Abstract
The α2δ-1 subunit of the voltage-gated Ca2+ channel (VGCC) is a molecular target of gabapentin (GBP), which has been used as a first-line drug for the relief of neuropathic pain. GBP exerts its anti-nociceptive effects by disrupting trafficking of the α2δ-1 subunit to the presynaptic membrane, resulting in decreased neurotransmitter release. We previously showed that GBP has an anti-allodynic effect in the first two weeks; but this is followed by insensitivity in the later stage after repeated administration in a rat model of central post-stroke pain (CPSP) hypersensitivity induced by intra-thalamic hemorrhage. To explore the mechanisms underlying GBP insensitivity, the cellular localization and time-course of expression of the α2δ-1 subunit in both the thalamus and spinal dorsal horn were studied in the same model. We found that the α2δ-1 subunit was mostly localized in neurons, but not astrocytes and microglia. The level of α2δ-1 protein increased in the first two weeks after injury but then decreased in the third week, when GBP insensitivity occurred. Furthermore, the α2δ-1 down-regulation was likely caused by later neuronal loss in the injured thalamus through a mechanism other than apoptosis. In summary, the present results suggest that the GBP receptor α2δ-1 is mainly expressed in thalamic neurons in which it is up-regulated in the early stage of CPSP but this is followed by dramatic down-regulation, which is likely associated with GBP insensitivity after long-term use.
Keywords: Central post-stroke pain, Calcium channel α2δ subunit, Gabapentinoid, Thalamic hemorrhagic stroke, Thalamus, Spinal dorsal horn
Introduction
Central post-stroke pain (CPSP) is a type of central neuropathic pain that is induced by a primary lesion of the central somatosensory system following ischemic or hemorrhagic stroke [1–3]. CPSP occurs most often after strokes that involve the thalamus [4–9]. The quality of life of patients with CPSP is very poor due to daily paroxysms of persistent spontaneous pain and hypersensitivity to noxious (hyperalgesia and allodynia) and non-noxious stimuli (paresthesia and dysesthesia) [10–13]. So far, the clinical treatment of CPSP has been inadequate due to resistance to both drug and non-drug therapies in about half of the affected patients [1, 2, 14–17].
The gabapentinoids, including gabapentin (GBP) and pregabalin, are a class of anticonvulsants originally approved by the U.S. Food and Drug Administration (FDA) for the treatment of epilepsy. In 2004, gabapentinoids were also approved as first-line drugs for the treatment of some types of neuropathic pain, such as painful diabetic neuropathy, post-herpetic neuralgia, and spinal cord injury-induced pain [3, 14, 15, 18]. Nonetheless, gabapentinoids are commonly used to treat other types of neuropathic pain including CPSP, although their safety and efficacy have not yet been approved by the FDA. In a recent placebo-controlled trial, pregabalin failed to show significant improvement over placebo in patients with CPSP [19]. More recently, we evaluated the anti-allodynic effects of GBP in rats with CPSP hypersensitivity induced by experimental thalamic hemorrhage and found that, although GBP had a dose-dependent anti-allodynic effect following a single systemic administration, the effectiveness was transient, gradually decreasing after repeated administration for 14 days, implying the existence of drug insensitivity [20]. The GBP insensitivity was shown by a decreased maximal possible effect and the shortened time-course of a single administration at a lower dose (10 mg/kg, i.p.) or by a shortened time-course of a single administration at a higher dose (100 mg/kg, i.p.) [20]. These results are evidence of both spatial and temporal changes in the molecular targets of GBP in the CNS of these rats.
Pharmacologically, gabapentinoids have high affinity for the α2δ-1 subunit of the voltage-gated Ca2+ channel (VGCC) in the CNS [21–23]. They inhibit presynaptic neurotransmitter release from hyperexcitable or abnormal neurons by blocking trafficking of the α2δ-1 subunit to the presynaptic membrane [24–27]. Experimentally, expression of α2δ-1, but not α2δ-2 (another binding site of GBP), is significantly increased in both the dorsal root ganglia (DRG) and the dorsal horn of the spinal cord in animal models of peripheral neuropathic pain. This up-regulation is thought to contribute to the development of allodynia and hyperalgesia [28–31]. It has thus been proposed that gabapentinoids alleviate neuropathic pain by blocking the trafficking of α2δ-1 to the presynaptic terminals of DRG neurons and this subsequently leads to reduced Ca2+ influx and neurotransmitter release in the spinal dorsal horn and the inhibition of central sensitization [27, 32].
Given that the α2δ-1 subunit of the VGCC is the molecular target of GBP, we proposed that the protein level of this subunit would increase shortly after intra-thalamic hemorrhage during the development of post-stroke pain. We then postulated that subsequent apoptotic loss of neurons surrounding the lesion focus that occurs at least 21 days after the initial injury would lead to reduced α2δ-1 subunit expression in the presynaptic terminals of excitatory interneurons, leading to reduced anti-allodynic effectiveness of the drug due to the loss of the GBP effect.
Thus, this study was designed to determine: (1) which cells (neurons, astrocytes, or microglia) express the α2δ-1 subunit after intra-thalamic hemorrhagic injury; (2) the time-course of changes in the protein level of the α2δ-1 subunit in rats with CPSP following intra-thalamic hemorrhagic injury; and (3) whether neuronal loss occurs in parallel with the changes in protein levels of the α2δ-1 subunit in rats with CPSP after intra-thalamic hemorrhagic injury. Finally, by examining the dorsal horn of the spinal cord, determine whether parallel changes occur within the ascending somatosensory system.
Materials and Methods
Animals
Male Sprague–Dawley rats weighing 280–320 g were provided by the Laboratory Animal Center of the Fourth Military Medical University (FMMU). Rats were housed in a climate-controlled room (22–26 °C) under a 12 h/12 h light/dark cycle with access to food and water ad libitum. Somatic functional evaluations were carried out between 09:00 and 18:30. The rats were acclimated to test boxes for >30 min on each day before the first test. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996), and followed the ethical guidelines for pain research in conscious animals of the International Association for the Study of Pain. This study was approved by the Animal Care and Use Committee of FMMU. The number of animals used and their suffering were minimized. Animals were randomly divided into two groups for the establishment of the CPSP model with thalamic hemorrhage: (1) rats receiving intra-thalamic microinjection of saline (ITS) (n = 24); and (2) rats receiving intra-thalamic microinjection of collagenase (ITC) (n = 42).
