Abstract
Given that lysophosphatidic acid (LPA) and the tetrodotoxin-resistant sodium channel Nav1.8 are both involved in bone cancer pain, the present study was designed to investigate whether crosstalk between the LPA receptor LPA1 (also known as EDG2) and Nav1.8 in the dorsal root ganglion (DRG) contributes to the induction of bone cancer pain. We showed that the EDG2 antagonist Ki16198 blocked the mechanical allodynia induced by intrathecal LPA in naïve rats and attenuated mechanical allodynia in a rat model of bone cancer. EDG2 and Nav1.8 expression in L4–6 DRGs was upregulated following intrathecal or hindpaw injection of LPA. EDG2 and Nav1.8 expression in ipsilateral L4–6 DRGs increased with the development of bone cancer. Furthermore, we showed that EDG2 co-localized with Nav1.8 and LPA remarkably enhanced Nav1.8 currents in DRG neurons, and this was blocked by either a protein kinase C (PKC) inhibitor or a PKCε inhibitor. Overall, we demonstrated the modulation of Nav1.8 by LPA in DRG neurons, and that this probably underlies the peripheral mechanism by which bone cancer pain is induced.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0060-7) contains supplementary material, which is available to authorized users.
Keywords: Lysophosphatidic acid, Bone cancer pain, Tetrodotoxin-resistant sodium channel Nav1.8, Dorsal root ganglion
Introduction
Bone cancer pain frequently manifests as severe spontaneous pain and breakthrough pain, which strongly impacts patients’ quality of life and is difficult to control completely [1–3]. Various cancer cells release algogenic substances such as tumor necrosis factor, bradykinin, nerve growth factor (NGF), and formaldehyde that may sensitize primary afferent neurons or destroy peripheral nerve fibers [4–8].
Lysophosphatidic acid (LPA), a potent signaling lipid secreted by activated blood platelets [9, 10], is found at high concentrations in cancer patients’ malignant ascites and blood plasma [11–14], promoting the progression of bone metastases [15]. There are five LPA receptor subtypes, LPA1-5, all of which are G protein-coupled receptors [15–17]. Dorsal root ganglion (DRG) neurons mainly express the LPA1 receptor (also known as EDG2) [18]. Our previous studies showed that LPA is involved in the initiation of bone cancer pain via sensitizing primary afferent C-fibers [19] and potentiates TRPV1 current via a PKC-dependent pathway in the DRG neurons of rats with bone cancer [20].
Nav1.8, a slow-inactivating tetrodotoxin-resistant (TTX-R) voltage-gated sodium channel, is mainly localized in nociceptive small and medium-sized DRG neurons and acts as a key component of the upstroke of the action potential in these neurons [21–27], thus influencing their excitability [22]. Nav1.8 knockdown rats show reduced pain behavior in models of neuropathic pain and inflammatory pain [26, 28–30]. Compelling studies have shown that this channel is involved in the development of bone cancer pain [31] and that the Nav1.8 currents are regulated by many inflammatory factors such as prostaglandin E2, NGF, and serotonin [24, 32–34]. What is more, LPA increases TTX-R currents [35].
Taken together, it is reasonable to assume that Nav1.8 may contribute to LPA mechanism underlying the induction of bone cancer pain. Therefore, we designed the present study to investigate whether there is crosstalk between Nav1.8 and the LPA receptor EDG2 in the development of bone cancer pain.
Materials and Methods
Animals
Female Sprague-Dawley rats (from the Experimental Animal Center, Nanchang University, China) weighing 80–120 g were used in the patch clamp recording experiments and female Sprague-Dawley rats weighing 180–200 g were used in the rest of the experiments. The 180–200 g rats were divided into three groups: LPA + Ki16198 (1 mmol/L, 50 μL LPA and 1 mmol/L, 50 μL Ki16198); LPA + Control (LPA and 1% DMSO in saline), and Control + Control (saline + 1% DMSO). All the rats were housed three per cage and maintained on a 12:12 h light/dark cycle at ~23 °C with free access to water and food. In all experiments, rats were used only once. All animal handling and experimental procedures were reviewed and approved by the Animal Care Committee of Nanchang University and carried out according to the guidelines of the International Association for the Study of Pain. Animal care, use, and treatment were in accordance with the guidelines and regulations. All efforts were made to minimize the number and suffering of the rats.
Establishment of Bone Cancer Model
The abdominal cavity of 80-g rats was injected with Walker 256 rat mammary gland carcinoma cells (Walker 256 rat mammary gland carcinoma cells used in the previous study were the same line with those used in our previous papers, provided by the Department of Integrative Medicine and Neurobiology, School of Medicine, Fudan University) for cancer cell culture. To induce bone tumors, carcinoma cells (107) in 4 μL phosphate-buffered saline (PBS) or 4 μL PBS alone (sham) was injected through the knee joint into the left tibial cavity in Chloral Hydrate anesthetized [300 mg/kg, intraperitoneal (i.p.)] animals.
von-Frey Test for Mechanical Allodynia
Rats were first placed individually into a Plexiglas chamber for 30 min acclimation as described previously [36]. As in our previous studies [19, 20, 37], the hindpaw withdrawal threshold (PWT) was determined by a calibrated series of von Frey hairs (1, 2, 4, 6, 8, 10, 15, and 26 g; Stoelting, Wood Dale, IL), applied in ascending order for 3 s to the center of the plantar surface of the left hindpaw. A positive response was considered only when the hindpaw was completely lifted off the platform. Each force was repeated 5 times at 10-s intervals. The lowest force to induce at least 3 responses out of 5 tests was defined as the PWT.
