Abstract
Neurons and glia are the two major cell types in the nervous system and work closely with each other to program neuronal interplay. Traditionally, neurons are thought to be the major cells that actively regulate processes like synapse formation, plasticity, and behavioral output. Glia, on the other hand, serve a more supporting role. To date, accumulating evidence has suggested that glia are active participants in virtually every aspect of neuronal function. Despite this, fundamental features of how glia interact with neurons, and their spatial relationships, remain elusive. Here, we describe the glial cell population in Drosophila adult brains. Glial cells extend and tightly associate their processes with major structures such as the mushroom body (MB), ellipsoid body (EB), and antennal lobe (AL) in the brain. Glial cells are distributed in a more concentrated manner in the MB. Furthermore, subsets of glia exhibit distinctive association patterns around different neuronal structures. Whereas processes extended by astrocyte-like glia and ensheathing glia wrap around the MB and infiltrate into the EB and AL, cortex glia stay where cell bodies of neurons are and remain outside of the synaptic regions structured by EB or AL.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0014-0) contains supplementary material, which is available to authorized users.
Keywords: Glia, Drosophila, Mushroom body
Introduction
Higher-order behavioral tasks and cognitive functions are mediated by complex neural circuits composed of two major cell types in the nervous system: neurons and glia [1, 2]. While neurons have been considered to be the leading players in transducing inputs, glia, once thought to be passive, are now known to be active in virtually all aspects of nervous system function [3]. For instance, during axonal growth, glia regulate pathfinding and direct axons to their targets [1, 4]. When axons overgrow and further pruning is required, glia detect and clean up the degenerative axon debris [5–7]. During synaptic information flow, glia are crucial for the formation of synapses, engulfment of synaptic debris, and plasticity changes [8, 9]. In addition, glia are traditionally recognized as major cells in the brain despite the fact that controversies have arisen about the ratio of glia to neurons [10, 11]. These lines of evidence have established glia as important players in the nervous system [12]. Despite this, many fundamental aspects of glia, such as their spatial proximity and relative distribution to neurons, remain mysterious. Thus, a comprehensive analysis of glial cell number, morphology, and their location in proximity to neurons would be extremely valuable for understanding glia-mediated neuronal functions and their detailed roles in circuitry [2, 8, 13].
The insect Drosophila melanogaster is not only well known for its sophisticated genetic tools and diverse behavioral features, but also its evolutionarily conserved sequences and genomes, making it an excellent animal model in which to study general signal transduction pathways and identify newly involved factors [7]. Remarkably, despite the difference in size, the Drosophila adult brain exhibits structures with functions similar to mammals. For instance, the mushroom body (MB) [14], a structure found in other arthropods, has been suggested to be loosely analogous to the mammalian hippocampus, mainly for their common function in learning and memory [15]. Furthermore, Drosophila glia are morphologically and functionally similar to their mammalian counterparts [14]. A plethora of studies have given thorough descriptions of the diverse types of Drosophila adult glia [16–18]. Particularly, glia associated with the antennal lobes (ALs) have been discussed [19]. These studies provide an excellent foundation for understanding the general anatomy of Drosophila glia. Three major types of glia have been described in the adult Drosophila brain. While ensheathing glia in Drosophila function similarly to oligodendrocytes in wrapping around the neuropil, astrocyte-like glia infiltrate into the neuropil and are in close contact with synaptic regions like mammalian astrocytes [20]. A third type of glia, cortex glia, localize around the cell bodies of neurons and also act like mammalian astrocytes. Here we describe the glial population in the Drosophila adult brains. Using antibodies and genetic tools that specifically label glia and neuronal structures, we were able to analyze how glia construct a working environment for neuronal function. In addition, numbers of glia in close proximity to the MB and other structures were analyzed and quantified. These results suggest that glia are closely associated with the MB and other neuronal structures, providing substantial support and tightly interacting with neurons for brain function.
Materials and Methods
Fly Strains
Flies were maintained at 25 °C on normal food. All strains were obtained from the Bloomington Stock Center or the Vienna Drosophila Resource Center. NP6520-Gal4, NP577-Gal4, and Alrm-Gal4 were gifts from R. Jackson and M. Freeman [21, 22]. All fly crosses were carried out at 25 °C under standard laboratory conditions unless noted otherwise.