Surgery
Surgery was performed as described previously [20, 33, 34]. Rats were anesthetized with chloralose (0.3 g/kg, i.p.), and then securely fixed in a stereotaxic instrument (Narishige Scientific Instrument Lab, Tokyo, Japan). After a midline incision, an opening was made in the right skull with a dental drill. Collagenase type IV (Sigma-Aldrich China, Shanghai) or saline was microinjected into the ventrobasal complex and posterior thalamic nucleus (stereotaxic coordinates: bregma −3.48 mm anteroposterior; 3.6 mm lateral to the midline, and 6.2 mm ventral to the brain surface) on the right side [35]. The needle on a 0.5-μL microinjection syringe filled with collagenase or saline was lowered into the region of interest, followed (5 min later) by slow ITC (0.025 IU collagenase dissolved in 0.25 μL saline) or ITS (0.25 μL saline) over a period of 10 min. The syringe was left in place for 5 min after each injection to prevent spread of the agent to the brain surface. Then the needle was slowly withdrawn, the skin closed using 4.0 sutures, and all rats were allowed to recover in individual cages for at least 7 days.
Measurement of Mechanical Pain Sensitivity
Mechanical sensitivity was evaluated with von Frey monofilaments as described previously [20, 36]. Rats were placed on the metal mesh floor of a plastic chamber and mechanical stimuli were applied using monofilaments with ascending bending forces of 0.8 g, 2–20 g at 2-g increments, and then 25, 30, 45, and 60 g. Each monofilament was applied 10 times (once every several seconds) to the plantar area of each hind-paw to induce a withdrawal reflex. The bending force of the monofilament able to elicit a 50% withdrawal response was expressed as the paw withdrawal mechanical threshold (PWMT, g).
Double Immunofluorescent Labeling
Double immunofluorescent labeling was performed to determine the cellular distribution of the VGCC α2δ-1 subunit in the thalamus. The primary antibodies were mouse monoclonal anti-dihydropyridine receptor (anti-Cavα2δ-1 subunit) (1:200, Novus Biologicals, Littleton, CO), rabbit anti-NeuN (1:200, abCam, Cambridge, UK), rabbit anti-GFAP (1:250, Millipore, Billerica, MA), and rabbit anti-Iba-1 (1:250, WAKO, Osaka, Japan). Secondary antibodies were FITC-conjugated goat anti-mouse IgG (1:200, Sigma, St. Louis, MO) and Cy3-conjugated sheep anti-rabbit IgG (1:200, Sigma). The animals were deeply anesthetized with urethane (2 g/kg, i.p.) on day 7 after intra-thalamic hemorrhage. They were perfused intracardially with saline followed by a phosphate-buffered solution of 4% paraformaldehyde. The brain was removed and post-fixed in the same fixative overnight, followed by cryoprotection in 30% phosphate-buffered sucrose. Transverse frozen sections (45 μm thick) were cut on a CM1900 freezing microtome (Leica, Wetzlar, Germany) and collected in 0.01 mol/L PBS. These sections were treated with Tris–HCl buffer and 3% H2O2 for 10 min to quench the endogenous peroxidase. Non-specific protein was blocked by incubation in PBS containing 1% bovine serum albumin (Sigma-Aldrich) and 0.1% Triton X-100 (Sigma-Aldrich) for 2 h at room temperature. Then the sections were incubated with the primary antibodies overnight at 4 °C. After three washes with PBS, the secondary antibodies were conjugated for 3 h at room temperature with agitation. Then the sections were rinsed, mounted on slides, and cover-slipped. Photomicrographs were obtained under a laser scanning confocal fluorescence microscope (Olympus FV1000, Tokyo, Japan) and processed with Image-Pro Plus digitizing software (Olympus). For details of the procedures see our previous reports [37, 38].
Western Blotting
The bilateral thalamus and the lumbar spinal cord (L3–L5) were obtained under deep anesthesia with urethane (2 g/kg, i.p.) on days 7, 14, and 21 after ITC or ITS, as in our previous report [20]. The tissues were homogenized in an ice-cold mixture of protease inhibitors and RIPA lysis buffer containing 50 mmol/L Tris (pH 7.4), 150 mmol/L NaCl, 1% NP-40, and 0.1% sodium dodecyl sulfate (SDS) (Applygen Technologies Inc., Beijing, China). Total proteins were extracted by centrifugation at 12,000 g for 10 min at 4 °C. Protein concentrations were determined using a BCA protein assay kit (Thermo Scientific, Waltham, MA). The same amounts of proteins (60 μg) were heated for 10 min at 98 °C, separated on 10% SDS-PAGE by electrophoresis (Bio-Rad), and transferred onto PVDF membranes (Immobilon P, Millipore). The membranes were blocked with 5% skim milk for 3 h at room temperature and incubated with mouse monoclonal anti-dihydropyridine receptor (anti-Cavα2δ-1 subunit) (1:200, Novus Biologicals) or mouse anti-β-tubulin antibody (1:20000, Sigma) overnight at 4 °C. The membranes were displayed with enhanced chemiluminescence reagents and images captured with FluorChem FC2 (Alpha Innotech Corp.). The density of the band area was quantified with AlphaImager software. All western blot analyses were performed at least three times from more than three rats.
