Abstract
17β-estradiol (E2) has been shown to have neuroprotective effects in different central nervous system diseases. The mechanisms underlying estrogen neuroprotection in spinal cord injury (SCI) remain unclear. Previous studies have shown that autophagy plays a crucial role in the course of nerve injury. In this study, we showed that E2 treatment improved the restoration of locomotor function and decreased the loss of motor neurons in SCI rats. Real-time PCR and western blot analysis revealed that the protective function of E2 was related to the suppression of LC3II and beclin-1 expression. Immunohistochemical study further confirmed that the immunoreactivity of LC3 in the motor neurons was down-regulated when treated with E2. In vitro studies demonstrated similar results that E2 pretreatment decreased the autophagic activity induced by rapamycin (autophagy sensitizer) and increased viability in a PC12 cell model. These results indicated that the neuroprotective effects of E2 in SCI are partly related to the suppression of excessive autophagy.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0017-x) contains supplementary material, which is available to authorized users.
Keywords: 17β-estradiol, Spinal cord injury, Autophagy, Motor neuron
Introduction
Spinal cord injury (SCI) is a common neurological problem that significantly impacts quality of life and life expectancy [1]. 17β-estradiol (E2) has been implied to be a neuroprotectant in experimental animal models such as those of neurodegenerative disease, cerebral ischemia, and traumatic brain injury [2–5]. In different SCI models, estradiol treatment improves motor function and reduces autonomic dysfunction [6, 7]. However, due to the peripheral actions of the hormone, the clinical application of E2 as a neuroprotective agent in humans has numerous limitations. In the past few years, selective estrogen receptor modulators have emerged as an alternative to estradiol to exert E2-like neuroprotective effects in experimental animal models of traumatic injury to the central nervous system [8, 9]. Thus, it is necessary to further investigate the neuroprotective mechanism of estrogen against SCI.
Autophagy is an essential process for the maintenance of cellular homeostasis in the physiology and pathology of the central nervous system [10]. Many studies have reported that autophagy can lead to progressive degeneration of the spinal cord and thus increase or decrease secondary injury in both cellular [11] and animal models [12]. Recently, the relationship between estrogen receptor (ER) signals and the mammalian target of rapamycin (mTOR) signals has been confirmed, and this is mediated by the PI3K/Akt signaling pathway to regulate autophagy [13]. The objective of the current study was to investigate the link between E2-mediated neuroprotection and autophagy in a rat model of SCI.
Previous studies have shown that continuous infusion of a high dose of estradiol rather than a physiological dose or a single application increases the availability of this neuroprotective agent and might further stimulate a neuroprotective response after SCI [14]. Therefore, we used a rat model of SCI to investigate the characteristics of autophagy in SCI as well as the effect of high doses of E2 on autophagy in vivo. PC12 cells were used to identify the protective effect of E2 in vitro. The physiological dose of 20 nmol/L was used in consideration of the reduction of cell viability caused by high doses of E2 [15].
Materials and Methods
Animals
A total of 120 adult female Sprague–Dawley rats (200–220 g) were purchased from the Animal Centre of the Chinese Academy of Sciences in Shanghai, China. All procedures and experimental operations were approved by the Animal Care and Use Committee of Wenzhou Medical University and conformed to the Guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health.
Ovariectomy
To eliminate gonadal estradiol and cyclical variability, a bilateral ovariectomy was carried out under aseptic and sterile conditions. Following 10% chloral hydrate anesthesia (4 mL/kg, i.p.), the ovaries were removed through a 1-cm incision in the dorsum of the animal. The muscle was then sutured and the wound closed with staples. Half of the animals received an intramuscular injection of estradiol benzoate (0.5 mg/kg; Ningbo Hormone Factory, Ningbo, China) every other day until sacrifice and the other half received a vehicle (soybean oil). Animals were allowed 1 week for recovery prior to experimental manipulations.
Spinal Cord Injury
SCI was performed 7 days after ovariectomy to allow for recovery and to achieve steady levels of the hormone. The rat was positioned on a cork platform, the dorsum was shaved and disinfected with iodine and alcohol, an incision was made, and a laminectomy was performed at T9. The exposed spinal cord was subjected to a crush injury by compression with a vascular clip (15 g force; Oscar, China) for 1 min. The sham group received a laminectomy but the spinal cord was left intact. Postoperative care involved manual evacuation of the urinary bladder thrice daily until recovery of spontaneous voiding. The BBB (Basso, Beattie, Bresnahan) open field locomotor scale and inclined plane test were performed before injury and at 7, 14, 21, and 28 days after operation by two investigators in a blinded fashion to evaluate the functional recovery of locomotion after SCI.
Estradiol Measurement
The plasma levels of estradiol were measured to confirm the hormone replacement therapy. Blood samples (300–500 μL) were obtained from the tail weekly and placed in a 1.5-mL microtube. Samples were centrifuged at 3000 g for 5 min at 4 °C and plasma was extracted. Total plasma estradiol levels were determined using a 17-beta estradiol ELISA kit (ab108667; Abcam, Cambridge, UK).
