Abstract
Accumulating evidence indicates that α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors (AMPARs) are involved in the relapse to abused drugs. However, the role of AMPARs containing the GluR2 subunit in opiate addiction is still unclear. GluR2-3Y, an interfering peptide, prevents the endocytosis of AMPARs containing the GluR2 subunit. In this study, we explored the effect of intravenous injection of GluR2-3Y on the acquisition, expression, and reinstatement of morphine-induced conditioned place preference (mCPP) in rats. We found that infusion of GluR2-3Y (1.5 nmol/g) one hour before morphine during the conditioning phase inhibited the acquisition of mCPP, while an identical injection one hour before the post-conditioning test had no influence on the expression of mCPP. Injection of GluR2-3Y (1.5 nmol/g) after mCPP extinction blocked the morphine-induced reinstatement of mCPP. Our results strongly support the hypothesis that inhibition of AMPAR endocytosis provides a new target for the treatment of opiate addiction.
Keywords: GluR2-3Y, AMPA receptors, Morphine, Conditioned place preference, Acquisition of mCPP, Reinstatement of mCPP
Introduction
Opioids, which are the most effective clinically-used analgesics, are seriously limited in use as repeated treatment produces addiction. Conditioned associations between environmental stimuli and the effects of addictive drugs play an important role in drug addiction. It is very important to understand the mechanisms underlying these powerful associations between drugs of abuse and environmental stimuli for developing effective treatments to deal with addiction [1, 2].
The alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) is one of the three types of ionotropic glutamate receptors. Most of the AMPARs contain GluR1 and GluR2 subunits, and GluR2 subunits control Ca2+ influx and regulate synaptic plasticity [3]. Chronic morphine treatment changes the composition and properties of AMPARs, and the expression and distribution of AMPAR subunits. For example, repeated morphine administration elevates the level of GluR1 [4], increases synaptic GluR1 labeling in the ventral tegmental area (VTA) [5], increases the expression of AMPARs lacking GluR2 in hippocampal synaptic fractions, and affects long-term depression (LTD) in hippocampal neurons [6]. Combined with other studies reporting that the proportion of membrane GluR2 decreases after chronic morphine treatment [7], repeated morphine administration may induce the endocytosis of AMPARs containing GluR2, while AMPARs lacking GluR2 insert into the membrane.
Chronic morphine treatment results in the alteration of postsynaptic levels of AMPARs, which is believed to underlie morphine addiction. Repeated morphine treatment produces behavioral sensitization along with decreased GluR2 mRNA expression in both the amygdala and hippocampus [8], and the synaptic expression of AMPARs in the hippocampus also plays critical roles in the acquisition and expression of context-dependent behavioral sensitization [9]. GluR1 on the plasma membrane of dendrites increases in the basolateral amygdala (BLA) in rats self-administering morphine [10]. Moreover, systemic administration of the AMPAR antagonist LY293558 blocks the development of morphine sensitization [11], and attenuates morphine-induced tolerance in mice [12] and in rats [13]. Recently, a few studies have also shown that the endocytosis of AMPARs is crucial for cue-induced reinstatement of heroin-seeking and the extinction of morphine-induced conditioned place preference (mCPP) [14, 15].
Although AMPARs play important roles in morphine-induced addiction, the effects of those containing GluR2 are poorly understood. In the present study, mCPP was used as a model [16–19]. GluR2-3Y, an interfering peptide that blocks both the endocytosis of AMPARs containing GluR2 and the induction of LTD [20, 21], was injected intravenously into rats to determine whether GluR2-3Y modulates the acquisition, expression, and reinstatement of morphine-related memory.
Materials and Methods
Male Sprague-Dawley rats (220–250 g on arrival; Experimental Animal Center, Academy of Military Medical Sciences, Beijing, China) were habituated to the animal facility for one week before use. Rats were housed under a reversed 12-h light/dark cycle with food and water ad libitum. All procedures were approved by the Animal Care and Use Committee of Peking University.
