Abstract
Abnormal approach-avoidance behavior has been linked to deficits in the mesolimbic dopamine (DA) system of the brain. Recently, increasing evidence has indicated that toll-like receptor 4 (TLR4), an important pattern-recognition receptor in the innate immune system, can be directly activated by substances of abuse, resulting in an increase of the extracellular DA level in the nucleus accumbens. We thus hypothesized that TLR4-dependent signaling might regulate approach-avoidance behavior. To test this hypothesis, we compared the novelty-seeking and social interaction behaviors of TLR4-deficient (TLR4 −/−) and wild-type (WT) mice in an approach-avoidance conflict situation in which the positive motivation to explore a novel object or interact with an unfamiliar mouse was counteracted by the negative motivation to hide in exposed, large spaces. We found that TLR4 −/− mice exhibited reduced novelty-seeking and social interaction in the large open spaces. In less stressful test apparatuses similar in size to the mouse cage, however, TLR4 −/− mice performed normally in both novelty-seeking and social interaction tests. The reduced exploratory behaviors under approach-avoidance conflict were not due to a high anxiety level or an enhanced fear response in the TLR4 −/− mice, as these mice showed normal anxiety and fear responses in the open field and passive avoidance tests, respectively. Importantly, the novelty-seeking behavior in the large open field induced a higher level of c-Fos activation in the nucleus accumbens shell (NAcSh) in TLR4 −/− mice than in WT mice. Partially inactivating the NAcSh via infusion of GABA receptor agonists restored the novelty-seeking behavior of TLR4 −/− mice. These data suggested that TLR4 is crucial for positive motivational behavior under approach-avoidance conflict. TLR4-dependent activation of neurons in the NAcSh may contribute to this phenomenon.
Keywords: Toll-like receptor 4, Novelty-seeking, Social interaction, Approach-avoidance conflict, Nucleus accumbens shell
Introduction
Approaching positive and avoiding negative stimuli facilitate the adaptation of an organism to its environment and are fundamental for survival [1]. Abnormal approach-avoidance behavior has been linked to many neuropsychiatric and neurological disorders, including drug addiction [2–4], eating disorders [5], and autism [6, 7]. For example, the addicted individual shows compulsive drug-seeking behavior, manifested by continuing to use the drug of abuse despite the negative health, economic, and social consequences [4]. Therefore, investigating approach-avoidance behavior has attracted increasing attention, particularly in the drug addiction field [4, 8–10]. But the detailed mechanisms underlying approach-avoidance behavior are still largely unknown.
Increasing evidence suggests that toll-like receptor 4 (TLR4), an important pattern-recognition receptor in the innate immune system, is critical in affective and motivational behaviors. TLR4 is widely expressed in both glial cells and neurons throughout brain development [11–13]. It functions in neurogenesis, axonal growth, and structural plasticity [12, 14, 15], and regulates the cognitive and affective behaviors associated with depression [16, 17] and drug addiction [18, 19]. Notably, TLR4 can be activated directly by opioids [19] and cocaine [20], and this activation contributes to drug reinforcement via increasing the level of extracellular dopamine (DA) in the nucleus accumbens (NAc) [20]. It is well established that the mesolimbic DA system is closely associated with approach-avoidance behavior [21, 22]. We thus hypothesized that TLR4 signaling might play a critical role in mediating this behavior.
In this study, we tested the hypothesis by comparing the novelty-seeking and social interaction behaviors of TLR4-deficient (TLR4 −/−) and wild-type (WT) mice under approach-avoidance conflict.
Materials and Methods
Animals
We used male congenic TLR4 −/− (C57BL/10ScNJNju) mice and their WT littermates at 10–15 weeks of age. The mice were housed in a temperature-controlled room on a 12-h light: 12-h dark cycle (lights on at 07:00). Food and water were available ad libitum. All animal use procedures were reviewed and approved by the Animal Care and Use Committee at Zhejiang University, following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Novelty-Seeking Test
The open field apparatus was an open white plastic box (40 × 40 × 40 cm3) with 250 lux of illuminance at the center. A central square region (20 × 20 cm2) was marked as the central area. After 3 days of 5-min habituation, mice were placed in the center of the box without any novel object and allowed to explore for 180 s. The time spent in the central area was recorded as the pretest score. Then a novel object (a colored bottle, 5 cm in diameter and 15 cm high) was placed in the center of the open field, and the test continued for another 180 s, during which the time spent in the central area was recorded as the test score. The location and movement of mice were monitored by an overhead webcam (Pro C920, Logitech, Suzhou, China) and analyzed for time spent in the central area and the locomotor distance using a custom tracking script written in MatLab (MathWorks, Natick, MA). The apparatus was cleaned with 75% ethanol between tests.
To test novelty-seeking in a less anxiety-provoking way, a smaller apparatus (30 × 25 × 20 cm3) similar in size and color to the animals’ home cages was used. The illuminance was set at 250 lux at the center of the apparatus. The novel object was replaced by a black-and-white bottle (3 cm in diameter and 12 cm high). The pretest and test scores were recorded using the same procedure as above.
Social Interaction Test
To measure the social interaction behavior, the social choice test was conducted in two three-chamber apparatuses [23]. The large apparatus was a non-transparent Plexiglas box (60 × 40 × 25 cm3) composed of three equal-sized chambers separated by two dividers with a central opening of 10 × 20 cm2. The small apparatus was one third the size of the large one. The social target cage was a transparent and hollow plastic cylinder (8 cm inner diameter) in both the left and right chambers of the two apparatuses. The social interaction test was conducted in a room lit at about 250 lux. Mice were tested in the small apparatus on day 1, and retested in the large one on the next day. Different social target mice were used on the day 1 and day 2 tests for each test mouse to ensure that the test mouse had never encountered these social target mice before.