Immunohistochemistry and Microscopy
Adult brains were dissected from one-week-old flies without mouthparts and fixed with 4% formaldehyde in 1 × phosphate-buffered saline following standard protocols as previously described. Reagents used included: normal donkey serum (0.5%; Jackson Lab), mouse anti-Repo (1:100, DSHB#8D12), mouse anti-FasII (1:100, DSHB#1D4), rat anti-Elav (1:500, DSHB#7E8A10), and mouse anti-nc82 (1:100, DSHB#AB_2314866). The other secondary antibodies (mouse-Cy3 and rat-Cy3) were from the Jackson Lab. Images were captured using Leica TCS SP5 confocal microscopy and a Zeiss Imager M2 microscope and processed with Adobe Photoshop and Illustrator. Z-stack projection was the projection of serial optical sections of the specimen (2 μm per section).
Results and Discussion
Glia Extend Processes Around Major Structures in the Drosophila Adult Brain
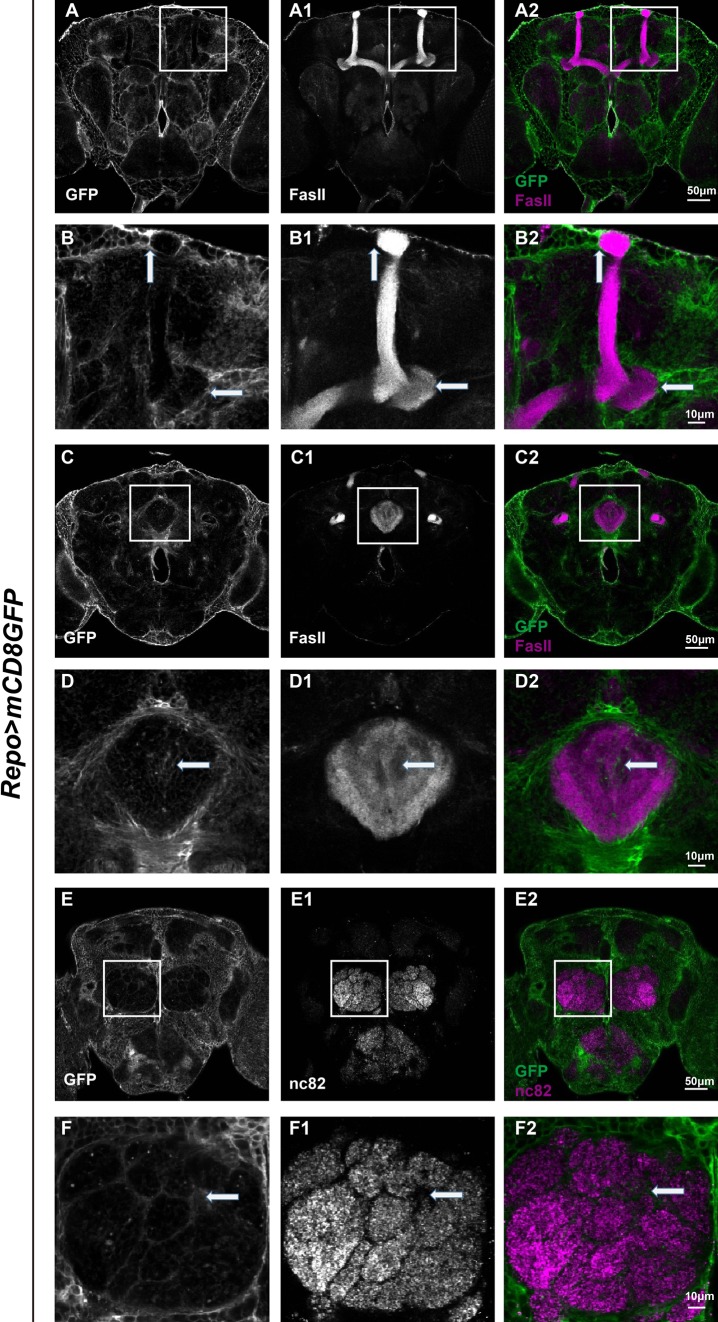
We were interested in how glia interact with neurons and their spatial relationships in the Drosophila adult brain. The brain consists of important neuronal structures such as the MB, ellipsoid body (EB), and AL [23]. These structures regulate activities such as locomotion, olfactory learning, and memory. Especially, MB is generally recognized as a vital center for information collection and integration [24], with the EB located posteriorly and the AL located anteriorly. To investigate the glial distribution associated with these structures, adult brains expressing UAS-mCD8GFP under the control of Repo-Gal4 (Repo>mCD8GFP) were dissected and immunostained with FasII or nc82 to reveal the MB, EB, and AL (Figs. 1 and S1). All z-stack projections of images from the whole brain (total thickness ~80–100 μm, taken in 40–50 sections of 2 μm each) were shown in Supplementary Fig. 1. To visualize these neuronal structures with clearer resolution, confocal sections capturing the majority of these structures (~10–15 sections each) were taken and merged separately (as in all Figures). Interestingly, green fluorescent protein (GFP) signals representing glial processes were seen clearly around the lobes of the MB, suggesting that glia are in close proximity to the MB axon bundles (Fig. 1B–B2). These glia were closely associated with the MB lobes, yet little infiltration was seen within the lobes. In addition, more glia were localized at the tip and the peduncle of the MBs (arrows in Fig. 1B–B2).
