Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder in which amyloid β plaques are a pathological characteristic. Little is known about the physiological functions of amyloid β precursor protein (APP). Based on its structure as a type I transmembrane protein, it has been proposed that APP might be a receptor, but so far, no ligand has been reported. In the present study, 9 proteins binding to the extracellular domain of APP were identified using a yeast two-hybrid system. After confirming the interactions in the mammalian system, mutated PLP1, members of the FLRT protein family, and KCTD16 were shown to interact with APP. These proteins have been reported to be involved in Pelizaeus-Merzbacher disease (PMD) and axon guidance. Therefore, our results shed light on the mechanisms of physiological function of APP in AD, PMD, and axon guidance.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0021-1) contains supplementary material, which is available to authorized users.
Keywords: Amyloid precursor protein, Alzheimer’s disease, Yeast two-hybrid screening, Cell death, Pelizaeus-Merzbacher disease
Introduction
Alzheimer’s disease (AD), a common form of dementia, is a neurodegenerative disorder characterized by senile plaques composed of beta-amyloid widely scattered in the brain. The beta-amyloid is produced from amyloid precursor protein (APP) by proteolytic processing. APP is a type I transmembrane protein with a long extracellular domain and a short intracellular domain and is involved in a variety of cellular processes such as cell adhesion [1–4], and axon guidance [5, 6]. Although the mechanisms underlying AD are far from being fully understood, it is possible that disturbance of APP function may contribute to the disease process.
An important approach to the study of APP function is to explore its binding partners. So far, many proteins including F-spondin [7], apolipoprotein E [8], Fe65 [9, 10], and JNK-interacting protein 1 [11] have been reported to interact with APP. However, the physiological function of APP is not fully understood due to its unknown binding proteins. Also, the structure of APP indicates that it may function as a cellular receptor. Therefore, finding the possible ligands of APP is of importance in revealing its physiological and pathological functions. The yeast two-hybrid method is a powerful tool for discovering unknown protein-protein interactions, so we used the extracellular domain of APP695 as the bait to identify possible membrane or secreted binding proteins.
Materials and Methods
Materials
The plasmid pGBKT7 was from Shanghai Jiran Biotech Co., Ltd. (Shanghai, China). The Y2HGold and Y187 yeast strains, as well as pGBKT7-Lam, pGBKT7-53, and pGADT7-T plasmids were from 2ndLab (http://www.2ndlab.org/; Shanghai, China). The Mate & Plate™ Human Brain cDNA Library was from Takara Biotechnology Co., Ltd. (#630486, Dalian, China). The yeast protein extraction kit was from Beijing ComWin Biotech Co., Ltd. (CW0003, Beijing, China). Normal mouse IgG was from Santa Cruz Biotechnology (Shanghai) Co., Ltd. (#sc-2025, Shanghai, China). Protein A+G agarose was from Beyotime Institute of Biotechnology Ltd. (#P2012, Haimen, China). Anti-c-myc, anti-His, anti-mCherry, and anti-EGFP primary antibodies were from Easy Bio (Beijing, China). Clean-Blot IP Detection Reagent (HRP, 21230) was from Thermo Fisher Scientific (Beijing, China). The full-length fibronectin-like domain-containing leucine-rich transmembrane protein 3 (FLRT3) and full-length proteolipid protein 1 (PLP1) were from Vigene Bioscience (Shandong, China) and the full-length potassium channel tetramerization domain-containing protein 16 (KCTD16) and FLRT1 were gifts from Prof. Chen Zhang (College of Life Sciences, Peking University, Beijing, China).
Yeast small-scale transformation was performed following the YeastmakerTM Yeast Transformation System 2 User Manual. Control experiments testing for auto-activation and toxicity and yeast mating were performed following the Matchmaker® Gold Yeast Two-Hybrid System User Manual.