The test comprised habituation, baseline, and social interaction phases. In the habituation phase, mice were allowed to explore the chambers freely for 5 min to habituate to the test environment. In the baseline phase, the mice were placed in the central chamber and allowed to explore the apparatus freely for 5 min, during which the time spent in each chamber was recorded as the pretest score. In the social interaction phase, the social interaction chamber and the control chamber were randomly chosen from the left and right chambers. A stranger male conspecific mouse was placed in the cage of the social interaction chamber, and the cage in the control chamber remained empty. The mice were placed in the central chamber and observed for another 5 min. Test mice were recorded with the overhead webcam (Pro C920) and the time spent in each chamber was automatically scored with a custom script written in MatLab. The preference score (time spent in the social interaction chamber − time spent in the control chamber) was calculated. The apparatus was cleaned with 75% ethanol between tests.
Open Field Test
The open field apparatus was an open white plastic box (40 × 40 × 40 cm3) with 250 lux of illuminance at the center. Mice were placed in the center of the open field and allowed to explore for 300 s. The time spent in the central area and the total locomotor distance were recorded. The apparatus was cleaned with 75% ethanol between tests.
Passive Avoidance Test
This test was performed in a two-compartment plastic box with a lit compartment (15 × 15 × 13 cm3) connected to a dark compartment of the same size by a guillotine door. As soon as the mice entered the dark compartment, the door was closed and they received a punishing electric foot shock (0.75 mA, 1 s). The latency to enter the dark compartment was recorded in the training test and in the retention test 24 h later. The apparatus was cleaned with 75% ethanol between tests.
Immunohistochemistry
Approximately 90 min after the novelty-seeking test, the mice were deeply anaesthetized by isoflurane inhalation and transcardially perfused with phosphate buffer saline (PBS), followed by 4% paraformaldehyde (PFA) in PBS. The brains were dissected and post-fixed in 4% PFA overnight and then moved to 30% sucrose in PBS. After they were saturated in 30% sucrose (36 h), coronal sections (30 μm) were cut on a freezing microtome (CM30503, Leica Microsystems, Wetzlar, Germany). The sections were first washed in PBS (3 × 5 min) and then incubated in a blocking solution containing 10% normal donkey serum (017-000-121, Jackson ImmunoResearch, West Grove, PA), 1% bovine serum albumen (A2153, Sigma, St. Louis, MO), 0.3% Triton X-100 in PBS for 1 h at room temperature. Next, the sections were incubated with an anti-c-Fos antibody (1:800, 2250, Cell Signaling Technology, Beverly, MA) for 72 h at 4 °C. The sections were then washed with PBS (7 × 5 min) and incubated with the secondary antibody, donkey anti-rabbit Alexa Fluor 488 (1:1000, A-21206, Life Technologies, Carlsbad, CA), at room temperature for 1 h. After washing with PBS (7 × 5 min), the sections were mounted onto glass slides with 30% glycerol in PBS and the glass edges were sealed with nail polish. All images were captured using a Nikon A1 laser-scanning confocal microscope (Nikon, Tokyo, Japan) with fixed settings. The numbers of immunopositive cells were counted and analyzed using ImageJ (NIH, Baltimore, MD).
Microinfusion of GABA Receptor Agonists
Two weeks prior to the novelty-seeking test, mice were deeply anesthetized by isoflurane inhalation and placed in a stereotaxic apparatus (REW Life Science, Shenzhen, China). Stainless-steel guide cannulae [outer diameter (o.d.) 0.48 mm, inner diameter (i.d.) 0.34 mm] were implanted bilaterally, ending 1 mm above the injection site [nucleus accumbens shell (NAcSh): bregma + 1.34 mm, midline ± 0.5 mm, 4.7 mm ventral to the skull] [24]. A mixture of baclofen and muscimol (Bac-Mus mix, 0.06/0.006 nmol/μL or 0.12/0.012 nmol/μL, Sigma) was injected bilaterally in a volume of 0.5 μL into the NAcSh on each side 10–15 min prior to the novelty-seeking test. The Bac-Mus mix was delivered through an injection cannula (o.d. 0.3 mm, i.d. 0.14 mm) coupled with a 1.0 μL Hamilton microsyringe driven by a microinfusion pump (Stoelting, Wood Dale, IL) at a rate of 0.25 μL/min. The injection cannulae were lowered into the NAcSh to a depth of 1 mm beyond the tips of the guide cannulae. After infusion, the injection cannulae remained in the guide cannulae for 1 min to prevent fluid backflow.
Statistical Analysis
Analyses of the novelty-seeking, social interaction, and passive avoidance tests were performed using two-way ANOVA with a Bonferroni post-hoc test. We used Grubbs’ test to determine the outliers in data sets. The independent t-test was used to analyze the open field and the c-Fos expression data. All data are expressed as mean ± SEM and were analyzed using SPSS 22.0 software for Windows (SPSS, Chicago, IL). The significance level was set at P < 0.05.
