Abstract
The outcome of early intravenous thrombolysis for ischemic stroke in patients with atrial fibrillation (AF) is worse than that without thrombosis. How to increase the efficacy of intravenous thrombolysis for AF-related ischemic stroke remains largely unknown. In this study, we investigated factors that influence the effect of intravenous thrombolysis in these patients. Our results showed that thrombolysis was independently associated with a favorable outcome (P < 0.001) and did not influence the mortality of AF-related ischemic stroke, although it increased the risk of hemorrhage within 24 h after treatment. Risk factors for a poor outcome at admission were: heart failure (P = 0.045); high systolic pressure (P = 0.039); high blood glucose (P = 0.030); and a high National Institutes of Health Stroke Scale (NIHSS) score (P < 0.001). Moreover, high systolic pressure at admission (P = 0.007), high blood glucose (P = 0.027), and a high NIHSS score (P < 0.001) were independent risk factors for mortality at 3 months. Besides thrombolysis, a high NIHSS score (P = 0.006) and warfarin taken within 48 h before stroke onset (P = 0.032) were also independent risk factors for symptomatic hemorrhage within 24 h after treatment. Ischemic stroke patients with AF benefited from intravenous thrombolysis with recombinant tissue plasminogen activator within 4.5 h after stroke.
Keywords: Ischemic stroke, Atrial fibrillation, Intravenous recombinant tissue plasminogen activator, Intravenous thrombolysis, Favorable outcome, Risk factors
Introduction
Atrial fibrillation (AF)-related ischemic stroke is often severe and has a poor prognosis [1, 2]. AF contributes to a poor outcome in ischemic stroke patients and is an independent predictor of high mortality [3]. Intravenous thrombolysis within 4.5 h can significantly improve the prognosis of ischemic stroke patients [4, 5] and an increasing number of retrospective studies have demonstrated that AF-related stroke patients also benefit from intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) compared to those without rt-PA [3, 6]. A recent prospective study enrolling 734 ischemic stroke patients, including 155 with AF, supported the use of rt-PA intravenous thrombolysis for acute cases [7]; however, ischemic stroke patients with AF benefited less than those without AF [6, 8–10]. Compared to non-AF patients, stroke patients with AF had worse outcomes after rt-PA intravenous thrombolysis, including higher mortality and disability rates and lower recanalization due to the resistance of previous thrombi to rt-PA in patients with AF [8, 11].
Previous studies on the independent prognostic factors of intravenous rt-PA thrombolysis in AF-induced stroke patients are limited. In this study, 267 AF-related ischemic stroke patients were enrolled from 15 hospitals in Jiangsu Province, China; 151 received rt-PA thrombolytic therapy and all received follow-up examinations at 3 months. The aim was to determine whether intravenous rt-PA thrombolytic therapy is an independent beneficial factor for AF-related acute stroke patients. Meanwhile, potential factors influencing the outcome in thrombolytic patients were also investigated. The results will help physicians to determine appropriate individual thrombolysis therapy for AF-related ischemic stroke patients.
Methods
Patients and Materials
This was an open-label, multicenter, retrospective study approved by the Ethics Committee of the Affiliated Drum Tower Hospital of Nanjing University Medical School, China. Patients with acute ischemic stroke were selected from the 15 hospitals of the Jiangsu Stroke Research Collaborative Group between November 2009 and July 2013. AF-related ischemic stroke patients who were eligible for thrombolysis were included. All patients gave written informed consent.
Inclusion criteria: (1) >18 years old; (2) clinical diagnosis of ischemic stroke causing a significant neurological deficit; (3) time from symptom onset to initiation of rt-PA or other treatment <4.5 h; (4) computed tomography (CT) or magnetic resonance imaging (MRI) indicating no intracranial hemorrhage; (5) AF on admission or diagnosed before onset; and (6) signed informed consent.
