnight culture was transferred to 10 ml of fresh TH medium, and that TH culture was incubated for ∼16 h at 37°C. An aliquot (0.2 ml) of the overnight culture was then inoculated into 10 ml of fresh TH medium, and a 1-ml sample was removed every 2 h (for up to 8 h) for measurement of the optical density at 600 nm (OD600), using a Bio-Rad Smartspec microplate reader. Supernatants from the same, ∼16-h overnight TH cultures were also tested for sialidase activity. For this purpose, a 20-μl aliquot of supernatant was added to 40 μl of 0.05 M Tris-HCl buffer (pH 7.2) in a microtiter plate. After a 40-μl aliquot of substrate (4 mM 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid) was added, the mixture was incubated at 37°C for 30 min. The absorbance at 595 nm was then measured using a Bio-Rad microplate reader.
For analysis of C. perfringens growth in DMEM supplemented with various sialic acid or glucose concentrations, a 0.2-ml aliquot of an overnight FTG culture was inoculated into 10 ml of TGY medium at 37°C. The next day, a 0.2-ml aliquot of the overnight culture was transferred to 10 ml of fresh TGY medium, and that culture was incubated overnight, for ∼16 h, at 37°C. Forty-microliter aliquots of TGY overnight cultures were then inoculated into multiple 1-ml wells containing DMEM that was or was not supplemented with different concentrations of sialic acid or d-glucose, and those cultures were incubated at 37°C. At intervals of up to 24 h, the OD600 of the cultures were measured. Supernatants from the cultures were also evaluated for sialidase activity. For this purpose, a 60-μl aliquot of supernatant was added to a 40-μl aliquot of substrate (4 mM 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid), and the mixture was incubated at 37°C for 30 min. The absorbance at 595 nm was then measured using a Bio-Rad microplate reader.
For testing of the F4969 nanR reversed mutant, all media and procedures used were the same, except that the culture temperature was 30°C to facilitate LtrA-mediated splicing removal of the intron from nanR mRNA (34), which restored partial nanR expression to the mutant. In comparison studies against the reversed mutant, F4969 and the isogenic nanR mutant were also grown at 30°C.
DNA isolation, PCR, and Southern blot analysis.
As described previously (11), DNAs were isolated from C. perfringens strains by use of a MasterPure Gram-positive DNA purification kit (Epicentre), using pelleted cells from 3-ml aliquots of 16-h overnight TGY cultures. PCRs were performed using the following amplification program: cycle 1, 95°C for 5 min; cycles 2 through 35, 95°C for 30 s, 55°C for 40 s, and 68°C for 80 s; and a final extension cycle of 5 min at 68°C. An aliquot (20 μl) of each PCR sample was then electrophoresed in a 1.5% agarose gel and visualized by staining with ethidium bromide.
To confirm that a single intron was inserted in the F4969 nanR null mutant, an intron-specific digoxigenin (DIG)-labeled probe was used in a Southern blot analysis with DNA isolated from wild-type F4969 or F4969::nanR. Purified DNA (3 μg) from each strain was digested with EcoRI overnight at 37°C, according to the manufacturer's instructions (New England BioLabs). Digested DNA was then electrophoresed in a 1% agarose gel. Using alkali transfer, the DNA on the gel was transferred to a nylon membrane (Roche), and the blot was hybridized with a DIG-labeled, intron-specific probe. CSPD substrate (Roche Applied Science) was used for detection of the DIG-labeled hybridized intron probe according to the manufacturer's instructions, as described previously (11).
RNA extraction, RT-PCR, and qRT-PCR.
Total RNAs were extracted from pelleted cells of 4-h DMEM or TH cultures of specified strains by using saturated phenol (Fisher Scientific) with TRIzol (Life Technologies). Briefly, after the RNA was extracted with saturated phenol, supernatants from the extractions were twice treated with TRIzol and then extracted with chloroform. Purified RNA (free of DNA contamination) was then quantified by determining the absorbance at 260 nm and was stored in a −80°C freezer.