Calcium Phosphate Transfection of HEK293T Cells
HEK293T cells were seeded in 6-well plates at 4 × 105 cells/well. The medium was changed 24 h later. Two Eppendorf tubes were prepared: one contained 100 μL 2 × Hepes buffered saline (HBS) and the other contained a mixture of 4 μg plasmid, 10 μL 2 mol/L CaCl2, and sterile water to a final volume of 100 μL. The DNA/CaCl2 mixture was gently mixed and added dropwise into HBS, followed by 20 episodes of air-bubbling through the mixture. The mixture was finally added dropwise to the medium.
Co-immunoprecipitation
Co-immunoprecipitation (Co-IP) was performed following the protocol with the Protein A+G Agarose system (Beyotime Institute of Biotechnology Ltd.). Briefly, HEK293T cells were harvested 24 h post-transfection and centrifuged at 13,000 rpm for 15 min at 4 °C. The supernatant was separated into two parts: one part served as the positive control (input), and the other was treated with normal mouse IgG and Protein A+G Agarose to eliminate nonspecific binding before incubation at 4 °C with Protein A+G Agarose and primary antibody overnight. After that, the mixture was centrifuged and the pellet was washed with pre-chilled phosphate-buffered saline. The pellet was heated at 99 °C for 5 min in 1 × loading buffer before centrifugation at 13,000 rpm at 4 °C for 1 min. The supernatant was assessed by Western blot together with the positive control.
Results and Discussion
cDNA Library Screening by Yeast Mating
Gal4 DNA-BD fused with mouse p53 was encoded by pGBKT7-53, and Gal4 AD fused with SV40 large T-antigen was encoded by pGADT7-T. It has been reported that p53 and large T-antigen interact in yeast two-hybrid assays [12, 13]. Therefore, mating between Y2HGold [pGBKT7-53] and Y187 [pGADT7-T] generated diploid cells with both plasmids that activated the reporter genes enabling the yeasts to grow on SD-Leu/Trp/His/Ade with X-α-Gal plates as well as aureobasidin A (QDO/X/A) plates. The negative control was performed by mating between pGBKT7-Lam and pGADT7-T (note that Gal4 AD fused with lamin was encoded by pGBKT7-Lam). Therefore, diploid yeast cells containing pGBKT7-Lam and pGADT7-T grew on SD-Leu, SD-Trp, and SD-Leu/Trp (DDO) plates, but not on QDO/X/A plates (Table S1). Blue colonies were observed on QDO/X/A plates in positive controls (Fig. S1A, red circles) but not in negative controls, indicating that the QDO/X/A plates were suitable for the subsequent yeast two-hybrid screen (Fig. S1A).
The coding sequence of APP contains 2088 base pairs. Base pairs 1–51 encode the signal sequence, 52–1872 encode the extracellular domain, 1873–1944 encode the transmembrane domain, and 1945–2088 encode the intracellular domain. Base pairs 52–1872 encoding the extracellular domain were cloned into the pGBKT7 vector, constructing the APPex-pGBKT7 clone (Fig. S1B).
APPex-pGBKT7 was transformed into the Y2HGold strain in a small-scale transformation according to the YeastmakerTM user’s manual. Then the yeast was plated on an SD-Trp plate (SD plate without tryptophane). After 4 days of incubation, colonies were inoculated with 50 mL SD-Trp medium and incubated for 3 days with shaking. Then the yeast proteins were extracted using a kit (Beijing ComWin Biotech Co.,Ltd., China) and the expression of APP was assessed by Western blotting using anti c-myc (Fig. S1C).
To determine whether APPex-pGBKT7 was toxic, APPex-pGBKT7 or pGBKT7 alone was transformed into Y2HGold strain on a small-scale according to the user’s manual, and the transformations were plated on SD-Trp plates. Three days after incubation at 30 °C, the sizes of the colonies from the two plates were compared and no significant difference was found (Fig. S1D). Therefore, APP was considered to have little toxicity.