Morphine hydrochloride injection (10 mg/mL; Shenyang First Pharmaceutical Factory, Shenyang, China) was diluted to 1 mg/mL in sterile saline. GluR2-3A (YGRKKRRQRRR-AKEGANVAG) and GluR2-3Y (YGRKKRRQRRR-YKEGYNVYG) (synthesized by GL Biochem Ltd, Shanghai, China) were dissolved in sterile saline.
The mCPP apparatus consisted of two main compartments (29 × 22 × 27 cm3) connected by a third neutral compartment (13 × 22 × 27 cm3) with optional manual guillotine doors. The two contexts differed in color (black or white) and floor texture (grid or mesh). The black compartment was paired with a grid floor and the white compartment with a mesh floor. The mCPP procedure consisted of four stages: acquisition, expression, extinction, and reinstatement.
After accommodation for five days, all rats received two baseline tests. Rats were placed in the central compartment without receiving an injection and were allowed free access to all three compartments for 15 min. On the next day, the initial baseline preference was evaluated by calculating the preference score as the time spent in the morphine-paired side divided by the time spent in both the morphine- and saline-paired sides. Morphine-paired side was assigned in a counterbalanced fashion, whereby half of rats were paired with black and the other half were paired with the white compartments. The preference score was about 0.5 and rats were discarded if they displayed a strong initial preference for either of the main compartment (preference score > 70% or < 30%). Conditioning training was conducted twice daily for 5 consecutive days, once in each context. Sessions were spaced 10 h apart (starting at 08:00 and 18:00), and the context sequence was balanced. Rats received an intraperitoneal (i.p.) injection of saline (1 ml/kg) followed by confinement in the saline-paired compartment for 45 min and received an injection of morphine (10 mg/kg, i.p.) followed by confinement in the morphine-paired compartment for 45 min. GluR2-3Y or GluR2-3A was infused one hour [14] before each morphine conditioning to explore the effects of AMPAR endocytosis on morphine conditioning.
One day after the last conditioning session, the rats underwent a post-conditioning test. They were placed in the central compartment and allowed to freely explore the chambers for 15 min. GluR2-3Y or GluR2-3A was infused one hour before the post-conditioning test.
One day after the post-conditioning test, an extinction test was conducted once daily without any treatment. Rats were placed in the neutral compartment and the guillotine doors were opened to allow free exploration of the three compartments for 15 min. This test was terminated when the preference score was significantly different from that of the post-conditioning test for three consecutive days.
One day after the last extinction test, the rats received an injection of morphine (3 mg/kg, i.p.) and 10 min later, they were placed in the central compartment and tested for 15 min. Rats were divided into two groups to receive injection of GluR2-3Y or GluR2-3A respectively, and 24 h later, reinstatement was induced again. GluR2-3Y or GluR2-3A was infused one hour before the second reinstatement.
Locomotor activity was measured in a Plexiglas chamber (AniLab, Ningbo, China) [22]. Each 8-beam infrared chamber (29 × 29 × 26 cm3) was placed in an opaque sound-proof box equipped with exhaust fans. Each rat was monitored for 45 min to measure the locomotor activity for five consecutive days. GluR2-3Y or GluR2-3A was intravenously infused one hour before each test. The number of beam breaks was recorded and data were collected with AniLab software (AniLab, Ningbo, China).
All the data are presented as mean ± SEM. The differences were determined by Student’s t-test (two-tailed) or one-way/two-way repeated measures analysis of variance (ANOVA) with the Bonferroni post-hoc test. *P <0.05, **P <0.01 and ***P <0.001 were considered as statistically significant.