Results
TLR4−/− Mice Exhibited Reduced Exploratory Behaviors Under Approach-Avoidance Conflict
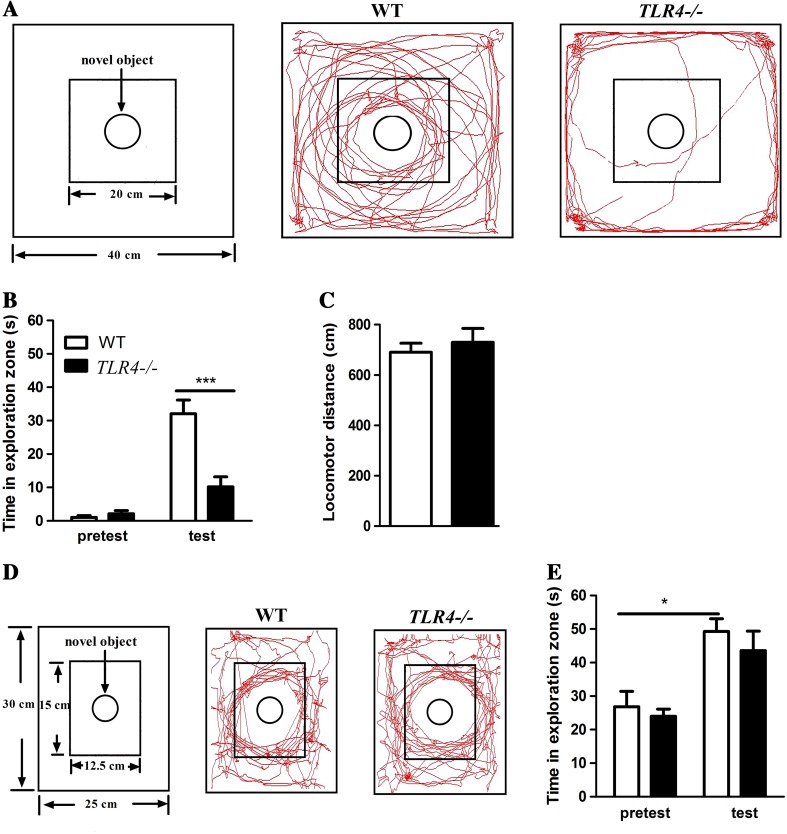
To determine the role of TLR4 deficiency in positive motivation-related exploration, we first tested the novelty-seeking behavior of the mice in an exposed, large field with a central object (Fig. 1A). Two-way ANOVA revealed a significant interaction effect between test type (pretest vs test) and genotype (WT vs TLR4 −/−) (F (1, 13) = 21.809, P < 0.001). The main effects of test type (F (1, 13) = 63.006, P < 0.001) and genotype (F (1, 13) = 13.550, P < 0.01) were both significant. Bonferroni post-hoc analysis showed that during the test phase, the TLR4 −/− mice (n = 7) spent less time in the exploration zone than the WT mice (n = 8) (P < 0.001) (Fig. 1B). Besides, TLR −/− mice showed normal locomotor activity, as they traveled the same distance as WT mice during the test phase (Fig. 1C). These data indicated that TLR4 −/− mice exhibit impaired novelty-seeking behavior in a large open space.
Fig. 1.
TLR4 deficiency caused a reduction in novelty-seeking behavior in mice. A. Representative tracings of WT (middle panel) and TLR4 −/− mice (right panel) performing the novelty-seeking task in the large apparatus (left panel). B. Bar graphs showing the time WT (n = 8) and TLR4 −/− (n = 7) mice spent in the exploration zone in the 3-min novelty-seeking test phase in the large apparatus. C. The locomotor distances of WT and TLR4 −/− mice in the 3-min novelty-seeking test phase in the large apparatus. D. Representative tracings of WT (middle panel) and TLR4 −/− mice (right panel) performing the novelty-seeking task in the small apparatus (left panel). E. Bar graphs showing the time WT (n = 8) and TLR4 −/− (n = 6) mice spent in the exploration zone in the 3-min novelty-seeking test in the small apparatus. *P < 0.05, ***P < 0.001.
However, the decreased novelty-seeking behavior of TLR4 −/− mice in the open field apparatus may have been due to an impairment of exploratory behavior or an increased anxiety level in the exposed, large field. To investigate these possibilities, we retested the same mice in a less stressful apparatus (Fig. 1D), a small apparatus similar in size and wall color to the mouse cage. Two-way ANOVA showed no significant interaction effects between test type and genotype (F (1, 12) = 0.040, P > 0.05). The main effect of genotype was also not significant (F (1, 12) = 1.52, P > 0.05) (Fig. 1E). However, we found a significant main effect of test type (F (1, 12) = 19.311, P < 0.01). Bonferroni post-hoc analysis showed that both TLR4 −/− (n = 6, P < 0.05) and WT mice (n = 8, P < 0.05) spent more time in the exploration zone in the novelty-seeking test than in the baseline test (Fig. 1E), indicating that the novel object was capable of eliciting clear exploratory behavior of TLR4 −/− mice in the small apparatus. These results indicated that TLR4 −/− mice still possess a drive to explore novel objects.