Statistical Analysis
Data are expressed as mean ± SEM. The unpaired Student’s t test was used for single comparisons. One-way ANOVA followed by the Dunnett’s or Tukey’s test was used for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Establishment of a Rat Model of CPSP Hypersensitivity
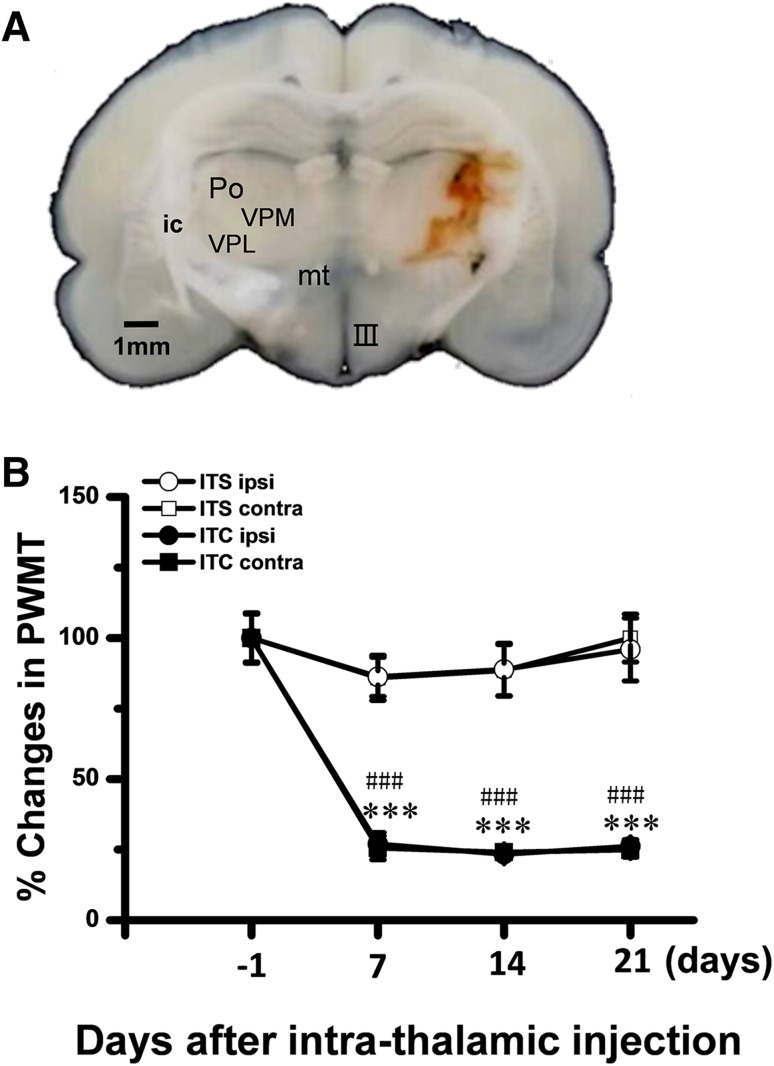
Similar to our previous report [20] unilateral ITC injections confined to the medial lemniscus-ventrobasal complex-posterior thalamic nucleus (Fig. 1A) resulted in bilateral reductions of the PWMT, suggesting the occurrence of bilateral mechanical pain hypersensitivity after intra-thalamic hemorrhage (Fig. 1B). Out of the total 42 rats, 64.29% (27/42) displayed persistent bilateral mechanical hypersensitivity from day 7 to the end of behavioral measurements (Fig. 1B).
Fig. 1.
Development of hemorrhagic central post-stroke pain hypersensitivity induced by intra-thalamic collagenase microinjection. A Photomicrograph of a 600-μm slice showing the hemorrhagic lesion site in the thalamus following unilateral intra-thalamic collagenase (ITC) microinjection. Scale bar, 1 mm. B Development of central post-stroke pain hypersensitivity to mechanical stimuli applied to both hind paws induced by ITC microinjection. Intra-thalamic saline (ITS) injection served as the control. III, third ventricle; contra, contralateral; ic, internal capsule; ipsi, ipsilateral; mt, mammillothalamic tract; Po, posterior thalamic nuclear group; PWMT, paw-withdrawal mechanical threshold; VPL, ventral posterolateral nucleus of the thalamus; VPM, ventral posteromedial nucleus of the thalamus. ***P < 0.001 ITC ipsi vs ITS ipsi; ### P < 0.001 ITC contra vs ITS contra, n = 6–8 animals/group.
Cellular Localization of α2δ-1 Subunit in the Thalamus of Rats with CPSP Hypersensitivity
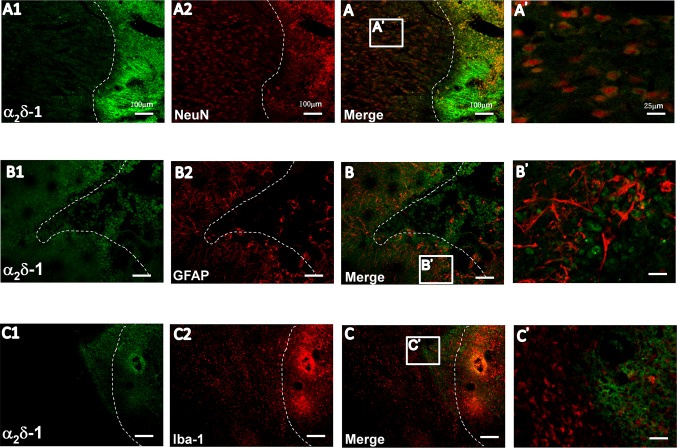
To identify the cellular localization of the α2δ-1 subunit, sections containing both thalami were obtained from rats with CPSP hypersensitivity 7 days after ITC when GBP has a stable anti-allodynic effect as shown in our previous report [20]. The α2δ-1 subunit was localized primarily in neurons surrounding the hemorrhagic focus (Fig. 2A). Labeling of astrocytes and microglia was also detected adjacent to the edge of the lesion (Fig. 2B2, C2). Double immunofluorescence labeling showed that the α2δ-1 subunit mainly co-localized with NeuN (Fig. 2A and A’), but scarcely with GFAP and Iba-1 (Fig. 2B, B’, C, C’), suggesting that the α2δ-1 subunit is mainly expressed in neurons surrounding the hemorrhagic center.
Fig. 2.