Western Blotting
The L4–6 DRGs from sham, cancer, and drug-treated rats were rapidly collected after the animals were anesthetized with chloral hydrate (400 mg/kg, i.p.) and homogenized in lysis buffer (12.5 μL/mg tissue) containing protease inhibitor (Roche, Mannheim, Germany) and phenylmethylsulphonyl fluoride (Sigma, St. Louis, MO). The protein concentrations were assessed with BCA assays (Pierce Biotechnology Inc., Rockford, IL). A protein sample (20 μg) was loaded onto each lane, separated using 8% SDS-PAGE, and then transferred to polyvinylidene fluoride membranes. After blocking in 5% nonfat dry milk for 2 h at room temperature (RT), the membranes were incubated overnight at 4°C with rabbit anti-EDG2 primary antibody (1:400, Novus Biologicals Inc., Littleton, CO), rabbit anti-Nav1.8 primary antibody (1:2000, Alomone Labs Ltd, Jerusalem, Israel), or mouse anti-tubulin primary antibody (1:5000, Sigma) and then incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (1:5000, Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at RT. Finally, signals were detected with enhanced chemiluminescence (Pierce Biotechnology Inc., Rockford, IL) and visualized with the ChemiDoc XRs system (Bio-Rad Laboratories Inc., Richmond, CA). The tubulin level was used as loading control, and EDG2 or Nav1.8 expression was normalized against the tubulin level. EDG2/tubulin or Nav1.8/tubulin in the DRGs from the treatment group was normalized against those in the control group. All Western blot analysis was repeated at least 3 times.
Immunohistochemistry
After an overdose of urethane (2 g/kg, i.p.), animals were perfused intracardially with normal saline followed by 4% paraformaldehyde in 0.1 mol/L PBS (pH 7.4, 4 °C). The DRGs of the L4–6 segments were removed, post-fixed in the same fixative (4 h, 4 °C), and then immersed in a 10%–30% gradient of sucrose in PBS for cryoprotection (24–48 h, 4 °C). DRG sections at 7 μm (to detect Nav1.8 and EDG2 co-localization) were cut on a cryostat (Leica 1900, Leica, Wetzlar, Hesse, Germany) and processed for immunofluorescence. After blocking with 10% donkey serum in 0.01 mol/L PBS (pH 7.4) with 0.3% Triton X-100 for 1 h at RT, two adjacent sections were each incubated overnight at 4 °C with rabbit anti-Nav1.8 (1:2000, Alomone) and rabbit anti-EDG2 (1:50, Novus Biologicals) primary antibodies in PBS with 1% normal donkey serum and 0.3% Triton X-100. Following three 15-min rinses in 0.01 mol/L PBS, the sections were incubated with Alex Fluor 546- and Alex Fluor 488-conjugated secondary antibodies for 2 h at 4 °C, respectively, and then washed in PBS. After coverslipping with 50% glycerin in 0.01 mol/L PBS, the sections were observed under a confocal laser scanning microscope (FV1000, Olympus, Tokyo, Japan). Images were captured with FV10-ASW software. Omission of primary antibody served as a negative control.
Preparation of DRG Neurons
L4–6 DRG neurons were acutely dissociated from 80–100 g rats as described previously [38–40]. Anesthetized with ether, the rats were rapidly decapitated. The DRGs were removed and incubated in Dulbecco’s modified Eagle’s medium (Gibco Life Technologies, Grand Island, NY) saturated with a CO2/O2 mixture, containing 2.67 mg/mL collagenase (type IA, Sigma, St. Louis, MO) and 1 mg/mL trypsin (type I, Sigma), for 35 min at 37 °C. After enzyme treatment, the DRGs were washed with standard external solution (in mmol/L, 150 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, and 10 glucose, pH 7.4) and gently triturated with a fine fired-polished Pasteur pipette to dissociate single cells. Neurons were plated onto glass coverslips in culture dishes, and then incubated in standard external solution for recording at RT. All experiments were carried out within 2–8 h after plating and each coverslip was used only once.
Patch-Clamp Recordings
Whole-cell voltage-clamp recordings were made at RT (23 ± 1°C) using an Axonpatch 200B amplifier (Molecular Devices, LLC Sunnyvale, CA). Only small (< 25 μm) DRG neurons with resting membrane potentials more negative than −50 mV were selected for study. Microelectrodes (N51A borosilicate glass, Sutter Instruments Co., Novato, CA) were pulled on a P97 puller (Sutter Instruments). Microelectrodes with resistances of 2–6 MΩ were selected and filled with (in mmol/L): 140 CsF, 1 MgCl2, 2.5 Na2ATP, 1 EGTA, and 10 HEPES, adjusted to pH 7.2 with CsOH. The data were sampled at 10 kHz and low-pass filtered at 2 kHz. The external solution contained (in mmol/L): 32 NaCl, 1 MgCl2, 20 TEA-Cl, 105 choline-Cl, 1 CaCl2, 0.1 CdCl2, 10 HEPES, 0.0005 TTX, and 10 glucose; pH was adjusted to 7.4 with NaOH. Nav1.8 currents were evoked by 50-ms depolarizing pulses in DRG neurons held at −60 mV. The peak Nav1.8 currents were determined by a voltage-clamp protocol of depolarizing steps from −55 mV to +40 mV (50 ms, at 5-mV increments).
