Abstract
Autism spectrum disorder (ASD) is defined by impairments of social interaction and the presence of obsessive behaviors. The “twin” nonapeptides oxytocin (OXT) and arginine-vasopressin (AVP) are known to play regulatory roles in social behaviors. However, the plasma levels and behavioral relevance of OXT and AVP in children with ASD have seldom been investigated. It is also unknown whether their mothers have abnormal plasma peptide levels. Here, using well-established methods of neuropeptide measurement and a relatively large sample size, we determined the plasma levels of the two neuropeptides in 85 normal children, 84 children with ASD, and 31 mothers from each group of children. As expected, children with ASD had lower plasma OXT levels than gender-matched controls (P = 0.028). No such difference was found for plasma AVP concentrations. Correlation analysis showed that ASD children with higher plasma OXT concentrations tended to have less impairment of verbal communication (Rho = −0.22, P = 0.076), while those with higher plasma AVP levels tended to have lower levels of repetitive use of objects (Rho = −0.231, P = 0.079). Unlike the findings in children, maternal plasma OXT levels showed no group difference. However, plasma AVP levels in the mothers of ASD children tended to be lower than in the mothers of normal children (P = 0.072). In conclusion, our results suggest that the OXT system is dysregulated in children with ASD, and that OXT and AVP levels in plasma seem to be associated with specific autistic symptoms. The plasma levels of OXT or AVP in mothers and their ASD children did not seem to change in the same direction.
Keywords: Autism spectrum disorder, Oxytocin, Vasopressin, Behavioral relevance
Introduction
Autism spectrum disorder (ASD) is a pervasive neurodevelopmental disorder involving deficits in language, social interaction, and social communication, as well as the presence of restricted interests and repetitive and stereotypic patterns of behavior. Oxytocin (OXT) and arginine-vasopressin (AVP), closely-related nonapeptides, are mainly synthesized in the supraoptic and paraventricular nuclei of the hypothalamus. OXT plays key regulatory roles in affiliative and social behaviors as has been revealed by substantial studies in rodents [1–3] and other species [4, 5], including humans [6]. Similarly, AVP is also involved in the regulation of social behaviors including social preference [7], social memory [8], social bonding [9], and aggression [10].
Given the prosocial effects of OXT and AVP, recent studies have focused on whether and how these peptides are involved in the pathogenesis of ASD. An abnormal brain OXT system has been found in several mouse models of autism [11–13]. For example, neonatal mice deficient in Magel2 (MAGE family member L2), which may be involved in ASD, have significantly reduced OXT in the hypothalamus [11]. A higher OXT content in the hypothalamus has been reported in BTBR mice, a validated model of ASD [12]. Cntnap2 (contactin-associated protein-like 2) gene-knockout mice display autistic-like behaviors, and have reduced OXT expression in the PVN and an overall reduction in brain OXT levels [13], while exogenous OXT treatment partially restores normal function of the OXT system and leads to improved social behaviors [13, 14]. Human studies have focused on pharmacological administration and the measurement of plasma neuropeptides, mainly of OXT. It has been suggested that intranasal OXT is beneficial for the remission of autistic symptoms by improving cooperation and sense of trust [15] as well as by enhancing social responsiveness [16] and social reciprocity [17].
However, studies of neuropeptide content in the plasma of ASD sufferers have shown ambiguous results. Four studies have shown lower plasma OXT levels in children with ASD than in control individuals [15, 18–20], two studies have reported higher OXT levels [21, 22], and one has reported no overall group difference [23]. Relationships between plasma OXT levels and social behaviors were either not investigated [15, 21] or were analyzed in subsets or subtypes of ASD [22, 23]. One study with small samples reporting a positive correlation of plasma OXT levels and autism severity [19] is contradicted by another study [18]. Similarly, three studies of plasma AVP concentrations in individuals with ASD were also not in accord with each other [23–25]. It is crucial to note that these studies had several limitations, such as a large age range of participants, small study cohorts, lack of well-validated methods of neuropeptide measurement, and/or use of nonstandard methods for ASD diagnosis. Therefore, the question of whether changes in peripheral OXT or AVP levels are a potential biomarker of the symptoms of ASD remains uncertain.
Family members of children diagnosed with ASD have been reported to display more autism spectrum traits, including social skills and communication impairments, than family members of normal controls [26, 27]. Social deficiency in the relatives of people with ASD may therefore reflect the heritability of the underlying physiology important to social behaviors (i.e., OXT or AVP). As far as we know, whether changes of OXT or AVP levels in ASD sufferers are also present in their unaffected family members remains unclear.
In the present study, we recruited a relatively large number of children within a narrow age range, and used a reliable method (standardized ELISA kit) to assess the plasma levels of OXT and AVP. Furthermore, we analyzed the associations of plasma OXT and AVP values with autistic symptoms as evaluated using the Childhood Autism Rating Scale (CARS). In addition, we measured the levels of the two neuropeptides in the mothers of normal and ASD children to explore the relationship of OXT or AVP levels in mothers and their offspring.