To test the possibility of auto-activation of APPex-pGBKT7, it was transformed into Y2HGold on a small-scale transformation. A 1:10 dilution of the transformation product was plated on an SD-Trp with X-α-Gal (SD-Trp/X) plate, an SD-Trp with X-α-Gal and aureobasidin A (SD-Trp/X/A) plate, and an SD-Trp/His/Ade with X-α-Gal and aureobasidin A (SD-Trp/His/Ade/X/A) plate. Five days after incubation at 30 °C, the growth and color of the colonies were recorded. Both the SD-Trp/X and the SD-Trp/X/A plates had blue colonies, indicating that the auto-activation of APPex-pGBKT7 occurred under these conditions (Fig. S1E, red circles). Nevertheless, no colonies were observed on the SD-Trp/His/Ade/X/A plate, indicating that APPex-pGBKT7 was not able to activate 4 reporter genes at the same time. Therefore, the auto-activation of APPex-pGBKT7 was unlikely under SD-Trp/His/Ade/X/A conditions.
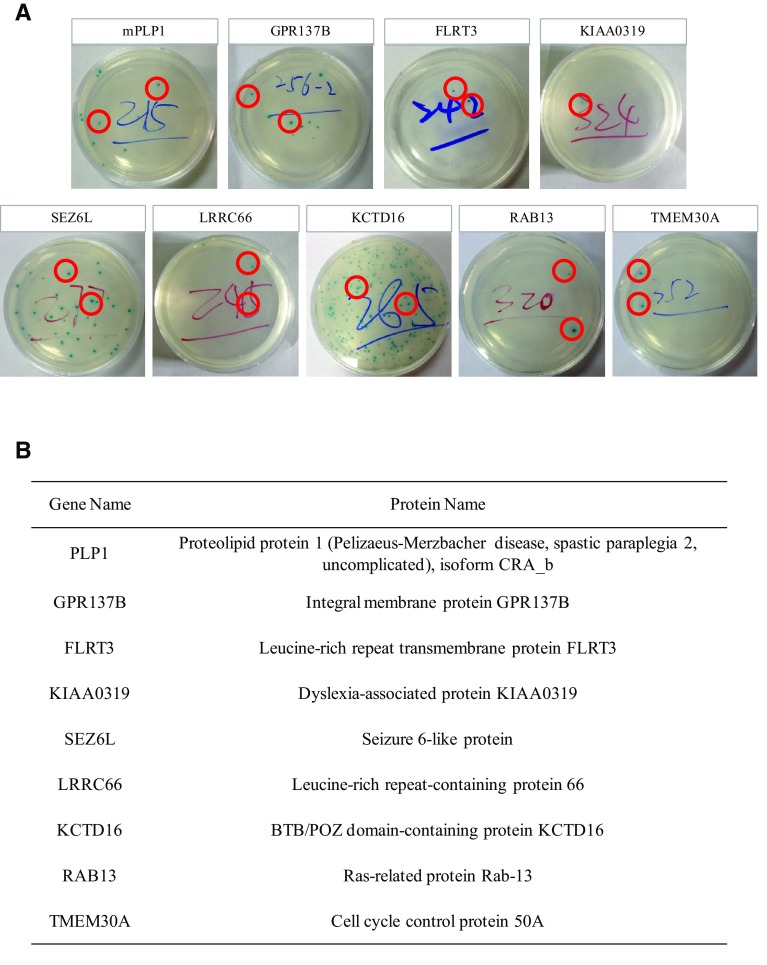
A mixture of bait APPex-pGBKT7 culture and cDNA library culture was made according to the Matchmaker® user’s manual. After 24 h of incubation, the mixture was spread on 150-mm diameter QDO/X/A plates and incubated at 30 °C for 5 days. Five days later, 192 colonies were obtained from the QDO/X/A plates. As blue and white colonies were found, they were then separated in order to obtain colonies that had interactions with bait plasmids. Pure blue colonies were acquired after 3–4 separations and 192 cDNA plasmids were recycled. Then the cDNA plasmids and APPex-pGBKT7 were co-transformed into Y2HGold according to the YeastmakerTM user’s manual. Among the 192 cDNA plasmids, 77 were positive. The sequencing results of these 77 candidates revealed that 9 were transmembrane proteins (Fig. 1; for DNA sequences, see supplementary materials). Of the 9 candidates, TMEM30A was demonstrated to bind APP by affinity capture-MS [14]. Although RAB13 has not been demonstrated to bind with APP, 19 other RAB family proteins (RAB1B, RAB2B, RAB3A, RAB3D, RAB5C, RAB7B, RAB8B, RAB10, RAB11B, RAB14, RAB26, RAB27A, RAB28, RAB35, RAB38, RAB39A, RAB40B, RAB43, and RABL3) all interact with amyloid β [15].