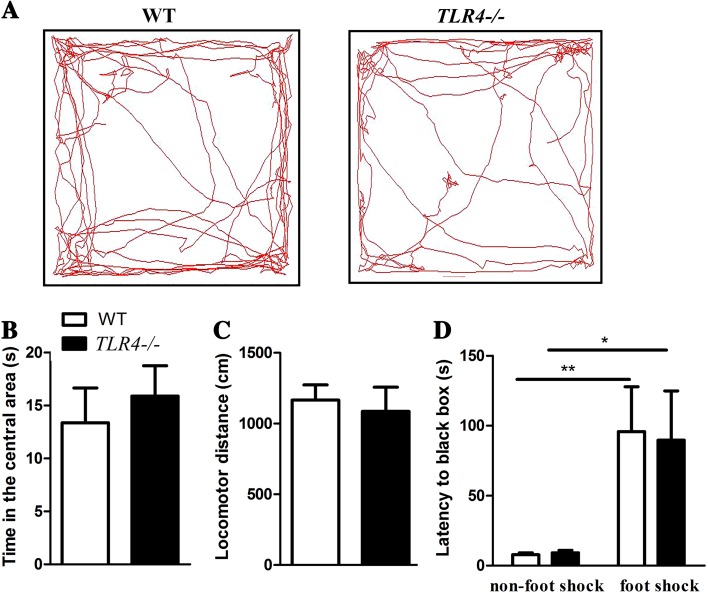
The reduced exploratory behavior in the large open field might have been due to a change in the anxiety level and/or fear responses of TLR4 −/− mice. To determine if the novelty-seeking change in TLR4 −/− mice was due to increased anxiety, the behaviors of a separate group of mice were evaluated in an open field apparatus (Fig. 2A). In the regular open field test, there was no difference in the time spent in the central area (Fig. 2B) and in the locomotor distance (Fig. 2C) between TLR4 −/− and WT mice (n = 11). These data indicated that TLR4 −/− mice have a normal anxiety response in the open field. To further test if the TLR4 −/− mice showed aggravated fear responses, we used the passive avoidance test to evaluate these responses by measuring the latency to enter the dark (shocked) compartment. Two-way ANOVA revealed no significant interaction effects between training type (non-foot shock vs foot shock) and genotype (F (1, 34) = 0.027, P > 0.05). Foot shocks induced a significant increase in the latency score in WT and TLR4 −/− mice (F (1, 34) = 14.021, P < 0.01), but the main effect of genotype was not significant (F (1, 34) = 0.011, P > 0.05) (Fig. 2D). These data demonstrated that TLR4 −/− mice exhibit normal fear-motivated responses in the passive avoidance test. Taken together, TLR4 −/− mice showed normal fear/anxiety responses. Neither a reduced motivation to explore nor a high level of fear/anxiety could explain the decreased novelty-seeking behavior of these mice in the large open field.
Fig. 2.
TLR4 −/− mice showed normal anxiety levels and fear responses in the open field and passive avoidance tests, respectively. A. Representative tracings of WT (left panel) and TLR4 −/− (right panel) mice in the open field. B. Bar graphs showing the time WT (n = 11) and TLR4 −/− (n = 11) mice spent in the central area in the 5-min test phase of the open field test. C. Bar graphs showing the locomotor distances of WT and TLR4 −/− mice in the test phase of the open field test. No significant differences in the central exploration time and the total distance traveled were found between WT and TLR4 −/− mice. D. The latency to enter the dark compartment in the passive avoidance test was similar in WT and TLR4 −/− mice. n = 8 for the non-foot shock WT group, 8 for the non-foot shock TLR4 −/− group, 10 for the foot shock WT group, and 8 for the foot shock TLR4 −/− group. *P < 0.05, **P < 0.01.
One possible explanation for the reduced novelty-seeking behavior in the open space is that the TLR4 −/− mice may respond differently in an approach-avoidance conflict environment. When the environmental novelty simultaneously evokes fear and curiosity, such as in the case of placing a novel object in an open field apparatus, a typical approach-avoidance conflict situation is generated. In this conflict situation, TLR4 −/− mice may “play it safe” and choose to hide instead of exploring, although they will explore the novel object if the environment is less stressful.
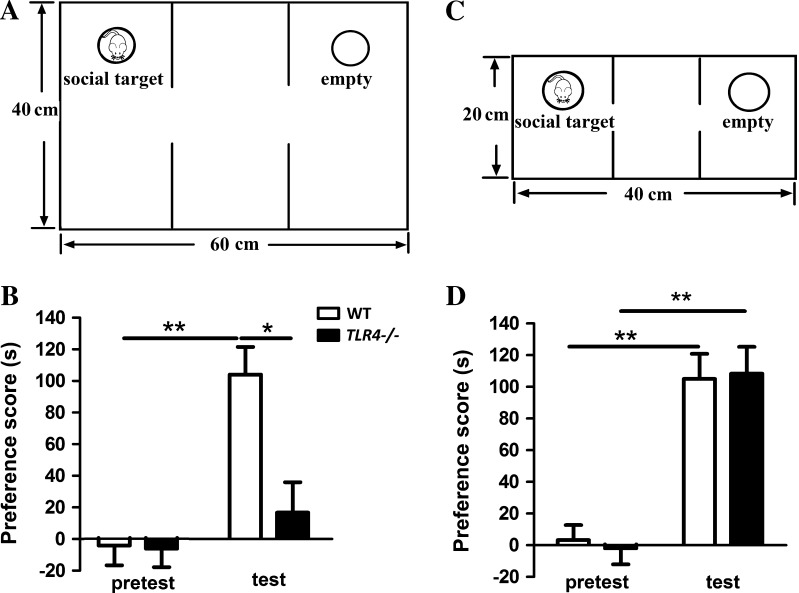
To further determine if the positive motivation-related behavior of TLR4 −/− mice is suppressed under approach-avoidance conflict, the social approach behavior of these mice was tested in a large social interaction test apparatus (Fig. 3A). We found a significant interaction effect between test type and genotype (F (1, 13) = 5.849, P < 0.05), and the main effects of test type (F (1, 13) = 13.774, P < 0.01) and genotype (F (1, 13) = 11.539, P < 0.01) were both significant. Bonferroni post-hoc analysis showed that the social interaction preference score was lower in TLR4 −/− mice than in WT mice during the test phase (P < 0.05) (Fig. 3B). We also tested the social interaction behavior in a small apparatus (one third the size of the large one) to remove the conflict (Fig. 3C). Two-way ANOVA did not reveal any significant interaction effects between test type and genotype (F (1, 13) = 0.083, P > 0.05) and the main effect of genotype (F (1, 13) = 0.007, P > 0.05), although the main effect of test type was significant (F (1, 13) = 50.433, P < 0.001) (Fig. 3D). Bonferroni post-hoc analysis showed that both TLR4 −/− (n = 7, P < 0.01) and WT mice (n = 8, P < 0.01) showed a preference for the social compartment in the social interaction test (Fig. 3D). These data indicated that the TLR4 −/− mice prefer to stay in the compartment of the small apparatus where an unfamiliar mouse is present, and this preference is lost in the large apparatus. These results are consistent with the phenomenon observed in the novelty-seeking tests. Taken together, these results indicated that suppression of positive motivation-related behaviors in TLR4 −/− mice is manifested under approach-avoidance conflict.