Reagents
All reagents for patch-clamp recording and intrathecal (i.t.) and subcutaneous injections were from Sigma, except that the EDG2 inhibitor Ki16198 was from Selleck (Selleck Chemicals, Houston, TX) and the PKCε inhibitor εV1–2 was from Biomol (Plymouth Meeting, PA). All reagents were dissolved in saline (at least 1000-fold the working concentration) as stock solutions stored at −20 °C and the working concentrations were prepared on the day of the experiment. The reagent concentrations used were based on previous studies. LPA was continuously applied near the neurons for 1 min using an ALA-VM8 perfusion system (ALA Scientific Instruments, Westbury, NY). Ki16198 or the PKC inhibitor bisindolylmaleimide (BIM) was added to the chamber 30 min before and during the perfusion of LPA at a concentration based on a previous study [41]. εV1–2 was delivered intracellularly via the recording electrode.
Data Analysis
Student’s t-test was used to analyze all of the data. The criterion of significance was set at P <0.05 and all data are presented as mean ± SEM.
Results
LPA-Induced Pain Behavior and Upregulation of EDG2 and Nav1.8 in DRGs of Normal Rats
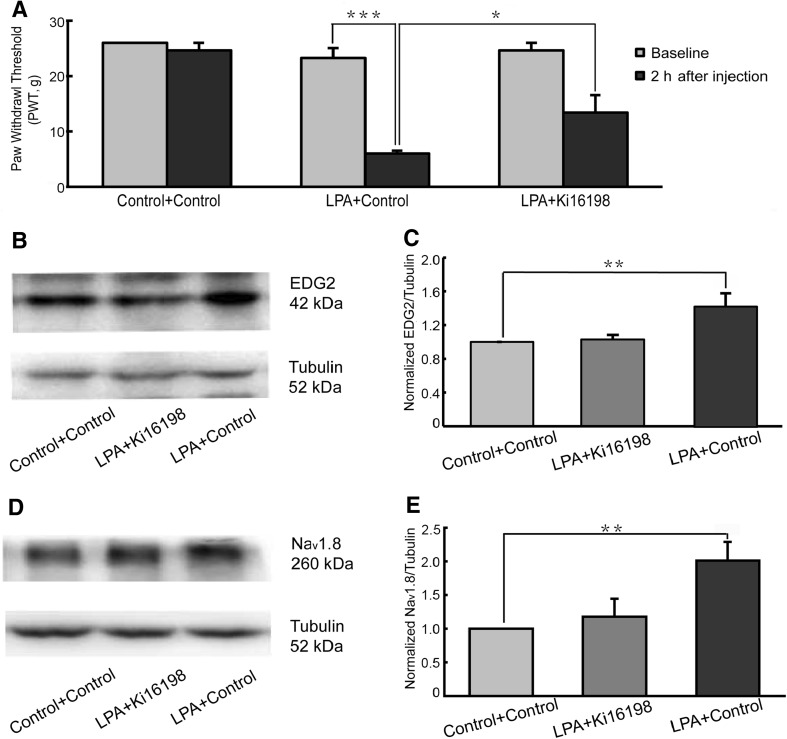
Two hours after i.t. administration, mechanical allodynia was tested using von Frey filaments. The results showed that PWTs in the Control + Control group did not differ before and after injection. In the LPA + Control group, compared with baseline, the PWT was significantly decreased 2 h after injection. Further, the LPA + Control group showed a lower PWT than the LPA + Ki16198 group (Fig. 1A).
Fig. 1.
Pain behavior and EDG2 and Nav1.8 expression after intrathecal injection of LPA. A LPA decreased PWTs (n = 8), and this was attenuated by the EDG2 antagonist Ki16198. B, C LPA up-regulated EDG2 expression in L4–6 DRGs (n = 6). D, E Nav1.8 expression on L4–6 DRGs increased after LPA injection (n = 6). *P <0.05, **P <0.01, ***P <0.001.
Meanwhile, EDG2 and Nav1.8 expression in L4–6 DRGs was examined 2 h after i.t. administration. Compared with the Control + Control group, EDG2 expression was up-regulated in the LPA + Control group, and this was blocked by the EDG2 antagonist Ki16198 (Fig. 1B, C). The level of EDG2 was similar in the LPA + Ki16198 and Control + Control groups. Similarly, Nav1.8 expression was significantly higher in the LPA + Control group than in the Control + Control group. Also, the upregulation of Nav1.8 expression was blocked by Ki16198; the LPA + Ki16198 group had an Nav1.8 level similar to that in the Control + Control group (Fig. 1D, E).
To investigate its direct action on peripheral afferent fibers, LPA was subcutaneously injected into the hindpaw and 2 h later, EDG2 and Nav1.8 expression was assessed in the ipsilateral L4–6 DRGs. EDG2 expression was significantly higher in the LPA + Control group than in the Control + Control group, and this was blocked by Ki16198. There was no statistical difference in the EDG2 level between the Control + Control group and the LPA + Ki16198 group (Supplemental Fig. 1A, B). Similar results were obtained for Nav1.8 expression. Nav1.8 expression was higher in the LPA + Control group than in the Control + Control group and its expression in the Control + Control group was similar to that in the LPA + Ki16198 group, indicating that LPA-induced upregulation of Nav1.8 expression was blocked by Ki16198 (Supplemental Fig. 1C, D).
