Abstract
Melittin is a basic 26-amino-acid polypeptide that constitutes 40–60% of dry honeybee (Apis mellifera) venom. Although much is known about its strong surface activity on lipid membranes, less is known about its pain-producing effects in the nervous system. In this review, we provide lines of accumulating evidence to support the hypothesis that melittin is the major pain-producing substance of bee venom. At the psychophysical and behavioral levels, subcutaneous injection of melittin causes tonic pain sensation and pain-related behaviors in both humans and animals. At the cellular level, melittin activates primary nociceptor cells through direct and indirect effects. On one hand, melittin can selectively open thermal nociceptor transient receptor potential vanilloid receptor channels via phospholipase A2-lipoxygenase/cyclooxygenase metabolites, leading to depolarization of primary nociceptor cells. On the other hand, algogens and inflammatory/pro-inflammatory mediators released from the tissue matrix by melittin’s pore-forming effects can activate primary nociceptor cells through both ligand-gated receptor channels and the G-protein-coupled receptor-mediated opening of transient receptor potential canonical channels. Moreover, subcutaneous melittin up-regulates Nav1.8 and Nav1.9 subunits, resulting in the enhancement of tetrodotoxin-resistant Na+ currents and the generation of long-term action potential firing. These nociceptive responses in the periphery finally activate and sensitize the spinal dorsal horn pain-signaling neurons, resulting in spontaneous nociceptive paw flinches and pain hypersensitivity to thermal and mechanical stimuli. Taken together, it is concluded that melittin is the major pain-producing substance of bee venom, by which peripheral persistent pain and hyperalgesia (or allodynia), primary nociceptive neuronal sensitization, and CNS synaptic plasticity (or metaplasticity) can be readily induced and the molecular and cellular mechanisms underlying naturally-occurring venomous biotoxins can be experimentally unraveled.
Keywords: Melittin, Algogen, Nociceptor, Spinal dorsal horn, Pain
Background
Melittin is a basic 26-amino-acid polypeptide [molecular formula, C131H229N39O31; molecular weight, 2846.46266 g/mol; and International Chemical Identifier Key, VDXZNPDIRNWWCW-UHFFFAOYSA-N (https://pubchem.ncbi.nlm.nih.gov/compound/16133648)]. It was originally separated and purified from honeybee (Apis mellifera) venom and sequenced as Gly-Ile-Gly-Ala-Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro-Ala-Leu-Ile-Ser-Trp-Ile-Lys-Arg-Lys-Arg-Gln-GlnNH2 [1]. It is the major active ingredient of honeybee venom, constituting 40–60% of whole dry venom. Melittin has various biological, pharmacological, and toxicological actions, including strong surface activity on cell lipid membranes, hemolyzing activity, antibacterial and antifungal activities, and potential anti-tumor properties [1–4]. Melittin is also known as a natural pore-forming peptide that can insert itself across the phospholipid bilayer, and interactions between biomembrane and proteins can be studied using this biological activity [5–15]. Melittin has also been used as an activator of phospholipase A2 (PLA2) after the discovery of its enhancing effects on bee venom PLA2 activity [16–20].
However, so far little is known about the pain-producing effects of melittin and its actions on the nervous system, although it is well known that a honey-bee sting and bee venom injection cause pain and inflammation [3, 4]. Nevertheless, in recent years, accumulating evidence strongly suggests that melittin is a unique and functionally identified pain-producing substance in bee venom and affects neuronal plasticity in the somatosensory system [4, 21–23]. In this review, strong lines of supporting evidence from behavioral, electrophysiological, pharmacological, and neurochemical studies in mammals are discussed. The molecular and cellular mechanisms underlying melittin-induced pain, primary nociceptor sensitization, and synaptic plasticity in the central nervous system are also considered.