Exclusion criteria: (1) NIHSS (National Institutes of Health Stroke Scale) score <4 or rapidly improving symptoms or signs; (2) NIHSS >20 and baseline MRI or CT showing extensive infarction; (3) history of intracranial hemorrhage (ICH) or symptoms suggestive of symptomatic intracranial hemorrhage (SICH); (4) myocardial infarction, stroke, or serious head trauma in the previous 3 months; (5) urinary tract, lung, or gastrointestinal hemorrhage within the 21 previous days; (6) serious trauma or major surgery within the previous 14 days; (7) lumbar or arterial puncture at a non-compressible site within 7 days; (8) received heparin within 48 h, resulting in an activated partial thromboplastin time greater than the upper limit of normal; (9) systolic pressure >185 mmHg or diastolic pressure >110 mmHg; (10) blood glucose <50 or >400 mg/dL; (11) current use of anticoagulants with an International Normalized Ratio >1.7 or prothrombin time >15 s; (12) platelet count <100,000/mm3; (13) pregnant; (14) serious heart, lung, kidney or other organ dysfunction; and (15) allergy to active ingredients of rt-PA.
AF was diagnosed using a 12-lead electrocardiogram at admission or diagnosed before admission. Rt-PA was administered by experienced neurologists; all information was systematically and rigorously recorded. The attending physician collected detailed information by inviting the patients to our department for follow-up or by telephone if they could not come, including details of the modified Rankin Scale (mRS) score and survival rate. Qualified, experienced observers completed the CT/MRI scanning and reading; and professional statisticians completed the follow-up analyses.
We reviewed the medical records of enrolled patients to obtain information about the demographics, baseline hemodynamics, medical history, vascular risk factors, stroke severity, and mRS score at 3 months. Hypertension was defined as a blood pressure >140/90 mmHg or the use of anti-hypertensive drugs. Diabetes mellitus was defined as the use of hypoglycemic drugs or insulin therapy. The criteria for a diagnosis of diabetes mellitus were symptoms of diabetes and casual plasma glucose ≥11.1 mmol/L (200 mg/dl), fasting plasma glucose ≥7.0 mmol/L (126 mg/dl), or 2-h postprandial blood glucose ≥11.1 mmol/L. Heart failure was defined as New York Heart Association functional class III to IV. Drinking was defined as a history of drinking alcohol.
To evaluate the efficacy of thrombolysis in ischemic stroke patients with AF, the enrolled patients were divided into the thrombolysis group and the control group according to whether or not they received rt-PA. In the thrombolysis group, 0.9 mg/kg rt-PA (maximum of 90 mg; 10% bolus with the remainder over 1 h) was administered. Patients who rejected thrombolysis received pharmaceutical treatment.
Measurement of Outcome
NIHSS and mRS scores were used to assess patients at admission, after rt-PA treatment, at discharge, and at 90 days by each patient’s attending physician. A favorable outcome was defined as an mRS score of 0–1 at 3 months, while a score >1 was defined as an unfavorable outcome. We also recorded the mortality within 3 months, as well as total ICH and SICH within 24 h after treatment (SICH was defined as the appearance of a new hemorrhage on imaging with neurologic deterioration ≥4 points on the NIHSS at 24 h after treatment). One patient in the thrombolysis group was lost to follow-up.
Statistical Analysis
To compare the categorical variables of the clinical features, χ 2 tests were used and the Mann-Whitney U-test or Student’s t-test was used to compare continuous variables of clinical characteristics. Univariate logistic regression analysis was used to identify potential factors influencing outcomes. Univariate predictors at a level of P < 0.10 were considered significant and were entered into a multivariate logistic regression model. All tests were two-tailed. P < 0.05 was considered to be statistically significant, but P < 0.01 was considered to suggest statistical significance in subgroup analysis to minimize the probability of Type I errors. All statistical analyses were performed using SPSS ver.16.0 (Chicago, IL) and Stata ver. 12.0 (StataCorp LP, College Station, TX) softwares.
Results
Baseline Information on Patients
A total of 267 ischemic stroke patients with AF (116 male, mean age 72.25 ± 9.10 years) were involved in this study. Baseline characteristics are summarized in Table 1. A total of 189 patients had hypertension and 81 suffered from heart failure. One patient did not receive follow-up.
Table 1.
