Abstract
An increasing body of neuroimaging and electrophysiological studies of the brain suggest that the insular cortex (IC) integrates multimodal salient information ranging from sensation to cognitive-affective events to create conscious interoception. Especially with regard to pain experience, the IC has been supposed to participate in both sensory-discriminative and affective-motivational aspects of pain. In this review, we discuss the latest data proposing that subregions of the IC are involved in isolated pain networks: the posterior sensory circuit and the anterior emotional network. Due to abundant connections with other brain areas, the IC is likely to serve as an interface where cross-modal shaping of pain occurs. In chronic pain, however, this mode of emotional awareness and the modulation of pain are disrupted. We highlight some of the molecular mechanisms underlying the changes of the pain modulation system that contribute to the transition from acute to chronic pain in the IC.
Keywords: Insular cortex, Pain, Emotion, Neural network, Dopamine, GABA, Molecular mechanism
Introduction
In rodents, the insular cortex (IC) is a portion of the cerebral cortex folded deeply within the lateral sulcus surrounding the rhinal fissure - hidden by the frontal and temporal opercula [1]. Based on its cytoarchitecture, the IC has been artificially divided into three main subregions [2], the agranular IC surrounding the rhinal fissure, the dysgranular IC situated dorsal to the rhinal fissure, and the granular IC located just ventral to the secondary somatosensory cortex. Along the ventro-dorsal and rostro-caudal axes, the cortex ranges from agranular to granular, the middle region representing transitional dysgranular cortex [3]. This cytoarchitecture of the IC corresponds with its connectivity patterns and functions in an internal disassociation in pain processing. The posterior IC (PI) participates in the somatosensory features of pain, while the anterior portion (AI) preferably mediates its affective aspects [4]. Despite its engagement in multiple types of information processing, the contribution of this region to pain modulation through bottom-up stimulus input or top-down conscious anticipation has been neglected. Yet disruption of the pain modulation circuitry in chronic pain involving the insula may in turn affect its involvement in cognitive and affective disorders. In parallel with our understanding, accumulating clinical evidence shows that chronic pain can lead to anatomical and functional alterations in the IC which are correlated with cognitive and affective disorders [5]. Furthermore, interventions such as meditation have significant protective effects on grey matter and the connectivity within these circuits [6, 7]. Particularly in the IC, altered grey matter is almost reversed to normal after effective therapy for trigeminal neuralgia [8].
To date, however, no insular functions have been associated with macroscopic changes in the emotional network and modulatory circuitry, and neural or molecular events that drive chronic pain. Here, we propose that ongoing noxious inputs lead to a series of molecular events that result in a quantitative imbalance of modulatory receptors such as GABAergic and dopamine receptors. Our previous study showed that persistent pain can bring about a series of neural plastic changes mediated by the synaptic GluA1 subunit of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) [9]. These molecular alterations may underpin the disturbance of modulatory circuitry in the insula.
Functional Profile of the Posterior Insular Cortex in Pain Sensation
The PI mainly receives nociceptive and thermoceptive afferents from the ventral posterior inferior nucleus and the ventromedial posterior nucleus of the thalamus, which receive input from spinothalamic neurons of lamine I [10]. Functional imaging data have shown that the caudal granular part of the PI is functionally connected to primary and secondary motor and somatosensory cortices in the macaque and this is consistent with its anatomical connectivity [11, 12]. It is worth noting that the PI also has intimate connections with the medial operculum. Neuroimaging and lesion studies have demonstrated that the two regions function as a whole, especially in thermo-nociceptive sensation [13, 14]. The connectivity profile between the IC and the cingulate cortex further demonstrates that the PI plays a role in the sensory dimension of pain. Distinct anatomical connections identified in non-human primates have shown that the PI is mainly connected with the mid-cingulate cortex [15], which is involved in pain sensation. In addition, brain imaging studies have revealed co-activation of the PI and the posterior mid-cingulate cortex across specific pain conditions [16, 17], suggesting that the PI is part of the cortical network specifically devoted to the sensory features of pain (Table 1). It is likely that the extent of activation of the PI reflects the intensity of a noxious stimulus independent of its quality [18]. In addition to evoked pain, it seems that the PI is also involved in chronic pain. A recent study tracking the evolving course of chronic pain in humans found that, among many pain-related areas, the degree of activation of the PI was most consistent with the progress of chronic pain [19]. Furthermore, the caudal granular IC (CGIC), a subregion of the PI, has been demonstrated to be a key supraspinal site that participates in the maintenance of persistent pain [20]. A lesion study has shown that lesioning the CGIC results in the long-term alleviation of allodynic manifestations in the rat [20]. Electrophysiological explorations have further suggested that a spinal-CGIC-primary somatosensory (S1)-spinal positive-feedback loop contributes to the maintenance of allodynia. However, since the spinal-insula pathway differs between rodents and humans, more clinical evidence is needed to determine whether such a positive-feedback loop applies to humans. Taken together, the PI is part of the pain-related cortical networks with a preference for the sensory aspect, and studies of both the development of chronic pain and the sequelae of lesions have demonstrated that the PI plays a fundamental role in the chronicity of pain. Nevertheless, the specific mechanisms by which pain emerges from nociception in the PI need further investigation.
Table 1.
Connectivity profile and functional network of the posterior insular cortex (PI).