Real-Time Polymerase Chain Reaction (PCR) Analysis
Total RNA was extracted from the spinal tissue three days after SCI using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse-transcribed into cDNA. Real-time PCR with SYBR Green (Sigma) detection was performed in the ABI Prism 7500 sequence detection system (Applied Biosystems, Carlsbad, CA). The primer sequences were synthesized by Sangon Biotech Co., Ltd. (Shanghai, China) as previously designed [16]: LC3, sense: 5’-GAGTGGAAGATGTCCGGCTC-3’ and antisense: 5’-CCAGGAGGAAGAAGGCTTGG-3’; beclin-1, sense: 5’-AGCACGCCATGTATAGCAAAGA-3’ and antisense: 5’-GGAAGAGGGAAAGGACAGCAT-3’; beta-actin, sense: 5’-CCCATCTATGAGGGTTACGC-3’ and antisense: 5’-TTTAATGTCACGCACGATTTC-3’. The expression of the target gene mRNA was standardized by beta-actin to ensure equal loading. The 2−ΔΔCt (cycle threshold) method was used to calculate relative gene expression levels.
Tissue Preparation
On days 1, 3, 7, and 28 after SCI, the rats were anesthetized with 10% chloral hydrate i.p. The rats were transcardially perfused with normal saline, followed by 4% paraformaldehyde in 0.1 mol/L PBS overnight. The spinal cord samples were then dehydrated in 30% sucrose in PBS until they sank. Next, the samples were frozen, and serial 10-μm transverse and longitudinal sections were cut around the SCI epicenter and mounted on slides. For immunohistochemical staining, the spinal cord was fixed in 10% formalin buffer and embedded in paraffin, then tissue (2 mm caudal to the epicenter of injury) was cut into 5-μm sections.
Hematoxylin–Eosin (HE) and Nissl Staining
For HE staining, the sections were washed three times with PBS for 5 min each, followed by hematoxylin for 5 min and eosin for another 5 min at room temperature. After another three 5-min washes in PBS, the sections were differentiated in 95% alcohol, permeabilized with xylene, and mounted with neutral resin. The sections were also incubated in 1% cresyl violet for Nissl staining and examined under a light microscope (BX53, Olympus, Japan).
Cell Culture and Viability Assay
PC12 cells were purchased from the Cell Storage Centre of Wuhan University (Wuhan, China). The cells were plated at 3 × 105 cells/well in 6-well plates or 1 × 104 cells/well in 96-well plates. Based on a previous study [15], the cells were divided into three groups: (1) cells treated with 1‰ DMSO for 24 h; (2) cells treated with 100 nmol/L rapamycin (Sigma) for 24 h; and (3) cells pre-incubated with 20 nmol/L estradiol (Sigma) for 24 h, followed by medium with rapamycin for another 24 h. Cell viability was determined using Cell Counting Kit-8 (CCK-8, Dojindo, Japan) according to the manufacturer’s instructions.
Immunohistochemistry
Immunohistochemical staining for LC3 was performed using spinal cord sections obtained on days 1, 3, and 7 after SCI. After incubation with 3% H2O2 for 10 min, the sections were washed with PBS, boiled in 0.1% trisodium citrate for 16 min for antigen retrieval and blocked with goat serum for 1 h. Then, the sections were incubated with rabbit anti-LC3 antibody (CST, Danvers, MA, 1:200) diluted in PBS overnight at 4 °C, followed by horseradish peroxidase-conjugated secondary antibodies (Zhongshan Golden Bridge Biotechnology, Beijing, China). Staining was visualized with DAB (Zhongshan Golden Bridge Biotechnology, Beijing, China), and sections were then counterstained with hematoxylin, dehydrated in graded ethanol, made transparent in dimethyl benzene, and sealed with neutral resin. Images of the ventral horn at 400× magnification were acquired using a biological imaging microscope (BX53, Olympus, Japan). For cellular immunofluorescence staining, PC12 cells were harvested, fixed with 4% paraformaldehyde, and adhered onto slides by centrifugation. The slides were incubated overnight at 4 °C with anti-LC3 antibody. After incubation with the secondary antibody (Invitrogen, Carlsbad, CA) for 1 h, slides were photographed using a CCD camera (DP70; Olympus, Tokyo, Japan).
Western Blot Analysis
Total proteins of the spinal cord and PC12 cells were suspended in lysis buffer and centrifuged. The equivalent of 20 μg protein was separated on a 12% gel and then transferred onto a PVDF membrane (Millipore, Bedford, MA). The membrane was blocked by incubation with 5% non-fat milk for 1 h and then incubated overnight at 4 °C with anti-beclin-1 (1:1000), anti-LC3 (1:1000), anti-ATG5 (1:1000), anti-ATG7 (1:1000), anti-p62 (1:1000), and anti-GAPDH (1:4000). All primary antibodies were from Cell Signaling Technology, Inc. (Danvers, MA). After washing in TBST, horseradish peroxidase was added for signal development. Immunoreactive proteins were visualized using an ECL kit (Bio-Rad, Hercules, CA) and quantitatively analyzed by densitometry using ImageJ software (Media Cybernetics, Bethesda, MD).