Fig. 3.
Effects of TLR4 deficiency on mouse social behavior. A. Schematic of the large 3-chamber social interaction apparatus with choice between a cage containing a novel mouse and an empty cage. B. Bar graphs showing the preference scores for a conspecific stranger mouse in the large apparatus. The preference score of TLR4 −/− mice (n = 7) was lower than that of WT mice (n = 8) in the test phase. C. Schematic of the small social interaction apparatus. D. Bar graphs showing the preference scores for a conspecific stranger mouse in the small apparatus. There was no difference in the preference score between WT and TLR4 −/− mice. *P < 0.05, **P < 0.01.
Partially Inactivating the Nucleus Accumbens Shell Rescued the Exploratory Behavior of TLR4−/− Mice Under Approach-Avoidance Conflict
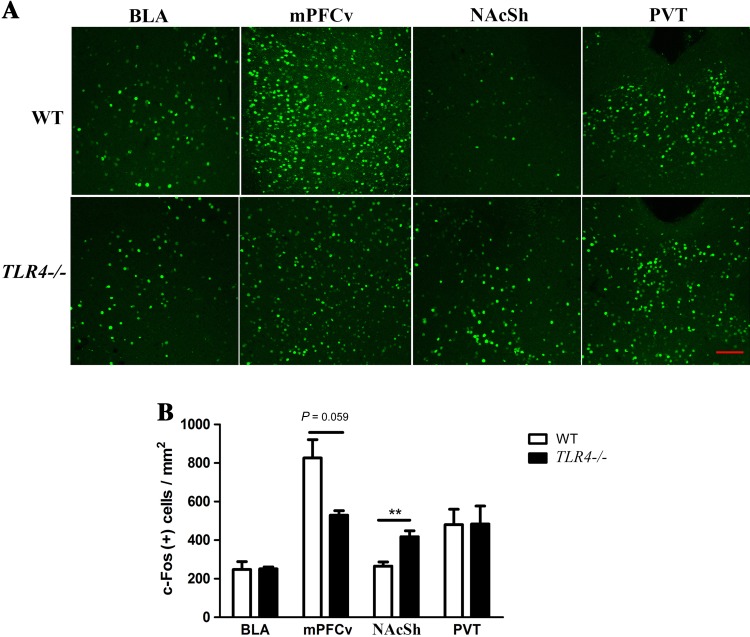
The reduced positive motivation-related behavior of TLR4 −/− mice under approach-avoidance conflict suggested that there might be changes in relevant neuronal circuitry in these mice. We next investigated if c-Fos activation was altered in the brain after the novelty-seeking task. In comparison to WT mice, we found that c-Fos expression was significantly increased in the NAcSh (P < 0.01) and reduced in the ventral medial prefrontal cortex (P = 0.059) in TLR4 −/− mice after the novelty-seeking test (Fig. 4A, B). The c-Fos expression in the basolateral amygdala and paraventricular thalamic nucleus, however, was similar in WT and TLR4 −/− mice after this test (Fig. 4A, B). These results suggested that the NAcSh plays a key role in decision-making under approach-avoidance conflict. Increased neuronal activity in the NAcSh might account for the reduction of exploratory activity induced by approach-avoidance conflict in TLR4 −/− mice.
Fig. 4.
The density of c-Fos-positive cells in the NAcSh was significantly higher in TLR4 −/− than in WT mice after performing novelty-seeking behavior. A. Representative photomicrographs showing c-Fos-positive cells in the BLA, mPFCv, NAcSh, and PVT of WT (n = 5) and TLR4 −/− (n = 3) mice. B. Histograms quantifying the density of c-Fos-positive cells in the BLA, mPFCv, NAcSh, and PVT of WT and TLR4 −/− mice. BLA, basolateral amygdala; mPFCv, ventral medial prefrontal cortex; NAcSh, nucleus accumbens shell; PVT, paraventricular thalamic nucleus. Scale bar, 100 μm. **P < 0.01.