| Brain level | Specific regions | Fiber connections | Possible functions |
|---|---|---|---|
| Thalamus | ventral posterior inferior and ventromedial posterior nuclei [4] | Afferent fibers from thalamus to PI | Path for nociceptive input |
| Supraspinal | Operculum [13, 14, 17] and mid-cingulate cortex [13, 14, 17] | Reciprocal fibers between mid-cingulate cortex/operculum and PI | Gating system involved in general salience and response selection |
| Cortices | Primary and secondary somatosensory (S1, S2), and motor [11, 12] | Reciprocal fibers between S1, S2/motor cortices and PI | Pathological function in persistence of allodynia |
Functional Profile of the Anterior Insular Cortex in Pain Perception
The AI is a portion of the insula that lies in the anterior part of the central sulcus in humans. The AI is divided into two subregions: an agranular region of the ventral AI and a dysgranular region of the dorsal anterior to middle IC. In general, the dysgranular area is gustatory cortex [21], whereas the agranular area is proposed to be involved in the regulation of physiological changes associated with emotional states [22]. Neural connections between the rostral agranular IC (RAIC) and nociception-related sites have been systemically explored using neural tracing methods [22]. Unlike the PI, of which the main connections are confined to thalamic nuclei (the ventromedial posterior nucleus) and somatosensory areas, most connections of the RAIC are with multiple sites involved in the affective aspects of pain as well as the descending inhibitory system (Table 2). The RAIC mainly receives input from the medial nuclei of the thalamus associated with the affective components of nociceptive processing [23]. A tracing study has also revealed that the RAIC is extensively interconnected with the ventral prelimbic and infralimbic cortices, which mediate both somatic nociceptive and visceral input [22]. In addition, the RAIC is cytoarchitectonically close to limbic cortex [7]. All this morphological evidence strongly indicates that the AI, especially the rostral part, is intimately associated with the emotional processing of nociception.
Table 2.
Connectivity profile and functional network of the anterior insular cortex (AI).
| Brain level | Specific regions | Fiber connections | Possible function |
|---|---|---|---|
| Thalamus | central lateral and submedial nuclei [23] | Afferent fibers from thalamus to AI | Receive emotional nociceptive and visceral input |
| Subcortical limbic areas | Amygdala [24] and accumbens [22] | Efferent fibers to amygdala and accumbens | Modulation of pain through direct (accumbens) and indirect (amygdala) impact on descending pain modulation system |
| Prelimbic and infralimbic cortices | Anterior cingulate (ACC) and medial prefrontal (PFC) [25] | Reciprocal fibers between ACC/PFC and AI | Gating nociceptive thalamic input to ventral prelimbic/infralimbic cortex [7] |
As stated in the James-Lange theory of emotion, our emotions are caused by our interpretation of bodily reactions [26]. That is, our nervous system responds to physiological changes, primarily mediated by the autonomic system, with emotional experiences. What are the specific processes underlying such emotional responses to painful experiences? Several brain regions are involved, among which the AI seems to be the critical site where emotional awareness and autonomic responses are represented cortically. However, the AI has to cooperate with other regions contributing to emotion to accomplish the emotional aspect of pain. The amygdala is one candidate structure; it interacts with the insula and shares many functions with the AI [24]. The AI possesses such extensive reciprocal projections with the basal nucleus of the amygdala that the two regions form the insula-amygdala circuit [24]. In this circuit, the IC receives nociceptive thalamic inputs to create emotional awareness and then sends its modulatory signal to the amygdala, which is in turn connected to the hypothalamic nuclei and the periaqueductal gray (PAG). Through this path, salient noxious information would arouse emotional responses and activate the descending pain modulatory system. This emotion-related circuit can partly explain some of the common autonomic reactions in daily life. As pain is a complex perception in which sensation, emotion, and cognition interweave, conscious perception and the unpleasantness of pain are mirrored by behavioral reactions (e.g. escape or sweating due to suffering), which require activity in wide brain regions. The cortex is a densely-connected network of regions, with reciprocal information flow between multiple hierarchically-organized groups of cells [27]. By analyzing the functional connectivity of resting-state activity, researchers have identified networks such as the task-negative network [i.e., the default mode network (DMN)] and anti-correlated task-positive networks [i.e., the central executive network (CEN)] that can be activated by salient stimuli such as a painful event [25, 28, 29]. Besides, there is another network called the salience network (SN), in which the AI and anterior cingulate cortex (ACC) comprise core nodes. It has been revealed that activation of these networks reflects specific types of sensory and cognitive functions [30]. Using Granger causality analyses, researchers have found that the right AI plays a core role in initiating the causal signal of switching between major networks [25, 31], which usually display competitive interactions in cognitive information processing. That pain is intrinsically salient (i.e., stands out relative to other stimuli) implies autonomic involvement of the SN. The process of filtering nociceptive information to higher levels involves the SN and CEN, during which the AI facilitates information of great valiance (e.g. a painful cue) access to attentional control and memory resources by activating the CEN and deactivating the DMN [32]. In this way, an individual can form a proper emotional awareness of pain.
Neural Mechanisms of Pain Modulation by Bottom-Up and Top-Down Information Modalities
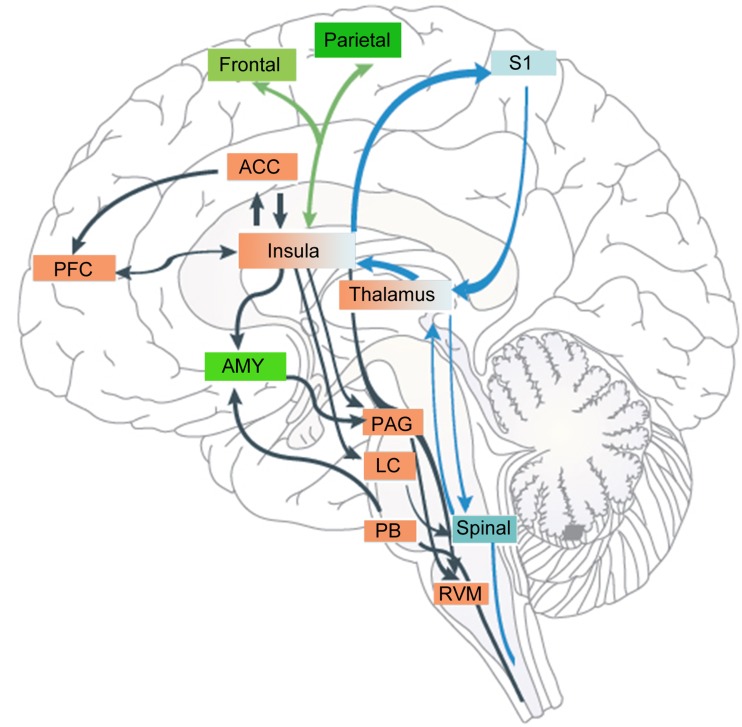
As the IC has abundant connections with other limbic areas and primary sensory areas, and is involved in empathy, attention, and pain [33], it is reasonable to speculate that it is also involved in the modulation of pain by various attention-demanding sources of information ranging from top-down cognition to bottom-up tactile input (Fig. 1).
