ABSTRACT
Exoenzyme Y (ExoY) is a type III secretion system effector found in 90% of the Pseudomonas aeruginosa isolates. Although it is known that ExoY is a soluble nucleotidyl cyclase that increases the cytoplasmic levels of nucleoside 3′,5′-cyclic monophosphates (cNMPs) to mediate endothelial Tau phosphorylation and permeability, its functional role in the innate immune response is still poorly understood. Transforming growth factor β-activated kinase 1 (TAK1) is critical for mediating Toll-like receptor (TLR) signaling and subsequent activation of NF-κB and AP-1, which are transcriptional activators of innate immunity. Here, we report that ExoY inhibits proinflammatory cytokine production through suppressing the activation of TAK1 as well as downstream NF-κB and mitogen-activated protein (MAP) kinases. Mice infected with ExoY-deficient P. aeruginosa had higher levels of tumor necrosis factor (TNF) and interleukin-6 (IL-6), more neutrophil recruitment, and a lower bacterial load in lung tissue than mice infected with wild-type P. aeruginosa. Taken together, our findings identify a previously unknown mechanism by which P. aeruginosa ExoY inhibits the host innate immune response.
KEYWORDS: nucleotidyl cyclase, ExoY, TAK1, MAPKs, NF-κB, proinflammatory cytokines
INTRODUCTION
Pseudomonas aeruginosa infection usually causes pneumonia that can progress to sepsis and acute lung injury, especially in immunocompromised patients (1–3). During infection, P. aeruginosa utilizes a type III secretion system (T3SS) to inject effector proteins directly into host cells (4, 5). Four T3SS effector proteins have been well characterized in P. aeruginosa, including exoenzymes U, T, S, and Y (5), and recently new effectors secreted by T3SS were identified (6). Exoenzyme Y (ExoY) is a soluble nucleotidyl cyclase with similarities to Bacillus anthracis edema factor (EF) and Bordetella pertussis CyaA (7, 8). Most recently, filamentous actin (F-actin) was identified as a eukaryotic cofactor that is responsible for activation of ExoY in host cells (9). Once injected into a host cell, ExoY is recruited to actin filaments (9) where it massively increases the cytoplasmic levels of cyclic GMP (cGMP) and cUMP and modestly increases the levels of cAMP and cCMP (10, 11). These intracellular signals activate protein kinase A (PKA), which increases Tau phosphorylation, causing microtubule breakdown (12, 13). In the endothelium, microtubule disassembly initiates the formation of interendothelial cell gaps and increases macromolecular permeability (13, 14). ExoY also can disrupt the actin cytoskeleton (15) and mediate bleb-niche formation (16) in epithelial cells.
Bacterial infection activates pattern recognition receptors (PRRs), including Toll-like receptor (TLR) signaling pathways (17). Upon recognition of a variety of molecular patterns specific for bacterial pathogens, TLRs recruit adaptor proteins such as MyD88, interleukin-1 (IL-1) receptor-associated kinase (IRAK), and tumor necrosis factor (TNF) receptor-associated factor 6 (TRAF6) (17). Recruitment of these adaptors in turn triggers the activation of nuclear factor kappaB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling cascades, including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38. Activation of these signaling pathways leads to the biosynthesis of a group of immunoregulatory molecules such as TNF, IL-6, and arachidonic acid metabolites. Recent reports indicate that P. aeruginosa induces lung inflammation via a TLR4- or TLR5-dependent process, and recognition of P. aeruginosa-associated lipopolysaccharide (LPS) or flagellin by TLRs is necessary for induction of Tnf and Il6 (18, 19).
Transforming growth factor β (TGF-β)-activated kinase 1 (TAK1, Map3k7) is a member of the MAPK kinase kinase family and was originally identified as a key regulator of MAPK activation in TGF-β-induced signaling pathways (20). Later, TAK1 was found to play a key role in the cellular response to a variety of stimuli (21–23). TAK1-deficient cells fail to activate transcription factor NF-κB and MAPK in response to IL-1β, TNF, and TLR ligands (24–26).
Much progress has been made to characterize ExoY in terms of the generation of nucleoside 3′,5′-cyclic monophosphates (cNMPs) and the regulation of integrity of the endothelial cell barrier. However, relatively little is known about the role of ExoY in the regulation of host innate immune responses. In this study, we demonstrate that ExoY inhibits TLR signaling pathways by downregulating the activation of TAK1.
RESULTS
ExoY inhibits cytokine production in epithelial cells.
Airway epithelial cells play an important role in sensing and signal transduction during P. aeruginosa infection (27–29). We examined the nucleotidyl cyclase activity of ExoY by measuring the abundance of cAMP in P. aeruginosa PAO1- or PAO1 ΔexoY-infected A549 cells. We observed that a deficiency in ExoY yielded almost complete blockage of cAMP generation in response to P. aeruginosa infection (see Fig. S1A in the supplemental material), suggesting that ExoY from PAO1 possesses nucleotidyl cyclase activity.
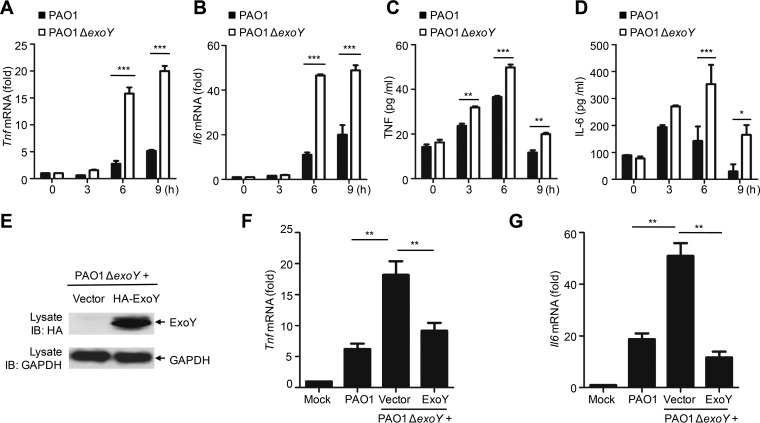
Investigators have shown previously that RAW264.7 macrophages (30) or Chinese hamster ovary (CHO) cells (31) infected with exoY deletion strains of P. aeruginosa released lactose dehydrogenase (LDH) at levels similar to those of cells infected with wild-type P. aeruginosa. In accordance with these results, we found that A549 cells infected with ExoY-deficient PAO1 had death rates similar to those of cells infected with wild-type strains, as measured by LDH release assay (Fig. S1B). However, cells infected with PAO1 ΔexoY exhibited higher mRNA and protein levels of Tnf and Il6 than cells infected with PAO1 (Fig. 1A to D). Similarly, infection of A549 cells with two other P. aeruginosa strains (PAK and PA388) in which ExoY had been deleted significantly enhanced mRNA expression of Tnf and Il6 (Fig. S2). Complementation of PAO1 ΔexoY with pUCPexoY was sufficient to restore inhibition of Tnf and Il6 expression (Fig. 1E to G).