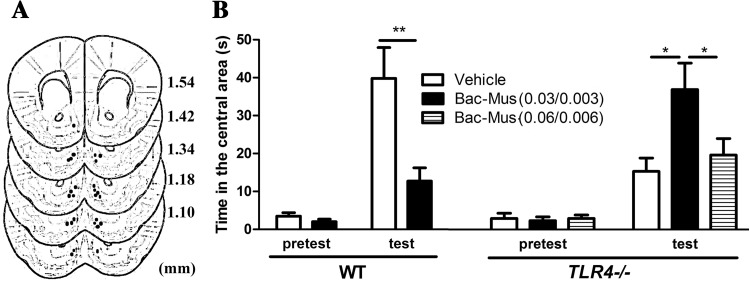
To further determine whether increased neuronal activity in the NAcSh is necessary to suppress the exploratory behavior of TLR4 −/− mice under approach-avoidance conflict, we next injected a cocktail of GABAergic agonists into the NAcSh to block neuronal activity (Fig. 5A), since the major projection neuron in the NAcSh, the medium spiny neuron (MSN), receives synaptic inputs from local GABAergic interneurons [25]. We found a significant interaction effect between test (pretest vs test) and drug type (vehicle vs Bac-Mus) (F (1, 12) = 8.625, P < 0.05) for WT mice, and the main effects of test (F (1, 12) = 29.121, P < 0.001) and drug type (F (1, 12) = 9.709, P < 0.01) were both significant (Fig. 5B). Bonferroni post-hoc analysis revealed that WT mice spent less time in the central area (P < 0.01) after the low dose of Bac (0.03 nmol)-Mus (0.003 nmol) in the test phase (Fig. 5B). On the other hand, partially increasing GABAergic transmission in the NAcSh of TLR4 −/− mice by injecting Bac (0.03 nmol)-Mus (0.003 nmol) into the NAcSh caused TLR4 −/− mice to spend significantly longer in the central area (P < 0.05) (Fig. 5B). These results indicated that partially inactivating the NAcSh rescues the novelty-seeking behavior of TLR4 −/− mice. Injecting a high dose of Bac (0.06 nmol)-Mus (0.006 nmol) into the NAcSh of TLR4 −/− mice caused them to explore a novel object placed in the center of the open field apparatus for a significantly shorter period than the low-dose Bac-Mus-treated mice (P < 0.05). These data implied that abnormal activation of the NAcSh might contribute to the impaired novelty-seeking behavior in TLR4 −/− mice.
Fig. 5.
Injecting a low dose of GABA receptor agonists into the NAcSh rescued the novelty-seeking behavior of TLR4 −/− mice placed in a large open space. A. Schematic of the intracranial cannula infusion sites in the NAcSh. Black dots indicate the locations of injector tips. Numbers on the right indicate the section position posterior to bregma. The figure was adapted from The Mouse Brain in Stereotaxic Coordinates (Franklin & Paxinos, 2008, Third Edition, Elsevier). B. Bar graphs summarizing the effects of NAcSh inactivation on the novelty-seeking behaviors of WT (n = 7) and TLR4 −/− (n = 6) mice. *P < 0.05, **P < 0.01.
Discussion
The main findings of the present study are: (1) TLR4 −/− mice displayed impaired novelty-seeking and social interaction behaviors in exposed, large spaces, though they showed normal interest in exploring a novel object in a less stressful environment as well as normal fear and anxiety responses, suggesting that their novelty-seeking deficiency was manifested in the approach-avoidance conflict environment; (2) the novelty-seeking behavior induced a higher number of c-Fos-positive cells in the NAcSh of TLR4 −/− mice, and partially inactivating the NAcSh rescued the reduced novelty-seeking behavior of these mice in the large open field. These data are the first to link TLR4 to positive motivation-related behaviors under approach-avoidance conflict conditions. Abnormal neuronal activity in the NAcSh might underlie this phenomenon.
TLR4 is best known for its essential roles in innate immunity under pathological conditions [26, 27]. It is also extensively expressed in the central nervous system [12, 28], and its activation has been shown to correlate with many neuropsychiatric conditions including depression and drug addiction [12, 29]. TLR4 activation triggers a cascade of signaling events leading to the release of many pro-inflammatory cytokines. These cytokines, such as tumor necrosis factor-α and interleukin-1β, not only exert their excitatory effects via upregulating excitatory synaptic transmission [30, 31], but also regulate neuronal functions via interacting with modulatory systems [32]. All these TLR4-mediated molecular and cellular consequences are pivotal to relevant neuropsychiatric diseases. In particular, it has recently been shown that substances of abuse such as opioids [33] and cocaine [20] function as xenobiotic ligands for TLR4 [34]. Xenobiotic activation of TLR4 increases the DA level in the NAc [20, 33]. We do not know if endogenous opioids are capable of activating TLR4 directly, nevertheless we speculate that TLR4 deficiency may lead to a decreased level of DA in the NAc, an area important in motivational conflict. Alternatively, TLR4 deficiency may interfere with the development of neuronal circuits associated with affective and motivational behaviors [35], leading to the observed impairment in exploratory behaviors under conflict. Additional studies are required to address these questions in the future.
In the present study, although many brain areas showed increased numbers of c-Fos-positive cells in WT and TLR4 −/− mice after the novelty-seeking task conducted in the large apparatus (Fig. 4), it was in the NAcSh that TLR4 −/− mice had more c-Fos reactivity than WT mice. It will be interesting to determine whether there is comparable c-Fos expression in both WT and TLR4 −/− mice after the novelty-seeking task in the small apparatus in future studies. Based on the relatively high density of c-Fos-positive cells in the NAcSh, we speculate that these cells may be MSNs which account for ~95% of all neurons in the NAc [36]. MSNs primarily express D1-like or D2-like dopamine receptors. Though we were not in a position to address the identity of c-Fos-positive neurons in TLR4 −/− mice in the current study, we think these neurons may be D2-MSNs as activation of these neurons mainly controls aversive behaviors [37–39]. It is well-known that D2-MSNs are inhibited by DA [40], and xenobiotic activation of TLR4 results in an increase in DA in the NAc [20, 33]. This may explain why TLR4 deficiency enhanced neuronal activity in the NAcSh after the novelty-seeking test. It will be interesting to investigate the cell type of these c-Fos-positive neurons and their functional regulation by DA in TLR4 −/− mice in future studies.