Fig. 1.
Screened products of APPex-pGBKT7. A QDO/X/A plates co-transformed with APPex-pGBKT7 and its screened products. The red circles indicated yeast colonies. B Gene and protein names of screened products.
Confirmation of Interactions in a Mammalian System
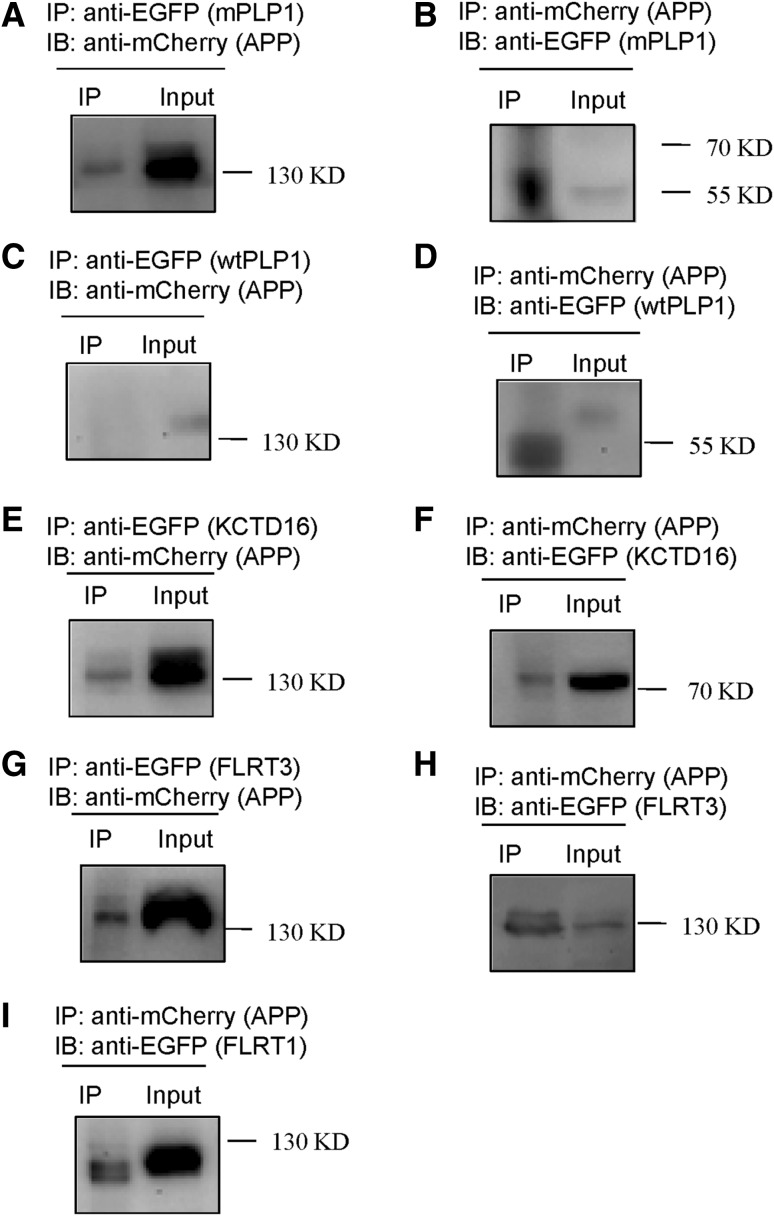
To assay for the interactions between APP and its screened products in a mammalian system by Co-IP, full-length APP was designed to express mCherry at its C-terminal (APP-mCherry). Meanwhile, mutated full-length PLP1, full-length FLRT3, and full-length KCTD16 were designed to express EGFP (enhanced green fluorescent protein) at C-terminal (mPLP1-EGFP, FLRT3-EGFP, and KCTD16-EGFP). Then APP-mCherry and mPLP1-EGFP were co-transfected into HEK293T cells. The cells were harvested 24 h later and Co-IP experiments were performed. mCherry and EGFP were each detected in EGFP and mCherry complexes, indicating that mutated PLP1 interacted with APP (Fig. 2A, B). However, wild-type PLP1 (wtPLP1-EGFP), in which the conformation was different from mutated PLP1, did not bind APP (Fig. 2C, D). Similarly, KCTD16-EGFP and FLRT3-EGFP interacted with APP-mCherry (Fig. 2E–H) (FLRT1 and FLRT3 are both members of the fibronectin leucine-rich transmembrane protein family). The Co-IP results showed that FLRT1 and APP interacted in HEK293T cells (Fig. 2I).