RT-PCR analysis of NanR expression was performed with the AccessQuick RT-PCR system (Promega), using 20-μl reaction volumes that contained 20 ng of purified RNA samples and primers nanRKOF and nanRKOR, which hybridize to sequences flanking the nanR intron insertion site. RT-PCR analysis of the 16S rRNA gene served as a positive control. For qRT-PCR, an aliquot (1 μg) of RNA was first reverse transcribed to cDNA by use of a Thermo Scientific Maxima First Strand cDNA synthesis kit according to the manufacturer's instructions. This cDNA was diluted 10 times, and qRT-PCR was performed using a StepOnePlus qRT-PCR instrument (Applied Biosystems). Power SYBR Green PCR master mix (Thermo Fisher Scientific) was used for this reaction. The reaction conditions were 1 cycle at 95°C for 5 min and 40 cycles of 95°C for 15 s and 60°C for 1 min. After qRT-PCR, quantitation of mRNA expression was normalized to the constitutive expression of the housekeeping 16S rRNA gene and calculated by the comparative threshold cycle method (2−ΔΔCT).
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 6. For more than two groups, ordinary one-way analysis of variance (ANOVA) was applied, with post hoc analysis using Dunnett's test. For comparison of two samples, Student's unpaired t test was used.
ACKNOWLEDGMENTS
This work was generously supported by grant R21 AI125796-1 (B.A.M., J.L., and Francisco Uzal) from the National Institute of Allergy and Infectious Disease.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We thank Anthony Richardson for helpful discussions during this work.
REFERENCES
- 1.Petit L, Gilbert M, Popoff M. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol 7:104–110. doi: 10.1016/S0966-842X(98)01430-9. [DOI] [PubMed] [Google Scholar]
- 2.Uzal FA, Freedman JC, Shrestha A, Theoret JR, Garcia J, Awad MM, Adams V, Moore RJ, Rood JI, McClane BA. 2014. Towards an understanding of the role of Clostridium perfringens toxins in human and animal disease. Future Microbiol 9:361–377. doi: 10.2217/fmb.13.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2011. CDC estimates of foodborne illness in the United States: Clostridium perfringens. CDC, Atlanta, GA: http://www.cdc.gov/foodborneburden/clostridium-perfringens.html. [Google Scholar]
- 4.Carman RJ. 1997. Clostridium perfringens in spontaneous and antibiotic-associated diarrhoea of man and other animals. Rev Med Microbiol 8(Suppl 1):S43–S45. doi: 10.1097/00013542-199712001-00023. [DOI] [Google Scholar]
- 5.Lawrence GW. 1997. The pathogenesis of enteritis necroticans, p 198–207. In Rood JI, McClane BA, Songer JG, Titball RW (ed), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom. [Google Scholar]
- 6.Ma M, Gurjar A, Theoret JR, Garcia JP, Beingesser J, Freedman JC, Fisher DJ, McClane BA, Uzal FA. 2014. Synergistic effects of Clostridium perfringens enterotoxin and beta toxin in rabbit small intestinal loops. Infect Immun 82:2958–2970. doi: 10.1128/IAI.01848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma M, Li J, McClane BA. 2012. Genotypic and phenotypic characterization of Clostridium perfringens isolates from Darmbrand cases in post-World War II Germany. Infect Immun 80:4354–4363. doi: 10.1128/IAI.00818-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawrence G. 2005. The pathogenesis of pig-bel in Papua New Guinea. 1979. P N G Med J 48:39–49. [PubMed] [Google Scholar]
- 9.Johnson S, Gerding DN. 1997. Enterotoxemic infections, p 117–140. In Rood JI, McClane BA, Songer JG, Titball RW (ed), The clostridia: molecular biology and pathogenesis. Academic Press, London, United Kingdom. [Google Scholar]