FIG 1.
ExoY inhibits cytokine production in epithelial cells. (A and B) qPCR analysis of Tnf or Il6 mRNA in A549 cells infected with PAO1 or PAO1 ΔexoY for 0 to 9 h. (C and D) ELISA of TNF or IL-6 release in supernatants of A549 cells infected as described for panels A and B. (E) Immunoblot (IB) of lysates from A549 cells infected with PAO1 ΔexoY(pUCP19) or PAO1 ΔexoY(pUCPexoY) for 6 h. (F and G) qPCR analysis of Tnf or Il6 mRNA in A549 cells infected with the indicated bacteria for 6 h. Multiplicity of infection, 10 (A to G). Values are expressed as means ± standard deviations from three technical replicates (A to D, F, and G). *, P < 0.05; **, P < 0.01; ***, P < 0.001. All data shown are representative of at least three independent experiments.
The inhibitory effect of ExoY depends on nucleotidyl cyclase activity.
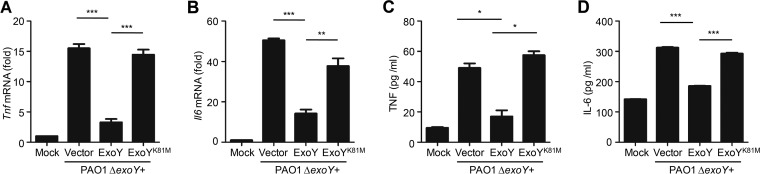
ExoY is a soluble bacterial nucleotidyl cyclase. Complementation of PAO1 ΔexoY with pUCPexoY, but not with its catalytically inactive mutant, pUCP expressing the K-to-M change at position 81 encoded by exoY (pUCPexoYK81M) (8), restored inhibition of Tnf and Il6 mRNA and protein expression in epithelial cells (Fig. 2). Further, we used pUCPexoY or pUCPexoYK81M to complement a PAK ΔexoS exoT exoY triple mutant strain and then infected A549 cells. Consistently, the PAK ΔexoS exoT exoY strain complemented with pUCPexoY but not with pUCPexoYK81M inhibited Tnf and Il6 mRNA expression in A549 cells (Fig. S3). Hence, the nucleotidyl cyclase activity of ExoY is required to suppress the host immune response.
FIG 2.
The inhibitory effect of ExoY on cytokine production is dependent on nucleotidyl cyclase activity. (A and B) qPCR analysis of Tnf or Il6 mRNA in A549 cells infected with PAO1 ΔexoY(pUCP19), PAO1ΔexoY(pUCPexoY) or PAO1 ΔexoY(pUCPexoY K81M) for 6 h. (C and D) ELISA of TNF or IL-6 release in supernatants of A549 cells infected as described for panels A and B. Multiplicity of infection, 10. All data shown are representative of at least three independent experiments, and values are expressed as mean values ± standard deviations from three technical replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ExoY inhibits cytokine production in macrophages.
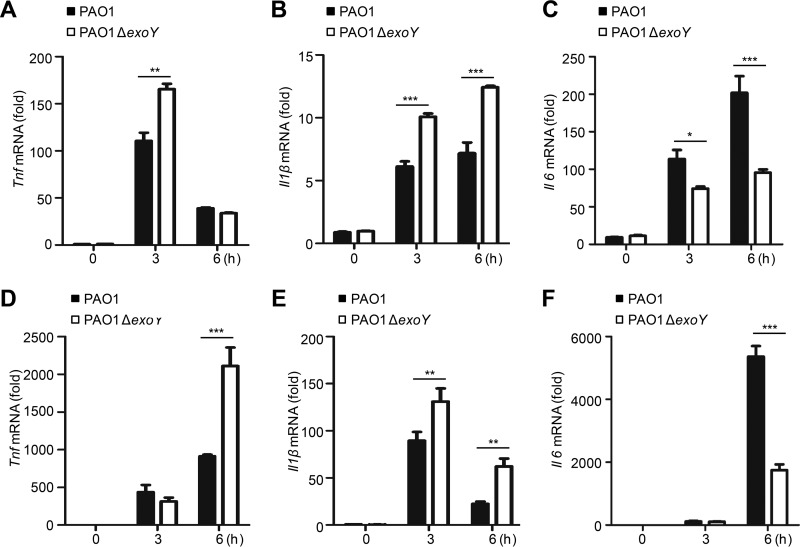
In response to P. aeruginosa infection, macrophages internalize and kill the pathogenic bacteria as well as induce the generation of proinflammatory cytokines (27, 32). To investigate the effect of ExoY on proinflammatory cytokine production in macrophages, we infected THP-1 cells (a human monocytic cell line) and mouse peritoneal macrophages with PAO1 or PAO1 ΔexoY. THP-1 cells infected with PAO1 ΔexoY exhibited higher levels of Tnf and Il1β mRNAs (Fig. 3A and B), but a lower level of Il6 mRNA (Fig. 3C), than cells infected with PAO1. Similarly, infection of mouse peritoneal macrophages by PAO1 ΔexoY resulted in higher levels of Tnf and Il1β (Fig. 3D and E) and a lower level of Il6 (Fig. 3F).
FIG 3.
ExoY inhibits cytokine production in macrophages. (A to C) qPCR analysis of Tnf, Il1β, or Il6 mRNA in THP-1 cells infected with PAO1 or PAO1 ΔexoY for 0 to 6 h. (D to F) qPCR analysis of Tnf, Il1β, or Il6 mRNA in mouse peritoneal macrophages infected as described for panels A to C. Multiplicity of infection, 10. All data shown are representative of at least three independent experiments, and values are expressed as mean values ± standard deviations from three technical replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ExoY suppresses the activation of TAK1.