Statistical Analysis
The results are expressed as mean ± SD unless otherwise specified. All experiments were repeated at least three times. Statistical significance was evaluated using Student’s t-test or one-way ANOVA. P <0.05 was considered statistically significant.
Results
E2 Decreases Motor Neuron Loss and Improves Functional Recovery after SCI In Vivo
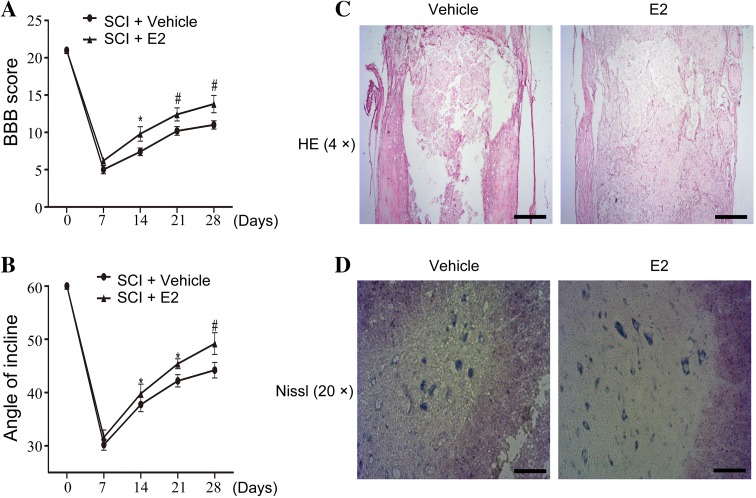
We first evaluated the neuroprotective effect of a high dose of E2 against traumatic SCI. The enzyme-linked immunosorbent assay revealed that the intramuscular injection of estradiol benzoate produced high plasma levels of E2 at different time points compared to the vehicle (Table S1). Both the BBB locomotor score test and the inclined plane test showed functional improvement in locomotor activity on day 28 after SCI (Fig. 1A, B).
Fig. 1.
A BBB scores. B Inclined plane test scores. *P < 0.05, # P < 0.01 vs SCI+ vehicle on the same day. C, D HE staining (C) and Nissl staining (D) of the SCI model treated with E2 or vehicle on day 28 after contusion (sections located 2 mm rostral to the epicenter). Scale bar 500 μm in C; 100 μm in D.