Fig. 2.
Co-immunoprecipitation of full-length APP with full-length PLP1, full-length KCTD16, and full-length FLRT in HEK293T cells. A Cell lysates were immunoprecipitated with an anti-EGFP antibody for mutated PLP1 and probed with an anti-mCherry antibody for APP (130 kD). B Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP and probed with an anti-EGFP antibody for mutated PLP1 (55 kD). C Cell lysates were immunoprecipitated with an anti-EGFP antibody for wild-type PLP1 and probed with an anti-mCherry antibody for APP (130 kD). D Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP and probed with an anti-EGFP antibody for wild-type PLP1 (57.5 kD). E Cell lysates were immunoprecipitated with an anti-EGFP antibody for KCTD16 and probed with an anti-mCherry antibody for APP (130 kD). F Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP and probed with an anti-EGFP antibody for KCTD16 (76 kD). G Cell lysates were immunoprecipitated with an anti-EGFP antibody for FLRT3 and probed with an anti-mCherry antibody for APP (130 kD). H Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP and probed with an anti-EGFP antibody for FLRT3 (130 kD). I Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP and probed with an anti-EGFP antibody for FLRT1 (130 kD).
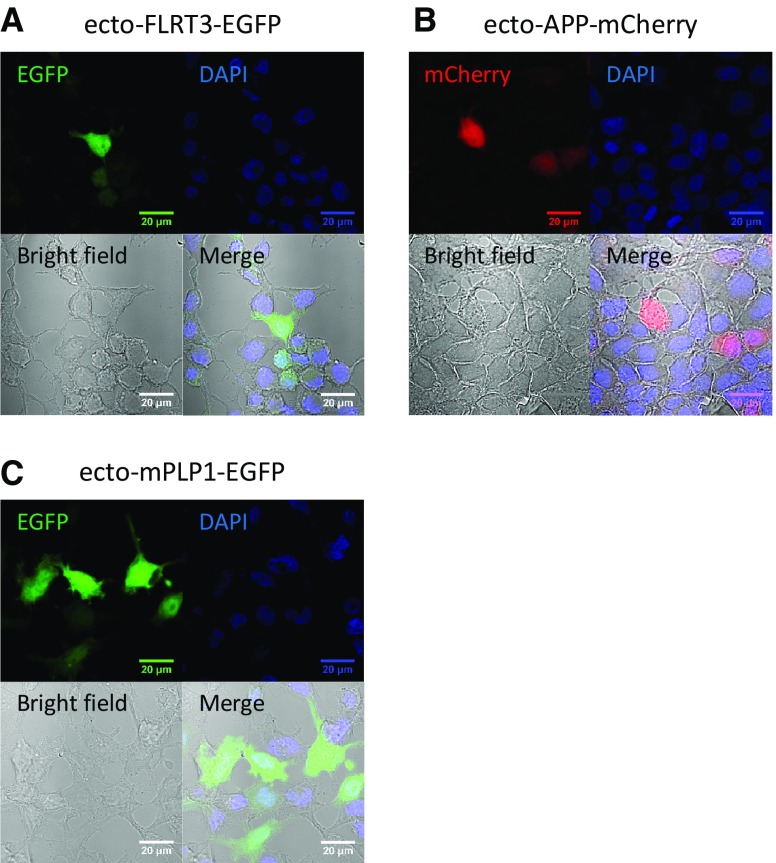
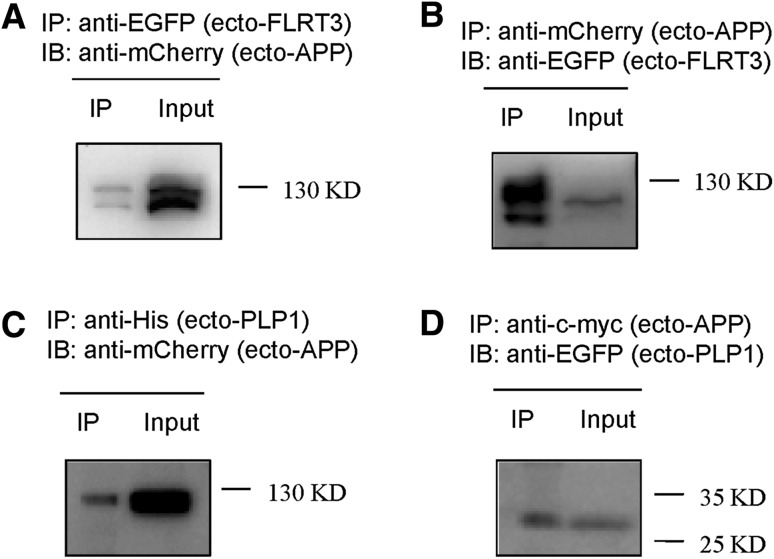
FLRT3 has been reported to promote neurite outgrowth in cultured neurons [16]. As a co-receptor for Robo 1, FLRT3 regulates the attractive response to the guidance molecule Netrin-1 together with Slit/Robo 1 [17]. During embryonic development, FLRT3 can be shed and release the ectodomain. Then this ectodomain can serve as a guidance cue for the axons of Unc5-positive neurons [18]. APP has been reported to mediate axon guidance [6]. To test whether the ectodomain of APP interacts with the ectodomain of FLRT3, the ectodomains