Pain-inducing Effects of Melittin
Experimental Evidence from Human Studies
A pain-producing substance, also called an algogen, is defined as a chemical with the ability to induce pain and hyperalgesia through the activation of primary nociceptor cells by opening ion channels [24–26]. A nociceptor (shortened from “noci-receptor”) is currently defined by the International Association for the Study of Pain as a receptor channel preferentially sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged (see Glossary in [26]). Endogenous algogens include protons (H+), adenosine triphosphate (ATP), 5-hydroxytryptamine (5-HT), histamine, K+, and bradykinin, while exogenous algogens are probably countless in number or type, but those that have been experimentally well-established are few, including capsaicin, paraformaldehyde, and bee venom [24–26]. There are also many endogenous pain-enhancing substances such as prostaglandin E2 (PGE2) and nerve growth factor that are not able to open the ion channels of nociceptors but can enhance their efficacy through modulation (see Glossary in [26]). To address whether melittin has a pain-producing effect, experimental studies have been performed on healthy human participants by intradermal (i.d.) injection of a small amount of melittin [27, 28]. A 0–10 visual analog scale in which scores of 0, 5, and 10 indicate ‘‘no pain’’, ‘‘moderate pain’’, and ‘‘intolerable pain’’ was used to evaluate the pain intensity perceived by the participants. After i.d. injection of melittin (5 μg in 50 μL saline) into the volar side of one forearm, a sharp pain sensation (score >8.0) was reported immediately in all seven participants (2 women and 5 men). The perceived pain was sustained and lasted 3 min after injection. Spatially on the skin, a flare surrounding the radial area of the injection site was detectable and this disappeared within 2 h. A local inflammatory response represented by an increase in skin temperature was also visualized by computer-assisted infrared thermography, which showed that a melittin-induced increase in skin temperature occurred slowly and peaked at 10 min. In another two experiments, the painful effects of two higher doses of melittin (10 μg and 50 μg in 50 μL saline) were also evaluated on healthy human participants [29, 30]. Although the time-course of pain intensity was similar to that in the lower-dose study, the intensity and duration of the perceived pain was greatly enhanced at the higher doses, demonstrating dose- and time-dependent pain induced by melittin. Moreover, i.d. injection of melittin produced heat and mechanical hyperalgesia in the zone of primary injury but only heat hyperalgesia was detected in an area surrounding the injection site [29, 30]. No anaphylactic and systemic reactions occurred in the volunteers receiving i.d. injection of melittin, and no itch sensation was induced by such treatment. As a single bee-sting contains ~140 μg of dried bee venom which is equivalent to 56–84 μg of melittin [31, 32], the dose used above was less than the amount of melittin naturally released from one bee sting.
Although some of the active substances such as H+, ATP, 5-HT, and histamine in bee venom have been demonstrated to be pain-producing substances by application of cantharidin in the human blister test [33–42], the time-course of spontaneous pain is transient and much shorter than that induced by melittin. Furthermore, no clear area of hyperalgesia and/or allodynia was identified in and surrounding the application site as seen in the melittin test. Together, these results strongly suggest that melittin is a unique pain-producing substance derived from bee venom.
Experimental Evidence from Animal Studies
Behavioral Assays
To avoid possible anaphylactic and systemic reactions in human participants, the painful and inflammatory effects of bee venom and the derived active substances, including melittin, have been examined in behaving animals. Painful behaviors can be rated quantitatively in animals by counting the number of spontaneous paw flinches or by measuring the time spent in licking, lifting, or biting the injected paw following subcutaneous (s.c.) injection of pain-inducing substances such as formalin, capsaicin, bee venom, and melittin [3, 4]. Moreover, pain hypersensitivity (hyperalgesia and allodynia) identified in human volunteers or patients can also be studied in animals by measuring changes in paw-withdrawal thermal latency and paw-withdrawal mechanical threshold with a radiant heat stimulator and von Frey filaments, respectively, applied to the injection site or the surrounding area [3, 4].
Before describing the nociceptive (surrogate of painful sensation) effects of melittin, we first introduce the behavioral responses of animals to s.c. bee venom injection. In rats, the behavioral responses to such an injection include both persistent spontaneous nociception and pain hypersensitivity. Similar to the above descriptions in human experiments, rats respond immediately to s.c. injection of bee venom into one hindpaw by displaying persistent spontaneous paw flinches and behaviors of licking, lifting, and biting the injected paw [43–46]. The bee venom-induced spontaneous nociception reaches a peak immediately after injection and lasts for at least 1 h when 200 μg bee venom (in 50 μL saline) is injected [43, 44]. Furthermore, such injections also result in primary heat and mechanical hyperalgesia as well as secondary and mirror-image heat hyperalgesia that lasts for 72–96 h [44–46]. Meanwhile, dramatic local inflammatory responses such as paw edema and plasma extravasation are induced by s.c. bee venom injection [4, 43]. However, similar to i.d. melittin injection in human volunteers, the peak of the bee venom-induced local inflammatory response occurs 10–20 min later than the spontaneous nociception [4, 43]. From the painful effects of melittin in humans and the nociceptive effects of bee venom in animals, it is easily deduced that melittin and other peptides might be unique in the production of long-term pain sensation.