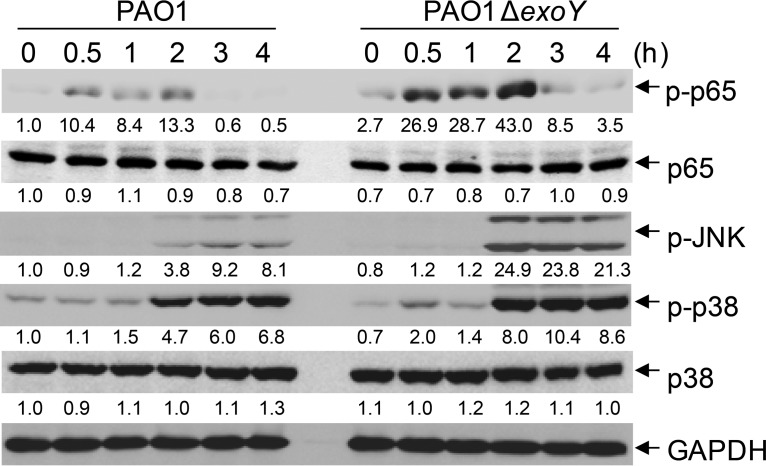
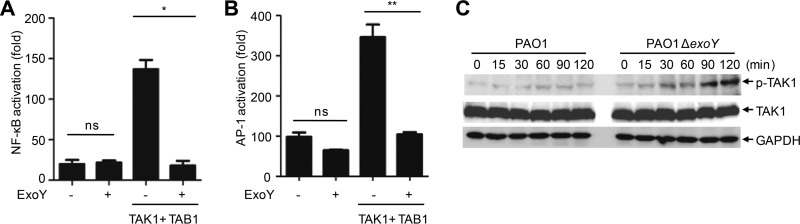
Expression of proinflammatory cytokines is induced by activation of NF-κB and MAPKs in TLR signaling pathways. Compared with A549 cells infected with PAO1, cells infected with PAO1 ΔexoY underwent high phosphorylation of p65, p38, and JNK (Fig. 4). The presence of ExoY efficiently blocked activation of NF-κB and AP-1 in HEK293T cells stimulated by coexpression of TAK1 and TAB1 in a luciferase reporter assay (Fig. 5A and B). In contrast, ExoY had no effect on TRAF6-stimulated activation of NF-κB (Fig. S4A). Infection of A549 cells with PAO1 ΔexoY also resulted in higher phosphorylation of TAK1 at Thr187 (33, 34) (Fig. 5C), indicating that ExoY disrupted activation of TAK1. In line with these results, A549 cells infected with the PAK ΔexoS exoT exoY strain complemented with pUCPexoY had lower activation of TAK1 and subsequent activation of NF-κB and MAPKs than cells infected with the PAK ΔexoS exoT exoY strain complemented with an empty vector or pUCPexoYK81M (Fig. S4B).
FIG 4.
ExoY inhibits the phosphorylation of p65, p38, and JNK. (A) Immunoblotting of lysates from A549 cells infected with PAO1 or PAO1 ΔexoY at the indicated times (multiplicity of infection, 10). Densitometry quantification (under the band) of results (ratio of the expression of the indicated protein to that of GAPDH) is presented relative to those of uninfected (0 h, PAO1) cells, set as 1. Data shown are representative of at least three independent experiments.
FIG 5.
ExoY suppresses the activation of TAK1. (A and B) Luciferase assay of NF-κB or AP-1 reporter activation in HEK293T cells transfected with various plasmids. (C) Immunoblotting of lysates from A549 cells infected with PAO1 or PAO1 ΔexoY at the indicated times (multiplicity of infection, 10). All data shown are representative of at least three independent experiments, and values are expressed as means ± standard deviations from three technical replicates. *, P < 0.05; **, P < 0.01; ns, nonsignificant.
TAK1 is essential for activation of NF-κB and MAPKs in TNF receptor (TNFR) and TLR/IL-1R signaling pathways (24, 25). To further confirm the inhibitory effect of ExoY on TAK1 activation, we transiently transfected the exoY or exoYK81M strain into HEK293T cells or A549 cells and then stimulated these cells with TNF or IL-1β, respectively. Expression of ExoY but not ExoYK81M efficiently blocked TNF- and IL-1β-stimulated TAK1 activation as well as downstream activation of NF-κB and MAPKs (Fig. S4C and D). These results indicated that ExoY had a generally inhibitory effect on TAK1 activation downstream of the TNFR and TLR/IL-1R signaling pathways.
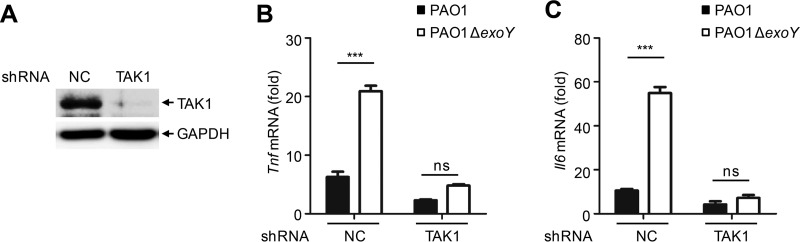
To investigate whether ExoY inhibited cytokine production through TAK1, we constructed a stable TAK1 knockdown in A549 cells via a lentiviral delivery system. Knockdown efficiency was determined by Western blotting using a TAK1-specific antibody (Fig. 6A). The production of Tnf and Il6 mRNA in TAK1 knockdown cells was significantly reduced upon PAO1 infection compared to the levels in A549 cells without the knockdown construct. Moreover, the effects of ExoY on cytokine production were absent in TAK1 knockdown cells (Fig. 6B and C). Therefore, ExoY may inhibit cytokine production through TAK1.
FIG 6.
ExoY inhibits cytokine production through TAK1. (A) Immunoblotting of lysates from TAK1 knockdown or control A549 cells. (B and C) qPCR analysis of Tnf or Il6 mRNA in control or TAK1 knockdown A549 cells infected with PAO1 or PAO1 ΔexoY for 6 h. Multiplicity of infection, 10. All data shown are representative of at least three independent experiments, and values in panels B and C are expressed as means ± standard deviations from three technical replicates. ***, P < 0.001; ns, nonsignificant. shRNA, short hairpin RNA; NC, negative control.