To link the enhanced neuronal activity in the NAcSh to the reduced exploratory behavior under conflict, we used low doses of GABAA and GABAB receptor agonists to partially inactivate the NAcSh. Although GABAergic interneurons make up about 2% of all neurons in the NAc [41], GABA receptor agonists have been widely used to inactivate the NAc [42, 43]. Infusion of one tenth of the regular doses of GABA receptor agonists into the NAcSh was effective in rescuing the novelty-seeking behavior of TLR4 −/− mice under motivational conflict. Interestingly, the same doses of GABA receptor agonists inhibited the exploratory behavior of WT mice performing the novelty-seeking task. Doubling the doses, on the other hand, suppressed the novelty-seeking behavior in TLR4 −/− mice. One possible explanation for these findings is that novelty-seeking behavior under conflict is dependent on the level of neuronal population excitability in the NAcSh. The higher the level, the more likely the neuronal circuit is to control the novelty-seeking behavior under conflict.
The novelty-seeking behavior we used represents a complex equilibrium between approach and avoidance behaviors [44], thus we reasoned that the manifestation of the aversive behavior in TLR4 −/− mice may be due to a shift in the equilibrium controlled by neuronal circuits with opposing functions [45]. The general consensus is that the NAcSh mainly controls aversive behavior, possibly via the indirect striato-pallidal pathway in which D2-MSNs are the major projection neurons. This indirect pathway may also be regulated by inputs from many areas in the prefrontal cortex and limbic regions [46, 47]. Compared to WT mice, we found that the novelty-seeking behavior induced an apparent decrease in the number of c-Fos-positive neurons in the medial prefrontal cortex (mPFC) in TLR4 −/− mice. No changes in c-Fos reactivity were observed in the basolateral amygdala. Thus, an abnormal mPFC-NAcSh circuit may underlie the reduced novelty-seeking behavior in TLR4-deficient mice. It has been shown that environmental stress modulates appetitive and defensive behaviors through the mPFC-NAcSh pathway. Blockade of AMPA receptors in the NAcSh elicits defensive behavior in a stressful environment [48]. This is consistent with our finding that reduced exploratory behavior in a stressful open field was associated with a decrease in mPFC neuronal activity in TLR4 −/− mice. Future studies are required to address the detailed circuit mechanism underlying the TLR4 deficiency-induced shift of approach-avoidance equilibrium. For example, it will be interesting to determine whether upregulating neuronal activity in the mPFC rescues the novelty-seeking behavior of TLR4 −/−mice under motivational conflict. It will also be interesting to clarify the crosstalk between the mPFC and NAcSh by evaluating the neuronal activity in the mPFC via c-Fos staining in response to infusion of GABA receptor agonists into the NAcSh.
Moreover, dysfunction in the mPFC-NAc circuit has been implicated in compulsive disorders. Repeated cortico-striatal activation generates persistent obsessive-compulsive disorder-like behavior [49]. Activation of cortical-NAc core inputs promotes compulsive alcohol intake in mice [50]. These studies raise the possibility that a TLR4-dependent pathway may account for other motivational disorders. In addition, abnormal novelty-seeking behavior has been linked to many neuropsychiatric disorders. For example, high novelty-seekers are more susceptible to drugs of abuse than low novelty-seekers [51, 52]. Reduced novelty-seeking is known to be associated with autism [6, 7] and Alzheimer’s disease [53]. These findings further imply that TLR4-mediated signaling is critical in many affective disorders. Thus, studying the interactions between Toll-like receptor-mediated immune signaling and motivational and affective disorders in the future will help our understanding of how immune activation affects relevant neuropsychiatric diseases.
In conclusion, the present study showed that TLR4 is involved in motivation-related behaviors under approach-avoidance conflict. Dysfunction of the mPFC-NAcSh neural circuit might contribute to this phenomenon. Future studies are required to address the detailed TLR4-mediated signaling in regulating this important behavior.
Acknowledgments
This work was supported by the National Basic Research Development Program (973 Program) of China (2013CB530902), the National Natural Science Foundation of China (91132712, 81571125, 81221003 and 81300979), the Natural Science Foundation of Zhejiang Province, China (LR12C09001 and Q13C090002), and the Fundamental Research Funds for the Central Universities of China (2014FZA7008). Chunlu Li was partly supported by a Chinese Postdoctoral Science Foundation grant (2015M570501).
Footnotes
Chunlu Li and Yixiu Yan have contributed equally to this work.
Contributor Information
Yi Shen, Email: yshen2@zju.edu.cn.
Yu-Dong Zhou, Email: yudongzhou@zju.edu.cn.