Demographic and clinical features of stroke patients with atrial fibrillation.
| Overall patients (n = 267) (%) |
|
|---|---|
| Gender, male | 116 (43.45%) |
| Age, mean±SD | 72.25±9.10 |
| Smoking | 56 (20.97%) |
| Drinking | 34 (12.73%) |
| Hypertension | 189 (70.79%) |
| Diabetes mellitus | 46 (17.23%) |
| Hyperlipidemia | 40 (14.98%) |
| Heart failure | 81 (30.34%) |
| History of stroke | 83 (31.09%) |
| Warfarin taken within 48 h | 12 (4.49%) |
| Time from stroke onset to treatment (min), mean±SD | 171.62±57.34 |
| Systolic pressure at admission (mmHg), mean±SD | 148.13±20.59 |
| Diastolic pressure at admission (mmHg), mean±SD | 85.60±13.43 |
| Blood glucose at admission (mmol/L), mean±SD | 7.61±2.58 |
| NIHSS score at admission, mean±SD | 14.55±7.69 |
| Thrombolysis treatment | 151 (56.6%) |
Independent Predictors of Outcome for Ischemic Stroke Patients with AF
Heart failure, systolic pressure at admission, blood glucose at admission, and a high NIHSS score were independent risk factors for a favorable outcome in AF-related ischemic stroke patients. However, thrombolysis was an independent predictor of a favorable outcome (adjusted OR: 5.73, 95%CI[2.40, 13.69], adjusted P < 0.001) (Table 2).
Table 2.
Univariate and multivariate logistic regression to predict primary outcome (mRS 0-1) of stroke patients with AF.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR[95%CI] | Unadjusted P value | OR[95%CI] | Adjusted P value | |
| Gender, male | 0.44[0.26–0.74] | 0.002 | 0.68[0.30–1.51] | 0.338 |
| Age | 0.98[0.95–1.01] | 0.122 | ||
| Smoking | 2.04[1.11–3.77] | 0.022 | 1.13[0.39–3.29] | 0.826 |
| Drinking | 1.94[0.93–4.05] | 0.076 | 0.77[0.25–2.37] | 0.643 |
| Hypertension | 1.30[0.73–2.34] | 0.375 | ||
| Diabetes mellitus | 0.86[0.43–1.74] | 0.679 | ||
| Hyperlipidemia | 0.96[0.46–1.99] | 0.902 | ||
| Heart failure | 0.44[0.24–0.83] | 0.011 | 0.44[0.19–0.98] | 0.045 |
| History of stroke | 0.69[0.39–1.24] | 0.220 | ||
| Warfarin taken within 48h | 1.13[0.33–3.85] | 0.848 | ||
| Time from stroke onset to treatment (min) | 1.00[0.99–1.01] | 0.413 | ||
| Systolic pressure at admission (mmHg) | 0.98[0.97–0.99] | 0.023 | 0.98[0.96–0.99] | 0.039 |
| Diastolic pressure at admission (mmHg) | 0.99[0.98–1.01] | 0.534 | ||
| Blood glucose at admission (mmol/L) | 0.85[0.75–0.97] | 0.012 | 0.83[0.70–0.98] | 0.030 |
| NIHSS score at admission | 0.81[0.76–0.86] | <0.001 | 0.76[0.70–0.82] | <0.001 |
| Thrombolysis | 1.77[1.03–3.04] | 0.039 | 5.73[2.40–13.69] | <0.001 |
NIHSS National Institutes of Health Stroke Scale.
Inpatients with AF-related ischemic stroke, high systolic pressure at admission (adjusted OR: 1.03, 95%CI[1.01, 1.04], adjusted P = 0.007), high blood glucose (adjusted OR: 1.16, 95%CI[1.02, 1.31], adjusted P = 0.027), and high NIHSS score (adjusted OR: 1.16, 95%CI[1.10, 1.22], adjusted P < 0.001) were independent risk factors for mortality at 3 months (Table 3). Thrombolysis (adjusted OR: 9.49, 95%CI[2.09, 43.06], adjusted P = 0.004), high NIHSS score (adjusted OR: 1.10, 95%CI[1.03, 1.17], adjusted P = 0.006), and warfarin taken within 48 h before stroke onset (adjusted OR: 6.31, 95%CI[1.18, 33.87], adjusted P = 0.032) were independent predictors for ICH in ischemic stroke patients with AF (Table 4).
Table 3.