Fig. 1.
The anterior insular cortex (AI) and the posterior insular cortex (PI) are involved in different pain circuits that mediate different aspects of pain. The AI serves as an interface where attention, anticipation, or belief shape pain perception by activating cognitive areas such as the prefrontal (PFC) and anterior cingulate (ACC) cortices and attention areas such as the frontal-parietal cortex and amygdala (AMY). Through the AI, the modulatory signal reaches the descending pain modulation system, whereas the PI plays a fundamental role in chronic pain maintenance through a spinal-CGIC-S1-spinal positive-feedback circuit. CGIC caudal granular insular cortex, LC locus coeruleus, Spinal spinal cord, PAG periaqueductal grey, PB parabrachial nucleus, RVM rostroventral medulla, S1 primary somatosensory cortex.
A pain-tactile interaction was previously thought to occur in a gate-control mechanism at the spinal level. Recently, using magnetoencephalography, researchers have found that cortical responses to noxious stimuli are inhibited by innocuous tactile stimuli [34]. They recorded cortical magnetic responses to paired noxious (intra-epidermal electrical stimulation, IES) and innocuous (transcutaneous electrical stimulation, TS) stimuli. Activity in the dorsal insula that creates the major magnetic component evoked by IES is almost completely suppressed by TS [35]. Apart from the interaction of touch and pain, clinical studies have shown that chronic post-stroke pain can be reduced by caloric vestibular stimulation [36]. The analgesic effect is attributed to vestibular activation of the PI, leading in turn to inhibition of the ACC and other pain-related regions. This bottom-up modulation of pain perception by other somatosensory modalities (e.g. touch) is common, and the IC serves as an interface for the interaction between pain and other bottom-up sensory modalities. Initial nociception is more or less weakened by tactile or vestibular stimuli in the competition for access to attentional and cognitive resources.
The subjective perception of painful stimulation can also be affected in a top-down pattern by emotional events that distract attention. It is known from animal studies that the AI is connected to brainstem structures such as the PAG, rostral ventromedial medulla, and parabrachial nucleus [22], which provides a morphological basis for how attention might influence pain perception. Anatomically, the SN is closely linked to the frontoparietal attention network [37]. Evidence for involvement of the frontoparietal attention network in the cross-modal shaping of pain comes from a recent fMRI study, which showed that pain-related modulation of hemodynamic responses by affective pictures involve interactions between the IC and other cortical structures, including the frontoparietal attention network [38]. An electrocorticographic study investigated the functional connectivity between the somatosensory cortex and the IC during the processing of attended and unattended noxious stimuli [39], during which the former is likely to involve both the attention [37] and the salience [40] networks. BOLD responses in the AI have a significantly shortened latency to painful laser stimuli versus non-painful laser stimulation, suggesting that the AI responds preferably to pre-noxious cues [41]. This anticipation of nociception often elicits analgesic effects that imply a process of cognitive–attentional integration in the AI. A recent study of top-down modulation of pain-induced oscillations suggests that attention to pain induces an increase in gamma band power, localized in the insula [42]. However, the analgesic effect depends largely on cognition and belief of the individual. For instance, in an experimental setting, the insula may encode a safety signal that tunes afferent sensory processing mechanisms to reflect the prior information that the noxious stimulus is safe and well-controlled.
Converging lines of evidence from studies of pain anticipation suggest that the IC may play a role in pain modulation by tuning the responsiveness of other areas via cortico-cortical interactions [43]. When a noxious stimulus is sensed by vision, pain-related regions and structures of the top-down attention network are recruited in an anticipatory manner through interactions with the visual cortex. Compared with the processing of unexpected pain stimuli, this leads to enhanced local oscillatory activity as well as to increased functional connectivity within pain-related regions among the sensorimotor cortex and the SN. Previous morphological studies have also shown that the dorsal visual system has direct anatomical projections to the AI [44]. This pathway may help to generate signals reflecting the saliency of visual stimuli in extrapersonal space. This pattern of pain modulation by the IC is also applicable to the analgesic effect of hypnosis, which has been confirmed by electrocorticographic studies investigating functional connectivity between the somatosensory, medial frontal, and IC during the processing of attended and unattended noxious stimuli [39]. However, whether anticipation produces analgesia or exacerbation of pain depends on the individual’s expectation of the upcoming noxious stimulus according to cues in the context of their own values. Apart from anticipation, it is likely that complex cognitive information like mood, maladaptive experience, and expectation may flow from various brain networks involving the amygdala, hippocampus, ACC, and PFC to the AI to be integrated with nociceptive information [3, 45, 46]. In such a way, the individual creates a comprehensive emotional awareness of the pain-related aversive state.
Taken together, both the bottom-up (touch-pain interaction) and top-down (anticipation-pain interaction) modulation of pain perception involve the IC [47]. In this process, the IC serves as an interface where multimodal information is synthesized and integrated with nociception. Given that the AI is the key node of the SN, the modulation of pain is indeed a process of enhancement or attenuation of the salience and quality of pain. One distinguishing feature of the IC is that it contains a special group of large, spindle-shaped neurons referred to as von Economo neurons (VENs) [48] in its anterior part. VENs are projection neurons ~4.6 times larger than neighboring pyramidal neurons and are proposed to be well-suited for the rapid, long-distance integration of information [49, 50]. A clinical lesion study of patients with damage to the IC found increased pain ratings relative to normal controls [47], indicating that the cognitive modulation of pain through top-down signal modulation may be disrupted by insular lesions. While the evidence on the lateralization of pain modulation is controversial, a lesion study in rodents has demonstrated that lesioning the RAIC produces a significant decrease in pain-related behaviors, regardless of the side of the lesion [51]. In humans, however, laterality is common in insular functions. Right AI activation is absent in empathy for positive emotions and is present only in empathy for negative emotions such as pain, whereas the left AI is activated by all valence categories [52]. Yet further investigations using causal modeling of neuroimaging data are needed to determine whether asymmetrical information flow between the left and right AI occurs.