Bone Cancer-Induced Upregulation of EDG2 and Nav1.8 in DRGs
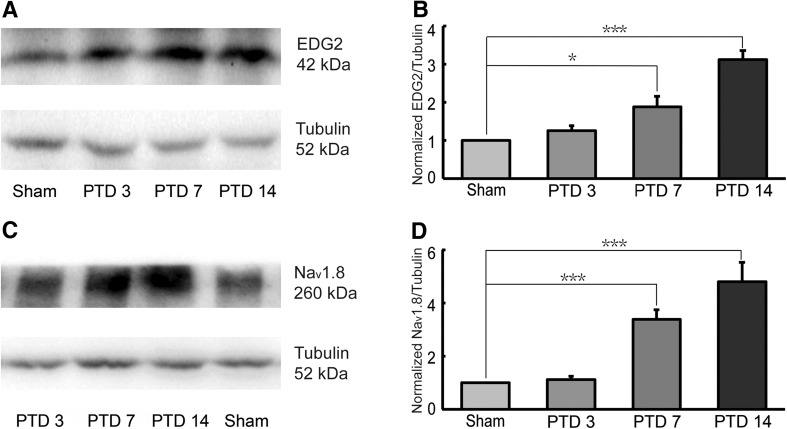
The levels of EDG2 and Nav1.8 expression were examined in the ipsilateral DRGs at the L4–6 spinal segments on post-tumor days (PTDs) 3, 7, and 14. Western-blotting results showed that expression of EDG2 was higher in rats with bone cancer than in sham rats on PTDs 7 and 14, but not on PTD 3 (Fig. 2A, B). Similarly, Nav1.8 was significantly upregulated on PTDs 7 and 14, but not on PTD 3, compared with sham rats (Fig. 2C, D).
Fig. 2.
Time-courses of EDG2 and Nav1.8 expression on ipsilateral L4–6 DRGs after cancer cell inoculation. EDG2 (A, B, n = 6) and Nav1.8 (C, D, n = 6) expression were both increased in PTD 7 and PTD 14 rats. *P <0.05, ***P <0.001.
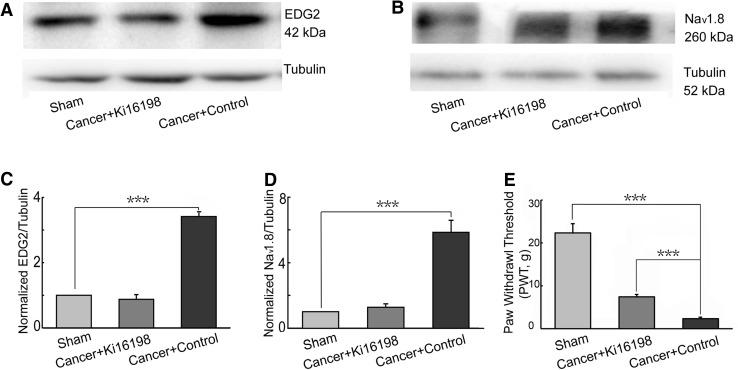
The EDG2 antagonist Ki16198 (1 mmol/L, 50 μL) or 50 μL of 1% DMSO (control) was injected i.t. the day before cancer cell implantation, as well as on PTDs 2, 4, 7, 10, 13, and 16. The sham rats received saline injections. On PTD 16, Nav1.8 and EDG2 expression were assessed in the ipsilateral L4–6 DRGs and pain behavior was assessed by ipsilateral PWTs with von Frey filaments. Compared with the sham group, EDG2 expression was strongly up-regulated in cancer rats that received control injections. In the Ki16198 group (Cancer + Ki16198), the upregulation of EDG2 was blocked (Fig. 3A, C). Similarly, Nav1.8 expression was increased in the Cancer + Control group, while it did not change significantly in the Cancer + Ki16198 group, compared to the sham group (Fig. 3B, D). Meanwhile, PWTs in the Cancer + Control and Sham groups were 2.35 ± 0.33 g and 22.33 ± 2.31 g, respectively (Fig. 3E), indicating a reduction of PWTs by bone cancer. However, this reduction was reversed by Ki16198 injection in the Cancer + Ki16198 group. These results suggested that Ki16198 significantly attenuated the upregulation of EDG2 and Nav1.8 expression and mechanical allodynia in rats with bone cancer.
Fig. 3.
Effects of Ki16198 on EDG2 and Nav1.8 upregulation and pain behavior of rats with bone cancer. A, C Ki16198 blocked the up-regulation of EDG2 expression on ipsilateral L4–6 DRGs of rats with cancer (n = 6). B, D Increased Nav1.8 expression on ipsilateral L4–6 DRGs of rats with cancer was also blocked by Ki16198 (n = 6). E Ki16198 attenuated mechanical allodynia in rats with cancer (n = 6). ***P <0.001.