Compared with the vehicle group, E2-treated rats showed a significant protective effect of treatment via reducing necrosis and area of defect (Fig. 1C). Nissl staining showed that motor neurons were significantly retained in the spinal cord of rats treated with E2 compared to the vehicle group following SCI (Fig. 1D). These results further strengthen the hypothesis that E2 has a neuroprotective effect on motor neurons in the SCI rat model in vivo.
Inhibition of Autophagy in the SCI Model is Involved in the Protective Function of E2
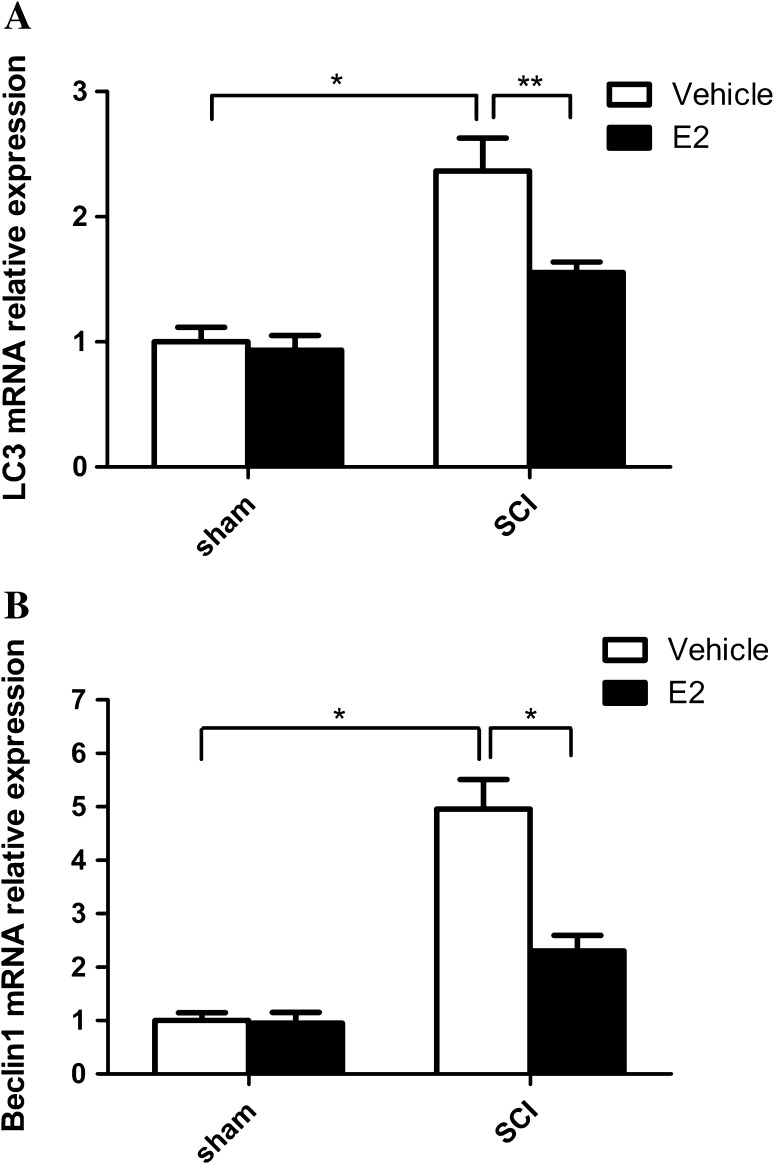
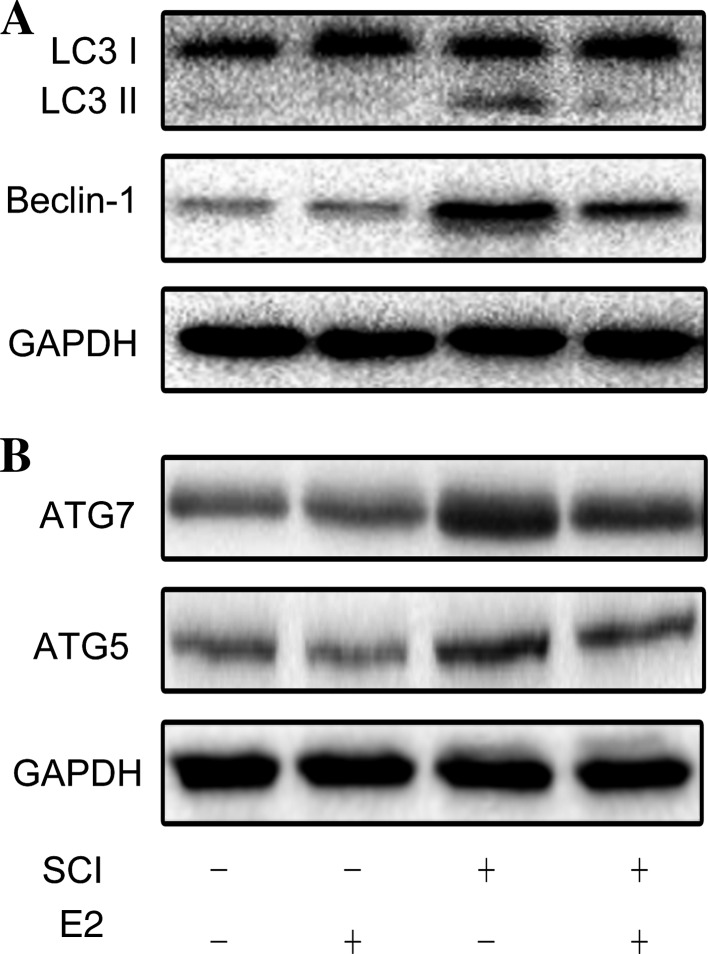
Previous studies have demonstrated that secondary damage principally occurs between 24 h and 3 days after the initial SCI [17, 18], so we investigated the changes in autophagy on day 3 after SCI. The RT-PCR results showed that the mRNA expression of the autophagy markers LC3 and beclin-1 in the SCI-vehicle group was markedly higher than that in the SCI-E2 group (Fig. 2A, B). Consistently, the protein levels of beclin-1 and LC3II increased in the SCI-vehicle group, whereas E2 delivery markedly restored the expression of beclin-1 and LC3II (Fig. 3A). ATG7 and ATG5 showed results similar to those of beclin-1 and LC3II (Fig. 3B).
Fig. 2.
Autophagy in the spinal cord. Real-time PCR for LC3 (A) and beclin1 (B). *P < 0.01, **P < 0.05, n = 6 per group.
Fig. 3.
Autophagy in the spinal cord. A, B Western blots for LC3, beclin1, and GAPDH (A) and for ATG5, ATG7, and GAPDH (B).