Results and Discussion
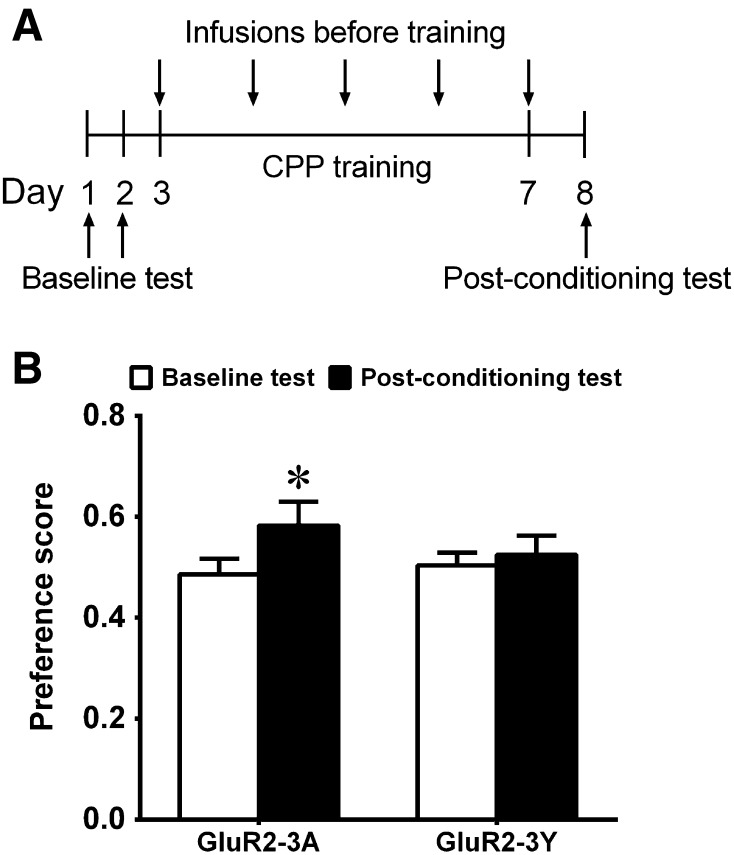
To measure the influence of GluR2-3Y on the acquisition of mCPP, we used two groups of rats: a GluR2-3Y group (n = 12) and a GluR2-3A group (n = 12). During the conditioning phase, rats were injected intravenously with 1.5 nmol/g of GluR2-3A or GluR2-3Y and kept in their home cages for one hour before each morphine conditioning test (Fig. 1A). The dosage of GluR2-3Y (1.5 nmol/g) was used because previous studies have demonstrated that it is an effective dose to inhibit LTD [21], prevent the sensitized behavioral response [21], inhibit cue-induced relapse [15], and facilitate the extinction of mCPP [14].
Fig. 1.
Effects of inhibiting AMPAR endocytosis on the acquisition of mCPP. A Timeline of protocol for mCPP acquisition. B Infusion of GluR2-3Y, but not GluR2-3A, one hour before each morphine conditioning inhibited the acquisition of mCPP. *P <0.05 compared with baseline.
One day after the last conditioning, the place preference was tested. The preference score of rats pretreated with GluR2-3A increased significantly compared with baseline (t = 2.57, P <0.05) (Fig. 1B), indicating that the rats preferred the morphine-paired compartment after training and GluR2-3A had no effect on the acquisition of mCPP. In the rats pretreated with GluR2-3Y, there was no significant change in preference for the morphine-paired compartment compared with baseline (t = 0.61, P = 0.56) (Fig. 1B), indicating that GluR2-3Y inhibited the acquisition of mCPP.