Univariate and multivariate logistic regression to predict death at 3 months for stroke patients with AF.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR[95%CI] | Unadjusted P value | OR[95%CI] | Adjusted P value | |
| Gender, male | 1.51[0.84–2.69] | 0.166 | ||
| Age | 1.05[1.02–1.09] | 0.004 | 1.02[0.98–1.06] | 0.349 |
| Smoking | 0.63[0.30–1.34] | 0.632 | ||
| Drinking | 0.65[0.26–1.64] | 0.358 | ||
| Hypertension | 1.32[0.70–2.50] | 0.396 | ||
| Diabetes mellitus | 0.99[0.47–2.10] | 0.996 | ||
| Hyperlipidemia | 0.14[0.03–0.60] | 0.140 | ||
| Heart failure | 1.84[1.02–3.31] | 0.042 | 1.73[0.83–3.60] | 0.145 |
| History of stroke | 1.22[0.67–2.21] | 0.515 | ||
| Warfarin taken within 48h | 2.37[0.73–7.75] | 0.153 | ||
| Time from stroke onset to treatment (min) | 1.00[0.99–1.01] | 0.867 | ||
| Systolic pressure at admission (mmHg) | 1.02[1.01–1.04] | 0.003 | 1.03[1.01–1.04] | 0.007 |
| Diastolic pressure at admission (mmHg) | 1.01[0.99–1.03] | 0.579 | ||
| Blood glucose at admission (mmol/L) | 1.21[1.09–1.35] | <0.001 | 1.16[1.02–1.31] | 0.027 |
| NIHSS score at admission | 1.17[1.11–1.22] | <0.001 | 1.16[1.10–1.22] | <0.001 |
| Thrombolysis | 0.91[0.51–1.59] | 0.730 | ||
AF atrial fibrillation, NIHSS National Institutes of Health Stroke Scale.
Table 4.
Univariate and multivariate logistic regression to predict symptomatic intracranial hemorrhage at 24 h after treatment in stroke patients with AF.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR[95%CI] | Unadjusted P value | OR[95%CI] | Adjusted P value | |
| Thrombolysis | 9.21[2.11–40.13] | 0.003 | 9.49[2.09–43.06] | 0.004 |
| Gender, male | 1.22[0.51–2.91] | 0.663 | ||
| Age | 1.01[0.96–1.06] | 0.679 | ||
| Smoking | 1.37[0.51–3.65] | 0.530 | ||
| Drinking | 0.29[0.04–2.23] | 0.235 | ||
| Hypertension | 0.94[0.37–2.38] | 0.893 | ||
| Diabetes mellitus | 0.70[0.20–2.47] | 0.580 | ||
| Hyperlipidemia | 0.52[0.12–2.29] | 0.385 | ||
| Heart failure | 1.54[0.64–3.71] | 0.340 | ||
| History of stroke | 0.97[0.38–2.45] | 0.944 | ||
| Warfarin taken within 48h | 3.92[0.98–15.63] | 0.053 | 6.31[1.18–33.87] | 0.032 |
| Time from stroke onset to treatment (min) | 0.99[0.986–1.001] | 0.994 | ||
| Systolic pressure at admission (mmHg) | 1.01[0.99–1.04] | 0.217 | ||
| Diastolic pressure at admission (mmHg) | 0.99[0.96–1.02] | 0.374 | ||
| Blood glucose at admission (mmol/L) | 1.13[0.98–1.30] | 0.086 | 1.09[0.93–1.28] | 0.295 |
| NIHSS score at admission | 1.08[1.02–1.14] | 0.006 | 1.10[1.03–1.17] | 0.006 |
AF atrial fibrillation, NIHSS National Institutes of Health Stroke Scale.
Efficacy and Safety of Thrombolysis in AF-Related Ischemic Stroke Patients
The baseline characteristics of the 151 patients in the thrombolysis group and 116 in the control group are summarized in Table 5. The mean NIHSS score was 15.06 ± 6.76 in the rt-PA group and 13.89 ± 8.75 in the control group (P = 0.054). The control group had fewer smokers than the thrombolysis group (P < 0.001). There were no significant differences between the two groups in the other baseline characteristics (Table 5).
Table 5.