of APP and FLRT3 were cloned and transfected into HEK293T cells. These ectodomains contained only the extracellular part without the signal sequence and the transmembrane domain. Therefore, the ectodomain proteins showed a clear cytoplasmic expression pattern (Fig. 3A, B), different from the membrane proteins (Fig. S2). The ectodomain of APP interacted with the ectodomain of FLRT3 (Fig. 4A, B). Therefore, it is possible that the ectodomain of FLRT3 functions as guidance cue by binding to its receptor APP. We also found that FLRT1 interacted with APP. These results indicate that the FLRT protein family is involved in cellular processes such as axon guidance and synaptic development by binding APP. However, whether APP works upstream or downstream of the FLRT protein family in mediating axon guidance needs further investigation.
Fig. 3.
Localization of ectodomain proteins in HEK293T cells. HEK293T cells were transfected with ecto-FLRT3-EGFP (A), ecto-APP-mCherry (B), and ecto-mPLP1-EGFP (C) and immunostained. Nuclei were stained with DAPI.
Fig. 4.
Co-immunoprecipitation of the APP extracellular domain with the FLRT3 extracellular domain or the mPLP1 extracellular domain in HEK293T cells. A Cell lysates were immunoprecipitated with an anti-EGFP antibody for FLRT3 extracellular domain and probed with an anti-mCherry antibody for APP extracellular domain. B Cell lysates were immunoprecipitated with an anti-mCherry antibody for APP extracellular domain and probed with an anti-EGFP antibody for FLRT3 extracellular domain. C Cell lysates were immunoprecipitated with an anti-His antibody for PLP1 extracellular domain and probed with an anti-mCherry antibody for APP extracellular domain. D Cell lysates were immunoprecipitated with an anti-c-myc antibody for APP extracellular domain and probed with an anti-EGFP antibody for PLP1 extracellular domain (32 kD).