Materials and Methods
Ethics Statement
This study was approved by the Review Board of Peking University (IRB00001052-13064). The parents or legal guardians of the child participants were informed of the purposes and detailed procedures of the study and gave informed consent before initiation of the experimental procedures.
Participants
Eighty-four children with ASD (71 boys, 13 girls) between the ages of 2 and 7 years were enrolled from autism rehabilitation centers in Beijing, China. All autistic participants were diagnosed by an experienced child psychiatrist (MXJ) according to the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR, 2000) criteria. Children with ASD taking psychotropic medications or with comorbid psychiatric disorders were excluded. Among the children diagnosed with ASD, 66 were given additional assessments using the CARS. Typically-developing children (71 boys, 14 girls) ranging in age from 2 to 7 years were recruited from a local kindergarten and community health center in Beijing, China. All normal children were free of neurological and psychiatric disorders as confirmed by evaluation by child psychiatrists. In addition, 31 mothers of normal children (NMs) and 31 age- and race-matched mothers of children with ASD (AMs) also participated in this study. The inclusion criteria for mothers in both groups were: (1) no present or past history of psychiatric disorders; (2) no significant medical illness; and (3) free of medication for at least 2 weeks at the time of the study. The characteristics of the participants are listed in Table 1.
Table 1.
Participant demographics.
| Control | ASD | P value | |
|---|---|---|---|
| Children | |||
| Sample size (male: female) | 85 (71:14) | 84 (71:13) | 0.860 |
| Race (Han: other) | 83:2 | 79:5 | 0.277 |
| Age, years | 4.80 ± 1.22 | 3.95 ± 1.26 | <0.01 |
| CARS (n) | 66 | ||
| Total score | 37.59 ± 4.71 | ||
| Mothers | |||
| Sample size (n) | 31 | 31 | |
| Age, years | 33.60 ± 3.95 | 33.72 ± 3.07 | 0.894 |
| Race (Han: other) | 31:0 | 28:3 | 0.238 |
Data are presented as mean ± SD unless otherwise noted. P values were calculated by unpaired Student’s t-test or χ 2 test.
ASD, autism spectrum disorder; CARS, Childhood Autism Rating Scale.
Assessment of Autistic Symptoms
Childhood Autism Rating Scale (CARS)
The CARS contains 15 items that provide qualitative assessments of a large range of issues for children with ASD [28]. The items are as follows: relating to people, imitative behavior, emotional response, body and object use, adaptation to change, visual response, listening response, perceptive response, fear or anxiety, verbal and non-verbal communication, activity level, level and consistency of intellective relations, and general impressions [29]. Among these items, “body use” and “object use” reflect the degree of repetitive use of the body (such as hand and finger mannerisms) and objects, respectively.
Blood Sampling and Plasma OXT and AVP Measurement
Children and mothers were instructed to fast overnight and allowed only a moderate amount of drinking water to minimize the potential effects of food and water intake. Blood samples were collected by a pediatric nurse at Peking University Sixth Hospital (Beijing, China) between 09:00 and 11:00 to control for potential circadian rhythmicity in plasma OXT and AVP concentrations [30]. Whole blood was drawn into chilled EDTA-treated tubes containing the protease inhibitor aprotinin (500 KIU/ml blood) (Sigma Aldrich, St. Louis, USA) and promptly centrifuged at 1,600×g for 15 min at 4°C. The plasma was then isolated and stored at −80°C until assay.
The OXT and AVP concentrations were measured using Oxytocin and Arg8-Vasopressin ELISA kits (both from Enzo Life Sciences, Farmingdale, USA). These kits are highly specific and have little cross-reactivity with other related peptides. The cross-reactivity with Arg8-vasopressin and Lys8-vasopressin was <0.02% for the oxytocin kit, and that with OXT was <0.001% for the arg8-vasopressin kit. The detection limits of the kits were 15.0 pg/mL for OXT and 3.39 pg/mL for AVP.
In order to make accurate measurements, all samples were extracted with acetone and petroleum ether according to the Product Manual and published methods [31, 32]. Extraction is necessary to eliminate interfering substances in the samples and to increase the low plasma concentrations of OXT and AVP for accurate measurements within the range of the assay. In this study, 400 μL plasma was extracted for OXT measurement and 650 μL for AVP measurement. The extracted samples were then freeze-dried and reconstituted with 120 μL assay buffer before quantification to provide a sufficient sample volume for the wells of microtiter plates (100 μL in each well). Samples were assayed with a Bio-Rad iMark™ microplate reader and read at 405 nm with correction between 570 and 590 nm. All assays were performed by an investigator who was blind to the diagnostic and behavioral data. The intra-assay coefficient of variation for OXT was 10.2% and for AVP was 5.9%. Among the samples, the plasma volume from 5 control children and 11 autistic children was not enough to measure both neuropeptides, so only the OXT levels were assessed.