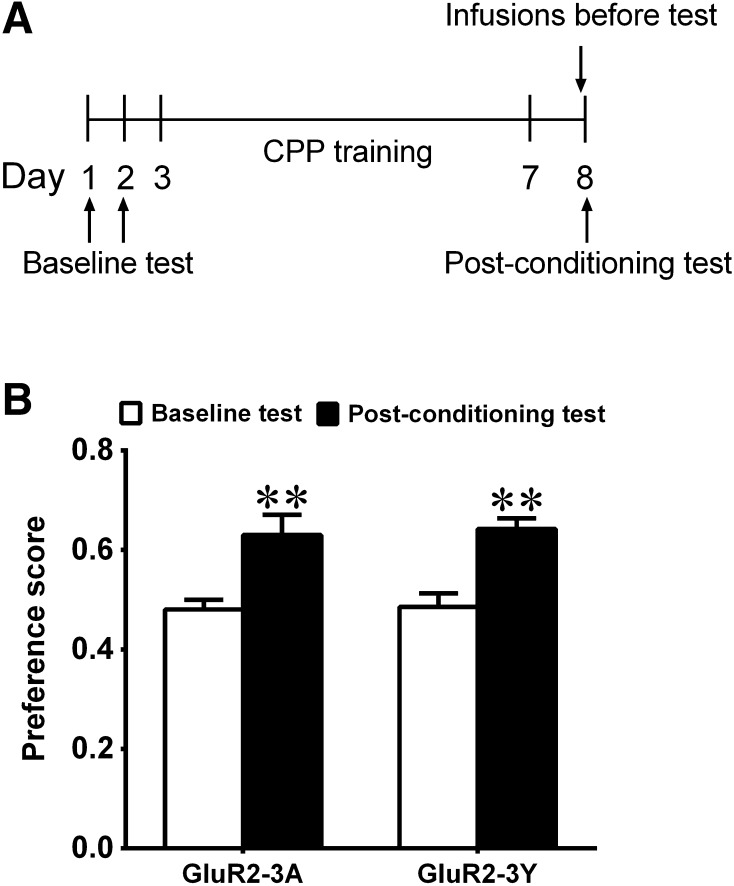
To measure the influence of GluR2-3Y on the expression of mCPP, rats were assigned to two groups after baseline tests: a GluR2-3Y group (n = 9) and a GluR2-3A group (n = 10). One day after the last conditioning, rats received intravenous injection of GluR2-3Y (1.5 nmol/g) or GluR2-3A (1.5 nmol/g) one hour before the post-conditioning test (Fig. 2A). The post-conditioning test was conducted, and the preference scores of the two groups increased significantly compared with the baseline tests (GluR2-3A group: t = 3.47, P <0.01; GluR2-3Y group: t = 4.26, P <0.01, Fig. 2B). These results indicate that blockade of the endocytosis of AMPARs by GluR2-3Y had no significant influence on the expression of mCPP.
Fig. 2.
Effects of inhibiting AMPAR endocytosis on the expression of mCPP. A Timeline of the protocol for expression of mCPP. B Neither GluR2-3Y nor GluR2-3A infused one hour before the post-conditioning test had any effect on the expression of mCPP (GluR2-3A, n = 10; GluR2-3Y, n = 9). **P <0.01 compared with baseline.