Co-localization of Nav1.8 with EDG2 and Potentiation of Nav1.8 Currents by LPA in DRG Neurons
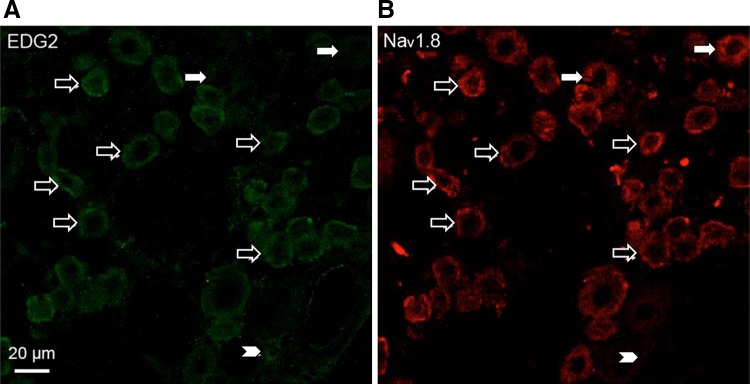
To explore the roles of EDG2 and Nav1.8 in the development of bone cancer pain, their co-localization and interaction were investigated. According to our previous study [27], immunofluorescence staining of two adjacent sections (7 μm) from an L4 DRG with Nav1.8 antibody and EDG2 antibody reveals their co-localization in the same neurons. With this method, we found that Nav1.8 and EDG2 were widely co-localized in DRG neurons (Fig. 4).
Fig. 4.
Co-localization of Nav1.8 and EDG2 in DRG neurons. A, B Double immunofluorescence staining showing Nav1.8 and EDG2 co-localized in DRG neurons (open arrows). Neurons expressing EDG2 but not Nav1.8 and Nav1.8-positive but EDG2-negative neurons are indicated by arrowheads and filled arrows, respectively.
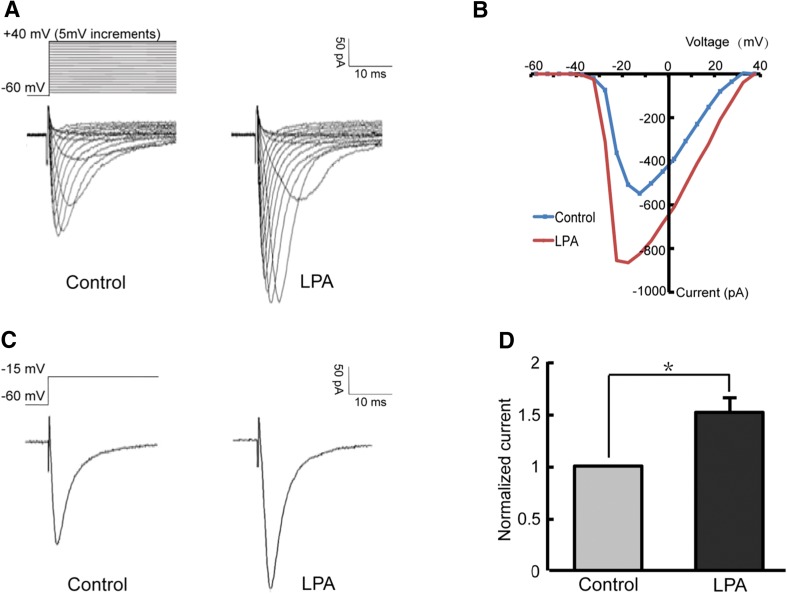
Given their co-localization, we explored whether LPA modulates the Nav1.8 channel. Whole-cell patch-clamp recordings were performed on isolated small-diameter (< 25 μm) DRG neurons in which the membrane potential was held at −60 mV to inhibit Nav1.9 currents and leave Nav1.8 intact [27]. A voltage-clamp protocol (depolarizing steps from −55 mV to +40 mV, 50 ms, 5 mV increments) was used to generate Nav1.8 currents (Fig. 5A). According to the current–voltage curve (Fig. 5B), the peak amplitude of Nav1.8 currents was elicited at −15 mV in most recordings. Three minutes after DRG neurons were perfused with 10 μmol/L LPA for 1 min, the Nav1.8 currents were potentiated by 51 ± 0.14% in the neurons recorded (Fig. 5C, D).
Fig. 5.
Potentiation of Nav1.8 currents by LPA. A Representative Nav1.8 currents recorded before (left) and after (right) LPA perfusion. B I-V curves of Nav1.8 currents. C, D Peak amplitude of Nav1.8 currents increased after LPA perfusion (n = 11). *P <0.05.
As a G-protein coupled receptor, EDG2 interacts with the Gi, Gq, and G12 families, activating their downstream mitogen-activated protein kinase (MAPK), PKC, and Rho-Rho kinase pathways, while inhibiting the protein kinase A (PKA) pathway [15]. Previous studies have suggested that the PKC-dependent pathways, especially the PKCε pathway, is involved not only in Nav1.8 channel modulation [34, 40, 42, 43], but also in the potentiation of the TRPV1 channel by LPA [20].