Demographics, baseline characteristics, and outcomes of patients in the thrombolysis and control groups.
| Baseline characteristics | Thrombolysis (n = 151) (%) | Control (n = 116) (%) | P value |
|---|---|---|---|
| Gender, male | 66 (43.7%) | 50 (43.1%) | 0.921 |
| Age, mean±SD | 71.32±7.77 | 73.47±10.05 | 0.066 |
| Smoking | 44 (29.1%) | 12 (10.3%) | <0.001 |
| Drinking | 19 (12.6%) | 15 (12.9%) | 0.933 |
| Hypertension | 111 (73.5%) | 78 (67.2%) | 0.264 |
| Diabetes mellitus | 24 (15.9%) | 22 (19.0%) | 0.510 |
| Hyperlipidemia | 26 (17.2%) | 14 (12.1%) | 0.243 |
| Heart failure | 44 (29.1%) | 37 (31.9%) | 0.627 |
| History of stroke | 43 (28.5%) | 40 (34.5%) | 0.293 |
| Warfarin taken within 48h | 6 (4.0%) | 6 (5.2%) | 0.639 |
| Time from stroke onset to treatment (min), mean±SD | 165.70±54.50 | 179.28±60.20 | 0.055 |
| Systolic pressure at admission (mmHg), mean±SD | 148.07±20.94 | 148.21±20.20 | 0.958 |
| Diastolic pressure at admission (mmHg), mean±SD | 85.83±13.69 | 85.29±13.14 | 0.748 |
| Blood glucose at admission (mmol/L), mean±SD | 7.81±2.53 | 7.32±2.64 | 0.139 |
| NIHSS score at admission, mean±SD | 15.06±6.76 | 13.89±8.75 | 0.054 |
| Median (min, max) | 15 (4–38) | 12 (4–36) | |
| Clinical outcomes | |||
| Favorable outcome(mRS 0–1) at 3 months | 54 (36.0%) | 28 (24.1%) | 0.038 |
| Death at 3 months | 35 (23.2%) | 29 (25.0%) | 0.730 |
| ICH within 24 h after treatment | 40 (26.5%) | 2 (1.7%) | <0.001 |
| SICH within 24 h after treatment | 21 (13.9%) | 2 (1.7%) | <0.001 |
ICH intracranial hemorrhage, NIHSS National Institutes of Health Stroke Scale, SICH symptomatic intracranial hemorrhage.
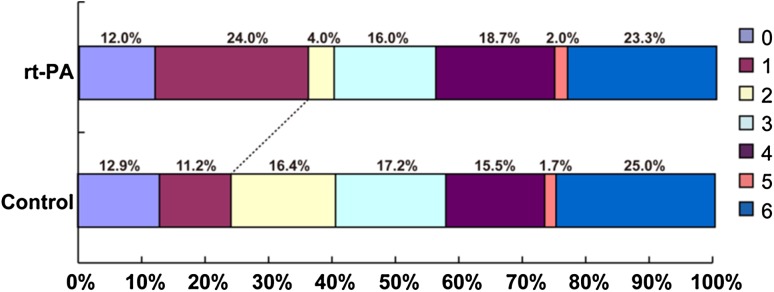
The distribution of mRS scores in the thrombolysis and control groups is summarized in Fig. 1. At 3 months, 54 patients (36.0%) in the thrombolysis group had a favorable outcome compared with 28 patients (24.1%) in the control group, representing an absolute improvement of 11.9% (P = 0.038) though there was no significant difference in neurological function between the two groups at discharge (data not shown). Mortality within 3 months in rt-PA patients was not significantly different from that in the control group (P = 0.730). Forty patients (26.5%) suffered from ICH within 24 h after rt-PA treatment, compared with only 2 patients (1.7%) in the control group (P < 0.001). Similarly, patients with rt-PA had a higher risk of SICH within 24 h after treatment than those in the control group (13.9% vs 1.7%, P < 0.001) (Table 5).
Fig. 1.
Outcome at 3 months: modified Rankin Scale (mRS) distribution in treatment and control groups.