PLP1, a multi-transmembrane protein, functions in myelin formation and maintenance. Mutations in the PLP1 gene cause dysmyelination in the central nervous system. Three kinds of PLP1 mutation have been reported: point mutations [19], duplications [20], and deletions [21]. The mutated PLP1 in our study had a 74-base-pair deletion that led to a premature stop codon. APP is a transmembrane protein expressed in neurons and oligodendrocytes and PLP1 is also a membrane-spanning protein that is abundantly expressed in oligodendrocytes. Similar to FLRT3, the ectodomain of mutated PLP1 contained only the extracellular part without the signal sequence and the transmembrane domain and showed a cytoplasmic expression pattern (Fig. 3C). We also found that the ectodomain of APP interacted with the ectodomain of mutated PLP1 (Fig. 4C, D). Therefore, the binding of APP with mutated PLP1 could affect the development of oligodendrocytes and the formation and maintenance of myelin sheaths in Pelizaeus-Merzbacher disease.
KCTD16 is an auxiliary subunit of the γ-aminobutyric acid B (GABAB) receptor, which is involved in many neurological and psychiatric disorders [22]. Its functions are affected in distinct ways by the auxiliary subunits [23]. By binding to the KCTD16 auxiliary subunit of the GABAB receptor, APP could be involved in neurological and psychiatric disorders.
In this study, three transmembrane proteins were found to bind with APP. The interaction of APP and mutated PLP1 shed light on the molecular mechanism of Pelizaeus-Merzbacher disease. Moreover, the interaction between APP and the FLRT protein family provided further evidence for a function of APP in axon guidance. Our results identified three transmembrane binding partners of APP and provide evidence for understanding its physiological and pathological functions.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 17,335 kb)
Acknowledgements
This work was supported by the National Natural Science Foundation of China for Distinguished Young Scholars (81425009), the Beijing Natural Science Foundation, China (7142085), and the Peking University Collaborative Fund, China (464-10606-00416).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0021-1) contains supplementary material, which is available to authorized users.
References
- 1.Breen KC, Bruce M, Anderton BH. Beta amyloid precursor protein mediates neuronal cell-cell and cell-surface adhesion. J Neurosci Res. 1991;28:90–100. doi: 10.1002/jnr.490280109. [DOI] [PubMed] [Google Scholar]
- 2.Storey E, Beyreuther K, Masters CL. Alzheimer’s disease amyloid precursor protein on the surface of cortical neurons in primary culture co-localizes with adhesion patch components. Brain Res. 1996;735:217–231. doi: 10.1016/0006-8993(96)00608-7. [DOI] [PubMed] [Google Scholar]
- 3.Beher D, Hesse L, Masters CL, Multhaup G. Regulation of amyloid protein precursor (APP) binding to collagen and mapping of the binding sites on APP and collagen type I. J Biol Chem. 1996;271:1613–1620. doi: 10.1074/jbc.271.3.1613. [DOI] [PubMed] [Google Scholar]
- 4.Kibbey MC, Jucker M, Weeks BS, Neve RL, Van Nostrand WE, Kleinman HK. beta-Amyloid precursor protein binds to the neurite-promoting IKVAV site of laminin. Proc Natl Acad Sci U S A. 1993;90:10150–10153. doi: 10.1073/pnas.90.21.10150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosa LJ, Bergman J, Estrada-Bernal A, Glorioso TJ, Kittelson JM, Pfenninger KH. Amyloid precursor protein is an autonomous growth cone adhesion molecule engaged in contact guidance. PLoS One. 2013;8:e64521. doi: 10.1371/journal.pone.0064521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rama N, Goldschneider D, Corset V, Lambert J, Pays L, Mehlen P. Amyloid precursor protein regulates netrin-1-mediated commissural axon outgrowth. J Biol Chem. 2012;287:30014–30023. doi: 10.1074/jbc.M111.324780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho A, Sudhof TC. Binding of F-spondin to amyloid-beta precursor protein: a candidate amyloid-beta precursor protein ligand that modulates amyloid-beta precursor protein cleavage. Proc Natl Acad Sci U S A. 2004;101:2548–2553. doi: 10.1073/pnas.0308655100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabo SL, Ikin AF, Buxbaum JD, Greengard P. The Alzheimer amyloid precursor protein (APP) and FE65, an APP-binding protein, regulate cell movement. J Cell Biol. 2001;153:1403–1414. doi: 10.1083/jcb.153.7.