References
- 1.Phaf RH, Mohr SE, Rotteveel M, Wicherts JM. Approach, avoidance, and affect: a meta-analysis of approach-avoidance tendencies in manual reaction time tasks. Front Psychol. 2014;5:378. doi: 10.3389/fpsyg.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-P. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 4.Baker TB, Piper ME, Mccarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 6.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–664. doi: 10.1016/S0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 7.Greco B, Manago F, Tucci V, Kao HT, Valtorta F, Benfenati F. Autism-related behavioral abnormalities in synapsin knockout mice. Behav Brain Res. 2013;251:65–74. doi: 10.1016/j.bbr.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betz C, Mihalic D, Pinto ME, Raffa RB. Could a common biochemical mechanism underlie addictions? J Clin Pharm Ther. 2000;25:11–20. doi: 10.1046/j.1365-2710.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- 9.Karim R, Chaudhri P. Behavioral Addictions: An Overview. J Psychoactive Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- 10.Marazziti D, Presta S, Baroni S, Silvestri S, Dell’osso L. Behavioral addictions: a novel challenge for psychopharmacology. CNS Spectr. 2014;19:486–495. doi: 10.1017/S1092852913001041. [DOI] [PubMed] [Google Scholar]
- 11.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci USA. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okun E, Griffioen KJ, Mattson MP. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34:269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barak B, Feldman N, Okun E. Toll-like receptors as developmental tools that regulate neurogenesis during development: an update. Front Neurosci. 2014;8:272. doi: 10.3389/fnins.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 15.Moraga A, Pradillo JM, Cuartero MI, Hernández-Jiménez M, Oses M, Moro MA, et al. Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia. FASEB J. 2014;28:4710–4718. doi: 10.1096/fj.14-252452. [DOI] [PubMed] [Google Scholar]
- 16.Painsipp E, Koefer MJ, Sinner F, Holzer P. Prolonged depression-like behavior caused by immune challenge: influence of mouse strain and social environment. PLoS One. 2011;6:e20719. doi: 10.1371/journal.pone.0020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Toll-like receptors in the depressed and suicide brain. J Psychiatr Res. 2014;53:62–68. doi: 10.1016/j.jpsychires.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascual, M, Baliño, P, Alfonso-Loeches, S, Aragón, CM, Guerri, C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun 2011, Suppl 1: S80–91 [DOI] [PubMed]
- 19.Jacobsen J, Watkins L, Hutchinson M. Discovery of a Novel Site of Opioid Action at the Innate Immune Pattern-Recognition Receptor TLR4 and its Role in Addiction. Int Rev Neurobiol. 2014;118:129–163. doi: 10.1016/B978-0-12-801284-0.00006-3. [DOI] [PubMed] [Google Scholar]
- 20.Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, et al. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry. 2015;20:1525–1537. doi: 10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enter D, Colzato L, Roelofs K. Dopamine transporter polymorphisms affect social approach–avoidance tendencies. Genes Brain Behav. 2012;11:671–676. doi: 10.1111/j.1601-183X.2012.00791.x. [DOI] [PubMed] [Google Scholar]
- 22.Brooks AM, Berns GS. Aversive stimuli and loss in the mesocorticolimbic dopamine system. Trends Cogn Sci. 2013;17:281–286. doi: 10.1016/j.tics.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Brodkin ES, Hagemann A, Nemetski SM, Silver LM. Social approach–avoidance behavior of inbred mouse strains towards DBA/2 mice. Brain Res. 2004;1002:151–157. doi: 10.1016/j.brainres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3. San Diego: Academic Press; 2008. pp. 45–49. [Google Scholar]
- 25.Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- 26.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 28.Kaul D, Habbel P, Derkow K, Kruger C, Franzoni E, Wulczyn FG, et al. Expression of Toll-like receptors in the developing brain. PLoS One. 2012;7:e37767. doi: 10.1371/journal.pone.0037767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carty M, Bowie AG. Evaluating the role of Toll-like receptors in diseases of the central nervous system. Biochem Pharmacol. 2011;81:825–837. doi: 10.1016/j.bcp.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-α. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H, et al. Interleukin-1β alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci. 2013;33:12105–12121. doi: 10.1523/JNEUROSCI.5369-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long-Smith CM, Collins L, Toulouse A, Sullivan AM, Nolan YM. Interleukin-1β contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J Neuroimmunol. 2010;226:20–26. doi: 10.1016/j.jneuroim.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Hutchinson M, Northcutt A, Hiranita T, Wang X, Lewis S, Thomas J, et al. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J Neurosci. 2012;32:11187–11200. doi: 10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hutchinson MR, Watkins LR. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology. 2014;76:218–227. doi: 10.1016/j.neuropharm.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okun E, Barak B, Saada-Madar R, Rothman SM, Griffioen KJ, Roberts N, et al. Evidence for a developmental role for TLR4 in learning and memory. PloS One. 2012;7:e47522. doi: 10.1371/journal.pone.0047522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meredith G. The synaptic framework for chemical signaling in nucleus accumbens. Ann NY Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- 37.Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hikida T, Yawata S, Yamaguchi T, Danjo T, Sasaoka T, Wang Y, et al. Pathway-specific modulation of nucleus accumbens in reward and aversive behavior via selective transmitter receptors. Proc Natl Acad Sci USA. 2013;110:342–347. doi: 10.1073/pnas.1220358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pothuizen HH, Jongen-Rêlo AL, Feldon J. The effects of temporary inactivation of the core and the shell subregions of the nucleus accumbens on prepulse inhibition of the acoustic startle reflex and activity in rats. Neuropsychopharmacology. 2005;30:683–696. doi: 10.1038/sj.npp.1300643. [DOI] [PubMed] [Google Scholar]
- 43.Ghods-Sharifi S, Floresco SB. Differential effects on effort discounting induced by inactivations of the nucleus accumbens core or shell. Behav Neurosci. 2010;124:179–191. doi: 10.1037/a0018932. [DOI] [PubMed] [Google Scholar]
- 44.Johansson K, Hansen S. Novelty seeking and harm avoidance in relation to alcohol drinking in intact rats and following axon-sparing lesions to the amygdala and ventral striatum. Alcohol. 2002;37:147–156. doi: 10.1093/alcalc/37.2.147. [DOI] [PubMed] [Google Scholar]
- 45.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “Accumbens” part of the rat ventral striatum: Immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 47.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seif T, Chang S-J, Simms JA, Gibb SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–1100. doi: 10.1038/nn.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 52.Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daffner KR, Rentz DM, Scinto LFM, Faust R, Budson AE, Holcomb PJ. Pathophysiology underlying diminished attention to novel events in patients with early AD. Neurology. 2001;56:1377–1383. doi: 10.1212/WNL.56.10.1377. [DOI] [PubMed] [Google Scholar]