Decreased LC3 Immunoreactivity in Motor Neurons is Involved in the Protective Effect of E2 in SCI Recovery
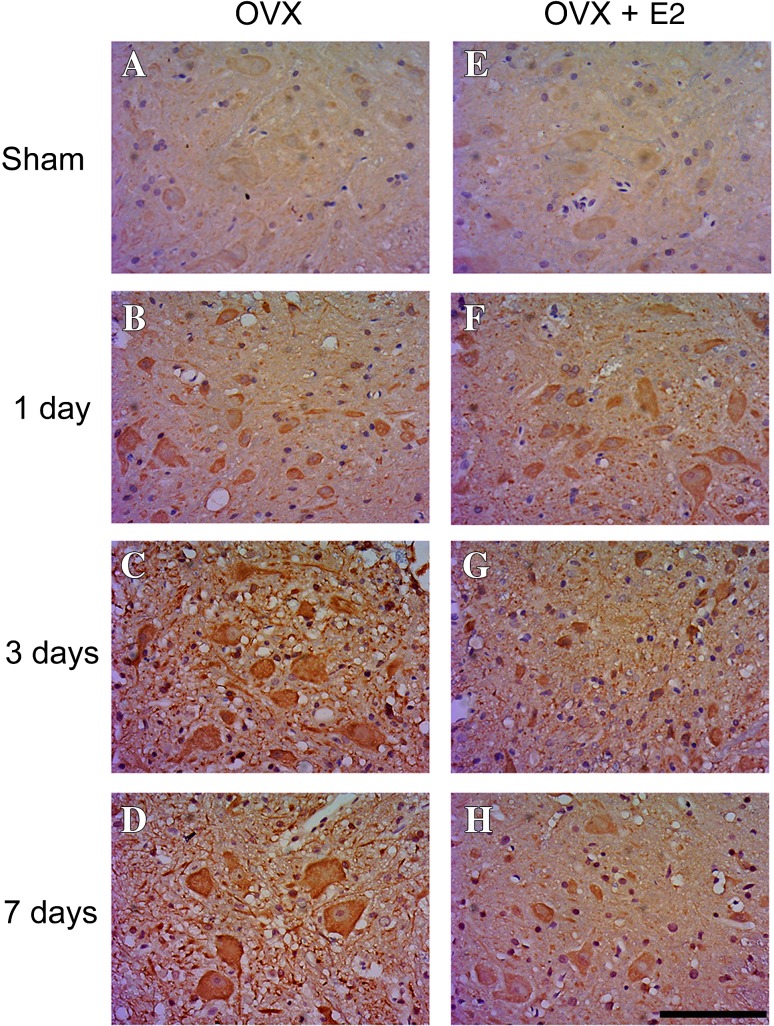
The motor neurons of sham-operated animals showed slight LC3 immunoreactivity in the cytoplasm (Fig. 4A, E). One day after SCI, LC3 expression was upregulated in the cytoplasm of motor neurons (Fig. 4B, F). In the SCI-vehicle group, the immunoreactivity for LC3 in the cytoplasm peaked on day 3 and was maintained to day 7 after SCI in motor neurons (Fig. 4C, D). In the SCI-E2 group, the immunoreactivity of LC3 in motor neurons was lower than in the SCI-vehicle group on day 3 (Fig. 4G) and remained at a low level on day 7 after SCI (Fig. 4H).
Fig. 4.
Immunostaining against LC3 in motor neurons of rats in the sham group (A, E) and in the SCI-vehicle and SCI-E2 groups on days 1 (B, F), 3 (C, G), and 7 (D, H) after SCI. Scale bar 100 μm; OVX, ovariectomy.
E2 Treatment Suppresses Acute SCI-Induced Autophagic Flux
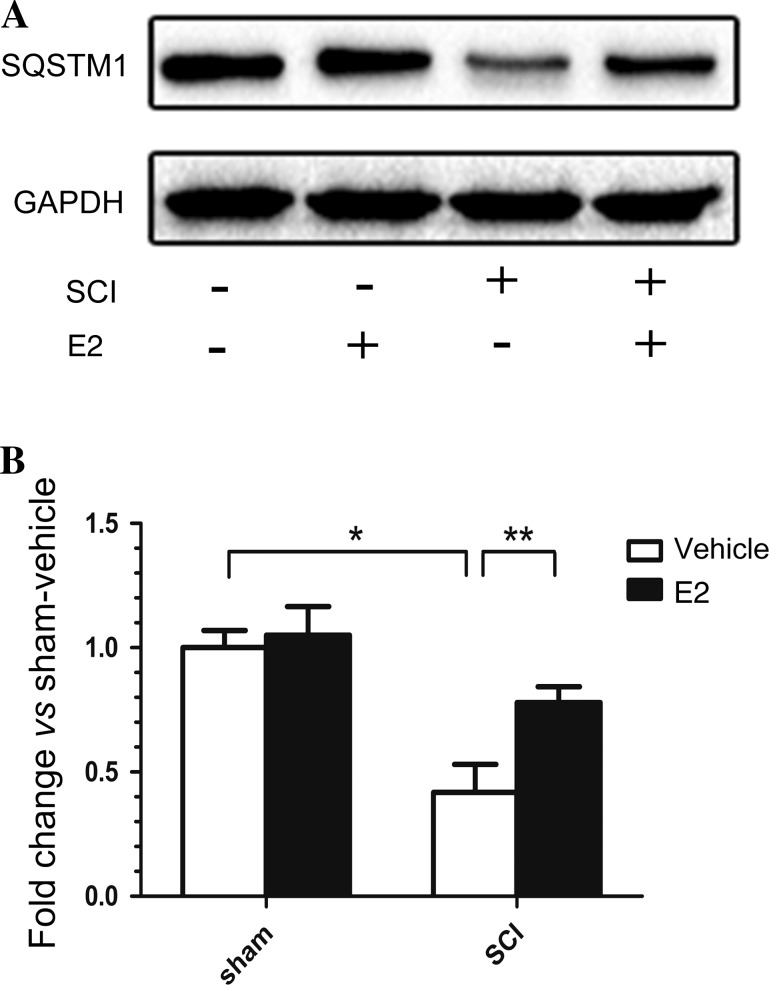
Autophagy is a dynamic mechanism that turns over proteins and organelles through a lysosome-associated degradation process [19]. During this process, these proteins and organelles accumulate, become packed, and fuse with lysosomes, in which they are digested by acidic enzymes to release nutrients back into the cell. The protein sequestosome-1 (SQSTM1), also known as p62, is a substrate of the autophagic process, and its level reflects the level of autophagic flux in vivo [20]. Western blotting showed that, compared with the control SCI-vehicle group, SQSTM1 expression was significantly increased in the group treated with E2 (Fig. 5).