Disruption of the Insula-Mediated Functional Network in Chronic Pain
A recent meta-analysis suggests that clinical pain processing differs from experimental pain processing [53], and the question arises whether the perceived intensity of clinical and experimental pain is differently encoded or modulated. Some clinical studies have suggested that clinical pain is represented significantly more rostrally in the insula [54], and the neural representation of aversive emotion is located close to that of clinical pain in the IC. Intriguingly, in healthy volunteers, pain can be shifted to the right AI only when there is either a cognitive or emotional modulation of the experience: for example, explicitly paying attention to a painful stimulus.
Essentially, this abnormal activation pattern of the IC in clinical chronic pain is associated with the disruption of networks involving the insula. Studies have described selective alterations in white matter connectivity between atrophied regions (including the AI) in complex regional pain syndrome [55]. However, gray matter volume in patients with trigeminal neuropathic pain is reduced in the AI, but an increased volume has been noted in the PI [56]. The abnormalities in local gray and white matter restructuring under chronic pain conditions may underlie disruptions of the intrinsic system that maintains normal pain modulation. Just as distinct neurodegenerative diseases involve the atrophy and degradation of their corresponding resting state networks [57], the specific condition of chronic pain results in unique patterns of network reorganization. Data adapted from Cauda et al. show that the DMN displays increased connectivity to the AI and PI in patients suffering from diabetic neuropathic pain [58]. Similar results have been reported from lab research on the resting state in patients with chronic back pain [59]. However, other results show that female patients with irritable bowel syndrome (a chronic pain condition) display greater negative functional connectivity of the dorsal AI with the medial PFC [60]. The discrepancy here is largely attributable to differences in techniques and methods, but it remains to be determined whether aberrant functional connectivity between the DMN and the insula reflects increased attention on pain. If so, uneven activation of CEN and DMN from the insula may partly account for the altered encoding of nociception in chronic pain.
With the chronification of pain, individuals are likely to develop mood disorders such as depression. The affective component of pain includes feelings of anxiety and depression in response to a noxious stimulus, and depression is one of the most common disorders in chronic pain patients [61]. In particular, depression and pain share a high degree of comorbidity. A recent review covering emotional impact of neuropathic pain addresses the question of whether the anxio-depressive consequences of neuropathic pain result from a long-lasting exposure to aversive emotion [62]. However, the functions of the insula in pain aversion and the anxio-depressive consequences of chronic pain are still unclear. Nevertheless, neuroimaging evidence has shown that the functional connectivity of the nucleus accumbens, core to the IC, and the primary and secondary somatosensory cortex (S1/S2) is significantly decreased in depressive rats, in parallel with its functional connectivity to the insula and the S1/S2 cortices in neuropathic pain model. In fact, the process of pain chronification is associated with a shift of brain activation from sensory to affective-emotional circuitry [63]. In a study on patients with neurogenic pain, enhanced EEG power has been reported in the high theta (6–9 Hz) and low beta frequency ranges (12–16 Hz) localized to the insula and other pain- and emotion-associated cortical areas [64]. Investigations of the functional organization of the IC in depressed patients complicated by the allodynia sign have revealed that emotion-related activation peaks are shifted to the dorsal AI that are associated with physical pain in healthy individuals [65]. This result suggests that emotional processing in the IC of patients with major depressive disorder is topologically shifted towards the area involved in pain processing, which makes this group of patients feel as if they are in pain—“emotional allodynia”. However, whether this shift in emotional processing circuitry towards pain-processing circuitry within the IC is a vulnerability factor for the development of psychiatric and/or psychosomatic disorders remains to be investigated.
Taken together, whether in the dynamic evolution of pain chronification or the emotional consequence of chronic pain (depression, anxiety), the IC displays discrepant activation pattern and anatomical changes. Essentially, these alterations largely reflect dysfunction of pain modulation system involving the insula that encompasses its connectivity with the CEN and DMN. Recently, some studies on chronic pain suggest that anatomical changes in the brain can be partly reversed by psychological intervention [8]. Pain perception has been shown to be modulated by cognitive measures such as cognitive behavior therapy [66] through a modulation system mediated by the AI. Using real-time functional magnetic resonance imaging (rt-fMRI), volitionally influencing activation of the AI by operant conditioning has been shown to result in a decrease of subjective pain perception [67]. A clinical study found that among the changes in gray matter in the ACC and PFC across numerous chronic pain conditions, the ventral AI is the only region returning to normal following effective treatment of trigeminal neuralgia [8].
The Neurochemical and Molecular Mechanisms Underlying Pain-Related Changes in the Insular Cortex
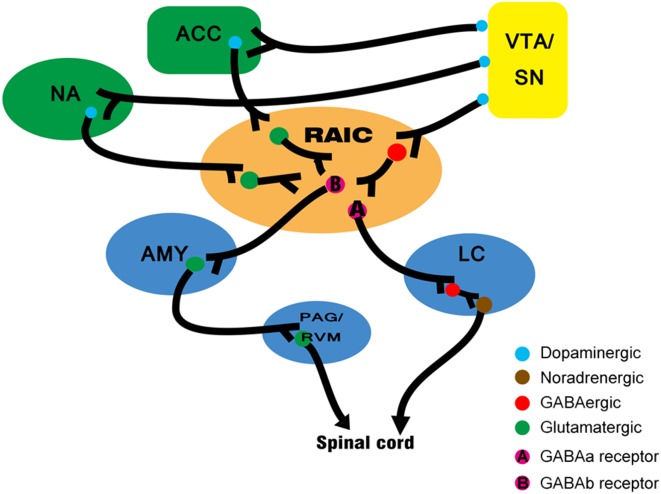
As noted above, pain can be altered by mood, attention, and cognition. However, the neurochemical basis of the cortical modulation of pain are obscure [68]. Synthesizing recent study on the cerebral modulation of pain, we conjecture that a ventral tegmental area (VTA)/SN-insula-amygdala/PAG circuit may participate in this process (Fig. 2).
Fig. 2.