Data Analysis and Statistics
Statistical analyses were performed with SPSS Statistics 19 (IBM spss, Armonk,USA) and graphs were generated using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, USA). The demographic characteristics of healthy participants and ASD sufferers were compared using Student’s t-test (for continuous variables) or χ 2 analysis (for categorical data). Comparisons of plasma OXT or AVP levels between control and ASD groups were analyzed by one-way analysis of covariance (ANCOVA). In ANCOVA, age was included as a covariate to control for a possible confounding effect. Before running ANCOVA analysis, Levene’s test for equality of error variances and tests for homogeneity of regression slopes were conducted. Spearman’s rank correlation was used to assess the associations of OXT or AVP levels and behavioral scores of ASD, as well as mother–child relationships of the neuropeptides.
Results
Decreased Plasma OXT Levels in Children with ASD
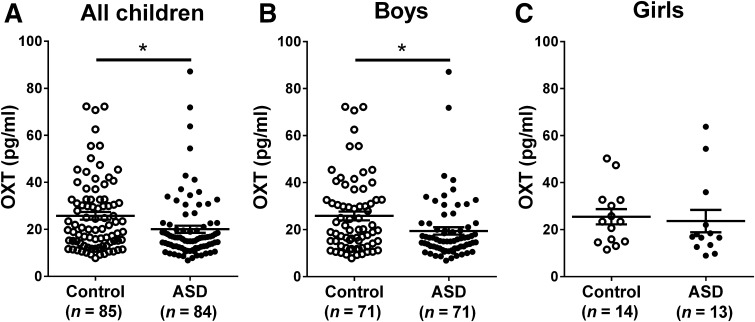
Given the possible confounding effect of age, which differed between the two groups (P < 0.01), we conducted ANCOVA with age as the covariate. The variances were homogeneous (P = 0.057) and the slope of the covariate was equal across control and ASD groups (P = 0.244). ANCOVA analysis showed that the relationships between the covariate and the dependent variable (OXT level) were non-significant, indicating that the OXT levels of children are not influenced by age. The group difference was statistically significant (P = 0.028), suggesting that the plasma level of OXT in children with ASD was markedly lower than that in control children (Fig. 1A). Broken down by gender, a lower plasma OXT concentration was found in autistic boys (P = 0.028, Fig. 1B), but not in autistic girls (P = 0.42, Fig. 1C). The number of girls recruited was only one fifth that of boys, so we inferred that the smaller sample size was at least partially responsible for the absence of a statistical difference in girls.
Fig. 1.
Plasma concentrations of OXT in control and ASD children. Children with ASD showed a lower plasma OXT levels than control children (P = 0.028) after adjusting for covariates (A). Boys with ASD had lower plasma OXT concentrations than control boys (P = 0.028) (B). No significant difference in OXT levels was found between control and autistic girls (C). All values are expressed as mean ± SEM (*P < 0.05; OXT, oxytocin; ASD, autism spectrum disorder).
Normal Plasma AVP Levels in Children with ASD
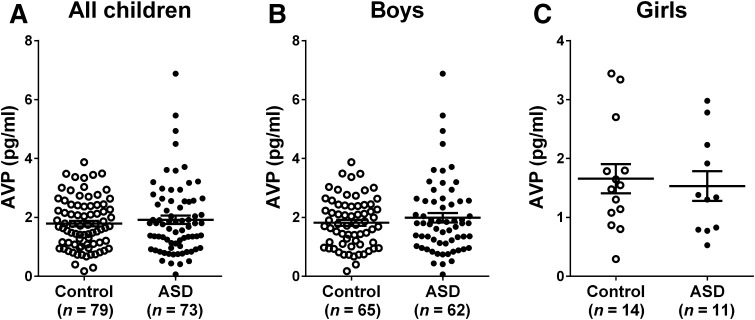
Since the plasma volume from some participants was insufficient, we ultimately measured the AVP concentrations in 79 control and 73 autistic participants. The variances were homogeneous (P = 0.058) and interaction of group and age in ANCOVA was not significant (P = 0.22). Unlike OXT, the plasma AVP concentration was comparable in the two groups (P = 0.477, Fig. 2A) after adjusting for age. Broken down by gender, there were no significant differences in AVP levels between control and autistic boys or girls (Fig. 2B, C).
Fig. 2.
Plasma concentrations of AVP in control and ASD children. The levels in the two groups were comparable (A). Boys with ASD did not differ from normal boys with respect to plasma AVP (B), nor were there significant difference between girls (C). All values are presented as mean ± SEM (AVP, arginine-vasopressin; ASD, autism spectrum disorder).
Relationship Between Plasma OXT and AVP Levels
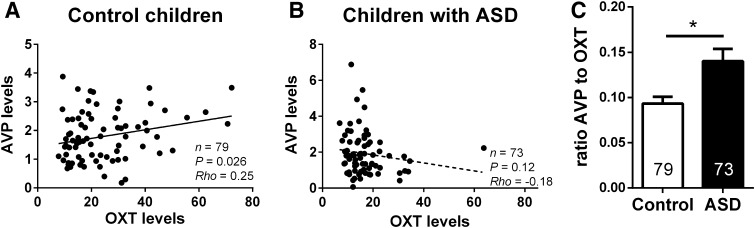
Spearman correlation analysis revealed that OXT levels showed a positive correlation with AVP levels in normal individuals (rho = 0.25, P = 0.026, Fig. 3A). After adjusting for age, gender, and race, this correlation was still significant (rho = 0.236, P = 0.04). For people with ASD, intriguingly, no such correlation was found between the two neuropeptides (rho = −0.18, P = 0.12, Fig. 3B), even after controlling for age, sex, and race (rho = −0.163, P = 0.180). Interestingly, individuals with ASD had a higher ratio AVP to OXT than control children (Fig. 3C, P = 0.010).