Fig. 1.
Glia extend processes around the MB, EB, and AL. Adult brains carrying Repo>mCD8GFP (green) were co-stained with FasII (magenta, A–D2) to reveal the structure of the MB and EB. E–F2 Adult brains carrying Repo>mCD8GFP (green) were co-stained with nc82 (magenta) to reveal the structure of the AL. Confocal projections of sections that exhibit most of each structure (~10–15 sections) were shown (A–A2, C–C2, E–E2) and corresponding enlarged views were shown below (B–B2, D–D2, F–F2). Note that GFP-positive glial processes were seen around the MB, particularly at the tip and the peduncle (arrows in B2), and the EB (arrow in D2). These processes also infiltrated into the EB (D2) and AL (arrow in F2).
On the other hand, the FasII-labeled EB was also surrounded by glial processes (arrows in Fig. 1D–D2). Distinctively, glial processes infiltrated into the EB ring and interconnected with EB axons rather than only surrounding the outer area. Infiltration of glial processes was further evident in the case of the AL, where olfactory processing is mediated. Glomerular interactions of glial processes and the AL were evident, suggesting that these processes infiltrate and interact closely with AL glomerular synapses (arrows in Fig. 1F–F2).
Glial Cells are More Concentrated in the Vicinity of the MB
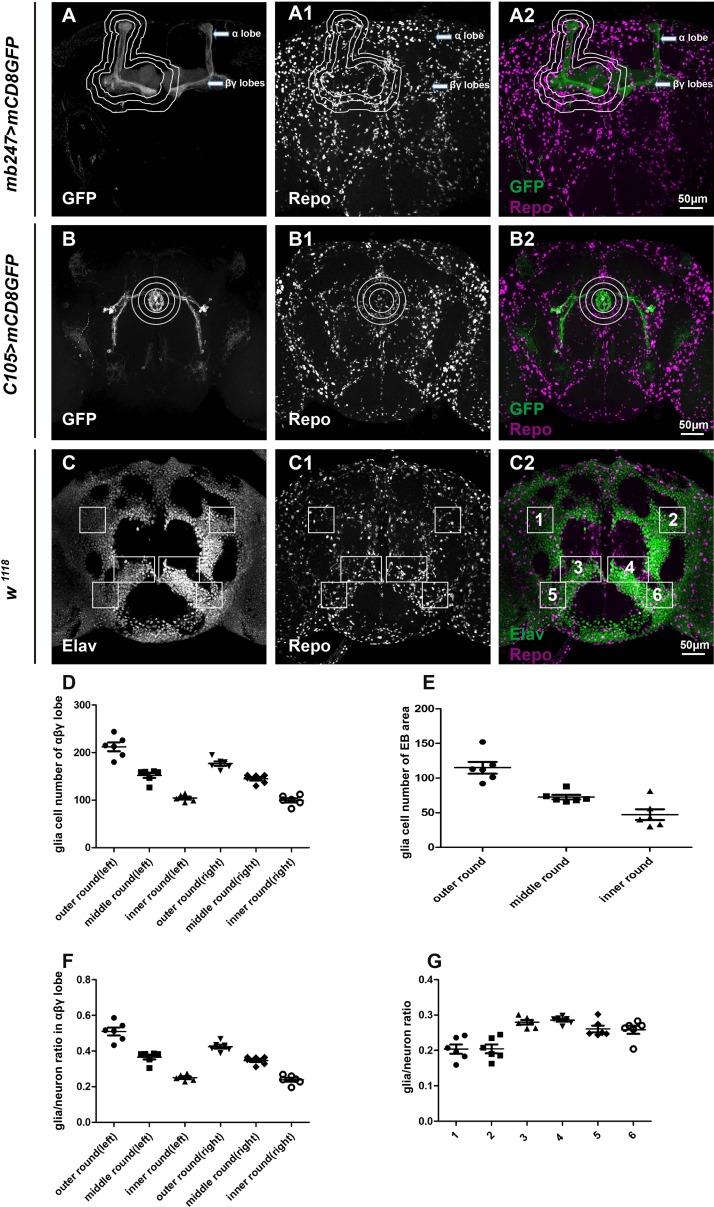
To further analyze the spatial relationship between glia and neurons, the α, β, and γ lobes of the MB labeled with mb247-Gal4-driven UAS-mCD8GFP (mb247>mCD8GFP) were dissected and stained with the glial marker Repo (Fig. 2A–A2). To quantify the number of glial cells, areas of 5, 15, or 25 μm away from the α, β, and γ lobes were defined and Repo-positive cells were counted within these areas for the vertical α and horizontal β + γ lobes (Fig. 2D). Interestingly, ~100–200 glial cells were found to be in close proximity to the vertical α lobe and the horizontal β + γ lobes, depending on the area chosen (Fig. 2D). These are likely to be neuropil-associated glia such as ensheathing glia and astrocyte-like glia. Similar numbers of these MB-associated glial cells were detected on the left and right sides of the brains. More glial cells were concentrated locally at the tip of α lobe and at the peduncle (arrows in Fig. 2A2). A glia/MB neuron ratio of 0.6 was calculated by dividing the sum of MB-associated glial cells by the number of MB neurons at the calyces that differentiate into the α, β, and γ lobes (~400 on either side; Fig. 2F).
Fig. 2.