Neural mechanisms underlying pain modulatory circuits involving the rostral agranular IC (RAIC). Neurons in the RAIC receive dopaminergic fibers from the VTA/SN. In the RAIC, there are close appositions between dopaminergic fibers and GABAergic interneurons. Then, GABAa neurons project to the locus coeruleus (LC), while GABAb neurons project to the amygdala (AMY). Altogether, by the activation of D1 and D2 receptors, dopaminergic modulation of the RAIC occurs through different types of GABAergic interneurons. The circuit provides potential access for reward-motivation information to pain modulation system. PAG periaqueductal gray, RVM rostroventral medulla, SN salience network, VTA ventral tegmental area.
The presence of D1 and D2 dopamine receptors in the RAIC has been documented [69, 70]. Morphological studies have shown that the RAIC has a close relationship with the mesolimbic dopaminergic system and it receives projections from the VTA [71]. Also, this dopaminergic system is involved in the modulation of chronic nociception [72, 73]. A previous study investigated the specific effects of D1 and D2 in modulating neuropathic pain and found a pro-nociceptive opponent role of insular D1 and D2 receptors in the development of neuropathic pain. Application of antagonists and agonists of D1 and D2 receptors in a rat modal of neuropathic pain showed that D2 receptor activation and D1 receptor blockade in the IC both diminish the score and incidence of autotomy due to neuropathic pain and delay its onset.
Another known modulatory element is the GABA receptor. GABA receptors in the RAIC act on at least two independent subcortical systems to modulate the nociceptive threshold [68]. The first, from the RAIC to the locus coeruleus, influences the noradrenergic bulbospinal projections and is modulated predominantly by GABAa receptors. The pyramidal neurons in the RAIC send excitatory projections to the ventrolateral PAG, through which the modulation signal arrives in the spinal via the descending pain modulatory system [74]. The second, from the RAIC to the amygdala, is modulated predominantly by GABAb receptors. Because GABAb receptors are metabotropic, their function is probably to keep cortical afferents to the amygdala under prolonged inhibition. Generally, the cerebral cortex modulates pain by acting on both pro-nociceptive and anti-nociceptive circuits. This dual effect is a defining feature of endogenous pain modulation system [75, 76].
Intriguingly, GABAergic and dopaminergic modulatory systems are not isolated. Using immunocytochemistry and path-tracing techniques, researchers have found close appositions between dopamine fibers and GABAergic interneurons in the RAIC. This suggests that part of the dopaminergic modulation of the RAIC occurs through GABAergic interneurons. This crucial connection provides a promising link between the reward and pain modulation systems. However, more evidence is required concerning their functional connections under specific conditions.
Under chronic pain conditions, however, the RAIC mainly modulates pain perception through GABAb projections to the amygdala, which belongs to a pro-nociception (pain-facilitating) circuit [53]. Do persistent noxious inputs alter modulatory systems in insula? There is now accumulating evidence that some brain abnormalities may indeed develop as a consequence of ongoing nociceptive input [77]. Neuroplastic change in chronic pain is a type of aversive learning, which drives changes in brain structure and function in the long run. Just like hippocampal neuroplastic changes during learning, the insula undergoes analogous synaptic changes in pain chronification. Our previous study suggested that activity-dependent plasticity occurs in the IC after nerve injury [78]. The plastic changes are associated with a long-term increase in the amount of synaptic N-methyl-D-aspartate receptors (NMDARs), which initiate the long-term potentiation (LTP) of AMPAR-mediated synaptic responses. Such plasticity triggered by nerve injury shares common mechanisms with electrically-induced LTP. Nevertheless, NMDARs cannot account for all the plasticity after nerve injury, as application of the NMDAR antagonist AP-5 does not completely block LTP in the insula. Moving forward, our work has shown that peripheral nerve ligation triggers the enhancement of AMPAR-mediated excitatory synaptic transmission in the mouse IC. And this enhancement relies on an increased amount and sensitivity of the synaptic GluA1 subunit of AMPARs, but not the GluA2/3 subunit [9]. The specific process may be phosphorylation of GluA1 at the Ser845 site attributable to the activation of adenylyl cyclase 1 and protein kinase A (PKA) that may enhance synaptic AMPARs by inhibiting their endocytosis [79] or increasing the exocytosis of GluA1 to extrasynaptic sites on the plasma membrane and priming AMPARs for synaptic delivery [80].
In central synapses, AMPAR-mediated responses undergo rapid and long-term increases or decreases in an activity-dependent manner, whereas NMDAR-mediated responses are relatively stable [81–83]. Recent reports have suggested that NMDA receptor activation results in a rapid decrease in the affinity of GluR2-containing AMPARs in chronic pain models [84, 85]. Collectively, both an increase in GluR1-containing AMPARs and the absence of GluR2-containg AMPARs at the postsynaptic membrane boost the synaptic permeability of Ca2+. This enhanced synaptic transmission cannot completely account for the changes caused by chronic pain, however, as chronic pain may alter the cognitive and emotional modulation of pain.
A previous study on the organization of dynamic mechanical allodynia processing in the rat IC under neuropathic conditions showed that an innocuous air-puff induces a dramatic increase in the number of pERK-1/2-immunopositive neurons in the IC on both sides [86]. The results indicate that trigeminal nerve injury significantly alters the IC processing of tactile stimuli and that ERK phosphorylation contributes to the mechanisms underlying abnormal pain perception. It has been demonstrated that pERK signaling is involved in the affective dimension of pain disorders as well as the pathophysiology and treatment of anxio-depressive disorders [87]. Thus, hyper-reactivity of pERK in the IC may reflect the enhanced signaling of insular neurons, which causes altered processing of normal tactile stimuli as aversive. Furthermore, neuronal ERK-1/2 phosphorylation is confined to layers II–III of the rostral dysgranular IC and agranular IC, where nociception-specific neurons are found. In parallel, clinical human imaging has also revealed that brush-evoked allodynia mainly activates the RAIC in patients with neuropathic pain. However, possible implications of activating this pERK signaling on neurotransmitter release and function remain to be investigated.