ExoY suppresses cytokine production and enhances P. aeruginosa pathogenicity in mice.
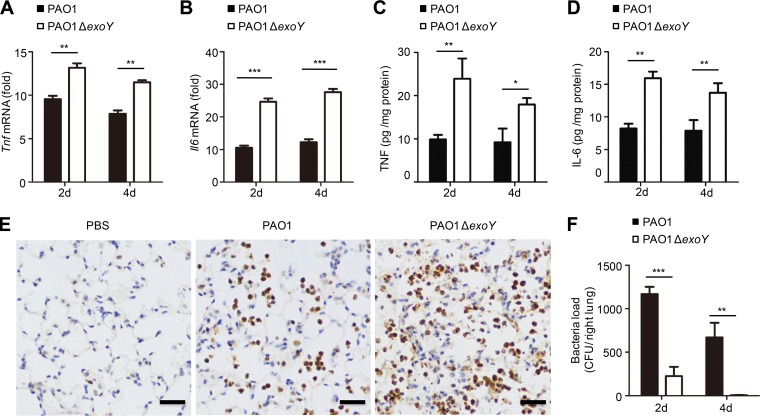
To explore the functional role of ExoY in an animal model, mice underwent intratracheal instillation of 1 × 106 CFU of PAO1 or PAO1 ΔexoY. Infection with PAO1 ΔexoY resulted in elevated levels of Tnf and Il6 in mouse lung homogenates compared to levels with PAO1 infection (Fig. 7A to D). Complementation of PAO1 ΔexoY with pUCPexoYK81M eliminated the inhibitory effect of ExoY on cytokine production in the mouse lung (Fig. S5).
FIG 7.
ExoY suppresses cytokine production and enhances P. aeruginosa pathogenicity in mice. (A and B) qPCR analysis of Tnf or Il6 mRNA in lung homogenates from mice infected with PAO1 or PAO1 ΔexoY (1 × 106 CFU) for 2 or 4 days (d) (n = 5 per group). (C and D) ELISA of TNF or IL-6 release in supernatants of mouse lung homogenates infected as described in panels A and B. (E) MPO staining of mouse lung infected with PAO1 or PAO1 ΔexoY (1 × 106 CFU) for 24 h. Scale bar, 50 μm. n = 3 per group. (F) Bacterial load in lung tissue of mice infected as described in panels A and B. All data shown are representative of three independent experiments. Values in panels A to D and F are expressed as mean values ± standard deviations from five mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Neutrophils are recruited into infected airways via inflammatory mediators during lung infection (35, 36), and they play an essential role in the clearance of P. aeruginosa from the lung (37, 38). We evaluated infiltration of neutrophils into the lung after P. aeruginosa infection by means of immunohistochemical staining of myeloperoxidase (MPO). Lung tissue infected with PAO1 ΔexoY had substantially higher neutrophil infiltration (Fig. 7E) and lower bacterial load (Fig. 7F) than lung tissue infected with PAO1. Therefore, P. aeruginosa ExoY inhibits the host immune response to allow the bacteria to establish a successful infection.
DISCUSSION
P. aeruginosa strains that harbor exoY can induce severe lung damage in the host (1). Despite considerable evidence that ExoY is an essential effector for the disassembly of microtubules (12, 13), relatively little is known about how ExoY regulates the innate inflammatory response. Here, the results obtained from our study demonstrate that ExoY disrupts activation of TAK1, thereby suppressing the production of proinflammatory cytokines during P. aeruginosa infection.
Appropriate inflammatory responses are crucial for controlling acute P. aeruginosa infection (39–41). Proinflammatory cytokines, induced following pathogen stimulation, recruit and activate factors of the acute-phase response to stimulate T and B cells for pathogen clearance (42). Inhibition of cytokine production by ExoY could block the innate and adaptive immune responses and improve the likelihood of a bacterial infection. Indeed, mice infected with PAO1 ΔexoY had a smaller bacterial load in their lungs than mice infected with PAO1. Consistently, results from a study involving a mouse model of burn injury showed that mice infected with a P. aeruginosa strain that carried a knockout of ExoY had a higher survival rate than mice infected with wild-type P. aeruginosa (strain 388; 103 CFU, injected subcutaneously) (43). In a BALB/c mouse model of acute pneumonia, Lee et al. deleted ExoY in the PAK strain and found only minor effects on the bacterial load in the lungs of infected female mice (31). Vance et al. reported that infection with an ExoY-deficient PAO1 strain had no significant effect on bacterial colonization in the lungs of neutropenic C57BL/6 mice (30). The differences between the results of our study and those of others may be due to the use of a different mouse model. Sufficient recruitment of neutrophils to the site of infection was essential for clearance of P. aeruginosa. BALB/c mice have a lower inflammatory response than mice with a C57BL background and decreased accumulation of neutrophils upon bacterial stimulation (44, 45). In ΔexoY strain-infected BALB/c mice, recruitment of neutrophils to the lungs may be blunted compared with that of C57BL/6 mice infected with the ΔexoY strain. In the neutropenic C57BL/6 mouse model, the effects of ExoY on neutrophil recruitment and subsequent bacterial clearance may be masked.
P. aeruginosa ExoY produces four types of nucleoside 3′,5′-cyclic monophosphates (cNMPs) with a preference for cGMP and cUMP formation (10). cAMP and cGMP are well-characterized second messengers that regulate numerous cellular functions via specific effectors (46, 47). However, only in recent years have cCMP and cUMP been unequivocally identified as second messengers in mammalian cells by means of highly specific and sensitive mass spectrometry analyses (48, 49). The functional roles of cCMP and cUMP are poorly understood, and their effect on the immune response remains to be clarified (46, 50, 51). Research regarding cGMP focuses primarily on the cardiovascular system, neurophysiology, and metabolic diseases (52–54). Baldissera et al. reported that BAY 60-2770, an activator of soluble guanylate cyclase, could inhibit airway inflammation to relieve ovalbumin-induced allergic asthma (55). Chang et al. reported that plecanatide-mediated activation of guanylate cyclase could reduce the production of multiple proinflammatory cytokines in dextran sodium sulfate (DSS)-induced colonic inflammation (56). The results of these two studies support the idea that cGMP has an inhibitory effect on inflammation, but the exact mechanism remains unknown. Increases in intracellular cAMP have been shown to suppress the expression of inflammatory mediators, such as TNF and IL-12 (57–59). The cross talk between the cAMP pathway and NF-κB/MAPKs varies by cell type and stimulus, with many potential mechanisms and discrepancies described in the literature (60–62). Here, we found that second messengers produced by P. aeruginosa ExoY inhibited cytokine production via suppression of TAK1, which may shed light on the relationship between second messengers and the production of inflammatory mediators.