Analysis of glial cell numbers in the adult brain. Adult brains carrying mb247>mCD8GFP or C105>mCD8GFP (green) were co-stained with Repo (white, A1 and B1) to reveal glial nuclei. C–C2 Adult brains co-stained with Elav (green) and Repo (white, C1). Three different bilaterally symmetrical regions (total of 6) were randomly chosen with sizes of 0.14 mm2 (1, 2, 5, and 6) and 0.4 mm2 (3 and 4). D–F Statistical analysis of glial cell numbers in the vicinity of the MB and EB. Areas 5, 10, and 15 μm away from the α and β + γ lobes of the MB were drawn appropriately and quantified for glial cell number (D). Rings enclosing the EB of diameter 40 (inner), 60, and 80 μm (outer) were designated and the glial cell numbers counted (E). The sum of MB-associated glia was divided by the number of MB neurons that differentiated into the α, β, and γ lobes to obtain the glia/MB neuron ratio (F). Numbers of glia and neurons were counted in areas 1–6 to obtain the glia/neuron ratio (G). Data are shown as mean ± SEM. Eight to ten brains were dissected and the numbers of glia and neurons were counted.
In addition, EBs labeled with C105-Gal4-driven UAS-mCD8GFP (C105>mCD8GFP) were stained with Repo. Similar to the MB, three distances were chosen for the measurement of glial cell numbers. Rings surrounded the EB of diameter 40, 60, and 80 μm were defined and the numbers of glial cells enclosed were counted (Fig. 2B–B2, E). Interestingly, ~100–150 glial cells were detected within the outer EB ring excluding the inner ring, whereas ~50 glial cells were found within the inner EB ring (Fig. 2E). Furthermore, the total number of glial cells around the MB on one side was similar to the number around the whole EB ring (outer), indicating a more concentrated distribution of glial cells in the MB than the EB (Fig. 2D). Finally, brains were co-stained with Repo and Elav to reveal the nuclei of glia and neurons, respectively. In doing so, Repo- and Elav-positive cells were counted in 3 different bilaterally-symmetrical regions (total of 6) randomly chosen throughout the brain (Fig. 2C–C2). We found no distinguishable difference in any of these areas, i.e. the glia/neuron ratio was roughly the same for all of them (~0.2 in Fig. 2G), suggesting that glial cells, likely cortex glia, are distributed in a similar manner regardless of the location of neurons. Compared to a glia/MB neuron ratio of 0.6, however, these results suggest that more glial cells are distributed in the vicinity of the MB, while in other brain areas, such as the cortex, glial cells are distributed similarly.