Such a heightened signal level in the RAIC can also be elicited by aversive stimuli such as stress. A study on stress-pain interactions showed that the expression of pCREB and c-Fos is increased in the rat AI after forced swimming (FS), and this increase is correlated with the enhancement of CFA (complete Freund’s adjuvant)-induced thermal hyperalgesia [88]. It has been suggested that the higher the signal level in the AI before stimulus application, the more likely the subsequently applied stimulus is to be perceived as painful [89–91]. As the AI has access to information on the emotional aspects of an experience [92], neuroplastic changes in the AI after FS enhance the CFA-induced hyperalgesia. Here, activation of pCREB and c-Fos in the AI after FS elevate the signal level of pre-stimulus anxiety, which enhances the thermal hyperalgesia after injection of CFA [88]. In addition, the activation of pCREB is mainly confined to the RAIC [88], local changes in gamma-aminobutyric acid (GABA) and dopamine levels can alter the nociceptive threshold in awake animals [68, 93]. Thus, it is tempting to believe that in the process of stress-induced hyperalgesia, the RAIC may undergo neuroplastic changes via activation of pCREB and c-Fos in the AI after aversive stimuli, which leads to dysfunction of the endogenous pain modulatory system.
Altogether, continuous aversive inputs (pain) trigger NMDAR/AMPAR-dependent LTP, which results in synaptic permeability to Ca2+. The increased levels of intracellular Ca2+ activate spinal PKC and indirectly lead to the activation of PKA through increased calmodulin and cAMP [94]. Subsequently, cAMP activates PKA. PKA then translocates to the nucleus and phosphorylates CREB. The activation of PKA and PKC contributes to ERK activation, which is coupled to phosphorylation of CREB at the serine 133 site [95] and expression of c-Fos. Studies in patients with chronic pain have shown that altered neurochemistry in brain systems (including the insula) may be involved in psychologically-modulated analgesia [96]. We speculate here that the altered neurochemistry may be elicited by NMDAR-mediated neural maladaptation, because it has been shown that NMDAR activation is involved in trafficking of D1 receptors at the neuronal surface in striatal neurons [88, 97–99]. Hence, stress/persistent painful input-induced hyperalgesia through activation of pCREB and c-fos in the RAIC may affect pain modulation by remodeling local GABAergic and dopaminergic receptors.
Conclusions and Implications
In summary, evidence from animal, preclinical, and clinical studies suggests that the IC is involved in both the sensory and affective dimensions of pain. Particularly, in the shaping of pain perception, the AI serves as a critical site where multimodal information competes and integrates with nociception to create an awareness of the body state. The progression to chronic pain reflects not only impairment of the normal functional activity of the insula, but also the disruption of specific circuits that modulate pain-related emotional awareness involving the IC. It is likely that plastic changes involving glutamatergic receptors touch off the onset of pain chronification, and subsequent pERK signaling pathways underpin the abnormal activation of the pain-processing system through dysfunction of pain-modulating receptors such as GABAergic and dopaminergic receptors. However, this mode of action of the IC in pain requires longitudinal studies involving more preclinical studies based on clinical observations. Besides, although most studies propose involvement of the AI and PI in isolated functional networks of pain, as there are intimate reciprocal links between AI and PI, evidence on functional interactions within the insula is imperative for a deeper insight into the role of the insula in pain. Clinically, whether reversing changes within the IC can alleviate chronic pain is not yet exactly known, however, targeting this point might provide new therapies for chronic pain.
Acknowledgments
This review was supported by the National Natural Science Foundation of China (31371120) and the Foundation for Returned Overseas Students of Ministry of Education, China (HG3503).
Footnotes
Changbo Lu and Tao Yang have contributed equally to this review.
References
- 1.Ture U, Yasargil DC, Al-Mefty O, Yasargil MG. Topographic anatomy of the insular region. J Neurosurg. 1999;90:720–733. doi: 10.3171/jns.1999.90.4.0720. [DOI] [PubMed] [Google Scholar]
- 2.Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- 3.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 4.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty O, McGuire BE, Finn DP. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 2011;93:385–404. doi: 10.1016/j.pneurobio.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, et al. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011;191:36–43. doi: 10.1016/j.pscychresns.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lutz A, McFarlin DR, Perlman DM, Salomons TV, Davidson RJ. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSouza DD, Davis KD, Hodaie M. Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia. Pain. 2015;156:1112–1123. doi: 10.1097/j.pain.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 9.Qiu S, Zhang M, Liu Y, Guo Y, Zhao H, Song Q, et al. GluA1 phosphorylation contributes to postsynaptic amplification of neuropathic pain in the insular cortex. J Neurosci. 2014;34:13505–13515. doi: 10.1523/JNEUROSCI.1431-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD, Bushnell MC, Zhang ET, Blomqvist A. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770–773. doi: 10.1038/372770a0. [DOI] [PubMed] [Google Scholar]
- 11.Frot M, Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438–450. doi: 10.1093/brain/awg032. [DOI] [PubMed] [Google Scholar]
- 12.Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Cortical representation of pain in primary sensory-motor areas (S1/M1): a study using intracortical recordings in humans. Hum Brain Mapp. 2013;34:2655–2668. doi: 10.1002/hbm.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner U, Tiede W, Treede RD, Craig AD. Laser-evoked potentials are graded and somatotopically organized anteroposteriorly in the operculoinsular cortex of anesthetized monkeys. J Neurophysiol. 2006;96:2802–2808. doi: 10.1152/jn.00512.2006. [DOI] [PubMed] [Google Scholar]
- 14.Frot M, Rambaud L, Guenot M, Mauguiere F. Intracortical recordings of early pain-related CO2-laser evoked potentials in the human second somatosensory (SII) area. Clin Neurophysiol. 1999;110:133–145. doi: 10.1016/S0168-5597(98)00054-9. [DOI] [PubMed] [Google Scholar]
- 15.Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- 16.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 17.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peltz E, Seifert F, DeCol R, Dorfler A, Schwab S, Maihofner C. Functional connectivity of the human insular cortex during noxious and innocuous thermal stimulation. Neuroimage. 2011;54:1324–1335. doi: 10.1016/j.neuroimage.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. 2015;18:499–500. doi: 10.1038/nn.3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benison AM, Chumachenko S, Harrison JA, Maier SF, Falci SP, Watkins LR, et al. Caudal granular insular cortex is sufficient and necessary for the long-term maintenance of allodynic behavior in the rat attributable to mononeuropathy. J Neurosci. 2011;31:6317–6328. doi: 10.1523/JNEUROSCI.0076-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa H, Hasegawa K, Murayama N. Difference in taste quality coding between two cortical taste areas, granular and dysgranular insular areas, in rats. Exp Brain Res. 1992;91:415–424. doi: 10.1007/BF00227838. [DOI] [PubMed] [Google Scholar]
- 22.Jasmin L, Burkey AR, Granato A, Ohara PT. Rostral agranular insular cortex and pain areas of the central nervous system: a tract-tracing study in the rat. J Comp Neurol. 2004;468:425–440. doi: 10.1002/cne.10978. [DOI] [PubMed] [Google Scholar]
- 23.Cliffer KD, Burstein R, Giesler GJ., Jr Distributions of spinothalamic, spinohypothalamic, and spinotelencephalic fibers revealed by anterograde transport of PHA-L in rats. J Neurosci. 1991;11:852–868. doi: 10.1523/JNEUROSCI.11-03-00852.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stehberg RM-AaJ. The Insular Cortex and the Amygdala: Shared Functions and Interactions. InTech 2012.