Fig. 5.
Expression of SQSTM1 on day 3 after SCI. A Western blots of SQSTM1 and GAPDH. B Quantitative analysis of the levels of SQSTM1 expression. The band densities were normalized to GAPDH. *P < 0.01, **P < 0.05, n = 6 per group.
Exogenous E2 Protects PC12 Cells by Depressing Excessive Autophagy In Vitro
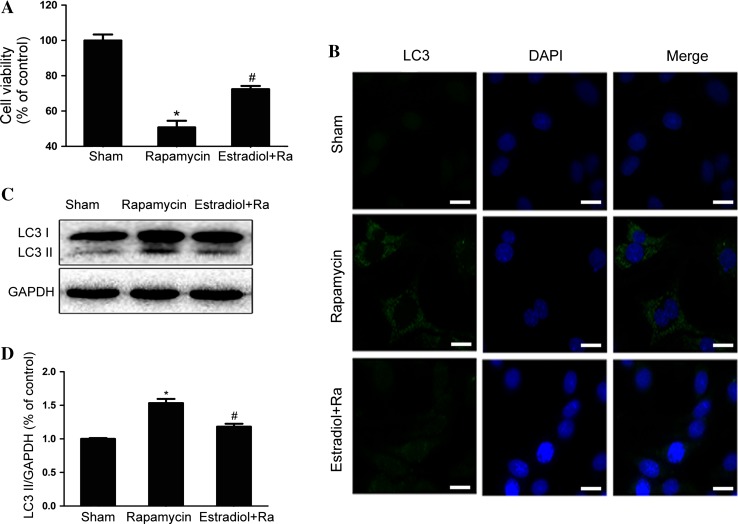
PC12 cells were incubated with rapamycin or pretreated with E2. In CCK8 assays, E2 pretreatment restored the rapamycin-inhibited cell viability (Fig. 6A). Immunofluorescence staining showed that the increased LC3 puncta in the rapamycin-incubated PC12 cells were reduced by pretreatment with E2 (Fig. 6B). Rapamycin incubation resulted in a dramatic elevation of LC3II, which was inhibited by E2 pretreatment (Fig. 6C, D). These findings supported the hypothesis that inhibition of autophagy is involved in the protective effect of E2 in vitro as well as in vivo.
Fig. 6.
A CCK8 results from PC12 cells treated by rapamycin with or without E2 pretreatment. B Immunofluorescence staining for LC3 (green), nuclei labeled with DAPI (blue). C Protein levels of LC3 in the sham, rapamycin, and rapamycin + E2 groups. D Optical density analysis of LC3II/GAPDH from (C). *P < 0.05 vs sham group, # P < 0.01 vs rapamycin group, n = 3 per group. Scale bar 10 μm.
Discussion
The occurrence of spinal cord injury due to different forms of trauma is very frequent and leads to a great burden on patients and society [1]. In the progression of SCI, the initial traumatic injury to spinal cord tissue is followed by long-term secondary damage including vascular changes, neurotransmitter (glutamate) accumulation, ionic disturbances, edema, inflammatory responses, free radical stress, and apoptosis [21–23]. Several studies have shown that E2 protects against traumatic central nervous system damage [24, 25]. However, as a multifunctional factor, investigations of the neuroprotective effect of E2 on the autophagy-induced cell death in secondary injury after SCI have not been reported. In this study, we treated SCI rats with estradiol and revealed that the protective effect of E2 is related to the suppression of autophagy in vivo.
Autophagy has been implicated in SCI, but its effect is not yet clear. Both increased and decreased autophagic activity contribute to cell survival in the spinal cord [26, 27]. By generating intracellular building blocks to purge damaged proteins, autophagy maintains the functions of vital organs to promote cell survival during harsh circumstances such as nutrient-limited conditions or stress states. In contrast, excessive activation of autophagy is detrimental to cells and may contribute to programmed cell death [28, 29]. E2 has been shown to be a potential therapeutic treatment for SCI through stimulating early astroglial responses and cytokine release, attenuating calpain activation, and inhibiting apoptosis [7, 30]. However, whether these protective effects are related to autophagy regulation has not yet been investigated. In our study, we first reported that E2 facilitated the survival of motor neurons and improved recovery from SCI by inhibiting excessive autophagy. The protein levels of LC3II and beclin-1 increased significantly on day 3 after SCI, and this up-regulation was alleviated by E2 treatment, as were ATG5 and ATG7. Notably, in the PC12 cell model, E2 pretreatment also decreased the autophagic activity induced by rapamycin (an autophagy activator) and increased cell viability. It is known that autophagy is a process of flux, which includes the formation of autophagosomes and the degradation of damaged organelles and long-lived proteins by acidic enzymes [31]; thus, in our model of acute SCI, we measured the expression of SQSTM1, which is a substrate of degradation, as a marker of autophagic flux [32]. Western blotting showed that the suppression of SQSTM1 expression in the SCI model was abolished by E2 treatment. The results of this comparison further confirmed that autophagy regulation is involved in the protective effect of E2.