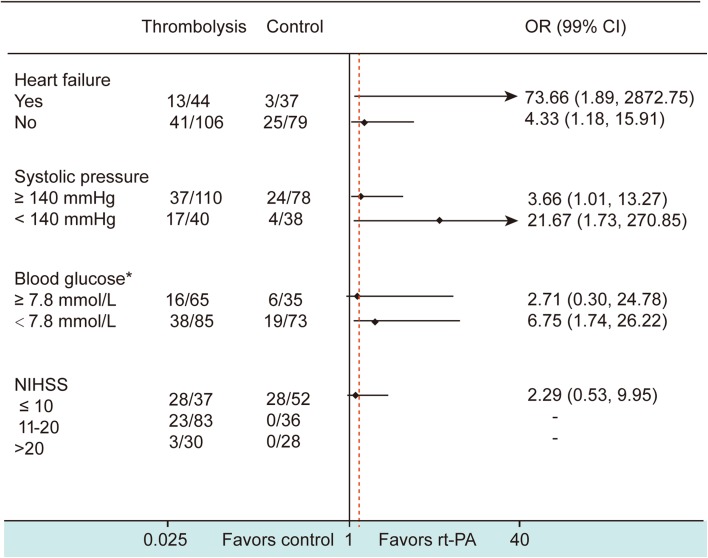
Subgroup analyses assessing the efficacy of thrombolysis in high-risk stroke patients with AF demonstrated that thrombolysis had an independent effect on a favorable outcome in patients with heart failure (adjusted OR: 73.66, 99%CI[1.89, 2872.75], P = 0.003); hypertension (adjusted OR: 3.66, 99%CI[1.01, 13.27], P = 0.009); and an NIHSS score of 11–20 (P < 0.001). There were no benefits for patients with blood glucose ≥7.8 mmol/L (P = 0.245) and an NIHSS score >20 (P = 0.261) at admission (Fig. 2).
Fig. 2.
Effect of thrombolysis on a favorable outcome (mRS 0–1 at 3 months) in stroke patients with atrial fibrillation. Data on the left represent the number of patients with mRS 0–1 at 3 months/total number of patients in each subgroup divided according to the variables heart failure, systolic pressure, blood glucose, and NIHSS score. Asterisk eight patients lacked blood glucose data at admission. The treatment OR in each subgroup has been adjusted for gender, smoking, drinking, and other key variables (heart failure, systolic pressure, blood glucose, and NIHSS score at admission). Diamonds represent the OR value. Lines without arrows indicate the range of 99%CI. Lines with arrows mean that the OR or 99%CI exceeds the upper limit of the scale (40) shown at the bottom. The red dash line shows the location of total OR of the effect of rt-PA therapy versus conventional therapy on favorable outcome.
Discussion
In this study, we found that (1) rt-PA thrombolysis within 4.5 h was an independent effect factor for AF-related ischemic stroke; (2) risk factors associated with death at 3 months after thrombolysis in AF-related ischemic stroke were high systolic pressure, high blood glucose, and a high NIHSS score at admission; (3) predictors for symptomatic ICH within 24 h after thrombolysis were warfarin-use within 48 h before thrombolysis and a high NIHSS score at admission; (4) AF-related stroke patients with heart failure, hypertension, and an NIHSS score between 11 and 20 at admission had more favorable outcomes with rt-PA thrombolysis than those without thrombolysis; and (5) no benefits of thrombolysis were found in AF-related ischemic stroke patients with blood glucose ≥7.8 mmol/L and an NIHSS score >20 at admission.
The efficacy of rt-PA in patients with stroke has been confirmed; however, results for the AF subgroup have not been well explored. A few large, randomized, controlled trials regarding the effectiveness of rt-PA treatment in AF patients have been conducted, but with apparently conflicting conclusions. In 1997, a National Institute of Neurological Disorders and Stroke trial found no difference in outcome, with or without thrombolysis treatment [12]. In the European Cooperative Acute Stroke Study III, no significant difference in response to rt-PA was found among patients with AF compared with the placebo group [13]. In 2012, the Third International Stroke Trial 3, which likely contained the largest number of AF cases, demonstrated a trend toward improved outcome in its rt-PA-treated group [14]. In the Virtual International Stroke Trials Archive, which documented results involving 1631 AF stroke patients, a similar magnitude of benefit with rt-PA compared with placebo for patients with AF and without AF was reported [6] and it was demonstrated that the presence of AF had no independent influence on stroke outcome relative to the untreated comparators. Similarly, Visnja et al. recently reported a prospective study of 734 ischemic stroke patients (including 155 with AF) who received rt-PA thrombolysis. The results indicated a trend that ischemic stroke patients with AF treated intravenously with rt-PA had worse outcomes because they were older and had more serious dysfunction at baseline; AF was not an independent predictor of a bad outcome or death [7]. Later, in 2013 [3], a study also supported a similar trend for a favorable outcome after rt-PA among stroke patients with AF,
In the present study, the rt-PA treatment group had a better outcome at 3 months and, as shown by logistic regression analysis, intravenous thrombolysis was independently associated with a better outcome (mRS 0–1). We again showed that intravenous thrombolysis was beneficial for cerebral infarction patients with AF. Heart failure, high systolic blood pressure, high blood glucose, and a high NIHSS score at admission were the risk factors for a poor prognosis. Congestive heart failure is known to increase mortality at 3 months in AF-related stroke patients [15, 16]. However, based on our subgroup analysis, AF-related ischemic stroke patients with heart failure still benefited from intravenous rt-PA thrombolysis.