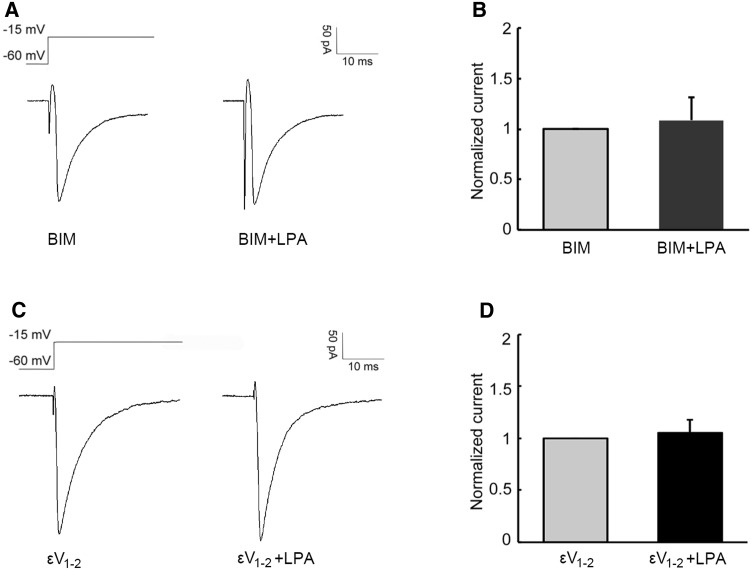
In the present study, the PKC inhibitor BIM and the PKCε inhibitor εV1–2 were used to explore the downstream molecules that contribute to potentiation of the Nav1.8 current by LPA. Among the 7 neurons tested, BIM (1 μmol/L, 30 min) incubation blocked the LPA-induced potentiation of Nav1.8 currents (Fig. 6A, B,). When εV1–2 (200 μmol/L) was delivered via the recording electrode 5 min before recording, LPA failed to increase the Nav1.8 currents (Fig. 6C, D) in all 8 of the neurons recorded.
Fig. 6.
PKC, especially PKCε, is involved in the LPA-induced potentiation of Nav1.8 currents. A, B After BIM incubation, LPA failed to increase the amplitude of Nav1.8 currents (P > 0.05). C, D With εV1–2 delivery, the amplitude of Nav1.8 currents remained unchanged after LPA perfusion (P > 0.05).
Discussion
LPA is secreted by activated platelets, as well as tumors and their surrounding tissues [15, 44, 45], mediating a wide range of effects such as the proliferation, migration, and survival of cancer cells [12, 46, 47]. Our previous study demonstrated that LPA is involved in the induction of bone cancer pain by interacting with TRPV1, an important pain-related factor widely expressed in small DRG neurons [20]. Nav1.8, another key pain signaling molecule in primary afferent neurons, is a TTX-R sodium channel primarily localized in nociceptors [21, 23]. In the present study, we showed that LPA facilitates the Nav1.8 channel in DRG neurons, providing a new peripheral LPA mechanism underlying the induction of bone cancer pain.
The present results showed that i.t. injection of LPA induced upregulation of both the LPA1 receptor EDG2 and Nav1.8 expression in the L4–6 DRGs of normal rats, and this was completely blocked by the EDG2 antagonist Ki16198. Functionally, i.t. injection of LPA decreased the PWT, and this was partially, but not completely, blocked by Ki16198, suggesting that LPA potentiates the excitability of the DRG neurons that innervate the hindpaw. This may be related to the upregulation of Nav1.8 in L4–6 DRGs, while other channels such as TRPV1 may also be involved in this effect. As EDG2 is also expressed in the spinal cord [15, 18], LPA injected i.t. may act directly on DRG neurons and/or via a spinal mechanism to up-regulate both EDG2 and Nav1.8 expression in L4–6 DRGs and induce allodynia.
It has been documented that bone innervation in the hind-limbs is predominantly from thinly-myelinated A-δ and unmyelinated C-fibers, originating from medium and small DRG nociceptor neurons, respectively [48]. Algogenic substances released by carcinoma cells and tumor stroma have been suggested to sensitize or directly activate peripheral nociceptive sensory neurons [49]. Our previous study revealed that LPA sensitizes sural C-fibers that innervate the hindpaw [19]. In the present work, we found that after intraplantar injection of LPA, the EDG2 and Nav1.8 levels were greatly increased, and this was blocked by Ki16198. These results suggested that LPA may amplify the C fiber-mediated nociceptive information, with the excitability of small-sized DRG neurons be enhanced and the EDG2 and Nav1.8 levels increased, thus sensitizing nociceptive DRG neurons and contributing to pain.
In addition to up-regulating the Nav1.8 level in DRGs, LPA also potentiated the Nav1.8 currents in isolated DRG neurons. This result for the first time identified the co-localization of EDG2 and Nav1.8 in DRG neurons, providing a basis for their interaction. In patch-clamp recordings, LPA greatly increased the Nav1.8 currents in small DRG neurons, reflecting that more Nav1.8 channels were opened after LPA perfusion, resulting in potentiation of the excitability of DRG neurons.
In rats with bone cancer, the up-regulated EDG2 and Nav1.8 expression had a similar time-course in ipsilateral DRGs. EDG2 and Nav1.8 expression was assessed at three time points, 3, 7, and 14 days after cancer cell inoculation, and they were significantly increased at 7 and 14 days. In particular, i.t. injection of Ki16198 blocked the upregulation of EDG2 and Nav1.8 expression in rats with bone cancer, further demonstrating that the up-regulation of EDG2 and Nav1.8 was associated with LPA. It has been reported that Nav1.8 influences the excitability of small and medium nociceptive DRG neurons [22], and an increase in Nav1.8 expression contributes to mechanical allodynia [26, 28, 30, 31, 50]. It is plausible that as cancer develops, LPA released by carcinoma cells and the tumor stroma increases the excitability of peripheral C-fibers, as well as the EDG2 and Nav1.8 expression, resulting in the enhancement of Nav1.8 currents, further increasing the excitability of DRG neurons and thus causing mechanical allodynia. Correspondingly, our results showed that cancer-induced bone pain was attenuated by i.t. injection of Ki16198, indicating that blocking the LPA1 receptor EDG2 partially prevents the sensitization of DRG neurons by LPA. However, as the cancer-induced bone pain was not totally blocked by i.t. Ki16198, other targets such as TRPV1 may also be involved in the effect of LPA. Taken together, it is conceivable that excessive LPA in rats with cancer activates unmyelinated peripheral sensory nerve fibers and sensitizes DRG neurons via increasing the activity and expression level of Nav1.8 channels.