The loss of motor neurons is closely related to functional recovery after secondary injury. Once neurons are damaged, neurons are first protected via various mechanisms, including reducing neuronal loss and discarding damaged cells to avoid toxic effects on healthy neurons [33]. The upregulation of LC3 in motor neurons after SCI may represent enhanced autophagy as a mechanism to protect cells under stress, or as a process leading to cell death. Thus, we used immunohistochemical analysis to explore the function of autophagy in motor neurons. In this study, LC3 was upregulated in motor neurons at an early phase after injury and remained at a high level for 7 days. However, the immunoreactivity of motor neurons for LC3 was markedly down-regulated by E2 treatment. This evidence supports the hypothesis that over-activation of autophagy in motor neurons resulting in neuronal loss is rescued by estradiol therapy in our model of acute SCI.
The purpose of our work was to preliminarily explore the mechanisms underlying estrogen’s protective effect on traumatic SCI. Previous studies have demonstrated that estradiol regulates mTOR signaling pathways. For instance, in a human renal tubular epithelial cell line (HK2), estradiol delivery elevates PTEN expression via ERβ signaling pathways, which negatively regulate the autophagy induced by the PI3K/AKT/mTOR pathway [13]. In osteoblasts, the protective effect of estradiol against serum-deprivation-induced apoptosis is associated with the up-regulation of autophagy through the ER-ERK-mTOR pathway [34]. The link between ERs and mTOR signaling pathways has also been demonstrated in an experimental animal model. In a rat model of iron-induced brain injury, E2 decreases the levels of autophagy and injury via ERα [24]. However, the distribution of classic ERs is limited in the dorsal horn of the spinal cord [35]. New evidence suggests that the protective effect of estrogen against SCI is probably not via nuclear ERs but through GPR30 [36]. As a partial ER antagonist, tamoxifen significantly enhances the functional recovery of SCI rats and can act as a GPER1 agonist [37]. Meanwhile, it has been reported in several studies that GPR30 modifies the PI3K/Akt pathway [38]. Here, it is likely that estradiol modifies the GREP1-PI3K/AKT-mTOR signaling pathway to regulate autophagy in SCI. Each part of the interactive mechanism needs further clarification, both in vivo and in vitro.
In conclusion, E2 distinctly reduced the extent of damage after SCI, minimizing the loss of motor neurons and promoting locomotor recovery. We report for the first time that the neuroprotective action of E2 in SCI is related to the restraint of excessive autophagy. Our current study suggests that a therapeutic strategy applying E2 may be a promising treatment for recovery from central nervous system injury.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Natural Science Foundation of Zhejiang Province, China (LY14H060009), National Natural Science Foundation of China (80215097), Science-technology Program of Wenzhou Municipal Sci-Tech Bureau, China (Y20150226) and the key Discipline Construction Project of Colleges and Universities in Zhejiang Province, China (2012-207).
Footnotes
Chao-Wei Lin and Bi Chen have contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0017-x) contains supplementary material, which is available to authorized users.
References
- 1.Silva NA, Sousa N, Reis RL, Salgado AJ. From basics to clinical: A comprehensive review on spinal cord injury. Prog Neurobiol. 2014;114:25–57. doi: 10.1016/j.pneurobio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29:217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 4.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 5.Khaksari M, Abbasloo E, Dehghan F, Soltani Z, Asadikaram G. The brain cytokine levels are modulated by estrogen following traumatic brain injury: Which estrogen receptor serves as modulator? Int Immunopharmacol. 2015;28:279–287. doi: 10.1016/j.intimp.2015.05.046. [DOI] [PubMed] [Google Scholar]
- 6.Webb AA, Chan CB, Brown A, Saleh TM. Estrogen reduces the severity of autonomic dysfunction in spinal cord-injured male mice. Behav Brain Res. 2006;171:338–349. doi: 10.1016/j.bbr.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Ritz MF, Hausmann ON. Effect of 17β-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- 8.Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L. The cancer drug tamoxifen: A potential therapeutic treatment for spinal cord injury. J. Neurotrauma. 2014;31:268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta SH, Dhandapani KM, De Sevilla LM, Webb RC, Mahesh VB, Brann DW. Tamoxifen, a selective estrogen receptor modulator, reduces ischemic damage caused by middle cerebral artery occlusion in the ovariectomized female rat. Neuroendocrinology. 2003;77:44–50. doi: 10.1159/000068332. [DOI] [PubMed] [Google Scholar]
- 10.Nikoletopoulou V, Papandreou ME, Tavernarakis N. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015;22:398–407. doi: 10.1038/cdd.2014.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ZY, Lin JH, Muharram A, Liu WG. Beclin-1-mediated autophagy protects spinal cord neurons against mechanical injury-induced apoptosis. Apoptosis. 2014;19:933–945. doi: 10.1007/s10495-014-0976-1. [DOI] [PubMed] [Google Scholar]