We found that ExoY inhibited Il6 production in infected A549 cells but promoted Il6 production in infected macrophages. Consistent with our results, Aronoff et al. reported that treatment with the cAMP analog 6-Bnz-cAMP promoted LPS-induced IL-6 production but suppressed TNF production in alveolar macrophages and peritoneal macrophages (58). These authors also showed that cAMP analogs decreased LPS-stimulated generation of TNF and IL-6 in dendritic cells (58). In addition, TAK1-deficient peritoneal macrophages were found to express similar levels of IL-6 but lower levels of TNF and IL-1β than wild-type peritoneal macrophages upon LPS stimulation (63). Therefore, modulation of IL-6 production by cAMP in different cell types may depend on different mechanisms.
It has been reported that ExoU can activate NF-κB and JNK signaling pathways to promote IL-8 production (64, 65). And recently ExoS and ExoT were reported to inhibit generation of reactive oxygen species in neutrophils by downregulating phosphatidylinositol 3-kinase (PI3K) signaling and thus decreasing bacterial clearance (66). Here, we demonstrated that the P. aeruginosa virulence factor ExoY, which is known to generate cNMPs in host target cells, downregulates the activation of TAK1, as well as downstream NF-κB and MAPKs, to inhibit production of proinflammatory cytokines during P. aeruginosa infection. These findings implicate ExoY as a negative regulator of the TLR signaling pathways to dampen the host's ability to clear bacteria in a mouse model. Our results provide a molecular basis for the pathological actions of ExoY in relation to the immune system and may inform the development of novel therapies for P. aeruginosa infection.
MATERIALS AND METHODS
Animals.
C57BL/6 mice were bred under specific-pathogen-free conditions at the Laboratory Animal Center of Tongji University. Female mice, aged 6 to 8 weeks, were used in the preparation of peritoneal macrophages; male mice, aged 12 to 14 weeks, were used for bacterial infection. All animal studies were conducted in accordance with institutional guidelines and complied with protocols that had been approved by the Animal Experiment Administration Committee of Tongji University.
Bacterium preparation and mouse infection.
PAO1 and its ΔexoY strains were kindly provided by K. M. Duan (Northwest University, Xi'an, China) (67). PAK, PA388, PAK ΔexoY, PA388 ΔexoY, and PAK ΔexoS exoT exoY strains were from S. G. Jin (University of Florida, USA). Electrocompetent PAO1 ΔexoY and PAK ΔexoS exoT exoY cells were prepared as described previously (68). A 3′ hemagglutinin (HA)-tagged exoY wild-type or K81M mutant gene was cloned into the pUCP19 vector and introduced into ΔexoY or ΔexoS exoT exoY competent cells by electroporation (capacitance [C], 25 μF; resistance [R] = 200, voltage [V], 2.5 kV). Bacteria were streaked from frozen cultures onto Luria-Bertani (LB) agar, and then a single colony was cultured for 16 to 18 h at 37°C with shaking in LB broth supplemented with 100 μg/ml of ampicillin. For mouse infection, the bacteria were washed at least three times with phosphate-buffered saline (PBS), and 50 μl of a suspension of P. aeruginosa in PBS was delivered into the lung of an anesthetized mouse via intratracheal instillation.
Antibodies.
Anti-TAK1 antibody (sc-7162) was obtained from Santa Cruz Biotech. Anti-p-TAK1 Thr187 (4536S), anti-p-p65 (3033S), anti-p-p38 (9215S), anti-p-JNK (9251S), anti-IκBα (4812S), and anti-p38 (9212S) antibodies were obtained from Cell Signaling Technology. Anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (G9545) and anti-HA (SAB1306169) antibodies were obtained from Sigma-Aldrich.
Cell culture.
All cell lines were tested to be mycoplasma negative by a commonly used PCR method. HEK293T and A549 cells obtained from the ATCC were cultured in Dulbecco's modified Eagle's medium (DMEM; HyClone). Human monocytic THP-1 cells (ATCC) and mouse peritoneal macrophages were cultured in RPMI 1640 medium (HyClone). All media were supplemented with 10% (vol/vol) fetal bovine serum (FBS; Gibco), 2 mM l-glutamine (HyClone), and 100 U/ml penicillin and streptomycin. Cells were grown at 37°C in a 5% CO2 incubator. To induce differentiation of THP-1 cells, 50 ng/ml phorbol 12-myristate 13-acetate (PMA) was added for 48 h. Thioglycolate-elicited peritoneal macrophages were generated by injecting 2 ml of 4% thioglycolate solution into the peritoneal cavity of mice. Two to three days later, macrophages were collected by peritoneal lavage with RPMI 1640 medium. Infection assays were performed with 1 × 106 cells unless otherwise noted. All infection assays were performed in medium without antibiotics.
Generation of knockdown cell lines.
To construct stable knockdown cell lines, pLKO.1 plasmids harboring the desired genes, together with the packing plasmids pSPAX2 and pMD2.G, were transfected into HEK293T cells at a ratio of 4:3:1 in a six-well plate for 6 h. Subsequently, the medium was replaced with 2 ml of fresh medium per well, and cells were maintained for another 48 h. Supernatants were collected, mixed with an equal volume of complete DMEM, and added to A549 cells. Cells were maintained for 24 h; the medium was removed, and the mixture was added again for another 24 h. Puromycin (2 μg/ml; Santa Cruz Biotech) was used to screen for positive cells.
Determination of cAMP.