Fig. 3.
A, B Scatter-plots showing correlations between OXT and AVP levels within the two groups. The two peptides were positively correlated in plasma of control children (A), but not in children with ASD (B). C The ratio of AVP to OXT in ASD children was higher than that in control children. *P < 0.05; OXT, oxytocin; AVP, arginine-vasopressin.
Behavioral Relevance of Plasma OXT and AVP Levels
Relationships between the behavior scores for children with ASD and the plasma neuropeptide levels were assessed by Spearman correlation analysis. The correlation coefficients and the statistical significance are listed in Table 2. Plasma OXT and AVP concentrations showed no correlation with the total CARS score. Among the CARS items, the plasma OXT levels tended to be negatively correlated with verbal communication (Rho = −0.22, P = 0.076) and positively related to adaptation to change (Rho = 0.233, P = 0.06), indicating that higher plasma OXT concentrations in ASD are associated with better verbal communication skills and with weaker adaptability. There was also a trend for negative correlation between plasma AVP levels and object use (Rho = −0.231, P = 0.079), suggesting that ASD children with higher plasma AVP levels tend to show less repetitive use of objects. Other CARS items showed no correlations with plasma OXT or AVP levels.
Table 2.
Correlation analysis of CARS scores of ASD children and their plasma neuropeptide levels.
| Children with ASD | ||||
|---|---|---|---|---|
| OXT level (n = 66) | AVP level (n = 59) | |||
| Rho | P | Rho | P | |
| CARS | ||||
| Total score | 0.026 | 0.835 | −0.102 | 0.444 |
| Relating to people | −0.022 | 0.860 | 0.123 | 0.354 |
| Imitative behavior | −0.017 | 0.894 | −0.175 | 0.186 |
| Emotional response | −0.061 | 0.629 | −0.025 | 0.853 |
| Body use | 0.168 | 0.178 | −0.186 | 0.158 |
| Object use | 0.056 | 0.656 | −0.231 | 0.079 |
| Adaptation to change | 0.233 | 0.060 | 0.028 | 0.834 |
| Visual response | 0.103 | 0.410 | 0.137 | 0.299 |
| Listening response | 0.199 | 0.110 | −0.066 | 0.619 |
| Perceptive response | 0.181 | 0.145 | 0.082 | 0.538 |
| Fear or anxiety | 0.013 | 0.917 | 0.021 | 0.876 |
| Verbal communication | −0.220 | 0.076 | 0.079 | 0.554 |
| Non-verbal communication | −0.031 | 0.802 | −0.078 | 0.556 |
| Activity level | −0.037 | 0.765 | −0.191 | 0.148 |
| Level/consistency of intellective relations | 0.020 | 0.871 | −0.063 | 0.637 |
| General impressions | −0.081 | 0.519 | −0.06 | 0.654 |
CARS, Childhood Autism Rating Scale; OXT, oxytocin; AVP, arg8-vasopressin.
Plasma OXT and AVP Levels in Mothers
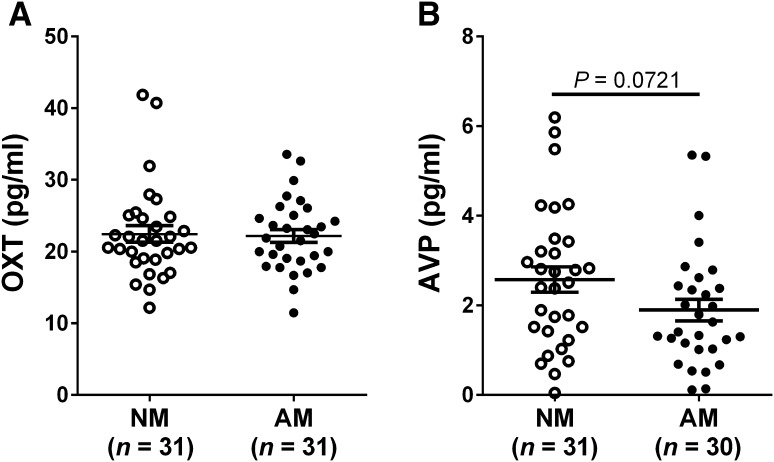
Plasma OXT and AVP concentrations in NMs and AMs were also measured. The results showed that AMs had plasma OXT levels similar to NMs after controlling for age (Fig. 4A). During AVP measurement, one AM sample was incorrectly processed and thus was excluded from the subsequent statistical analysis. There was a tendency for AVP concentrations to be lower in AMs than in NMs (P = 0.072, Fig. 4B).
Fig. 4.