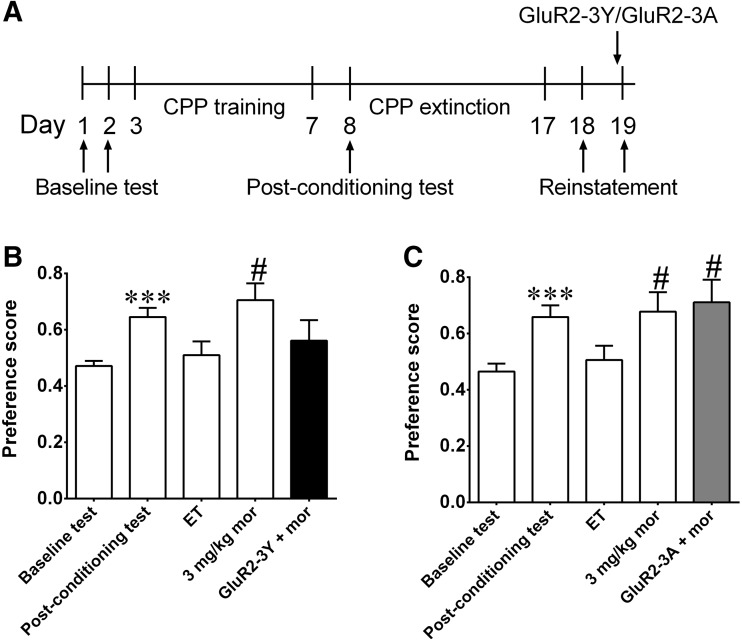
To measure the influence of GluR2-3Y on the reinstatement of mCPP, mCPP was established in two other groups of rats (GluR2-3Y group: n = 8, t = 7.29, P <0.001; GluR2-3A group: n = 8, t = 6.40, P <0.001). After 9 extinction sessions, the rats met the extinction criterion (preference score in extinction was significantly different from the post-conditioning test for three consecutive days; GluR2-3Y group: extinction session 7 (ET7): t = 2.55, P <0.05; ET8: t = 3.59, P <0.01; ET9: t = 4.45, P <0.01; GluR2-3A group: ET7: t = 2.46, P <0.05; ET8: t = 3.67, P <0.01; ET9: t = 2.45, P <0.05; note that only the results of ET9 are shown in Fig. 3). Reinstatement was induced by 3 mg/kg morphine. Two reinstatements were conducted, and rats were divided into two groups (a GluR2-3Y group and a GluR2-3A group) using a balanced protocol after the first reinstatement. In the first reinstatement test, the rats were returned to their cages for 10 min after injection of morphine and then placed in the neutral compartment and allowed to explore the three compartments for 15 min. The preference scores of the two groups were significantly higher than those in the last extinction test (GluR2-3Y group: t = 2.77, P <0.05; GluR2-3A group: t = 2.72, P <0.05), which indicated that 3 mg/kg morphine reinstated the preference for the morphine-paired compartment. On the second reinstatement test, the rats were intravenously injected with GluR2-3Y or GluR2-3A (1.5 nmol/g) in their cages, and one hour later were given 3 mg/kg morphine. As shown in Fig. 3B, the preference score of the GluR2-3Y group did not significantly differ from the last extinction test (t = 0.50, P = 0.63, Fig. 3B), suggesting that pretreatment with GluR2-3Y inhibited the morphine-induced reinstatement. However, the preference score of the GluR2-3A group was significantly higher than in the last extinction test (t = 2.74, P <0.05, Fig. 3C). These results indicated that inhibiting the endocytosis of AMPARs by intravenous injection of GluR2-3Y blocked the morphine-induced reinstatement of mCPP.
Fig. 3.
Effects of inhibiting AMPAR endocytosis on the reinstatement of mCPP. A Timeline of the experiment. B GluR2-3Y infusion one hour before morphine injection inhibited the morphine-induced reinstatement (n = 8). C GluR2-3A infusion one hour before morphine injection did not affect the morphine-induced reinstatement (n = 8). ***P <0.001 compared with baseline; # P <0.05 compared with the last extinction test. ET: extinction test; mor: morphine.