A549 cells were infected with PAO1 or PAO1 ΔexoY at the times indicated in the figures, and the level of cAMP was measured with a cAMP-Glo assay (V1501; Promega) according to the manufacturer's instructions. Briefly, 1 × 104 cells per well were infected in a 96-well plate. Cells were lysed with 20 μl of cAMP-Glo lysis buffer for 20 min at room temperature and then incubated with 40 μl of cAMP-Glo detection solution for another 20 min. Subsequently, 80 μl of cAMP-Glo reagent was added, and cells were incubated at room temperature for 10 min. Luminescence was measured with a plate-reading luminometer.
Dual-luciferase reporter assay.
HEK293T cells were transiently transfected with pRL-NF-κB-Luc (or pRL-AP-1-Luc), pRL-TK, and the plasmids indicated in the figures for 48 h in a 24-well plate (a total of 500 ng of DNA). Cells were lysed and measured with a Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions.
Preparation of mouse lung homogenates.
For RNA extraction, comparable sizes and sites of mouse lungs were collected in 500 μl of TRIzol reagent (Invitrogen). For cytokine measurement, whole lungs were harvested in 5 ml of cold PBS plus protease inhibitor cocktail. For determination of bacterial numbers, the right lungs of mice were aseptically removed and harvested in 1 ml of cold PBS. Lungs were homogenized on ice with a tissue homogenizer. The homogenizer was carefully cleaned and disinfected with 75% alcohol after each homogenization. The bacterial load was determined by plating serial 10-fold dilutions (in sterile PBS) of lung homogenates onto LB agar with ampicillin and incubation at 37°C for 24 h. For cytokine measurement, the homogenates were spun at 8,000 × g for 20 min at 4°C. Supernatants were collected for subsequent analysis.
Quantitative RT-PCR analysis.
Total RNA was extracted with 500 μl of TRIzol reagent (Invitrogen) in a 12-well plate. A total of 1 μg of RNA was reverse transcribed with a ReverTra Ace quantitative PCR (qPCR) reverse transcription (RT) kit (Toyobo). A SYBR RT-PCR kit (Toyobo) was used with diluted cDNA (10:1) and primers according to the manufacturer's instructions. An Applied Biosystems 7500 real-time PCR system was used for subsequent analyses. The mRNA levels were normalized to those of Gapdh. The primer sequences are as follows for human genes: Tnf-F, CTGGCCCAGGCAGTCAGAT; Tnf-R, AGCTGCCCCTCAGCTTGAG; Il-6-F, ACTCACCTCTTCAGAACGAATTG; Il-6-R, CCATCTTTGGAAGGTTCAGGTTG; Gapdh-F, CTGGGCTACACTGAGCACC; Gapdh-R, AAGTGGTCGTTGAGGGCAATG. The primer sequences are as follows for mouse genes: Tnf-F, GTCCCCAAAGGGATGAGAAGTT; Tnf-R, GTTTGCTACGACGTGGGCTACA; Il-6-F, AGATAAGCTGGAGTCACAGAAGGAG; Il-6-R, CGCACTAGGTTTGCCGAGTAG; Il-1β-F, CAACCAACAAGTGATATTCTCCATG; Il-1β-R, GATCCACACTCTCCAGCTGCA; Gapdh-F, TGGAGAAACCTGCCAAGTATGA; Gapdh-R, CTGTTGAAGTCGCAGGAGACAA.
Cell death assay.
Cell death was determined with an LDH assay using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega) according to the manufacturer's instructions.
Cytokine release assay.
Concentrations of cytokines in cell supernatants or mouse lung homogenates were measured with enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Westang Bio-Tech Co., Ltd.).
Transfection, immunoblot analysis, and densitometry quantification.
HEK293T cells and A549 cells were transiently transfected using Lipofectamine 2000 (Invitrogen) or ExFect transfection reagent (Vazyme) according to the manufacturers' instructions. The infected or transfected cells were harvested by 1× sodium dodecyl sulfate (SDS) loading buffer and were boiled at 95°C for 10 min. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose blotting membrane (GE Healthcare Life Science). The blots were blocked with 5% nonfat dry milk for 1 h at room temperature and subsequently incubated overnight at 4°C with the primary antibodies indicated in the figures. Following three washes (10 min each) with Tris-buffered saline plus Tween (TBST), the blots were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:5,000) for 1 h at room temperature. After three washes with TBST, the blots were developed with SuperSignal West Pico Plus chemiluminescent substrate (34578; Thermo Fisher Scientific) according to the manufacturer's instructions. Densitometries were measured with ImageJ software.
Immunohistochemistry.
The lung tissues of mice infected with PAO1 or PAO1 ΔexoY were fixed in 4% paraformaldehyde (PFA). The tissues were dehydrated, embedded in paraffin, and cut into 5-μm sections. Paraffin sections were dewaxed with xylene and a graded alcohol series. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and nonspecific binding sites were blocked with 3% bovine serum albumin (BSA). Slices were incubated with polyclonal rabbit antibody against MPO overnight at 4°C. Sections were then incubated with secondary antibody at room temperature for 1 h.
Statistical analysis.
Statistical significance between groups was determined by a two-tailed Student's t test and two-way analysis of variance (ANOVA). GraphPad Prism, version 5.0, software was used for all analyses. A P value of <0.05 was considered to indicate a significant difference.
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Duan (Northwest University) for PAO1 and its ΔexoY strains and members of Ge's laboratory for helpful discussions and technical assistance.
This work was supported by the National Natural Science Foundation of China (projects 91542111, 81330069, and 31030028).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00239-17.