Plasma OXT and AVP levels in mothers. A Maternal plasma OXT concentrations were comparable in NMs and AMs. B AVP levels in AMs approached significance versus NMs (P = 0.072). All values are expressed as mean ± SEM (OXT, oxytocin; AVP, arginine-vasopressin; NM, mothers of normal children; AM, mothers of children with ASD).
Correlation of Plasma Neuropeptides Between Children and Their Mothers
Spearman’s rank correlation was used to analyze the relationship between mothers and their children with respect to plasma OXT and AVP concentrations. As shown in Table 3, neither OXT nor AVP levels showed significant correlation between normal children and their mothers. Similarly, the neuropeptide levels in children with ASD were not associated with those in their mothers.
Table 3.
Spearman correlation analysis of plasma neuropeptides in children and their mothers.
| Plasma OXT levels | Plasma AVP levels | |||||
|---|---|---|---|---|---|---|
| n | P | Rho | n | P | Rho | |
| ASD-AM | 31 | 0.397 | −0.160 | 31 | 0.590 | 0.110 |
| Control-NM | 31 | 0.443 | 0.140 | 30 | 0.156 | 0.260 |
Rho, Spearman’s rank correlation coefficient; OXT, oxytocin; AVP, arginine-vasopressin; AM, mothers of children with ASD; NM, mothers of normal children.
Discussion
In this study, we investigated the plasma OXT and AVP levels in children with and without ASD, and the relationships between the two neuropeptides and autistic-like behaviors as evaluated by CARS scores. As predicted, OXT plasma levels in ASD children were lower than those in control children. However, plasma AVP concentrations showed no group difference. In children with ASD, low plasma OXT levels tended to predict impaired verbal communication, and plasma AVP values were correlated with repetitive use of objects at a trend level. The results indicated that plasma OXT and AVP concentrations in ASD children may reflect different aspects of autistic symptoms. For the first time, we explored the mother–child relationships of plasma OXT and AVP levels, but neither showed a significant correlation, suggesting that these neuropeptides may not be the common biological basis accounting for the autism phenotype in ASD and their mothers.
Modahl et al. (1998) first reported lower plasma levels of OXT in pre-pubertal autistic boys than in age-matched controls [20], and subsequent researchers reported a similar decrease in autistic children [18, 19, 25]. In accord with these findings, our results also showed significantly lower OXT levels in the plasma of ASD children relative to controls. However, two studies of ASD patients reached different conclusions. One showed higher plasma OXT levels and another suggested no change in plasma OXT levels in autistic individuals [22, 23]. The methods of OXT measurement used in these studies were radioimmunoassay (RIA) and enzyme-linked immunoassay (ELISA) without sample extraction. In the present study, ELISA was conducted to measure OXT levels following plasma sample extraction, which has been suggested to be more sensitive and accurate than RIA or ELISA without extraction [31, 32]. Therefore, the differences in assay methods for OXT may help to explain the inconsistent findings. Furthermore, patients in those two studies were adults or spanned pre-pubertal and pubertal age ranges. In contrast, the ages of participants in the present study were strictly restricted to 2–7 years. This may be one explanation for the discrepancy between the studies. Several possible mechanisms could lead to abnormal plasma OXT levels. Genetically, mutation of the oxt gene or variation in the genes encoding proteins regulating OXT synthesis or release, such as CD38 [33, 34], may be one reason. A mutation may be inherited from parents or occur de novo. On the other hand, prenatal factors (like prenatal stressors [35] and drug abuse [36]) and the postnatal environment (like aberrant social environments [37]) may also lead to alteration of the OXT system, characterized by decreased central OXT mRNA levels and lower plasma OXT levels.
Similar to OXT, studies on plasma AVP levels in ASD are limited and the results are controversial. Al-Ayadhi reported low plasma AVP levels in autistic children in Saudi Arabia [25]. Conversely, another study of adults with ASD showed elevated concentrations of AVP [24]. In the present study, we did not find a significant difference in plasma AVP levels between autistic and control participants, even with a relatively large sample size (>70 in each group) and a well-established AVP measurement method. A recent study supported our result, reporting no difference between ASD and normal controls, although with higher AVP levels in boys than in girls [23]. The absence of a group difference in plasma AVP levels in the current study implies normal hypothalamic AVP synthesis and/or release in ASD children.
OXT and AVP are closely-related neuropeptides with a shared evolutionary origin [38]. Both peptides consist of nine amino-acids, differing only in two of them at positions 3 and 8. A clinical study in our lab showed that the plasma concentrations of OXT and AVP are positively correlated in adult females [39]. Recent animal studies have demonstrated that a positive relationship between the two neuropeptides also exists in the rodent brain [40, 41]. In the present study, the plasma OXT and AVP levels in normal children showed a significant positive correlation. All the evidence points to the idea that these two neuropeptides are secreted synchronously and may share the same mechanisms of regulation of synthesis and release. Interestingly, we found no such positive correlation between plasma OXT and AVP in autistic children, suggesting that dysregulation of OXT in individuals with ASD may disrupt the normal relationship between OXT and AVP.