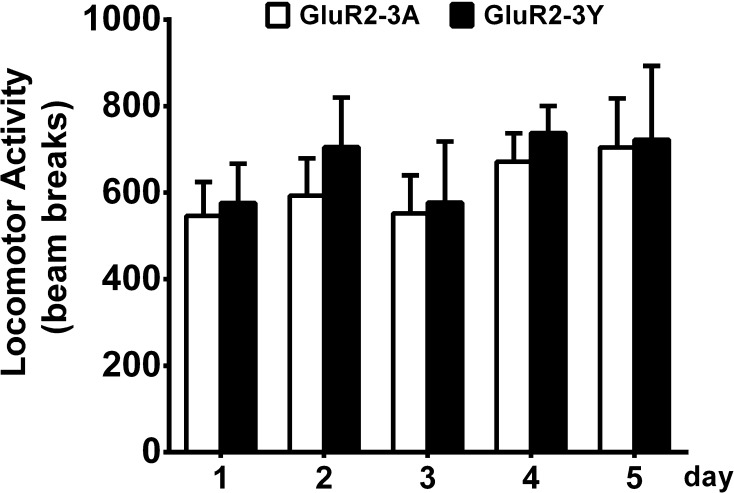
It is possible that GluR2-3Y affects general motor activity. Therefore, we performed mobility tests in a new cohort of rats for five consecutive days. GluR2-3Y (n = 6) or GluR2-3A (n = 6) was intravenously infused one hour before each test. No significant difference was found in locomotor activity (drug × time interaction: F (4,40) = 0.17, P = 0.95, drug effects: F (1,10) = 0.16, P = 0.70; Fig. 4). The results indicated that the effects of GluR2-3Y were unlikely to result from motor deficits.
Fig. 4.
Effects of inhibiting AMPAR endocytosis on locomotor activity. GluR2-3Y infusion did not affect the basal locomotor activity on the five days of testing (GluR2-3A, n = 6; GluR2-3Y, n = 6).
Repeated exposure to morphine changes the surface expression of AMPARs in many brain regions, such as prefrontal cortex (PFC), BLA, VTA, hippocampus, and nucleus accumbens (NAc) [5, 6, 10, 23, 24]. Systemic administration of the AMPAR antagonist LY293558 blocks the development of morphine sensitization [11]. These studies showed that AMPARs in several brain regions play important roles in opiate addiction. In this study, we used intravenous injection, which is widely used in clinical treatment, to simultaneously affect these regions. A critical problem in opiate addiction is the occurrence of relapse. Here, we found that blockade of AMPAR endocytosis by intravenous infusion of GluR2-3Y before morphine injection inhibited the reinstatement of mCPP, and this result was supported by previous evidence which suggests that GluR2-3Y decreases cue-induced heroin reinstatement [15]. Collectively, GluR2 endocytosis may be needed in both drug- and cue-induced opiate relapse. Considering another study which found that GluR2-3Y injection during the acquisition phase of mCPP does not influence the following morphine priming reinstatement [14], the injection timing is a key factor.
The results of this study also demonstrated that intravenous infusion of GluR2-3Y before the post-conditioning test did not influence the expression of mCPP, consistent with a previous study [14]. On the other hand, we found that infusion of GluR2-3Y before the morphine conditioning session inhibited the acquisition of mCPP, while Dias and colleagues found that GluR2-3Y had no effect on the acquisition of mCPP [14]. The explanation for this inconsistency may be that, as Dias and colleagues discussed, co-administration of GluR2-3Y with morphine during the conditioning phase might weaken the association between the contextual cues and morphine, which led to the rapid extinction of mCPP in their studies, and stronger weakening effects were induced in the present study, which led to inhibited acquisition. The difference in the levels of weakening effects might be due to the different number and/or interval of GluR2-3Y infusions. Dias and colleagues administered morphine and saline on alternate days for eight days, so GluR2-3Y affected morphine conditioning four times, 48 hours apart, while in the present study, conditioning training was conducted twice daily for five consecutive days, so GluR2-3Y affected morphine conditioning five times, 24 hours apart.
Previous experiments have indicated that GluR2-3Y blocks the induction of LTD, which is a proposed cellular substrate for learning and memory [21]. In this study, GluR2-3Y inhibition of the acquisition of mCPP may be due to the weakened association between the contextual cues and morphine, while GluR2-3Y blockade of the morphine-induced reinstatement of mCPP may be because of the inhibition of memory retrieval. Then in which brain regions does GluR2-3Y inhibit the addictive behavior? Previous studies found that the endocytosis of AMPARs containing GluR2 in the NAc plays an important role in the expression of amphetamine-induced behavioral sensitization [21] and the long-term maintenance of mCPP [25]. Moreover, endocytosis in the BLA and in the ventral medial PFC is crucial for the reconsolidation of methamphetamine reward memory [26] and cue-induced heroin-seeking [15], respectively. However, no evidence has identified the brain regions in which the endocytosis of AMPARs is required for the acquisition and reinstatement of mCPP. Based on previous studies of drug-related memory, the hippocampus and ventral medial PFC may be the key brain regions for acquisition and reinstatement, respectively [15, 27], and further investigations are needed.