- 25.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane RD, Schwartz GE. Levels of emotional awareness: a cognitive-developmental theory and its application to psychopathology. Am J Psychiatry. 1987;144:133–143. doi: 10.1176/ajp.144.2.133. [DOI] [PubMed] [Google Scholar]
- 27.Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb Cortex. 2006;16:355–365. doi: 10.1093/cercor/bhi114. [DOI] [PubMed] [Google Scholar]
- 35.Inui K, Tran TD, Qiu Y, Wang X, Hoshiyama M, Kakigi R. A comparative magnetoencephalographic study of cortical activations evoked by noxious and innocuous somatosensory stimulations. Neuroscience. 2003;120:235–248. doi: 10.1016/S0306-4522(03)00261-6. [DOI] [PubMed] [Google Scholar]
- 36.Ramachandran VS, McGeoch PD, Williams L, Arcilla G. Rapid relief of thalamic pain syndrome induced by vestibular caloric stimulation. Neurocase. 2007;13:185–188. doi: 10.1080/13554790701450446. [DOI] [PubMed] [Google Scholar]
- 37.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 38.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Flexible cerebral connectivity patterns subserve contextual modulations of pain. Cereb Cortex. 2011;21:719–726. doi: 10.1093/cercor/bhq146. [DOI] [PubMed] [Google Scholar]
- 39.Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123:244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Keil J, Muller N, Ihssen N, Weisz N. On the variability of the McGurk effect: audiovisual integration depends on prestimulus brain states. Cereb Cortex. 2012;22:221–231. doi: 10.1093/cercor/bhr125. [DOI] [PubMed] [Google Scholar]
- 41.Pomares FB, Faillenot I, Barral FG, Peyron R. The ‘where’ and the ‘when’ of the BOLD response to pain in the insular cortex. Discussion on amplitudes and latencies. Neuroimage. 2013;64:466–475. doi: 10.1016/j.neuroimage.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 42.Hauck M, Domnick C, Lorenz J, Gerloff C, Engel AK. Top-down and bottom-up modulation of pain-induced oscillations. Front Hum Neurosci. 2015;9:375. doi: 10.3389/fnhum.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia– imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- 44.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- 46.Friedman DP, Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol. 1986;252:348–373. doi: 10.1002/cne.902520305. [DOI] [PubMed] [Google Scholar]
- 47.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, et al. Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci. 2009;29:2684–2694. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seeley WW, Merkle FT, Gaus SE, Craig AD, Allman JM, Hof PR. Distinctive neurons of the anterior cingulate and frontoinsular cortex: a historical perspective. Cereb Cortex. 2012;22:245–250. doi: 10.1093/cercor/bhr005. [DOI] [PubMed] [Google Scholar]
- 49.Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, et al. The von Economo neurons in the frontoinsular and anterior cingulate cortex. Ann N Y Acad Sci. 2011;1225:59–71. doi: 10.1111/j.1749-6632.2011.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coffeen U. Manuel Ortega-Legaspi J, Lopez-Munoz FJ, Simon-Arceo K, Jaimes O, Pellicer F. Insular cortex lesion diminishes neuropathic and inflammatory pain-like behaviours. Eur J Pain. 2011;15:132–138. doi: 10.1016/j.ejpain.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Gu X, Hof PR, Friston KJ, Fan J. Anterior insular cortex and emotional awareness. J Comp Neurol. 2013;521:3371–3388. doi: 10.1002/cne.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Schweinhardt P, Glynn C, Brooks J, McQuay H, Jack T, Chessell I, et al. An fMRI study of cerebral processing of brush-evoked allodynia in neuropathic pain patients. Neuroimage. 2006;32:256–265. doi: 10.1016/j.neuroimage.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 55.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gustin SM, Peck CC, Wilcox SL, Nash PG, Murray GM, Henderson LA. Different pain, different brain: thalamic anatomy in neuropathic and non-neuropathic chronic pain syndromes. J Neurosci. 2011;31:5956–5964. doi: 10.1523/JNEUROSCI.5980-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pievani M, de Haan W, Wu T, Seeley WW, Frisoni GB. Functional network disruption in the degenerative dementias. Lancet Neurol. 2011;10:829–843. doi: 10.1016/S1474-4422(11)70158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, et al. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038. doi: 10.1001/archpsyc.57.11.1033. [DOI] [PubMed] [Google Scholar]
- 61.Doan L, Manders T, Wang J. Neuroplasticity underlying the comorbidity of pain and depression. Neural Plast. 2015;2015:504691. doi: 10.1155/2015/504691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yalcin I, Barthas F, Barrot M. Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev. 2014;47:154–164. doi: 10.1016/j.neubiorev.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain Res. 2013;136:2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sliz D, Hayley S. Major depressive disorder and alterations in insular cortical activity: a review of current functional magnetic imaging research. Front Hum Neurosci. 2012;6:323. doi: 10.3389/fnhum.2012.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mutschler I, Ball T, Wankerl J, Strigo IA. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520:204–209. doi: 10.1016/j.neulet.2012.03.095. [DOI] [PubMed] [Google Scholar]