Cellular localization of the α2δ-1 subunit on the injured side of the thalamus after intra-thalamic hemorrhage. Laser confocal fluorescent images of double-staining for the α2δ-1 subunit (A1–C1, green) with anti-NeuN, a marker for neurons (A2, red), anti-GFAP, a marker for astrocytes (B2, red), and anti-Iba-1, a marker for microglia (C2, red) on the injured side. A–C are merged images; A’–C’ are magnifications of the boxes in A–C. Dashed line indicates border between the hemorrhagic injury center (right) and the adjacent surround (left). Scale bars, 100 μm for A1–C1, A2–C2, A–C; 25 μm for A’–C’.
Time-Course of α2δ-1 Protein Expression in Rats with CPSP Hypersensitivity
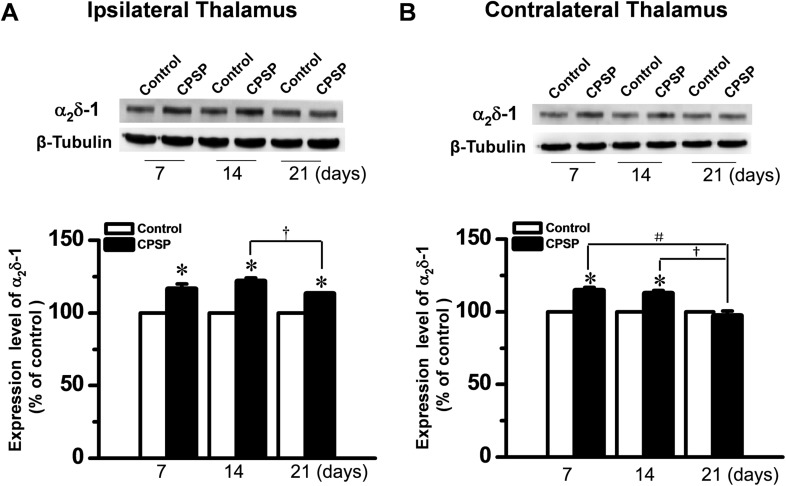
Rats with CPSP hypersensitivity were randomly allowed to survive for 7, 14, and 21 days after ITC. At the diencephalic level, the α2δ-1 protein was bilaterally up-regulated at 7 and 14 days after unilateral ITC injection (Fig. 3). However, the level of α2δ-1 protein on day 21 post-injection was significantly lower bilaterally than in matched sites on days 7 and 14 (Fig. 3).
Fig. 3.
Up-regulation of α2δ-1 protein in bilateral thalami after unilateral intra-thalamic hemorrhage. A, B Upper panels: Western blots for time-related changes in α2δ-1 protein levels in bilateral thalami 7, 14, and 21 days after unilateral intra-thalamic collagenase (ITC) microinjection. Lower panels: averaged data from 4–5 animals with central post-stroke pain (CPSP) hypersensitivity at the corresponding time points. Intra-thalamic saline (ITS) microinjection served as the control. *P < 0.05 vs Control; # P < 0.05, † P < 0.05; n = 4/time-point.
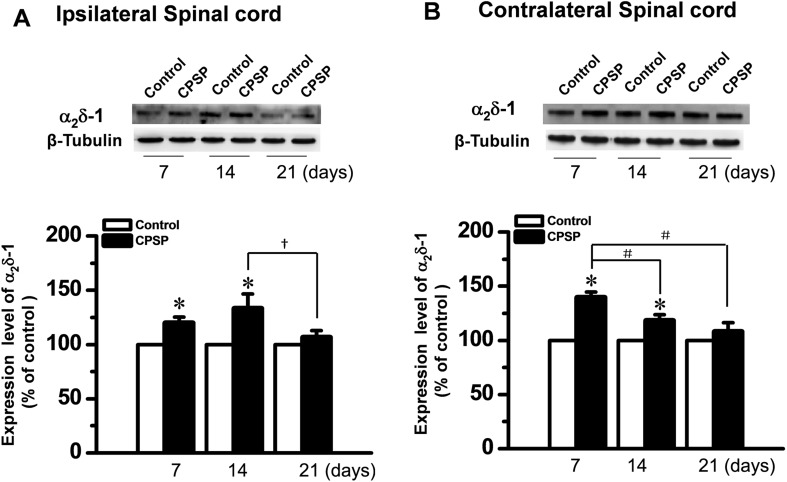
The expression of α2δ-1 following unilateral ITC injection on days 7 and 14 was up-regulated bilaterally in the dorsal horn of the spinal cord when compared to the ITS controls (Fig. 4). Similar to the results in the thalamus, the α2δ-1 protein level on day 21 was also lower than that on days 7 and 14 after ITC (Fig. 4).
Fig. 4.
Up-regulation of α2δ-1 protein in bilateral spinal dorsal horn after unilateral intra-thalamic hemorrhage. A, B Upper panels: Western blots for time-related changes in α2δ-1 protein levels in the bilateral dorsal horn 7, 14, and 21 days after unilateral intra-thalamic collagenase (ITC) microinjection. Lower panels: averaged data from 4–5 animals with central post-stroke pain (CPSP) hypersensitivity at the corresponding time points. Intra-thalamic saline (ITS) microinjection served as the control. *P < 0.05 vs Control; # P < 0.05, † P < 0.05.