Previous work in our lab has shown that injection of GluR2-3Y into the periaqueductal grey dose-dependently alleviates neuropathic pain [28]. In this study, only one dose of GluR2-3Y (1.5 nmol/g) was used because 1.5 and 2.25 nmol/g seem to have the same effects on the acquisition, extinction, and reinstatement of mCPP [14], and previous studies have shown that GluR2-3Y injection (1.5 nmol/g intravenously) prevents D-amphetamine-induced behavioral sensitization [21] and inhibits cue-induced relapse to heroin-seeking [15].
Our results showed that intravenous injection of GluR2-3Y led to suppression of the acquisition and reinstatement of mCPP, but had no effect on the expression of mCPP. Because AMPARs are widely distributed in many brain areas and the spinal cord, the mechanisms underlying the actions of GluR2-3Y are complicated. The present study revealed the roles of AMPAR endocytosis in morphine-related addiction. Moreover, although we did not investigate the possible brain regions responsible for the effects of GluR2-3Y, the intravenous injection we used raises the possibility of clinical use.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81171043, 31400880) and the Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, China (KLMH2014ZG02).
Footnotes
Xiao-Jing Lin and Jian-Jun Zhang have contributed equally to this work.
References
- 1.Gawel K, Labuz K, Jenda M, Silberring J, Kotlinska JH. Influence of cholinesterase inhibitors, donepezil and rivastigmine on the acquisition, expression, and reinstatement of morphine-induced conditioned place preference in rats. Behav Brain Res. 2014;268:169–176. doi: 10.1016/j.bbr.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Guo SJ, Cui Y, Huang ZZ, Liu H, Zhang XQ, Jiang JX, et al. Orexin A-mediated AKT signaling in the dentate gyrus contributes to the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Addict Biol 2015. [DOI] [PubMed]
- 3.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, et al. Enhanced LTP in mice deficient in the AMPA receptor GluR2. Neuron. 1996;17:945–956. doi: 10.1016/S0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 4.Granstrem O, Adriani W, Shumilina M, Izykenova G, Dambinova S, Laviola G. Specific changes in levels of autoantibodies to glutamate and opiate receptors induced by morphine administration in rats. Neurosci Lett. 2006;403:1–5. doi: 10.1016/j.neulet.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Lane DA, Lessard AA, Chan J, Colago EE, Zhou Y, Schlussman SD, et al. Region-specific changes in the subcellular distribution of AMPA receptor GluR1 subunit in the rat ventral tegmental area after acute or chronic morphine administration. J Neurosci. 2008;28:9670–9681. doi: 10.1523/JNEUROSCI.2151-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher P, et al. Increased insertion of glutamate receptor 2-lacking alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 8.Sepehrizadeh Z. Bahrololoumi Shapourabadi M, Ahmadi S, Hashemi Bozchlou S, Zarrindast MR, Sahebgharani M. Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol. 2008;35:1321–1330. doi: 10.1111/j.1440-1681.2008.05004.x. [DOI] [PubMed] [Google Scholar]
- 9.Xia Y, Portugal GS, Fakira AK, Melyan Z, Neve R, Lee HT, et al. Hippocampal GluA1-containing AMPA receptors mediate context-dependent sensitization to morphine. J Neurosci. 2011;31:16279–16291. doi: 10.1523/JNEUROSCI.3835-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, Pickel VM. Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse. 2005;58:1–12. doi: 10.1002/syn.20176. [DOI] [PubMed] [Google Scholar]