Subsets of Glia Exhibit Distinctive Expression Patterns
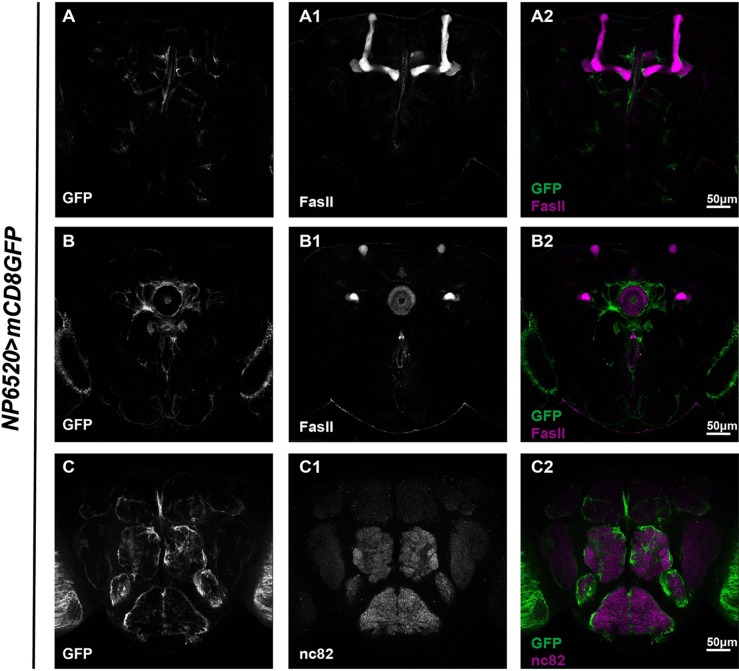
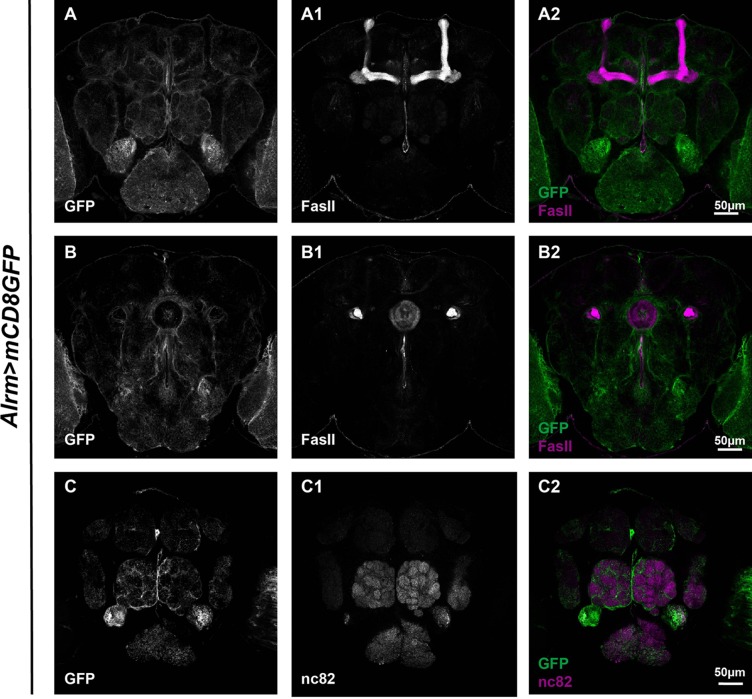
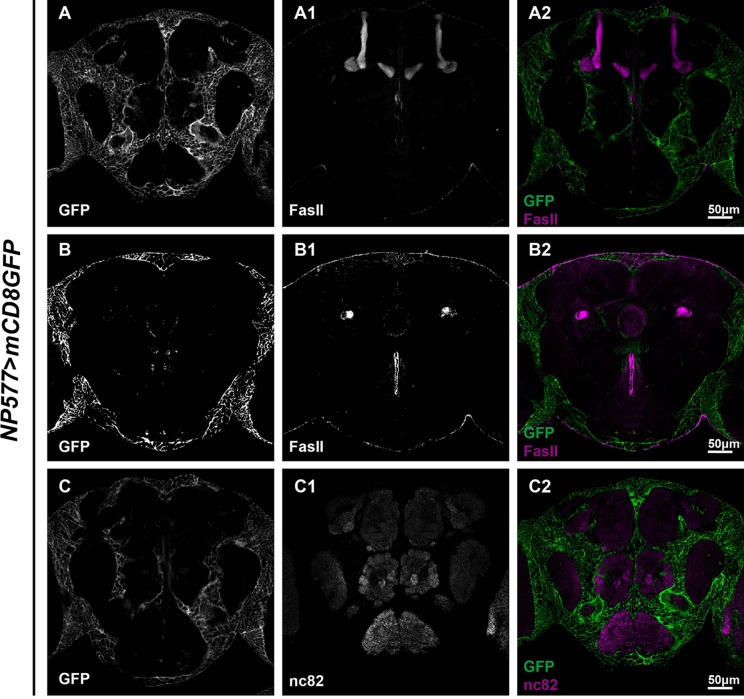
Three subsets of glia have been previously reported in the adult Drosophila brain: ensheathing glia, astrocyte-like glia, and cortex glia. To get a better idea of how these subsets are distributed, brains with corresponding Gal4s driving GFP expression were dissected and stained with FasII or nc82 to reveal the MB, EB, and AL. Using NP6520-Gal4, we were able to identify GFP expression in ensheathing glia [16] (Fig. 3). Our analysis revealed that ensheathing glial processes wrapped around the MB, EB, and AL, with an overall expression level lower than the astrocyte-like glia labeled by Alrm-Gal4 [23] (Fig. 4). Both types of glia wrapped around the MB lobes, and infiltrated into the EB ring and the AL. However, astrocyte-like glia infiltrated into AL to a greater extent than the ensheathing glia (compare Figs. 3C2 and 4C2), and there was no distinguishable difference in the EB infiltration of both types of glia (compare Figs. 3B2 and 4B2). In contrast, analysis of brains carrying NP577>mCD8GFP revealed that cortex glial expression was restricted to the locations of neuron cell bodies [16] (Fig. 5). Interestingly, unlike astrocyte-like glia and ensheathing glia, few cortex glial processes were seen to extend into the MB or infiltrate into the EB and AL (Fig. 5).
Fig. 3.
Ensheathing glia extend processes around the MB, EB, and AL. Adult brains carrying NP6520>mCD8GFP (green, A–C2) co-stained with FasII (white, A1 and B1) or nc82 (white, C1) to reveal the structure of the MB, EB, and AL. Confocal projections merging sections exhibiting most of each structure (~10–15 sections) were shown. Note that GFP-positive glial processes were seen around the MB (A) and EB (B). These processes also infiltrated into the EB (B) and AL (C).