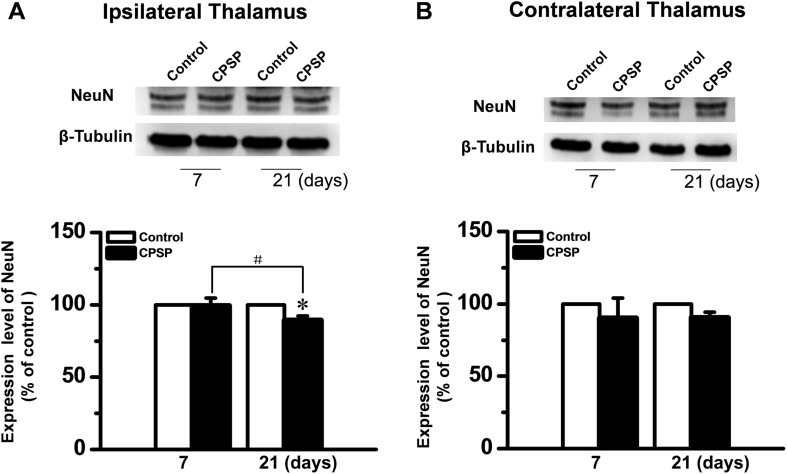
Later Neuronal Loss in the Hemorrhagic Thalamus of Rats with CPSP Hypersensitivity
Because the α2δ-1 subunit was mainly localized in thalamic neurons on the hemorrhagic side (Fig. 2), neuronal loss was suspected to account for the decreased labeling on day 21 post-ITC injection. Thus, the amount of NeuN protein, a neuronal marker, was assessed using Western blot. The amount of NeuN was lower on the lesioned side at 21 days than on day 7 post-injection (Fig. 5). Unlike α2δ-1, NeuN remained unchanged in the contralateral side on day 21.
Fig. 5.
NeuN protein levels in bilateral thalami after unilateral intra-thalamic hemorrhage. A, B Upper panels: Western blots for time-related changes in NeuN protein levels in bilateral thalami on days 7 and 21 after unilateral intra-thalamic collagenase (ITC) microinjection. Lower panels: averaged data from 3–4 animals with central post-stroke pain (CPSP) hypersensitivity at the corresponding time points. Intra-thalamic saline (ITS) microinjection served as the control. *P < 0.05 vs Control; # P < 0.05.
To determine whether the neuronal loss is caused by apoptosis, the caspase-3 levels were assessed. The caspase-3 protein level was significantly up-regulated on both sides of the thalamus 7 days after ITC, but returned to baseline by day 21 post-injection (Fig. 6).
Fig. 6.
Levels of caspase-3 protein, an effector of apoptosis, in bilateral thalami after unilateral intra-thalamic hemorrhage. A, B Upper panels: Western blots for time-related changes in caspase-3 protein levels in bilateral thalami on days 7 and 21 after unilateral intra-thalamic collagenase (ITC) microinjection. Lower panels: averaged data from 3–4 animals with central post-stroke pain (CPSP) hypersensitivity at the corresponding time points. Intra-thalamic saline (ITS) microinjection served as the control. *P < 0.05 vs Control; # P < 0.05.
Discussion
The major findings of the present study are as follows: (1) the VGCC α2δ-1 subunit was expressed mainly in neuronal cell bodies, but not in glial cells (astrocytes and microglia), of the thalamus in rats with CPSP hypersensitivity; (2) the α2δ-1 subunit was significantly up-regulated on both sides of the thalamus and the dorsal horn to much higher levels from day 7 up to day 14 after ITC injection in rats with CPSP hypersensitivity, but decreased on day 21 after ITC; and (3) the down-regulation of the α2δ-1 subunit in both sides of the thalamus and dorsal horn 21 days after ITC was likely caused by the later neuronal loss on the injured side of the thalamus. However, the apoptotic process was probably not directly involved in the neuronal death because caspase-3 was activated on day 7 but not day 21 after ITC. This result is consistent with the original description of neuronal apoptosis in the same rat model of CPSP [34], which demonstrated that apoptosis reflected by TUNEL-positive cells is dramatic between 6 h and 3 days after ITC and declines significantly between 3 and 7 days after ITC, suggesting that apoptotic cell death is an early phenomenon following intra-thalamic hemorrhage [34]. Nonetheless, whether autophagic or secondary necrotic cell death is involved in this later neuronal death remains to be determined [39]. Taken together, expression of the α2δ-1 subunit, a molecular target of GBP, is up-regulated in both the thalamus and dorsal horn in the first two weeks (7–14 days) after hemorrhagic stroke induced by ITC injection, while down-regulation of the α2δ-1 subunit may begin sometime between days 7 and 21 after ITC due to the later neuronal loss. This is likely to be responsible for the decreased anti-allodynic effectiveness of GBP described in our recent report [20].
Gabapentinoid Insensitivity in Central Neuropathic Pain of Thalamic Hemorrhage
Gabapentinoids can effectively relieve many types of peripheral neuropathic pain in patients, including painful diabetic peripheral neuropathy [40, 41] and post-herpetic neuralgia [40, 42, 43]. GBP and pregabalin are also effective in relieving pain associated with spinal cord injury, a type of central neuropathic pain [44–46]. However, so far no evidence has been provided to support the use of gabapentinoids in the treatment of central neuropathic pain of thalamic damage caused by either ischemic or hemorrhagic stroke. In a 13-week, randomized, double-blind, multicenter, placebo-controlled trial, treatment with pregabalin did not provide significant pain relief in patients with CPSP [19].
This phenomenon has also been noted in animal studies. For instance, the anti-nociceptive and anti-allodynic effectiveness of GBP has been widely supported by studies in animal models of many types of pain induced by peripheral nerve injury such as spinal nerve ligation [47–50], chronic compression injury [51–56] and spared nerve injury [57, 58]. Moreover, GBP has also been demonstrated to have anti-allodynic and anti-hyperalgesic effects in animal models of diabetic neuropathy [29] and post-herpetic neuralgia [59]. However, in our animal model of CPSP hypersensitivity, GBP delivered once daily via the intraperitoneal route only had a significant anti-allodynic effect during the first week of administration; GBP insensitivity occurred after the second week of treatment [20].
Taken together, it is likely that GBP is differentially effective in the relief of neuropathic pain of different origins. It may be effective in the treatment of peripheral neuropathic pain and spinal cord injury-associated pain, but may not be useful in the long-term treatment of central neuropathic pain due to thalamic hemorrhagic stroke.