- 11.Carlezon WA, Jr, Rasmussen K, Nestler EJ. AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse. 1999;31:256–262. doi: 10.1002/(SICI)1098-2396(19990315)31:4<256::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.McLemore GL, Kest B, Inturrisi CE. The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res. 1997;778:120–126. doi: 10.1016/S0006-8993(97)00985-2. [DOI] [PubMed] [Google Scholar]
- 13.Kest B, McLemore G, Kao B, Inturrisi CE. The competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. J Pharmacol Exp Ther. 1997;283:1249–1255. [PubMed] [Google Scholar]
- 14.Dias C, Wang YT, Phillips AG. Facilitated extinction of morphine conditioned place preference with Tat-Glu A2(3Y) interference peptide. Behav Brain Res. 2012;233:389–397. doi: 10.1016/j.bbr.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Zhang JJ, Yu LC. Post-retrieval extinction training enhances or hinders the extinction of morphine-induced conditioned place preference in rats dependent on the retrieval-extinction interval. Psychopharmacology (Berl) 2012;221:19–26. doi: 10.1007/s00213-011-2545-4. [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Wang Q, Cheng Y, Ji J, Yu LC. Changes of protein expression profiles in the amygdala during the process of morphine-induced conditioned place preference in rats. Behav Brain Res. 2011;221:197–206. doi: 10.1016/j.bbr.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Lin X, Wang Q, Ji J, Yu LC. Role of MEK-ERK pathway in morphine-induced conditioned place preference in ventral tegmental area of rats. J Neurosci Res. 2010;88:1595–1604. doi: 10.1002/jnr.22326. [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Niu H, Rizak JD, Li L, Wang G, Xu L, et al. Combined action of MK-801 and ceftriaxone impairs the acquisition and reinstatement of morphine-induced conditioned place preference, and delays morphine extinction in rats. Neurosci Bull. 2012;28:567–576. doi: 10.1007/s12264-012-1269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, et al. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, et al. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang N, Su P, Lu J, Wang Y. Disruption of dopamine D1 receptor phosphorylation at serine 421 attenuates cocaine-induced behaviors in mice. Neurosci Bull. 2014;30:1025–1035. doi: 10.1007/s12264-014-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mickiewicz AL, Napier TC. Repeated exposure to morphine alters surface expression of AMPA receptors in the rat medial prefrontal cortex. Eur J Neurosci. 2011;33:259–265. doi: 10.1111/j.1460-9568.2010.07502.x. [DOI] [PubMed] [Google Scholar]
- 24.Glass MJ, Lane DA, Colago EE, Chan J, Schlussman SD, Zhou Y, et al. Chronic administration of morphine is associated with a decrease in surface AMPA GluR1 receptor subunit in dopamine D1 receptor expressing neurons in the shell and non-D1 receptor expressing neurons in the core of the rat nucleus accumbens. Exp Neurol. 2008;210:750–761. doi: 10.1016/j.expneurol.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, et al. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu YJ, Chang CH, Gean PW. AMPA receptor endocytosis in the amygdala is involved in the disrupted reconsolidation of Methamphetamine-associated contextual memory. Neurobiol Learn Mem. 2013;103:72–81. doi: 10.1016/j.nlm.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JJ, Han J, Sui N. Okadaic acid blocks the effects of 5-aza-2-deoxycytidine on consolidation, acquisition and retrieval of morphine-induced place preference in rats. Neuropharmacology. 2014;86:282–293. doi: 10.1016/j.neuropharm.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Liu TY, Cheng Y, Qin XY, Yu LC. Pharmacologically inhibiting GluR2 internalization alleviates neuropathic pain. Neurosci Bull. 2015;31:611–616. doi: 10.1007/s12264-015-1556-2. [DOI] [PMC free article] [PubMed] [Google Scholar]