The behavioral data showed a tendency of negative correlation between ASD-related language communication and OXT levels, indicating that less impairment of social language is associated with higher levels of OXT. That is, higher OXT levels may protect the individual against defective social communication. This result was in accord with the outcome of a treatment study reporting improvement of speech comprehension after intravenous administration of OXT [42]. Meanwhile, correlations between plasma OXT levels and other CARS items, such as fear or anxiety, relating to people, or emotional response, were absent. This finding suggested a symptom-specific association of plasma OXT concentration. The item “Object use” in CARS, to some extent, reflects restricted interest and repetitive behavior of ASD individuals with regard to specific objects [43]. Plasma AVP concentrations were negatively associated with the “Object use” score at a trend level, suggesting that lower AVP levels are correlated with more repetitive behaviors in ASD. An animal study seems to support our result, reporting stereotypic behavior in Avp-deficient neonatal rats [44]. A clinical study also reported a similar finding, with a negative association between AVP and repetitive behavior symptoms in boys with ASD [23]. The behavioral relevance of plasma neuropeptides suggests that plasma OXT or AVP levels may be indicators of different aspects of autistic symptoms.
In the mothers, neither plasma OXT nor AVP levels showed significant differences between AMs and NMs. Our results differ from previous reports, with lower plasma OXT and AVP levels in AMs [39]. It should be noted that the prior study did not control for age and race of mothers when comparing group differences in OXT and AVP. Another possible explanation for the discrepancy is that the mothers recruited in this study were not confined to a specific phase of the menstrual cycle. Plasma OXT levels have been suggested to fluctuate throughout the menstrual cycle in both healthy women and non-human primates [45, 46]. Conversely, there are also studies showing that plasma OXT concentrations are unchanged during the follicular and luteal phases of the menstrual cycle in women with mental disorders and normal women [47–49]. Our finding was supported by a recent clinical study, reporting that salivary OXT levels in mothers and fathers do not differ between the parents of typically developing children and those with ASD [50].
There are several limitations of the current study. It remains unclear whether peripheral OXT or AVP levels represent the central neuropeptide levels and activities. Some studies in pregnant women [51], adult suicide attempters [52], and adult patients undergoing surgical procedures [53] have indicated a lack of correlation between OXT concentrations in plasma and cerebrospinal fluid (CSF). However, a recent study on children and adult patients have reported a positive correlation of OXT levels between the two compartments and this relationship is stronger when only children are included in the analysis [54]. Also in studies with children, higher peripheral OXT levels have been shown to correspond with greater interaction skills in normal individuals [20]. Recently-published studies on human neonates and children have also suggested that plasma AVP is a surrogate for brain AVP activity and a biomarker of social functioning in children with ASD [55, 56]. All these findings indicate that a correlation between OXT/AVP levels in plasma and CSF is more likely to occur in pediatric populations. The participants in our study were all in childhood, so we speculated that plasma OXT or AVP concentrations, to some extent, represent brain neuropeptide levels. Subsequent work, if possible, should focus on OXT and AVP levels in CSF, which are more directly relevant to behavioral effects or psychopathology.
The ASD rating scale used for assessment of behavioral and social outcomes was limited in the present study, though we found behavioral relevance of plasma OXT and AVP. Further studies should aim to conduct more behavioral assessment of ASD.
We have provided evidence that plasma neuropeptide levels are not correlated between ASD children and their mothers. However, the relationships of OXT concentrations between children with ASD and other relatives, including the father and unaffected siblings, remain unexplored. So it is important to extend the correlation analysis to other family members in subsequent work.
The physiological effects of OXT are mediated through its specific receptors (OXTRs), and numerous studies have shown that OXTRs are involved in the regulation of social cognition and behavior [57, 58]. Prior studies have indicated that genetic variability (single-nucleotide polymorphisms or haplotypes) in the OXTR gene is associated with an increased risk of ASD [58–60]. Therefore, in addition to assessing OXT peptide levels, it is interesting to know whether polymorphisms in the OXTR gene are also involved in the pathogenesis of ASD.
In summary, our study showed that children with ASD in China had lower plasma OXT levels, without concomitant changes in plasma AVP concentrations. The plasma levels of these neuropeptides reflected different aspects of autistic behaviors, with OXT possibly correlated with verbal communication and AVP with repetitive use of objects. Though some mothers of ASD patients also exhibited autism spectrum traits, the neuropeptides in mothers did not change in the same direction as those in their ASD children.
Acknowledgements
This work was supported by grants from the Research Special Fund for Public Welfare Industry of Health of China (201302002-11) and the National Natural Science Foundation of China (81271507).
Contributor Information
Rong Zhang, Email: zhangrong@bjmu.edu.cn.
Ji-Sheng Han, Email: hanjisheng@bjmu.edu.cn.