Loss of the Gabapentin Effect May Contribute to Gabapentinoid Insensitivity in the Central Neuropathic Pain of Thalamic Hemorrhage
Both GBP and pregabalin are ligands of the VGCC α2δ subunit. It has been demonstrated that the VGCC α2δ-1 subunit can be induced to overexpress in both DRG neurons and the dorsal horn in peripheral nerve injury models [26–28, 30, 60–62]. Overexpression of the α2δ-1 subunit has been thought to contribute to the development of neuropathic pain hypersensitivity through its trafficking to the presynaptic membrane that leads to increased presynaptic Ca2+ influx and neurotransmitter release [24–27, 31, 32, 62–64]. The von Willebrand factor-A domain is involved in trafficking the VGCC α2δ-1 subunit to the plasma membrane [23, 65, 66]. Knock-down of α2δ-1 reduces pain hypersensitivity in an animal model of nerve injury [30]. It has also been confirmed that the anti-nociceptive and anti-allodynic effects of GBP and pregabalin are due to disruption of trafficking of the VGCC α2δ-1 subunit to the presynaptic terminal membrane induced by nerve injury [25–27, 32]. Thus the pharmacological action of GBP against neuropathic pain could be dependent upon aberrant overexpression of the VGCC α2δ-1 subunit in the presynaptic membrane. Based on the anti-allodynic mechanisms of GBP and the results of the present study, we propose that the onset of insensitivity of GBP later in the process of CPSP hypersensitivity is largely due to the loss of the GBP effect resulting from down-regulation of the α2δ-1 subunit at both the thalamic and spinal levels of the somatosensory system.
However, other mechanisms of GBP insensitivity cannot be completely excluded because GBP insensitivity might be due to changes in the α2δ-1 expression level and the pharmacokinetic and pharmacodynamic characteristics in patients and animals with CPSP. As for the relationship between the level of α2δ-1 expression and anti-allodynic effectiveness, it has been demonstrated that the effectiveness of a certain dose of GBP can change due to circadian changes in both the mRNA and the protein levels of α2δ-1 in rats with partial sciatic nerve ligation (PSNL), and it was surprisingly noted that GBP sensitivity was augmented at a time when the α2δ-1 subunit protein was lower at 05:00, while it was attenuated at a time when the α2δ-1 subunit protein was abundant at 17:00 during 24 h of measurement [67]. Moreover, the maximal binding capacity of [3H]-GBP in the ipsilateral DRG of rats with PSNL was also demonstrated to be two-fold higher at 17:00 than at 05:00 but with the affinity constant value unchanged [67]. However, the pharmacokinetic and pharmacodynamic characteristics of GBP in rats with PSNL does not change with the circadian rhythm [67]. Whether the levels of α2δ-1 genes and proteins can be influenced by the circadian rhythm in rats with CPSP is not clear and remains to be further investigated. Nonetheless, because in our previous work [20] and the current study, the pharmacological experiments were performed between 09:00 and 15:00 and the perfusion of the animals subjected to immunoblotting was carried out between 15:00 and 17:00, our results were unlikely to be influenced by circadian effects. α2δ-1 has also been demonstrated to be a thrombospondin (astrocyte-secreted protein) receptor in CNS neurons that is responsible for excitatory synaptogenesis. So, blocking the interaction between thrombospondin and α2δ-1 by GBP could eliminate the thrombospondin-induced formation of excitatory synapses without affecting established synapses [68], and over-expression of α2δ-1 in rats with CPSP might be involved in excitatory synaptogenesis in the thalamus and dorsal horn following thalamic hemorrhage. Long-term administration of GBP could play an inhibitory role in the thrombospondin-induced formation of excitatory synapses involved in the repair of thalamic hemorrhage-induced tissue damage; however, the thrombospondin-induced aberrant formation of excitatory synapses, if it occurs, is not likely to be involved in the late process of CPSP hypersensitivity due to the appearance of GBP insensitivity after repeated administration.
In conclusion, the GBP receptor α2δ-1 is mainly expressed in thalamic neurons, in which it is up-regulated in the early process of CPSP but this is followed by dramatic down-regulation that is likely associated with GBP insensitivity after long-term use.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81171049), the National Basic Research Development Program of China (2011CB504100 and 2013CB835100), the National Key Technology R&D Program of China (2013BAI04B04), and the Twelfth Five-Year Project of China (AWS12J004).
Footnotes
Yan Yang and Fei Yang have contributed equally to this work.