- 12.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 2011;36:E1427–E1434. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 13.Zeng M, Chen B, Qing Y, Xie W, Dang W, Zhao M, et al. Estrogen receptor β signaling induces autophagy and downregulates Glut9 expression. Nucleosides Nucleotides Nucleic Acids. 2014;33:455–465. doi: 10.1080/15257770.2014.885045. [DOI] [PubMed] [Google Scholar]
- 14.Mosquera L, Colón JM, Santiago JM, Torrado AI, Meléndez M, Segarra AC, et al. Tamoxifen and estradiol improved locomotor function and increased spared tissue in rats after spinal cord injury: Their antioxidant effect and role of estrogen receptor alpha. Brain Res. 2014;1561:11–22. doi: 10.1016/j.brainres.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma YL, Qin P, Li Y, Shen L, Wang SQ, Dong HL, et al. The effects of different doses of estradiol (E2) on cerebral ischemia in an in vitro model of oxygen and glucose deprivation and reperfusion and in a rat model of middle carotid artery occlusion. BMC Neurosci. 2013;14:118. doi: 10.1186/1471-2202-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Zheng X, Li B, et al. Increased activity of osteocyte autophagy in ovariectomized rats and its correlation with oxidative stress status and bone loss. Biochem Biophys Res Commun. 2014;451:86–92. doi: 10.1016/j.bbrc.2014.07.069. [DOI] [PubMed] [Google Scholar]
- 17.Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 18.Yong C, Arnold PM, Zoubine MN, Citron BA, Watanabe I, Berman NE, et al. Apoptosis in cellular compartments of rat spinal cord after severe contusion injury. J Neurotrauma. 1998;15:459–472. doi: 10.1089/neu.1998.15.459. [DOI] [PubMed] [Google Scholar]
- 19.Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, Overvatn A, et al. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penas C, Guzman MS, Verdu E, Fores J, Navarro X, Casas C. Spinal cord injury induces endoplasmic reticulum stress with different cell-type dependent response. J Neurochem. 2007;102:1242–1255. doi: 10.1111/j.1471-4159.2007.04671.x. [DOI] [PubMed] [Google Scholar]
- 22.Cevikbas F, Steinhoff M, Ikoma A. Role of spinal neurotransmitter receptors in itch: New insights into therapies and drug development. CNS Neurosci Ther. 2011;17:742–749. doi: 10.1111/j.1755-5949.2010.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, Oh TH, et al. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J Neurosci Res. 2012;90:243–256. doi: 10.1002/jnr.22734. [DOI] [PubMed] [Google Scholar]
- 24.Chen CW, Chen TY, Tsai KL, Lin CL, Yokoyama KK, Lee WS, et al. Inhibition of autophagy as a therapeutic strategy of iron-induced brain injury after hemorrhage. Autophagy. 2012;8:1510–1520. doi: 10.4161/auto.21289. [DOI] [PubMed] [Google Scholar]
- 25.Azcoitia I, Arevalo MA, De Nicola AF, Garcia-Segura LM. Neuroprotective actions of estradiol revisited. Trends Endocrinol Metab. 2011;22:467–473. doi: 10.1016/j.tem.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Hao HH, Wang L, Guo ZJ, Bai L, Zhang RP, Shuang WB, et al. Valproic acid reduces autophagy and promotes functional recovery after spinal cord injury in rats. Neurosci Bull. 2013;29:484–492. doi: 10.1007/s12264-013-1355-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49:276–287. doi: 10.1007/s12035-013-8518-3. [DOI] [PubMed] [Google Scholar]
- 28.Kanno H, Ozawa H, Sekiguchi A, Itoi E. Spinal cord injury induces upregulation of Beclin 1 and promotes autophagic cell death. Neurobiol Dis. 2009;33:143–148. doi: 10.1016/j.nbd.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Button RW, Luo S, Rubinsztein DC. Autophagic activity in neuronal cell death. Neurosci Bull. 2015;31:382–394. doi: 10.1007/s12264-015-1528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosci Res. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- 31.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown KN, Chen S, Han Z, Lu CH, Tan X, Zhang XJ, et al. Clonal production and organization of inhibitory interneurons in the neocortex. Science. 2011;334:480–486. doi: 10.1126/science.1208884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang YH, Chen K, Li B, Chen JW, Zheng XF, Wang YR, et al. Estradiol inhibits osteoblast apoptosis via promotion of autophagy through the ER-ERK-mTOR pathway. Apoptosis. 2013;18:1363–1375. doi: 10.1007/s10495-013-0867-x. [DOI] [PubMed] [Google Scholar]
- 35.Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: Relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 36.Hu R1, Sun H, Zhang Q, Chen J, Wu N, Meng H et al. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit Care Med 2012, 40: 3230–3237. [DOI] [PubMed]
- 37.Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, Carpino A, et al. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006;70:1414–1423. doi: 10.1124/mol.106.026344. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Hu R, Ge H, Duanmu W, Li Y, Xue X, et al. G-protein-coupled receptor 30-mediated antiapoptotic effect of estrogen on spinal motor neurons following injury and its underlying mechanisms. Mol Med Rep. 2015;12:1733–1740. doi: 10.3892/mmr.2015.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.