References
- 1.Lukas M, Toth I, Reber SO, Slattery DA, Veenema AH, Neumann ID. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacol. 2011;36:2159–2168. doi: 10.1038/npp.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kent K, Arientyl V, Khachatryan MM, Wood RI. Oxytocin induces a conditioned social preference in female mice. J Neuroendocrinol. 2013;25:803–810. doi: 10.1111/jne.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calcagnoli F, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Chronic enhancement of brain oxytocin levels causes enduring anti-aggressive and pro-social explorative behavioral effects in male rats. Horm Behav. 2014;65:427–433. doi: 10.1016/j.yhbeh.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, et al. Social evolution. Oxytocin-gaze positive loop and the coevolution of human–dog bonds. Science. 2015;348:333–336. doi: 10.1126/science.1261022. [DOI] [PubMed] [Google Scholar]
- 5.Simpson EA, Sclafani V, Paukner A, Hamel AF, Novak MA, Meyer JS, et al. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc Natl Acad Sci U S A. 2014;111:6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 7.Ramos L, Hicks C, Caminer A, McGregor IS. Inhaled vasopressin increases sociability and reduces body temperature and heart rate in rats. Psychoneuroendocrinology. 2014;46:46–51. doi: 10.1016/j.psyneuen.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Cushing BS, Martin JO, Young LJ, Carter CS. The effects of peptides on partner preference formation are predicted by habitat in prairie voles. Horm Behav. 2001;39:48–58. doi: 10.1006/hbeh.2000.1633. [DOI] [PubMed] [Google Scholar]
- 10.Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Schaller F, Watrin F, Sturny R, Massacrier A, Szepetowski P, Muscatelli F. A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum Mol Genet. 2010;19:4895–4905. doi: 10.1093/hmg/ddq424. [DOI] [PubMed] [Google Scholar]
- 12.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, et al. Low stress reactivity and neuroendocrine factors in the BTBR T + tf/J mouse model of autism. Neuroscience. 2010;171:1197–1208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Penagarikano O, Lazaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271–278. doi: 10.1126/scitranslmed.3010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meziane H, Schaller F, Bauer S, Villard C, Matarazzo V, Riet F, et al. An Early Postnatal Oxytocin Treatment Prevents Social and Learning Deficits in Adult Mice Deficient for Magel2, a Gene Involved in Prader–Willi Syndrome and Autism. Biol Psychiatry. 2015;78:85–94. doi: 10.1016/j.biopsych.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Andari E, Duhamel JR, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 18.Alabdali A, Al-Ayadhi L, El-Ansary A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J Neuroinflammation. 2014;11:4. doi: 10.1186/1742-2094-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi: 10.1016/j.physbeh.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Modahl C, Green L, Fein D, Morris M, Waterhouse L, Feinstein C, et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43:270–277. doi: 10.1016/S0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson JD, Ellerbeck KA, Kelly KA, Fleming KK, Jamison TR, Coffey CW, et al. Evidence for alterations in stimulatory G proteins and oxytocin levels in children with autism. Psychoneuroendocrinology. 2014;40:159–169. doi: 10.1016/j.psyneuen.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jansen LM. Gispen-de Wied CC, Wiegant VM, Westenberg HG, Lahuis BE, van Engeland H. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36:891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- 23.Miller M, Bales KL, Taylor SL, Yoon J, Hostetler CM, Carter CS, et al. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: sex differences and associations with symptoms. Autism Res. 2013;6:91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boso M, Emanuele E, Politi P, Pace A, Arra M, Ucelli di Nemi S, et al. Reduced plasma apelin levels in patients with autistic spectrum disorder. Arch Med Res. 2007;38:70–74. doi: 10.1016/j.arcmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Al-Ayadhi LY. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences (Riyadh) 2005;10:47–50. [PubMed] [Google Scholar]
- 26.Wheelwright S, Auyeung B, Allison C, Baron-Cohen S. Defining the broader, medium and narrow autism phenotype among parents using the Autism Spectrum Quotient (AQ) Mol Autism. 2010;1:10. doi: 10.1186/2040-2392-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kose S, Bora E, Erermis S, Ozbaran B, Bildik T, Aydin C. Broader autistic phenotype in parents of children with autism: Autism Spectrum Quotient-Turkish version. Psychiatry Clin Neurosci. 2013;67:20–27. doi: 10.1111/pcn.12005. [DOI] [PubMed] [Google Scholar]
- 28.Schopler E, Reichler RJ, DeVellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS) J Autism Dev Disord. 1980;10:91–103. doi: 10.1007/BF02408436. [DOI] [PubMed] [Google Scholar]
- 29.Rellini E, Tortolani D, Trillo S, Carbone S, Montecchi F. Childhood Autism Rating Scale (CARS) and Autism Behavior Checklist (ABC) correspondence and conflicts with DSM-IV criteria in diagnosis of autism. J Autism Dev Disord. 2004;34:703–708. doi: 10.1007/s10803-004-5290-2. [DOI] [PubMed] [Google Scholar]