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiore F, Zambrano N, Minopoli G, Donini V, Duilio A, Russo T. The regions of the Fe65 protein homologous to the phosphotyrosine interaction/phosphotyrosine binding domain of Shc bind the intracellular domain of the Alzheimer’s amyloid precursor protein. J Biol Chem. 1995;270:30853–30856. doi: 10.1074/jbc.270.52.30853. [DOI] [PubMed] [Google Scholar]
- 11.Scheinfeld MH, Roncarati R, Vito P, Lopez PA, Abdallah M, D’Adamio L. Jun NH2-terminal kinase (JNK) interacting protein 1 (JIP1) binds the cytoplasmic domain of the Alzheimer’s beta-amyloid precursor protein (APP) J Biol Chem. 2002;277:3767–3775. doi: 10.1074/jbc.M108357200. [DOI] [PubMed] [Google Scholar]
- 12.Iwabuchi K, Li B, Bartel P, Fields S. Use of the two-hybrid system to identify the domain of p53 involved in oligomerization. Oncogene. 1993;8:1693–1696. [PubMed] [Google Scholar]
- 13.Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. FASEB J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- 14.Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell. 2015;162:425–440. doi: 10.1016/j.cell.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olah J, Vincze O, Virok D, Simon D, Bozso Z, Tokesi N, et al. Interactions of pathological hallmark proteins: tubulin polymerization promoting protein/p25, beta-amyloid, and alpha-synuclein. J Biol Chem. 2011;286:34088–34100. doi: 10.1074/jbc.M111.243907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji L, Yamashita T, Kubo T, Madura T, Tanaka H, Hosokawa K, et al. FLRT3, a cell surface molecule containing LRR repeats and a FNIII domain, promotes neurite outgrowth. Biochem Biophys Res Commun. 2004;313:1086–1091. doi: 10.1016/j.bbrc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 17.Leyva-Diaz E, del Toro D, Menal MJ, Cambray S, Susin R, Tessier-Lavigne M, et al. FLRT3 is a Robo1-interacting protein that determines Netrin-1 attraction in developing axons. Curr Biol. 2014;24:494–508. doi: 10.1016/j.cub.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 18.Yamagishi S, Hampel F, Hata K, Del Toro D, Schwark M, Kvachnina E, et al. FLRT2 and FLRT3 act as repulsive guidance cues for Unc5-positive neurons. EMBO J. 2011;30:2920–2933. doi: 10.1038/emboj.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osaka H, Koizume S, Aoyama H, Iwamoto H, Kimura S, Nagai J, et al. Mild phenotype in Pelizaeus-Merzbacher disease caused by a PLP1-specific mutation. Brain Dev. 2010;32:703–707. doi: 10.1016/j.braindev.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Madry J, Hoffman-Zacharska D, Krolicki L, Jakucinski M, Friedman A. PLP1 gene duplication as a cause of the classic form of Pelizaeus-Merzbacher disease - case report. Neurol Neurochir Pol. 2010;44:511–515. doi: 10.1016/s0028-3843(14)60142-0. [DOI] [PubMed] [Google Scholar]
- 21.Torisu H, Iwaki A, Takeshita K, Hiwatashi A, Sanefuji M, Fukumaki Y, et al. Clinical and genetic characterization of a 2-year-old boy with complete PLP1 deletion. Brain Dev. 2012;34:852–856. doi: 10.1016/j.braindev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Kantrowitz J, Citrome L, Javitt D. GABA(B) receptors, schizophrenia and sleep dysfunction: a review of the relationship and its potential clinical and therapeutic implications. CNS Drugs. 2009;23:681–691. doi: 10.2165/00023210-200923080-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gassmann M, Bettler B. Regulation of neuronal GABA(B) receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. doi: 10.1038/nrn3249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 17,335 kb)