The LPA1 receptor EDG2 is a G-protein coupled receptor [15], which is able to interact with three G protein families, Gi, Gq, and G12, generating their downstream propagation through the MAPK, PKC, and Rho-Rho kinase pathways, while inhibiting the PKA pathway [15], further triggering a wide range of signaling molecules underlying modulation of the excitability of DRG neurons. Compelling evidence has shown that the Nav1.8 channel is modulated by a PKC-dependent pathway [34, 42, 43], and PKC activation enhances TTX-R currents in DRG neurons[33]. Our previous results demonstrated that the PKC signal pathway is involved in the interaction between LPA and TRPV1 in the induction of bone cancer pain [20]. Also, neurokinin-1, a G-protein coupled receptor, potentiates Nav1.8 currents via the PKCε pathway [39, 40]. Therefore, it is reasonable to assume that the PKC signal pathway participates in the LPA-induced sensitization of the Nav1.8 channel and up-regulation of Nav1.8 expression in rats with bone cancer. Our supplemental experiments showed that both the PKC inhibitor BIM and the PKCε inhibitor εV1–2 blocked the potentiation of Nav1.8 currents by LPA. In addition, given that cancer pain is a complicated symptom with inflammatory and neuropathic components [48] and LPA is important for the initiation of neuropathic pain via the Rho-Rho kinase pathway [18, 51], it is probable that LPA is involved in bone cancer pain by modulating several effectors through different intracellular signal pathways.
Taken together, LPA is involved in bone cancer pain via facilitating the TTX-R sodium channel Nav1.8 in nociceptive primary sensory neurons, acting directly on DRG neurons and/or via a spinal mechanism, which probably constitutes a peripheral mechanism by which bone cancer pain develops.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Zhi-Qi Zhao for his helpful reading and revision of the manuscript. This work was supported by the National Natural Science Foundation of China (81200854), the Natural Science Foundation of Jiangxi Province, China (20122BAB215027), the Science Foundation of the Educational Committee of Jiangxi Province, China (GJJ12064), and the International Postdoctoral Exchange Fellowship Program, China (20150062).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0060-7) contains supplementary material, which is available to authorized users.
References
- 1.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/S0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 2.Mantyh P. Bone cancer pain: causes, consequences, and therapeutic opportunities. Pain. 2013;154(Suppl 1):S54–62. doi: 10.1016/j.pain.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 3.Fazzari J, Lin HX, Murphy C, Ungard R, Singh G. Inhibitors of glutamate release from breast cancer cells; new targets for cancer-induced bone-pain. Sci Rep. 2015;5:8380. doi: 10.1038/srep08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/S0306-4522(00)00110-X. [DOI] [PubMed] [Google Scholar]
- 5.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 6.Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- 7.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han Y, Li Y, Xiao X, Liu J, Meng XL, Liu FY, et al. Formaldehyde up-regulates TRPV1 through MAPK and PI3K signaling pathways in a rat model of bone cancer pain. Neurosci Bull. 2012;28:165–172. doi: 10.1007/s12264-012-1211-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002;277:48737–48744. doi: 10.1074/jbc.M206812200. [DOI] [PubMed] [Google Scholar]
- 10.Kostic I, Fidalgo-Carvalho I, Aday S, Vazao H, Carvalheiro T, Graos M, et al. Lysophosphatidic acid enhances survival of human CD34(+) cells in ischemic conditions. Sci Rep. 2015;5:16406. doi: 10.1038/srep16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast-cancer cells. Biochem J. 1995;309:933–940. doi: 10.1042/bj3090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang XJ, Schummer M, Mao ML, Yu SX, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biolphys Acta. 2002;1582:257–264. doi: 10.1016/S1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 13.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, et al. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–1725. doi: 10.1172/JCI200422123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spiegel S, Milstien S. Critical role of acylglycerol kinase in epidermal growth factor-induced mitogenesis of prostate cancer cells. Biochem Soc Trans. 2005;33:1362–1365. doi: 10.1042/BST0331362. [DOI] [PubMed] [Google Scholar]
- 15.Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. doi: 10.1016/j.semcdb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi K, Ishii S, Shimizu T. Identification of p2y(9)/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 17.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G(12/13)- and G(q)-coupled lysophosphatidic acid receptor that increases cAMP, LPA(5) J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 18.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- 19.Zhao J, Pan HL, Li TT, Zhang YQ, Wei JY, Zhao ZQ. The sensitization of peripheral C-fibers to lysophosphatidic acid in bone cancer pain. Life Sci. 2010;87:120–125. doi: 10.1016/j.lfs.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Pan HL, Zhang YQ, Zhao ZQ. Involvement of lysophosphatidic acid in bone cancer pain by potentiation of TRPV1 via PKC epsilon pathway in dorsal root ganglion neurons. Mol Pain. 2010;6:85. doi: 10.1186/1744-8069-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 22.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- 23.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550:739–752. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, et al. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain. 2006;7:S1–29. doi: 10.1016/j.jpain.2006.01.444. [DOI] [PubMed] [Google Scholar]