- 10.Petrillo TM, Beck-Sague CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, Dean AG, Lee H, Bueschel DM, Nesheim SR. 2000. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med 342:1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Sayeed S, Robertson S, Chen J, McClane BA. 2011. Sialidases affect the host cell adherence and epsilon toxin-induced cytotoxicity of Clostridium perfringens type D strain CN3718. PLoS Pathog 7:e1002429. doi: 10.1371/journal.ppat.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, McClane BA. 2014. The sialidases of Clostridium perfringens type D strain CN3718 differ in their properties and sensitivities to inhibitors. Appl Environ Microbiol 80:1701–1709. doi: 10.1128/AEM.03440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, McClane BA. 2014. Contributions of NanI sialidase to Caco-2 cell adherence by Clostridium perfringens type A and C strains causing human intestinal disease. Infect Immun 82:4620–4630. doi: 10.1128/IAI.02322-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Uzal FA, McClane BA. 2016. Clostridium perfringens sialidases: potential contributors to intestinal pathogenesis and therapeutic targets. Toxins (Basel) 8:E341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiarezza M, Lyras D, Pidot SJ, Flore-Diaz M, Awad MM, Kennedy CL, Cordner LM, Phumoonna T, Poon R, Hughes ML, Emmins JJ, Alape-Giron A, Rood JI. 2009. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence. Infect Immun 77:4421–4428. doi: 10.1128/IAI.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Therit B, Cheung JK, Rood JI, Melville SB. 2015. NanR, a transcriptional regulator that binds to the promoters of genes involved in sialic acid metabolism in the anaerobic pathogen Clostridium perfringens. PLoS One 10:e0133217. doi: 10.1371/journal.pone.0133217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Freedman JC, McClane BA. 2015. NanI sialidase, CcpA, and CodY work together to regulate epsilon toxin production by Clostridium perfringens type D strain CN3718. J Bacteriol 197:3339–3353. doi: 10.1128/JB.00349-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tailford LE, Crost EH, Kavanaugh D, Juge N. 2015. Mucin glycan foraging in the human gut microbiome. Front Genet 6:81. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Severi E, Hood DW, Thomas GH. 2007. Sialic acid utilization by bacterial pathogens. Microbiology 153:2817–2822. doi: 10.1099/mic.0.2007/009480-0. [DOI] [PubMed] [Google Scholar]
- 20.Juge N, Taiford L, Owen CD. 2016. Sialidases from gut bacteria: a mini-review. Biochem Soc Trans 44:166–175. doi: 10.1042/BST20150226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nees S, Schauer R. 1974. Induction of neuraminidase from Clostridium perfringens and the cooperation of this enzyme with acylneuraminate pyruvate lyses. Behring Inst Mitt 55:68–78. [Google Scholar]
- 22.Vimr ER, Kalivada KA, Deszo EL, Steenbergen SM. 2004. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev 68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walters DM, Stirewalt VL, Melville SB. 1999. Cloning, sequence, and transcriptional regulation of the operon encoding a putative N-acetyl-mannosamine-6-phosphate epimerase (nanE) and sialic acid lyase (nanA) in Clostridium perfringens. J Bacteriol 181:4526–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtani K, Hirakawa H, Tashiro K, Yoshizawa S, Kuhara S, Shimizu T. 2010. Identification of a two-component VirR/VirS regulon in Clostridium perfringens. Anaerobe 16:258–264. doi: 10.1016/j.anaerobe.