REFERENCES
- 1.Stevens TC, Ochoa CD, Morrow KA, Robson MJ, Prasain N, Zhou C, Alvarez DF, Frank DW, Balczon R, Stevens T. 2014. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am J physiology Lung cellular and molecular physiology 306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. 2002. Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Gaynes R, Edwards JR, National Nosocomial Infections Surveillance S . 2005. Overview of nosocomial infections caused by Gram-negative bacilli. Clin Infect Dis 41:848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 4.Engel J, Balachandran P. 2009. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol 12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Hauser AR. 2009. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol 7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burstein D, Satanower S, Simovitch M, Belnik Y, Zehavi M, Yerushalmi G, Ben-Aroya S, Pupko T, Banin E. 2015. Novel type III effectors in Pseudomonas aeruginosa. mBio 6:e00161-15. doi: 10.1128/mBio.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahuja N, Kumar P, Bhatnagar R. 2004. The adenylate cyclase toxins. Crit Rev Microbiol 30:187–196. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]
- 8.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. 1998. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A 95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belyy A, Raoux-Barbot D, Saveanu C, Namane A, Ogryzko V, Worpenberg L, David V, Henriot V, Fellous S, Merrifield C, Assayag E, Ladant D, Renault L, Mechold U. 2016. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat Commun 7:13582. doi: 10.1038/ncomms13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckert U, Wolter S, Hartwig C, Bahre H, Kaever V, Ladant D, Frank DW, Seifert R. 2014. ExoY from Pseudomonas aeruginosa is a nucleotidyl cyclase with preference for cGMP and cUMP formation. Biochem Biophys Res Commun 450:870–874. doi: 10.1016/j.bbrc.2014.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beckert U, Grundmann M, Wolter S, Schwede F, Rehmann H, Kaever V, Kostenis E, Seifert R. 2014. cNMP-AMs mimic and dissect bacterial nucleotidyl cyclase toxin effects. Biochem Biophys Res Commun 451:497–502. doi: 10.1016/j.bbrc.2014.07.134. [DOI] [PubMed] [Google Scholar]
- 12.Balczon R, Prasain N, Ochoa C, Prater J, Zhu B, Alexeyev M, Sayner S, Frank DW, Stevens T. 2013. Pseudomonas aeruginosa exotoxin Y-mediated tau hyperphosphorylation impairs microtubule assembly in pulmonary microvascular endothelial cells. PLoS One 8:e74343. doi: 10.1371/journal.pone.0074343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ochoa CD, Alexeyev M, Pastukh V, Balczon R, Stevens T. 2012. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. J Biol Chem 287:25407–25418. doi: 10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. 2004. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 15.Cowell BA, Evans DJ, Fleiszig SM. 2005. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol Lett 250:71–76. doi: 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Hritonenko V, Mun JJ, Tam C, Simon NC, Barbieri JT, Evans DJ, Fleiszig SM. 2011. Adenylate cyclase activity of Pseudomonas aeruginosa ExoY can mediate bleb-niche formation in epithelial cells and contributes to virulence. Microb Pathog 51:305–312. doi: 10.1016/j.micpath.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 18.Ramphal R, Balloy V, Jyot J, Verma A, Si-Tahar M, Chignard M. 2008. Control of Pseudomonas aeruginosa in the lung requires the recognition of either lipopolysaccharide or flagellin. J Immunol 181:586–592. doi: 10.4049/jimmunol.181.1.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. 2009. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One 4:e7259. doi: 10.1371/journal.pone.0007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. 1995. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science 270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 21.Ajibade AA, Wang HY, Wang RF. 2013. Cell type-specific function of TAK1 in innate immune signaling. Trends Immunol 34:307–316. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. 1999. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai H. 2012. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci 33:522–530. doi: 10.1016/j.tips.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. 2005. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 25.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev 19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omori E, Matsumoto K, Sanjo H, Sato S, Akira S, Smart RC, Ninomiya-Tsuji J. 2006. TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J Biol Chem 281:19610–19617. doi: 10.1074/jbc.M603384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavoie EG, Wangdi T, Kazmierczak BI. 2011. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect 13:1133–1145. doi: 10.1016/j.micinf.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. 2005. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun 73:7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tseng J, Do J, Widdicombe JH, Machen TE. 2006. Innate immune responses of human tracheal epithelium to Pseudomonas aeruginosa flagellin, TNF-alpha, and IL-1beta. Am J Physiol Cell Physiol 290:C678–C690. doi: 10.1152/ajpcell.00166.2005. [DOI] [PubMed] [Google Scholar]
- 30.Vance RE, Rietsch A, Mekalanos JJ. 2005. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun 73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee VT, Smith RS, Tummler B, Lory S. 2005. Activities of Pseudomonas aeruginosa effectors secreted by the type III secretion system in vitro and during infection. Infect Immun 73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. 1998. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun 66:3164–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singhirunnusorn P, Suzuki S, Kawasaki N, Saiki I, Sakurai H. 2005. Critical roles of threonine 187 phosphorylation in cellular stress-induced rapid and transient activation of transforming growth factor-beta-activated kinase 1 (TAK1) in a signaling complex containing TAK1-binding protein TAB1 and TAB2. J Biol Chem 280:7359–7368. doi: 10.1074/jbc.M407537200. [DOI] [PubMed] [Google Scholar]
- 34.Scholz R, Sidler CL, Thali RF, Winssinger N, Cheung PC, Neumann D. 2010. Autoactivation of transforming growth factor beta-activated kinase 1 is a sequential bimolecular process. J Biol Chem 285:25753–25766. doi: 10.1074/jbc.M109.093468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolbeling F, Munder A, Kerber-Momot T, Neumann D, Hennig C, Hansen G, Tummler B, Baumann U. 2011. Lung function and inflammation during murine Pseudomonas aeruginosa airway infection. Immunobiology 216:901–908. doi: 10.1016/j.imbio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Wagner JG, Roth RA. 2000. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol Rev 52:349–374. [PubMed] [Google Scholar]
- 37.Hirche TO, Benabid R, Deslee G, Gangloff S, Achilefu S, Guenounou M, Lebargy F, Hancock RE, Belaaouaj A. 2008. Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J Immunol 181:4945–4954. doi: 10.4049/jimmunol.181.7.4945. [DOI] [PubMed] [Google Scholar]