- 25.Medvedeva YV, Kim MS, Schnizler K, Usachev YM. Functional tetrodotoxin-resistant Na(+) channels are expressed presynaptically in rat dorsal root ganglia neurons. Neuroscience. 2009;159:559–569. doi: 10.1016/j.neuroscience.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annu Rev Neurosci. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 27.Gu XY, Liu BL, Zang KK, Yang L, Xu H, Pan HL, et al. Dexmedetomidine inhibits Tetrodotoxin-resistant Na(v)1.8 sodium channel activity through G(i/o)-dependent pathway in rat dorsal root ganglion neurons. Mol. Brain. 2015;8:15. doi: 10.1186/s13041-015-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, et al. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, Na(v)1.8. Pain. 2002;95:143–152. doi: 10.1016/S0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 29.Ekberg J, Jayamanne A, Vaughan CW, Aslan S, Thomas L, Mouldt J, et al. mu O-conotoxin MrVIB selectively blocks Na(v)1.8 sensory neuron specific sodium channels and chronic pain behavior without motor deficits. Proc Natl Acad Sci USA 2006, 103: 17030–17035. [DOI] [PMC free article] [PubMed]
- 30.Dong XW, Goregoaker S, Engler H, Zhou X, Mark L, Crona J, et al. Small interfering RNA-mediated selective knockdown of Na(v)1.8 tetrodotoxin-resistent sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience. 2007;146:812–821. doi: 10.1016/j.neuroscience.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Liu XD, Yang JJ, Fang D, Cai J, Wan Y, Xing GG. Functional upregulation of nav1.8 sodium channels on the membrane of dorsal root ganglia neurons contributes to the development of cancer-induced bone pain. Plos One. 2014;9:e114623. doi: 10.1371/journal.pone.0114623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495:429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda M, Yoshida S, Kadoi J, Nakano Y, Mastumoto S. The effect of PKC activity on the TTX-R sodium currents from rat nodose ganglion neurons. Life Sci. 2005;78:47–53. doi: 10.1016/j.lfs.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 35.Lee WS, Hong MP, Kim TH, Shin YK, Lee CS, Park M, et al. Effects of lysophosphatidic acid on sodium currents in rat dorsal root ganglion neurons. Brain research. 2005;1035:100–104. doi: 10.1016/j.brainres.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 36.Pitcher GM, Ritchie J, Henry JL. Paw withdrawal threshold in the von Frey hair test is influenced by the surface on which the rat stands. J Neurosci Meth. 1999;87:185–193. doi: 10.1016/S0165-0270(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Cao H, Han M, Li TT, Pan HL, Zhao ZQ, et al. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129:64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Li GD, Zhao ZQ. State-dependent phosphorylation of epsilon-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Cang CL, Kawasaki Y, Liang LL, Zhang YQ, Ji RR, et al. Neurokinin-1 receptor enhances TRPV1 activity in primary sensory neurons via PKC epsilon: A novel pathway for heat hyperalgesia. J Neurosci. 2007;27:12067–12077. doi: 10.1523/JNEUROSCI.0496-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cang CL, Zhang H, Zhang YQ, Zhao ZQ. PKCepsilon-dependent potentiation of TTX-resistant Nav1.8 current by neurokinin-1 receptor activation in rat dorsal root ganglion neurons. Mol Pain. 2009;5:33. doi: 10.1186/1744-8069-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komachi M, Sato K, Tobo M, Mogi C, Yamada T, Ohta H, et al. Orally active lysophosphatidic acid receptor antagonist attenuates pancreatic cancer invasion and metastasis in vivo. Cancer Sci. 2012;103:1099–1104. doi: 10.1111/j.1349-7006.2012.02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thio CL, Sontheimer H. Differential modulation of Ttx-sensitive and Ttx-resistant Na+ channels in spinal-cord astrocytes following activation of protein-kinase-C. J Neurosci. 1993;13:4889–4897. doi: 10.1523/JNEUROSCI.13-11-04889.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matsumoto S, Yoshida S, Ikeda M, Tanimoto T, Saiki C, Takeda M, et al. Effect of 8-bromo-cAMP on the tetrodotoxin-resistant sodium (Nav 1.8) current in small-diameter nodose ganglion neurons. Neuropharmacology. 2007;52:904–924. doi: 10.1016/j.neuropharm.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 44.Fukushima N. LPA in neural cell development. J Cell Biochem. 2004;92:993–1003. doi: 10.1002/jcb.20093. [DOI] [PubMed] [Google Scholar]
- 45.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Bioch P 2008, 160: 25–46. [DOI] [PubMed]
- 46.Radeff-Huang J, Seasholtz TM, Matteo RG, Brown JH. G protein mediated signaling pathways in lysophospholipid induced cell proliferation and survival. J Cell Biochem. 2004;92:949–966. doi: 10.1002/jcb.20094. [DOI] [PubMed] [Google Scholar]
- 47.Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, et al. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- 48.Mantyh PW. Bone cancer pain: from mechanism to therapy. Curr Opin Support Pa. 2014;8:83–90. doi: 10.1097/SPC.0000000000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjorkman R, Ullman A, Hedner J. Morphine-sparing effect of diclofenac in cancer pain. Eur J Clin Pharmacol. 1993;44:1–5. doi: 10.1007/BF00315271. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Guan SM, Sun W, Fu H. Melittin, the major pain-producing substance of bee venom. Neurosci Bull. 2016;32:265–272. doi: 10.1007/s12264-016-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008;152:296–298. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.