- 66.Gard T, Holzel BK, Sack AT, Hempel H, Lazar SW, Vaitl D, et al. Pain attenuation through mindfulness is associated with decreased cognitive control and increased sensory processing in the brain. Cereb Cortex. 2012;22:2692–2702. doi: 10.1093/cercor/bhr352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Emmert K, Breimhorst M, Bauermann T, Birklein F, De Van, Ville D, Haller S. Comparison of anterior cingulate vs. insular cortex as targets for real-time fMRI regulation during pain stimulation. Front. Behav Neurosci. 2014;8:350. doi: 10.3389/fnbeh.2014.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- 69.Richfield EK, Young AB, Penney JB. Comparative distributions of dopamine D-1 and D-2 receptors in the cerebral cortex of rats, cats, and monkeys. J Comp Neurol. 1989;286:409–426. doi: 10.1002/cne.902860402. [DOI] [PubMed] [Google Scholar]
- 70.Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur J Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 71.Ohara PT, Granato A, Moallem TM, Wang BR, Tillet Y, Jasmin L. Dopaminergic input to GABAergic neurons in the rostral agranular insular cortex of the rat. J Neurocytol. 2003;32:131–141. doi: 10.1023/B:NEUR.0000005598.09647.7f. [DOI] [PubMed] [Google Scholar]
- 72.Sotres-Bayon F, Torres-Lopez E, Lopez-Avila A, del Angel R, Pellicer F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Res. 2001;898:342–349. doi: 10.1016/S0006-8993(01)02213-2. [DOI] [PubMed] [Google Scholar]
- 73.Lopez-Avila A, Coffeen U, Ortega-Legaspi JM, del Angel R, Pellicer F. Dopamine and NMDA systems modulate long-term nociception in the rat anterior cingulate cortex. Pain. 2004;111:136–143. doi: 10.1016/j.pain.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 74.Floyd NS, Price JL, Ferry AT, Keay KA, Bandler R. Orbitomedial prefrontal cortical projections to distinct longitudinal columns of the periaqueductal gray in the rat. J Comp Neurol. 2000;422:556–578. doi: 10.1002/1096-9861(20000710)422:4<556::AID-CNE6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 75.Jung MJ, Lippert B, Metcalf BW, Bohlen P, Schechter PJ. gamma-Vinyl GABA (4-amino-hex-5-enoic acid), a new selective irreversible inhibitor of GABA-T: effects on brain GABA metabolism in mice. J Neurochem. 1977;29:797–802. doi: 10.1111/j.1471-4159.1977.tb10721.x. [DOI] [PubMed] [Google Scholar]
- 76.Watkins LR, Wiertelak EP, McGorry M, Martinez J, Schwartz B, Sisk D, et al. Neurocircuitry of conditioned inhibition of analgesia: effects of amygdala, dorsal raphe, ventral medullary, and spinal cord lesions on antianalgesia in the rat. Behav Neurosci. 1998;112:360–378. doi: 10.1037/0735-7044.112.2.360. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qiu S, Chen T, Koga K, Guo YY, Xu H, Song Q, et al. An increase in synaptic NMDA receptors in the insular cortex contributes to neuropathic pain. Sci Signal. 2013;6:ra34. doi: 10.1126/scisignal.2003778. [DOI] [PubMed] [Google Scholar]
- 79.Kam AY, Liao D, Loh HH, Law PY. Morphine induces AMPA receptor internalization in primary hippocampal neurons via calcineurin-dependent dephosphorylation of GluR1 subunits. J Neurosci. 2010;30:15304–15316. doi: 10.1523/JNEUROSCI.4255-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 81.Li HL, Huang BS, Vishwasrao H, Sutedja N, Chen W, Jin I, et al. Dscam mediates remodeling of glutamate receptors in Aplysia during de novo and learning-related synapse formation. Neuron. 2009;61:527–540. doi: 10.1016/j.neuron.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 83.Ahmad M, Polepalli JS, Goswami D, Yang X, Kaeser-Woo YJ, Sudhof TC, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73:260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, et al. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–3170. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- 85.Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, et al. Persistent inflammation induces GluR2 internalization via NMDA receptor-triggered PKC activation in dorsal horn neurons. J Neurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 87.Cao H, Ren WH, Zhu MY, Zhao ZQ, Zhang YQ. Activation of glycine site and GluN2B subunit of NMDA receptors is necessary for ERK/CREB signaling cascade in rostral anterior cingulate cortex in rats: implications for affective pain. Neurosci Bull. 2012;28:77–87. doi: 10.1007/s12264-012-1060-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Imbe H, Kimura A, Donishi T, Kaneoke Y. Repeated forced swim stress enhances CFA-evoked thermal hyperalgesia and affects the expressions of pCREB and c-Fos in the insular cortex. Neuroscience. 2014;259:1–11. doi: 10.1016/j.neuroscience.2013.11.045. [DOI] [PubMed] [Google Scholar]
- 89.Boly M, Balteau E, Schnakers C, Degueldre C, Moonen G, Luxen A, et al. Baseline brain activity fluctuations predict somatosensory perception in humans. Proc Natl Acad Sci USA. 2007;104:12187–12192. doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci USA. 2010;107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 2010;30:16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 93.Burkey AR, Carstens E, Jasmin L. Dopamine reuptake inhibition in the rostral agranular insular cortex produces antinociception. J Neurosci. 1999;19:4169–4179. doi: 10.1523/JNEUROSCI.19-10-04169.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, Van Der Meer C, et al. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24:8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, et al. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- 98.Jahr CE, Stevens CF. Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. J Neurosci. 1990;10:3178–3182. doi: 10.1523/JNEUROSCI.10-09-03178.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Imbe H, Kimura A. Repeated forced swim stress prior to complete Freund’s adjuvant injection enhances mechanical hyperalgesia and attenuates the expression of pCREB and DeltaFosB and the acetylation of histone H3 in the insular cortex of rat. Neuroscience. 2015;301:12–25. doi: 10.1016/j.neuroscience.2015.05.065. [DOI] [PubMed] [Google Scholar]