- 38.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. 2009. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun 77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Faure K, Sawa T, Ajayi T, Fujimoto J, Moriyama K, Shime N, Wiener-Kronish JP. 2004. TLR4 signaling is essential for survival in acute lung injury induced by virulent Pseudomonas aeruginosa secreting type III secretory toxins. Respir Res 5:1. doi: 10.1186/1465-9921-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mijares LA, Wangdi T, Sokol C, Homer R, Medzhitov R, Kazmierczak BI. 2011. Airway epithelial MyD88 restores control of Pseudomonas aeruginosa murine infection via an IL-1-dependent pathway. J Immunol 186:7080–7088. doi: 10.4049/jimmunol.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skerrett SJ, Liggitt HD, Hajjar AM, Wilson CB. 2004. Cutting edge: myeloid differentiation factor 88 is essential for pulmonary host defense against Pseudomonas aeruginosa but not Staphylococcus aureus. J Immunol 172:3377–3381. doi: 10.4049/jimmunol.172.6.3377. [DOI] [PubMed] [Google Scholar]
- 42.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 43.Holder IA, Neely AN, Frank DW. 2001. Type III secretion/intoxication system important in virulence of Pseudomonas aeruginosa infections in burns. Burns 27:129–130. doi: 10.1016/S0305-4179(00)00142-X. [DOI] [PubMed] [Google Scholar]
- 44.Marley SB, Hadley CL, Wakelin D. 1994. Effect of genetic variation on induced neutrophilia in mice. Infect Immun 62:4304–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morissette C, Skamene E, Gervais F. 1995. Endobronchial inflammation following Pseudomonas aeruginosa infection in resistant and susceptible strains of mice. Infect Immun 63:1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seifert R. 2015. cCMP and cUMP: emerging second messengers. Trends Biochem Sci 40:8–15. doi: 10.1016/j.tibs.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Wolter S, Golombek M, Seifert R. 2011. Differential activation of cAMP- and cGMP-dependent protein kinases by cyclic purine and pyrimidine nucleotides. Biochem Biophys Res Commun 415:563–566. doi: 10.1016/j.bbrc.2011.10.093. [DOI] [PubMed] [Google Scholar]
- 48.Bahre H, Hartwig C, Munder A, Wolter S, Stelzer T, Schirmer B, Beckert U, Frank DW, Tummler B, Kaever V, Seifert R. 2015. cCMP and cUMP occur in vivo. Biochem Biophys Res Commun 460:909–914. doi: 10.1016/j.bbrc.2015.03.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneider EH, Seifert R. 2015. Report on the third symposium “cCMP and cUMP as New Second Messengers.” Naunyn Schmiedebergs Arch Pharmacol 388:1–3. doi: 10.1007/s00210-014-1072-3. [DOI] [PubMed] [Google Scholar]
- 50.Seifert R. 2017. cCMP and cUMP across the tree of life: from cCMP and cUMP generators to cCMP- and cUMP-regulated cell functions. Handb Exp Pharmacol 238:3–23. doi: 10.1007/164_2016_5005. [DOI] [PubMed] [Google Scholar]
- 51.Seifert R, Schneider EH, Bahre H. 2015. From canonical to non-canonical cyclic nucleotides as second messengers: pharmacological implications. Pharmacol Ther 148:154–184. doi: 10.1016/j.pharmthera.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Friebe A, Sandner P, Seifert R. 2015. From bedside to bench—meeting report of the 7th International Conference on cGMP “cGMP: generators, effectors and therapeutic implications” in Trier, Germany, from June 19th to 21st 2015. Naunyn Schmiedebergs Arch Pharmacol 388:1237–1246. doi: 10.1007/s00210-015-1176-4. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann F, Wegener JW. 2013. cGMP-dependent protein kinases (cGK). Methods Mol Biol 1020:17–50. doi: 10.1007/978-1-62703-459-3_2. [DOI] [PubMed] [Google Scholar]
- 54.Pfeifer A, Kilic A, Hoffmann LS. 2013. Regulation of metabolism by cGMP. Pharmacol Ther 140:81–91. doi: 10.1016/j.pharmthera.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Baldissera L Jr, Squebola-Cola DM, Calixto MC, Lima-Barbosa AP, Renno AL, Anhe GF, Condino-Neto A, De Nucci G, Antunes E. 2016. The soluble guanylyl cyclase activator BAY 60-2770 inhibits murine allergic airways inflammation and human eosinophil chemotaxis. Pulm Pharmacol Ther 41:86–95. doi: 10.1016/j.pupt.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 56.Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, Palejwala VA, Clapper ML, Shailubhai K. 2017. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc+/Min-FCCC mice. World J Gastrointest Pharmacol Ther 8:47–59. doi: 10.4292/wjgpt.v8.i1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. 2005. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol 174:595–599. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 58.Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. 2006. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res 26:827–833. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- 59.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. 2008. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerlo S, Kooijman R, Beck IM, Kolmus K, Spooren A, Haegeman G. 2011. Cyclic AMP: a selective modulator of NF-κB action. Cell Mol Life Sci 68:3823–3841. doi: 10.1007/s00018-011-0757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. 1996. Elevated cyclic AMP inhibits NF-κB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem 271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 62.Gerits N, Kostenko S, Shiryaev A, Johannessen M, Moens U. 2008. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: comradeship and hostility. Cell Signal 20:1592–1607. doi: 10.1016/j.cellsig.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 63.Ajibade AA, Wang Q, Cui J, Zou J, Xia X, Wang M, Tong Y, Hui W, Liu D, Su B, Wang HY, Wang RF. 2012. TAK1 negatively regulates NF-κB and p38 MAP kinase activation in Gr-1+ CD11b+ neutrophils. Immunity 36:43–54. doi: 10.1016/j.immuni.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuzick A, Stirling FR, Lindsay SL, Evans TJ. 2006. The type III pseudomonal exotoxin U activates the c-Jun NH2-terminal kinase pathway and increases human epithelial interleukin-8 production. Infect Immun 74:4104–4113. doi: 10.1128/IAI.02045-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Lima CD, Calegari-Silva TC, Pereira RM, Santos SA, Lopes UG, Plotkowski MC, Saliba AM. 2012. ExoU activates NF-κB and increases IL-8/KC secretion during Pseudomonas aeruginosa infection. PLoS One 7:e41772. doi: 10.1371/journal.pone.0041772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vareechon C, Zmina SE, Karmakar M, Pearlman E, Rietsch A. 2017. Pseudomonas aeruginosa effector ExoS inhibits ROS production in human neutrophils. Cell Host Microbe 21:611–618e5. doi: 10.1016/j.chom.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Yang L, Zhao X, Shen L, Duan K. 2010. Identification of Pseudomonas aeruginosa genes associated with antibiotic susceptibility. Sci China Life Sci 53:1247–1251. doi: 10.1007/s11427-010-4071-8. [DOI] [PubMed] [Google Scholar]
- 68.Choi KH, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.