References
- 1.Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009;8:857–868. doi: 10.1016/S1474-4422(09)70176-0. [DOI] [PubMed] [Google Scholar]
- 2.Kumar G, Soni CR. Central post-stroke pain: current evidence. J Neurol Sci. 2009;284:10–17. doi: 10.1016/j.jns.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Haanpaa M, Attal N, Backonja M, Baron R, Bennett M, Bouhassira D, et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27. doi: 10.1016/j.pain.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Bogousslavsky J, Regli F, Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988;38:837–848. doi: 10.1212/WNL.38.6.837. [DOI] [PubMed] [Google Scholar]
- 5.Wessel K, Vieregge P, Kessler C, Kompf D. Thalamic stroke: correlation of clinical symptoms, somatosensory evoked potentials, and CT findings. Acta Neurol Scand. 1994;90:167–173. doi: 10.1111/j.1600-0404.1994.tb02700.x. [DOI] [PubMed] [Google Scholar]
- 6.Kumral E, Kocaer T, Ertubey NO, Kumral K. Thalamic hemorrhage. A prospective study of 100 patients. Stroke. 1995;26:964–970. doi: 10.1161/01.STR.26.6.964. [DOI] [PubMed] [Google Scholar]
- 7.Chung CS, Caplan LR, Han W, Pessin MS, Lee KH, Kim JM. Thalamic haemorrhage. Brain. 1996;119(Pt 6):1873–1886. doi: 10.1093/brain/119.6.1873. [DOI] [PubMed] [Google Scholar]
- 8.Nasreddine ZS, Saver JL. Pain after thalamic stroke: right diencephalic predominance and clinical features in 180 patients. Neurology. 1997;48:1196–1199. doi: 10.1212/WNL.48.5.1196. [DOI] [PubMed] [Google Scholar]
- 9.Paciaroni M, Bogousslavsky J. Pure sensory syndromes in thalamic stroke. Eur Neurol. 1998;39:211–217. doi: 10.1159/000007936. [DOI] [PubMed] [Google Scholar]
- 10.Henry JL, Lalloo C, Yashpal K. Central poststroke pain: an abstruse outcome. Pain Res Manag. 2008;13:41–49. doi: 10.1155/2008/754260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry JL. Central poststroke pain: An animal model. In: Henry JL, Panju A, Yashpal K, editors. Central Neuropathic Pain: Focus on poststroke pain. Seattle: IASP Press; 2007. [Google Scholar]
- 12.Henry JL, Panju A, Yashpal K. Central Neuropathic Pain: Focus on poststroke pain. Seattle: IASP Press; 2007. [Google Scholar]
- 13.Jensen TS, Finnerup NB. Central pain. In: McMahon SB, Koilzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Textbook of Pain. 6. Philadelphia: Elsevier Saunders; 2013. [Google Scholar]
- 14.Dworkin RH, O’Connor AB, Audette J, Baron R, Gourlay GK, Haanpaa ML, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85:S3–14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dworkin RH, O’Connor AB, Backonja M, Farrar JT, Finnerup NB, Jensen TS, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS. Post-stroke pain. Expert Rev Neurother. 2009;9:711–721. doi: 10.1586/ern.09.19. [DOI] [PubMed] [Google Scholar]
- 17.Kumar B, Kalita J, Kumar G, Misra UK. Central poststroke pain: a review of pathophysiology and treatment. Anesth Analg. 2009;108:1645–1657. doi: 10.1213/ane.0b013e31819d644c. [DOI] [PubMed] [Google Scholar]
- 18.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122:S22–32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS, Bashford G, Murphy TK, Martin A, Dror V, Cheung R. Safety and efficacy of pregabalin in patients with central post-stroke pain. Pain. 2011;152:1018–1023. doi: 10.1016/j.pain.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Yang F, Fu H, Lu YF, Wang XL, Yang Y, Yang F, et al. Post-stroke pain hypersensitivity induced by experimental thalamic hemorrhage in rats is region-specific and demonstrates limited efficacy of gabapentin. Neurosci Bull. 2014;30:887–902. doi: 10.1007/s12264-014-1477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–647. doi: 10.1023/B:JOBB.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- 22.Maneuf YP, Gonzalez MI, Sutton KS, Chung FZ, Pinnock RD, Lee K. Cellular and molecular action of the putative GABA-mimetic, gabapentin. Cell Mol Life Sci. 2003;60:742–750. doi: 10.1007/s00018-003-2108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha2delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, et al. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci. 2009;29:4076–4088. doi: 10.1523/JNEUROSCI.0356-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer CS, Rahman W, Tran-van-Minh A, Lujan R, Dickenson AH, Dolphin AC. The anti-allodynic alpha2delta ligand pregabalin inhibits the trafficking of the calcium channel alpha2delta-1 subunit to presynaptic terminals in vivo. Biochem Soc Trans. 2010;38:525–528. doi: 10.1042/BST0380525. [DOI] [PubMed] [Google Scholar]
- 28.Luo ZD, Chaplan SR, Higuera ES, Sorkin LS, Stauderman KA, Williams ME, et al. Upregulation of dorsal root ganglion alpha2delta calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats. J Neurosci. 2001;21:1868–1875. doi: 10.1523/JNEUROSCI.21-06-01868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel alpha2delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- 30.Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel alpha2delta-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, et al. Calcium channel alpha2delta-1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor CP. Mechanisms of analgesia by gabapentin and pregabalin-calcium channel alpha2-delta [Cavalpha2-delta] ligands. Pain. 2009;142:13–16. doi: 10.1016/j.pain.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Ren LY, Lu ZM, Liu MG, Yu YQ, Li Z, Shang GW, et al. Distinct roles of the anterior cingulate cortex in spinal and supraspinal bee venom-induced pain behaviors. Neuroscience. 2008;153:268–278. doi: 10.1016/j.neuroscience.2008.01.067. [DOI] [PubMed] [Google Scholar]
- 34.Wasserman JK, Koeberle PD. Development and characterization of a hemorrhagic rat model of central post-stroke pain. Neuroscience. 2009;161:173–183. doi: 10.1016/j.neuroscience.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 35.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsteerdam: Elsevier Academic Press; 2005. [Google Scholar]
- 36.Chen J, Luo C, Li H, Chen H. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain. 1999;83:67–76. doi: 10.1016/S0304-3959(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 37.Yu YQ, Zhao F, Guan SM, Chen J. Antisense-mediated knockdown of NaV1.8, but not NaV1.9, generates inhibitory effects on complete Freund’s adjuvant-induced inflammatory pain in rat. PLoS One. 2011;6:e19865. doi: 10.1371/journal.pone.0019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu YQ, Zhao ZY, Chen XF, Xie F, Yang Y, Chen J. Activation of tetrodotoxin-resistant sodium channel NaV1.9 in rat primary sensory neurons contributes to melittin-induced pain behavior. Neuromol Med. 2013;15:209–217. doi: 10.1007/s12017-012-8211-0. [DOI] [PubMed] [Google Scholar]
