Oscillatory neuronal activity is the fundamental signature of neural networks. In the EEG literature, the oscillations are described as belonging to different frequency bands that are commonly used in clinical monitoring. EEG activity at different frequencies is associated with different states of alertness. Generation of the oscillations depends on the internal states of cell assemblies within the neural network. Oscillatory activity in the theta band (4–12 Hz) is linked to memory processes and higher frequencies in the gamma band (30–80 Hz) are thought to be involved in higher cognitive functions [1]. In addition, oscillations in different frequency bands can couple together to engage in temporal synchronization and network dynamics [2]. Oscillations can coordinate different brain areas to synchronize their activities and such cross-area interactions may contribute to neuronal communication and information processing [3]. In vivo electrophysiological studies have shown that the hippocampus exhibits prominent theta activity and that the theta oscillation could play pivotal roles in spatial representation and synaptic plasticity [4].
Using the completely isolated rat hippocampus in vitro, Wang and colleagues [5] showed that local field potentials and whole-cell recordings in CA1 exhibit a spontaneous low-frequency oscillation around 0.5–1.5 Hz. This oscillation consisted of membrane potential depolarizations and bursting activity. Using paired patch recordings from two neurons simultaneously, the authors further demonstrated that pairs of CA1 principal neurons show synchronized oscillatory activity.
Previous studies have shown that GABAergic neurons contribute to low-frequency oscillations, so the authors then did a series of pharmacological manipulations on GABAA receptor-mediated transmission. First, they blocked GABAergic transmission by applying a GABAA receptor antagonist and found that the hippocampal oscillatory frequency decreased (the frequency of membrane potential depolarization was restored when the antagonist was washed out). In addition, they found that the peak amplitude of the membrane potential depolarization was not changed by the antagonist. Furthermore, neither the threshold for generating spikes nor the synchronization between CA1 principal neurons changed after antagonist application. These data revealed a role of GABAA receptor-mediated synaptic transmission in the generation of low-frequency oscillations.
Next, the authors recorded from the hippocampal fast-spiking interneurons and investigated how the GABAA receptor antagonist affected the CA1 interneuron oscillation. The fast-spiking interneurons exhibited a low-frequency oscillation and, similar to the excitatory CA1 neurons, this oscillation slowed when bicuculline was applied to the bath. Apart from GABAA receptor-mediated GABAergic transmission, GABAB receptors may also play a role in the oscillatory activity, so the authors blocked these receptors but found no effect on the oscillatory frequency of CA1 principal cells. Then they examined how the CA1 oscillation responded to an increase in GABAA receptor activity. In line with the data that blockade of GABAA receptors slows the CA1 oscillation, application of the GABAA agonist midazolam increased the oscillatory frequency in CA1 principal neurons. The amplitude of the membrane potential was not changed by the midazolam.
Using paired recordings from CA1 principal cells and fast-spiking interneurons, the authors were able to determine the relationship between these two cell types and showed that they were synchronized. Further, they measured the timing relationship between the firing in the two types and found that fast-spiking interneurons fired spikes earlier than the principal cells within a single cycle of oscillation. This synchronization indicates that the two cell groups interact with a preferred timing relationship. The authors then examined how excitatory transmission contributed to the CA1 oscillation. When the Schaffer collaterals were stimulated in hippocampal slices, blockade of AMPA receptors with different concentrations of DNQX reduced the EPSP frequency and amplitude accordingly. The CA1 oscillation was blocked by high concentrations of DNQX and slowed by low concentrations. These results provide a role for excitatory synaptic transmission in the CA1 oscillation.
This study points to the phenomenon that the rat CA1 network by itself exhibits a slow oscillation in vitro (Fig. 1). Previously, GABAergic interneurons had been shown to inhibit themselves or other GABAergic neurons to promote disinhibition [6]. This study complements the previous studies by providing interesting findings of a slow oscillation in CA1 principal cells and interneurons mediated by GABAA receptors [7]. This work reveals important aspects of the interneuron circuit for generating slow oscillations and provides insights for understanding GABAergic transmission in hippocampal networks.
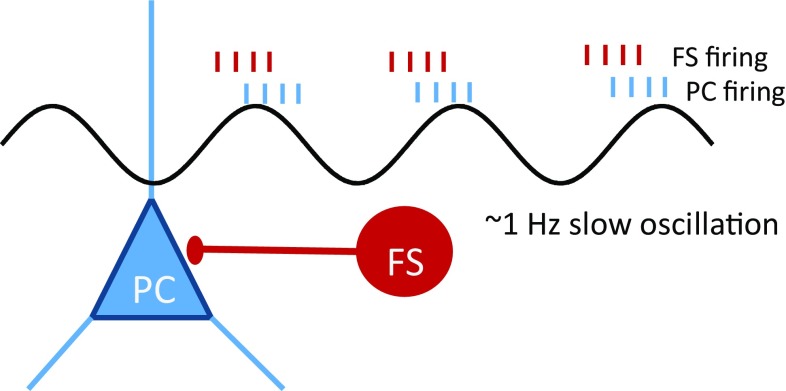
Fig. 1.
Schematic showing the main findings that principal cells (PC) and fast-spiking interneurons (FS) show spontaneous slow oscillations and FS cells fire earlier than PCs.
References
- 1. Buzsaki G. Rhythms of the Brain. Oxford University Press, 2011.
- 2.Wulff P, Ponomarenko AA, Bartos M, Korotkova TM, Fuchs EC, Bahner F, et al. Hippocampal theta rhythm and its coupling with gamma oscillations require fast inhibition onto parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009;106:3561–3566. doi: 10.1073/pnas.0813176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon JA. Oscillations and hippocampal–prefrontal synchrony. Curr Opin Neurobiol. 2011;21:486–491. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 5.Xu Y, Wang L, Liu Y-z, Xue X, Wang Z. GABAergic Interneurons are required for generation of slow CA1 oscillation in rat hippocampus. Neurosci Bull. 2016;32:363–373. doi: 10.1007/s12264-016-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature. 2013;503:521–524. doi: 10.1038/nature12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Perez Velazquez JL, Tian GF, Wu CP, Skinner FK, Carlen PL, et al. Slow oscillations (</=1 Hz) mediated by GABAergic interneuronal networks in rat hippocampus. J Neurosci. 1998;18:9256–9268. doi: 10.1523/JNEUROSCI.18-22-09256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]