Moreover, data from our study suggested that thrombolysis is beneficial for AF-related ischemic stroke patients with heart failure, high BP, or an NIHSS score of 11–20. This finding may guide physicians to administer rt-PA sooner and more aggressively to this type of patient.
In the baseline characteristics of this study, more smoking patients appeared in the rt-PA treatment group than those without. Anna et al. found that smoking is probably independently associated with recanalization and reperfusion in rt-PA therapy [17]. However, this study did not have sufficient weight to support this assumption (OR 0.63, 95%CI 0.30–1.34, P = 0.632) by univariate and multivariate logistic regression analyses. Similar to previous studies [12, 13, 18–20], we found a higher rate of ICH (26.5% vs 1.7%) and SICH (13.9% vs 1.7%) in AF-related ischemic stroke with thrombolysis than without, which could result in a higher mortality in AF-related ischemic stroke patients with rt-PA treatment. However, the AF ischemic stroke patients with rt-PA who were alive and independent (mRS 0–1) at 3 months were increased (36% vs 24.1%) in contrast to previous reports. This may be due to early arterial recanalization, which is recognized as a marker of a favorable prognosis after thrombolysis with rt-PA [21–23].
The current study has some limitations: (1) it was a small-scale and non-randomized study so it may be difficult to evaluate the real efficacy of rt-PA in every patient; (2) no classification of AF types and related response differences was carried out (one study found a relationship between patients with chronic AF and risk of poor prognosis compared with other types of AF [24]); (3) the follow-up time was short and did not provide data on longer-term prognosis; long-term survival (e.g., 2 years) may be a better predictor of response to intravenous thrombolysis with rt-PA; (4) no analysis of other factors that may influence prognosis, such as intracranial infarct size, biochemical indicators, the region of stroke, and history of other diseases, was carried out; and (5) basic differences between enrolled patients that may affect prognosis could not be excluded (for instance, different smoking patterns between the two groups). Additional studies are needed to clarify these problems.
We conclude that intravenous rt-PA thrombolysis is beneficial for ischemic stroke patients with AF, including those with heart failure, high systolic blood pressure, and a high NIHSS score at admission.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81230026 and 81171085), the Natural Science Foundation of Jiangsu Province, China (BL2012013), and the Science Foundation of the Bureau of Health of Jiangsu Province, China (LJ201101).
Footnotes
Qiuyun Zhao, Xiaobo Li, Wanli Dong, and Min Ye contributed equally to this work.
Contributor Information
Yan Chen, Email: chenyan20141014@163.com.
Yun Xu, Email: xuyun20042001@aliyun.com.