- 39.Krysko DV, Vanden Berghe T, Parthoens E, D’Herde K, Vandenabeele P. Methods for distinguishing apoptotic from necrotic cells and measuring their clearance. Methods Enzymol. 2008;442:307–341. doi: 10.1016/S0076-6879(08)01416-X. [DOI] [PubMed] [Google Scholar]
- 40.Chou R, Carson S, Chan BK. Gabapentin versus tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia: discrepancies between direct and indirect meta-analyses of randomized controlled trials. J Gen Intern Med. 2009;24:178–188. doi: 10.1007/s11606-008-0877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009;9:6. doi: 10.1186/1471-2377-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice AS, Maton S. Postherpetic Neuralgia Study G. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 43.Stacey BR, Glanzman RL. Use of gabapentin for postherpetic neuralgia: results of two randomized, placebo-controlled studies. Clin Ther. 2003;25:2597–2608. doi: 10.1016/S0149-2918(03)80320-X. [DOI] [PubMed] [Google Scholar]
- 44.To TP, Lim TC, Hill ST, Frauman AG, Cooper N, Kirsa SW, et al. Gabapentin for neuropathic pain following spinal cord injury. Spinal Cord. 2002;40:282–285. doi: 10.1038/sj.sc.3101300. [DOI] [PubMed] [Google Scholar]
- 45.Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–157. doi: 10.1016/j.pain.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 46.Amr YM. Multi-day low dose ketamine infusion as adjuvant to oral gabapentin in spinal cord injury related chronic pain: a prospective, randomized, double blind trial. Pain Physician. 2010;13:245–249. [PubMed] [Google Scholar]
- 47.Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahm TS, Ahn HJ, Bae CD, Kim HS, Lim SW, Cho HS, et al. Protective effects of gabapentin on allodynia and alpha2delta1-subunit of voltage-dependent calcium channel in spinal nerve-ligated rats. J Korean Med Sci. 2009;24:146–151. doi: 10.3346/jkms.2009.24.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu LC, Tsaur ML, Lin CS, Hung YC, Wang TY, Chen CC, et al. Chronic intrathecal infusion of gabapentin prevents nerve ligation-induced pain in rats. Br J Anaesth. 2011;106:699–705. doi: 10.1093/bja/aer063. [DOI] [PubMed] [Google Scholar]
- 50.Dias QM, Silveira JW, Reis GM, Costa KA, Rossaneis AC, Fais RS, et al. The effect of intrathecal gabapentin on neuropathic pain is independent of the integrity of the dorsolateral funiculus in rats. Life Sci. 2012;91:837–842. doi: 10.1016/j.lfs.2012.08.032. [DOI] [PubMed] [Google Scholar]
- 51.Roeska K, Doods H, Arndt K, Treede RD, Ceci A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain. 2008;139:349–357. doi: 10.1016/j.pain.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Gunduz O, Oltulu C, Guven R, Buldum D, Ulugol A. Pharmacological and behavioral characterization of the saphenous chronic constriction injury model of neuropathic pain in rats. Neurol Sci. 2011;32:1135–1142. doi: 10.1007/s10072-011-0761-7. [DOI] [PubMed] [Google Scholar]
- 53.Kayser V, Christensen D. Antinociceptive effect of systemic gabapentin in mononeuropathic rats, depends on stimulus characteristics and level of test integration. Pain. 2000;88:53–60. doi: 10.1016/S0304-3959(00)00307-9. [DOI] [PubMed] [Google Scholar]
- 54.Ma LL, Liu W, Huang YG, Yang N, Zuo PP. Analgesic effect of gabapentin in a rat model for chronic constrictive injury. Chin Med J (Engl) 2011;124:4304–4309. [PubMed] [Google Scholar]
- 55.Gregoire S, Michaud V, Chapuy E, Eschalier A, Ardid D. Study of emotional and cognitive impairments in mononeuropathic rats: effect of duloxetine and gabapentin. Pain. 2012;153:1657–1663. doi: 10.1016/j.pain.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 56.De la OAM, Diaz-Reval MI, Cortes-Arroyo AR, Dominguez-Ramirez AM, Lopez-Munoz FJ. Anti-nociceptive synergism of morphine and gabapentin in neuropathic pain induced by chronic constriction injury. Pharmacol Biochem Behav. 2009;92:457–464. doi: 10.1016/j.pbb.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Hama AT, Borsook D. The effect of antinociceptive drugs tested at different times after nerve injury in rats. Anesth Analg. 2005;101:175–179. doi: 10.1213/01.ANE.0000155247.93604.62. [DOI] [PubMed] [Google Scholar]
- 58.Folkesson A, Honore PH, Andersen LM, Kristensen P, Bjerrum OJ. Low dose of donepezil improves gabapentin analgesia in the rat spared nerve injury model of neuropathic pain: single and multiple dosing studies. J Neural Transm. 2010;117:1377–1385. doi: 10.1007/s00702-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 59.Chen SR, Pan HL. Effect of systemic and intrathecal gabapentin on allodynia in a new rat model of postherpetic neuralgia. Brain Res. 2005;1042:108–113. doi: 10.1016/j.brainres.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 60.Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A, et al. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL, et al. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience. 2002;114:529–546. doi: 10.1016/S0306-4522(02)00341-X. [DOI] [PubMed] [Google Scholar]
- 62.Li KW, Yu YP, Zhou C, Kim DS, Lin B, Sharp K, et al. Calcium channel alpha2delta-1 proteins mediate trigeminal neuropathic pain states associated with aberrant excitatory synaptogenesis. J Biol Chem. 2014;289:7025–7037. doi: 10.1074/jbc.M114.548990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boroujerdi A, Kim HK, Lyu YS, Kim DS, Figueroa KW, Chung JM, et al. Injury discharges regulate calcium channel alpha2delta-1 subunit upregulation in the dorsal horn that contributes to initiation of neuropathic pain. Pain. 2008;139:358–366. doi: 10.1016/j.pain.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nguyen D, Deng P, Matthews EA, Kim DS, Feng G, Dickenson AH, et al. Enhanced pre-synaptic glutamate release in deep-dorsal horn contributes to calcium channel alpha2delta-1 protein-mediated spinal sensitization and behavioral hypersensitivity. Mol Pain. 2009;5:6. doi: 10.1186/1744-8069-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whittaker CA, Hynes RO. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol Biol Cell. 2002;13:3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, Richards MW, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci USA. 2005;102:11230–11235. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kusunose N, Koyanagi S, Hamamura K, Matsunaga N, Yoshida M, Uchida T, et al. Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Mol Pain. 2010;6:83. doi: 10.1186/1744-8069-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Özkan E, et al. The gabapentin receptor α2δ-1 is the neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]