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Hiscox TJ, Harrison PF, Chakravorty A, Choo JM, Ohtani K, Shimizu T, Cheung JK, Rood JI. 2013. Regulation of sialidase production in Clostridium perfringens by the orphan sensor histidine kinase ReeS. PLoS One 8:e73525. doi: 10.1371/journal.pone.0073525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiscox TJ, Chakravorty A, Choo JM, Ohtani K, Shimizu T, Cheung JK, Rood JI. 2011. Regulation of virulence by the RevR response regulator in Clostridium perfringens. Infect Immun 79:2145–2153. doi: 10.1128/IAI.00060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Ma M, Sarker MR, McClane BA. 2013. CodY is a global regulator of virulence-associated properties for Clostridium perfringens type D strain CN3718. mBio 4:e00770-13. doi: 10.1128/mBio.00770-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraris RP, Yasharpour S, Lloyd KC, Mirzayan R, Diamond JM. 1990. Luminal glucose concentrations in the gut under normal conditions. Am J Physiol 259:G822–G837. [DOI] [PubMed] [Google Scholar]
- 29.Almagro-Moreno S, Boyd EF. 2010. Bacterial catabolism of nonulosonic (sialic) acid and fitness in the gut. Gut Microbes 1:45–50. doi: 10.4161/gmic.1.1.10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruteser N, Marin K, Kramer R, Thomas GH. 2012. Sialic acid utilization by the soil bacterium Corynebacterium glutamicum. FEMS Microbiol Lett 336:131–138. doi: 10.1111/j.1574-6968.2012.02663.x. [DOI] [PubMed] [Google Scholar]
- 31.Olson ME, King JM, Yahr TL, Horswill AR. 2013. Sialic acid catabolism in Staphylococcus aureus. J Bacteriol 195:1779–1788. doi: 10.1128/JB.02294-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubin JB, Kingston JJ, Chowdhury N, Boyd EF. 2012. Sialic acid catabolism and transport gene clusters are lineage specific in Vibrio vulnificus. Appl Environ Microbiol 78:3407–3415. doi: 10.1128/AEM.07395-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P. 2005. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl Environ Microbiol 71:7542–7547. doi: 10.1128/AEM.71.11.7542-7547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, Gupta P, Rood JI, McClane BA. 2008. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol 67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- 35.McClane BA, Uzal FA, Miyakawa MF, Lyerly D, Wilkins TD. 2006. The enterotoxic clostridia, p 688–752. In Dworkin M, Falkow S, Rosenburg E, Schleifer H, Stackebrandt E (ed), The prokaryotes, 3rd ed Springer Press, New York, NY. [Google Scholar]
- 36.Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, DeBoy RT, Ren Q, Varga J, Awad MM, Brinkac LM, Daugherty SC, Haft DH, Dodson RJ, Madupu R, Nelson WC, Rosovitz MJ, Sullivan SA, Khouri H, Dimitrov GI, Watkins KL, Mulligan S, Benton J, Radune D, Fisher DJ, Atkins HS, Hiscox T, Jost BH, Billington SJ, Songer JG, McClane BA, Titball RW, Rood JI, Melville SB, Paulsen IT. 2006. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res 16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimizu T, Ohtani K, Hirakawa H, Ohshima K, Yamashita A, Shiba T, Ogasawara N, Hattori M, Kuhara S, Hayashi H. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc Natl Acad Sci U S A 99:996–1001. doi: 10.1073/pnas.022493799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502:96–99. doi: 10.1038/nature12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley RE. 2014. Harnessing microbiota to kill a pathogen: the sweet tooth of Clostridium difficile. Nat Med 20:248–249. doi: 10.1038/nm.3494. [DOI] [PubMed] [Google Scholar]
- 40.Kim BS, Hwang J, Kim MH, Choi SH. 2011. Cooperative regulation of the Vibrio vulnificus nan gene cluster by NanR protein, cAMP receptor protein, and N-acetylmannosamine 6-phosphate. J Biol Chem 286:40889–40899. doi: 10.1074/jbc.M111.300988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hwang J, Kim BS, Jang SY, Lim JG, You D, Jung HS, Oh T, Lee J, Choi SH, Kim MH. 2013. Structural insights into the regulation of sialic acid catabolism by the Vibrio vulnificus transcriptional repressor NanR. Proc Natl Acad Sci U S A 110:E2829–E2837. doi: 10.1073/pnas.1302859110. [DOI] [PMC free article] [PubMed] [Google Scholar]