Fig. 4.
Astrocyte-like glia extend processes around the MB, EB, and AL. Adult brains carrying Alrm>mCD8GFP (green, A–C2) were co-stained with FasII (white, A1 and B1) or nc82 (white, C1) to reveal the structure of the MB, EB, and AL. Confocal projections merging sections exhibiting most of each structure (~10–15 sections) were shown. Note that the overall GFP expression for astrocyte-like glia was higher than that for ensheathing glia. GFP-positive glial processes were seen around the MB (A) and EB (B). These processes also infiltrated into the EB (B) and AL (C).
Fig. 5.
Cortex glial processes extend around the cell bodies of neurons. Adult brains carrying NP577>mCD8GFP (green, A–C2) were co-stained with FasII (white, A1 and B1) or nc82 (white, C1) to reveal the structure of the MB, EB, and AL. Confocal projections merging sections exhibiting most of each structure (~10–15 sections) were shown. Note that GFP-labeled glial processes were mainly seen near the cell bodies of neurons. No specific processes were detected around the MB and they hardly infiltrated into the EB and AL.
To further explore these glial sub-types, we stained brains carrying these three glial Gal4 s with nc82 that labels the neuropil. By imaging the whole brain in stacks (totally ~45 confocal sections), we merged the 10 sections exhibiting most of the ALs and the remaining 35 sections separately (Fig. S6A and B, C and D, E and F). Consistent with our observations using Repo>GFP and nc82 (Figs. 1 and 3, 4, 5), ALs were mostly infiltrated by GFP-labeled glial processes from Alrm-Gal4 and NP6520-Gal4. On the other hand, NP577-Gal4-labeled glial processes infiltrated the ALs less. In the rest of the brain areas, we did not see prominent nc82-labeled structures that were infiltrated or co-labeled by these glial Gal4s (Fig. S2B2, D2, F2, S3–S5).