- 30.Forsling ML, Montgomery H, Halpin D, Windle RJ, Treacher DF. Daily patterns of secretion of neurohypophysial hormones in man: effect of age. Exp Physiol. 1998;83:409–418. doi: 10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- 31.Szeto A, McCabe PM, Nation DA, Tabak BA, Rossetti MA, McCullough ME, et al. Evaluation of enzyme immunoassay and radioimmunoassay methods for the measurement of plasma oxytocin. Psychosom Med. 2011;73:393–400. doi: 10.1097/PSY.0b013e31821df0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCullough ME, Churchland PS, Mendez AJ. Problems with measuring peripheral oxytocin: can the data on oxytocin and human behavior be trusted? Neurosci Biobehav Rev. 2013;37:1485–1492. doi: 10.1016/j.neubiorev.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- 34.Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, et al. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry. 2012;72:175–181. doi: 10.1016/j.biopsych.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 35.Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaiserman AM. Long-term health consequences of early-life exposure to substance abuse: an epigenetic perspective. J Dev Orig Health Dis. 2013;4:269–279. doi: 10.1017/S2040174413000123. [DOI] [PubMed] [Google Scholar]
- 37.Wismer Fries AB Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proc Natl Acad Sci U S A. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 39.Xu XJ, Shou XJ, Li J, Jia MX, Zhang JS, Guo Y, et al. Mothers of autistic children: lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS One. 2013;8:e74849. doi: 10.1371/journal.pone.0074849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang HF, Li HX, Dai YC, Xu XJ, Han SP, Zhang R, et al. Electro-acupuncture improves the social interaction behavior of rats. Physiol Behav. 2015;151:485–493. doi: 10.1016/j.physbeh.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Xu XJ, Zhang HF, Shou XJ, Li J, Jing WL, Zhou Y, et al. Prenatal hyperandrogenic environment induced autistic-like behavior in rat offspring. Physiol Behav. 2015;138:13–20. doi: 10.1016/j.physbeh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, et al. Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 43.Park EY, Kim J. Factor structure of the Childhood Autism Rating Scale as per DSM-5. Pediatr Int. 2016;58:139–145. doi: 10.1111/ped.12770. [DOI] [PubMed] [Google Scholar]
- 44.Schank JC. Early locomotor and social effects in vasopressin deficient neonatal rats. Behav Brain Res. 2009;197:166–177. doi: 10.1016/j.bbr.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, et al. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Moscovice LR, Ziegler TE. Peripheral oxytocin in female baboons relates to estrous state and maintenance of sexual consortships. Horm Behav. 2012;62:592–597. doi: 10.1016/j.yhbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Challinor SM, Winters SJ, Amico JA. Pattern of oxytocin concentrations in the peripheral blood of healthy women and men: effect of the menstrual cycle and short-term fasting. Endocr Res. 1994;20:117–125. doi: 10.3109/07435809409030403. [DOI] [PubMed] [Google Scholar]
- 48.Rubin LH, Carter CS, Drogos LL, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Effects of sex, menstrual cycle phase, and endogenous hormones on cognition in schizophrenia. Schizophr Res. 2015;166:269–275. doi: 10.1016/j.schres.2015.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubin LH, Carter CS, Drogos L, Pournajafi-Nazarloo H, Sweeney JA, Maki PM. Peripheral oxytocin is associated with reduced symptom severity in schizophrenia. Schizophr Res. 2010;124:13–21. doi: 10.1016/j.schres.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldman R, Golan O, Hirschler-Guttenberg Y, Ostfeld-Etzion S, Zagoory-Sharon O. Parent-child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. Br J Psychiatry. 2014;205:107–112. doi: 10.1192/bjp.bp.113.137513. [DOI] [PubMed] [Google Scholar]
- 51.Altemus M, Fong J, Yang R, Damast S, Luine V, Ferguson D. Changes in cerebrospinal fluid neurochemistry during pregnancy. Biol Psychiatry. 2004;56:386–392. doi: 10.1016/j.biopsych.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Jokinen J, Chatzittofis A, Hellstrom C, Nordstrom P, Uvnas-Moberg K, Asberg M. Low CSF oxytocin reflects high intent in suicide attempters. Psychoneuroendocrinology. 2012;37:482–490. doi: 10.1016/j.psyneuen.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 53.Kagerbauer SM, Martin J, Schuster T, Blobner M, Kochs EF, Landgraf R. Plasma oxytocin and vasopressin do not predict neuropeptide concentrations in human cerebrospinal fluid. J Neuroendocrinol. 2013;25:668–673. doi: 10.1111/jne.12038. [DOI] [PubMed] [Google Scholar]
- 54.Carson DS, Berquist SW, Trujillo TH, Garner JP, Hannah SL, Hyde SA, et al. Cerebrospinal fluid and plasma oxytocin concentrations are positively correlated and negatively predict anxiety in children. Mol Psychiatry. 2015;20:1085–1090. doi: 10.1038/mp.2014.132. [DOI] [PubMed] [Google Scholar]
- 55.Carson DS, Garner JP, Hyde SA, Libove RA, Berquist SW, Hornbeak KB, et al. Arginine Vasopressin Is a Blood-Based Biomarker of Social Functioning in Children with Autism. PLoS One. 2015;10:e0132224. doi: 10.1371/journal.pone.0132224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carson DS, Howerton CL, Garner JP, Hyde SA, Clark CL, Hardan AY, et al. Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides. 2014;61:12–16. doi: 10.1016/j.peptides.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 57.Insel TR, O’Brien DJ, Leckman JF. Oxytocin, vasopressin, and autism: is there a connection? Biol Psychiatry. 1999;45:145–157. doi: 10.1016/S0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 60.Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]