References
- 1.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 2.Asberg S, Henriksson KM, Farahmand B, Asplund K, Norrving B, Appelros P, et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke. 2010;41:1338–1342. doi: 10.1161/STROKEAHA.110.580209. [DOI] [PubMed] [Google Scholar]
- 3.Saposnik G, Gladstone D, Raptis R, Zhou L, Hart RG, et al. Atrial fibrillation in ischemic stroke: predicting response to thrombolysis and clinical outcomes. Stroke. 2013;44:99–104. doi: 10.1161/STROKEAHA.112.676551. [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Ye M, Chen BL, Chen GF, Gao ZQ, Zhou JS, et al. Thrombolysis on Ischemic Stroke Patients with Decreased Level of Consciousness within 4.5 h. CNS Neurosci Ther. 2013;9:48–52. doi: 10.1111/cns.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank B, Fulton R, Weimar C, Shuaib A, Lees KR, et al. Impact of atrial fibrillation on outcome in thrombolyzed patients with stroke: evidence from the Virtual International Stroke Trials Archive (VISTA) Stroke. 2012;3:1872–1877. doi: 10.1161/STROKEAHA.112.650838. [DOI] [PubMed] [Google Scholar]
- 7.Padjen V, Bodenant M, Jovanovic DR, Ponchelle-Dequatre N, Novakovic N, Cordonnier C, et al. Outcome of patients with atrial fibrillation after intravenous thrombolysis for cerebral ischaemia. J Neurol. 2013;260:3049–3054. doi: 10.1007/s00415-013-7119-4. [DOI] [PubMed] [Google Scholar]
- 8.Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Yamashita S, Aoki J, et al. IV t-PA therapy in acute stroke patients with atrial fibrillation. J NeurolSci. 2009;276:6–8. doi: 10.1016/j.jns.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Sanak D, Herzig R, Kral M, Bártková A, Zapletalová J, Hutyra M, et al. Is atrial fibrillation associated with poor outcome after thrombolysis? J Neurol. 2010;257:999–1003. doi: 10.1007/s00415-010-5452-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JB, Ding ZY, Yang Y, Sun W, Hai F, Sui XN, et al. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol Res. 2010;32:353–358. doi: 10.1179/016164110X12656393665206. [DOI] [PubMed] [Google Scholar]
- 11.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, et al. Timing of recanalization after tissue plasminogen activator therapy determined by transcranialdoppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31:1812–1816. doi: 10.1161/01.STR.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 12.Generalized efficacy of t-PA for acute stroke Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997;28:2119–2125. doi: 10.1161/01.STR.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 13.Bluhmki E, Chamorro A, Davalos A, Machnig T, Sauce C, Wahlgren N, et al. Stroke treatment with alteplase given 3.0-4.5 h after onset of acute ischaemic stroke (ECASS III): additional outcomes and subgroup analysis of a randomised controlled trial. Lancet Neurol. 2009;8:1095–1102. doi: 10.1016/S1474-4422(09)70264-9. [DOI] [PubMed] [Google Scholar]
- 14.Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomized controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 16.Wahlgren N, Ahmed N, Eriksson N, Aichner F, Bluhmki E, Dávalos A, et al. Multivariable analysis of outcome predictors and adjustment of main outcome results to baseline data profile in randomized controlled trials: Safe Implementation of Thrombolysis in Stroke-Monitoring STudy (SITS-MOST) Stroke. 2008;39:3316–3322. doi: 10.1161/STROKEAHA.107.510768. [DOI] [PubMed] [Google Scholar]
- 17.Kufner A, Nolte CH, Galinovic I, Brunecker P, Kufner GM, Endres M, et al. Smoking-thrombolysis paradox: recanalization and reperfusion rates after intravenous tissue plasminogen activator in smokers with ischemic stroke. Stroke. 2013;44:407–413. doi: 10.1161/STROKEAHA.112.662148. [DOI] [PubMed] [Google Scholar]
- 18.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 19.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 20.Xing Yingqi, Guo Zhen-Ni, Yang Yi, Jin H, Wang S, Yang Y, et al. Increased globulin and its association with hemorrhagic transformation in patients receiving intra-arterial thrombolysis therapy. Neurosci Bull. 2014;6:469–476. doi: 10.1007/s12264-013-1440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado-Mederos R, Rovira A, Alvarez-Sabin J, Ribó M, Munuera J, Rubiera M, et al. Speed of tPA-induced clotlysis predicts DWI lesion evolution in acute stroke. Stroke. 2007;38:955–960. doi: 10.1161/01.STR.0000257977.32525.6e. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Garami Z, Mikulik R, et al. Early recanalization rates and clinical outcomes in patients with tandem internal carotid artery/middle cerebral artery occlusion and isolated middle cerebral artery occlusion. Stroke. 2005;36:869–871. doi: 10.1161/01.STR.0000160007.57787.4c. [DOI] [PubMed] [Google Scholar]
- 23.Zangerle A, Kiechl S, Spiegel M, Molina CA, Alexandrov AV, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology. 2007;68:39–44. doi: 10.1212/01.wnl.0000250341.38014.d2. [DOI] [PubMed] [Google Scholar]
- 24.Seet RCS, Zhang Y, Wijdicks EF, Rabinstein AA, et al. Relationship between chronic atrial fibrillation and worse outcomes in stroke patients after intravenous thrombolysis. Arch Neurol. 2011;68:1454–1458. doi: 10.1001/archneurol.2011.248. [DOI] [PubMed] [Google Scholar]