Taken together, these results suggest that subsets of glia are distributed differently and possibly possess distinct functions. Whereas ensheathing glia and astrocyte-like glia both infiltrate into the EB and AL and extend processes around the MB, cortex glia are mainly localized in regions with neuron cell bodies. Our results provide a comprehensive analysis of glial cell numbers and their spatial relationships to neurons in the Drosophila adult brain. These findings lay a foundation for future studies elucidating glial cell function in terms of the ways they interact with neurons and how they act as essential partners in neuronal interplay.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank the Bloomington Stock Center, the Vienna Drosophila RNAi Center, and the Developmental Studies Hybridoma Bank for fly stocks and antibodies. We thank R. Jackson and M. Freeman for sharing the fly strains. This work was supported by grants from the National Basic Research Program of China (973 Program 2013CB945602) and the National Natural Science Foundation of China (31270825 and 31171043).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0014-0) contains supplementary material, which is available to authorized users.
References
- 1.Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts RJ, Schuldiner O, Perrino J, Larsen C, Luo L. Glia engulf degenerating axons during developmental axon pruning. Curr Biol. 2004;14:678–684. doi: 10.1016/j.cub.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 5.Awasaki T, Ito K. Engulfing action of glial cells is required for programmed axon pruning during Drosophila metamorphosis. Curr Biol. 2004;14:668–677. doi: 10.1016/j.cub.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Seid MA, Wehner R. Delayed axonal pruning in the ant brain: a study of developmental trajectories. Dev Neurobiol. 2009;69:350–364. doi: 10.1002/dneu.20709. [DOI] [PubMed] [Google Scholar]
- 7.Ou J, He Y, Xiao X, Yu TM, Chen C, Gao Z, et al. Glial cells in neuronal development: recent advances and insights from Drosophila melanogaster. Neurosci Bull. 2014;30:584–594. doi: 10.1007/s12264-014-1448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booth GE, Kinrade EF, Hidalgo A. Glia maintain follower neuron survival during Drosophila CNS development. Development. 2000;127:237–244. doi: 10.1242/dev.127.2.237. [DOI] [PubMed] [Google Scholar]
- 9.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- 11.Hilgetag CC, Barbas H. Are there ten times more glia than neurons in the brain? Brain Struct Funct. 2009;213:365–366. doi: 10.1007/s00429-009-0202-z. [DOI] [PubMed] [Google Scholar]
- 12.Oland LA, Tolbert LP. Roles of glial cells in neural circuit formation: insights from research in insects. Glia. 2011;59:1273–1295. doi: 10.1002/glia.21096. [DOI] [PubMed] [Google Scholar]
- 13.Corty MM, Freeman MR. Cell biology in neuroscience: Architects in neural circuit design: glia control neuron numbers and connectivity. J Cell Biol. 2013;203:395–405. doi: 10.1083/jcb.201306099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones TA, Donlan NA, O’Donnell S. Growth and pruning of mushroom body Kenyon cell dendrites during worker behavioral development in the paper wasp, Polybia aequatorialis (Hymenoptera: Vespidae) Neurobiol Learn Mem. 2009;92:485–495. doi: 10.1016/j.nlm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs JR. The midline glia of Drosophila: a molecular genetic model for the developmental functions of glia. Prog Neurobiol. 2000;62:475–508. doi: 10.1016/S0301-0082(00)00016-2. [DOI] [PubMed] [Google Scholar]
- 16.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards TN, Meinertzhagen IA. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol. 2010;90:471–497. doi: 10.1016/j.pneurobio.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartenstein V. Morphological diversity and development of glia in Drosophila. Glia. 2011;59:1237–1252. doi: 10.1002/glia.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oland LA, Biebelhausen JP, Tolbert LP. Glial investment of the adult and developing antennal lobe of Drosophila. J Comp Neurol. 2008;509:526–550. doi: 10.1002/cne.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakim Y, Yaniv SP, Schuldiner O. Astrocytes play a key role in Drosophila mushroom body axon pruning. PLoS One. 2014;9:e86178. doi: 10.1371/journal.pone.0086178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng FS, Tangredi MM, Jackson FR. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol. 2011;21:625–634. doi: 10.1016/j.cub.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renn SC, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, et al. Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol. 1999;41:189–207. doi: 10.1002/(SICI)1097-4695(19991105)41:2<189::AID-NEU3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Li X, Guo J, Li Y, Guo A. Two clusters of GABAergic ellipsoid body neurons modulate olfactory labile memory in Drosophila. J Neurosci. 2013;33:5175–5181. doi: 10.1523/JNEUROSCI.5365-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.