Bee venom is complex, with more than ten peptide constituents [3, 4]. To evaluate the nociceptive and inflammatory effects of these peptides, the crude venom of Georgian gray honey bees (A. mellifera; kindly provided by Floret Ltd. and its partner company New Techniques Laboratory Ltd., Tbilisi, Georgia) was separated and purified by gel chromatography and reverse-phase high-pressure liquid chromatography [22]. Four active polypeptides (melittin, apamin, mast-cell degranulating peptide, and a novel PLA2-related peptide) were successfully isolated, purified, and finally identified by both matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and amino-acid sequence analysis. Then inflammation- and pain-related behaviors were evaluated and quantified in rats receiving s.c. injection of each peptide. All four polypeptides resulted in dramatic local inflammatory responses (edema) 1 h after injection. However, they had different nociceptive and hyperalgesic effects. Although melittin, mast-cell degranulating peptide, and PLA2-related peptide induced distinct nociceptive paw flinches following s.c. injection (100 μg in 50 μL saline), only the melittin-induced spontaneous nociception lasted for nearly 1 h. Furthermore, although both melittin and apamin resulted in heat and mechanical hypersensitivity at the primary injury site, only the melittin-induced primary mechanical hypersensitivity lasted >48 h. Accordingly, melittin is likely to be the major polypeptide responsible for the prolonged painful stimulation by bee venom injection, while the other polypeptides contribute only to the early nociceptive responses within 10–20 min after injection [4, 43]. The melittin-induced nociceptive responses can be partially inhibited by both pre- and post-treatment with capsazepine (CPZ), a potent antagonist of the thermal nociceptor transient receptor potential vanilloid receptor (TRPV1), implying involvement of this molecular target in the melittin-induced nociception [22]. CPZ also reverses the melittin-induced primary heat hypersensitivity, but has no effect on the melittin-induced primary mechanical hypersensitivity [22]. Based on the unique nociceptive effects of melittin and exclusion of the other compounds [47, 48], melittin is proposed to play a central role in the production of the long-lasting pain, hyperalgesia, and local inflammation following s.c. injection of bee venom [4, 43].
Electrophysiological Recordings
A unique property of algogens is that they are able to activate primary nociceptors in the periphery, and then result in activation and sensitization of the nociceptive (pain-related) neurons in the CNS [24, 26]. Thus, both in vitro and in vivo electrophysiological recording techniques have been used to assess the effects of melittin on the nociceptive neurons in the dorsal root ganglia (DRG) and the dorsal horn of the spinal cord.
To understand how primary nociceptors respond to melittin, its direct effects have been examined using in vitro whole-cell patch-clamp recording from primary sensory neurons acutely dissociated from the rat DRG [23]. Topical application of melittin resulted in a distinct rise in the intracellular calcium concentration ([Ca2+]i) in 69% of the small-to-medium (20–35 μm in diameter) DRG cells. In current-clamp mode, following topical application of melittin, 55% of the recorded DRG neurons depolarized, and this was followed by tonic firing for 70–1200 s. Most of the melittin-responsive DRG cells were nociceptors because the action potentials (APs) exhibited electrophysiological characteristics of typical primary nociceptor cells: (1) a long AP duration and a prolonged after-hyperpolarization; (2) an inflection on the falling phase, which is generally considered characteristic of nociceptors. They were also sensitive to capsaicin or IB4-positive for immunofluorescent labeling. In in vivo recordings from single fibers, melittin activates physiologically-identified primary mechanical nociceptors and mechano-heat nociceptors when injected into the cutaneous receptive field of a single fiber [49]. This report strongly supports the in vitro studies. Together, these results show that melittin can activate and sensitize primary nociceptors.
In extracellular single-unit recording, injection of melittin into the cutaneous receptive field of spinal wide-dynamic-range (WDR) neurons, a class of pain-signaling neurons, results in a dose-dependent increase in AP firing in anesthetized rats [21]. This melittin-induced AP firing is completely blocked by local injection of CPZ, suggesting that melittin can activate the spinal dorsal horn pain-signaling neurons through direct actions on the thermal nociceptor TRPV1 at the peripheral injection site. Local injection of melittin also results in hyper-responsiveness of spinal WDR neurons to both thermal and mechanical stimuli, leading to a leftward shift of the stimulus-response curve relative to controls. This suggests that s.c. melittin can cause a functionally-sensitized state in spinal pain-signaling neurons. The melittin-induced activation and sensitization of spinal dorsal horn WDR neurons is quite similar to that induced by s.c. bee venom injection in terms of both intensity and time-course [21, 50, 51]. The melittin-induced long-lasting activation of spinal WDR neurons can be suppressed by inhibition of the extracellular signal-regulated kinases (ERKs) at both the spinal and peripheral levels [52–54].
Molecular Targets of Melittin on Primary Nociceptor Cells
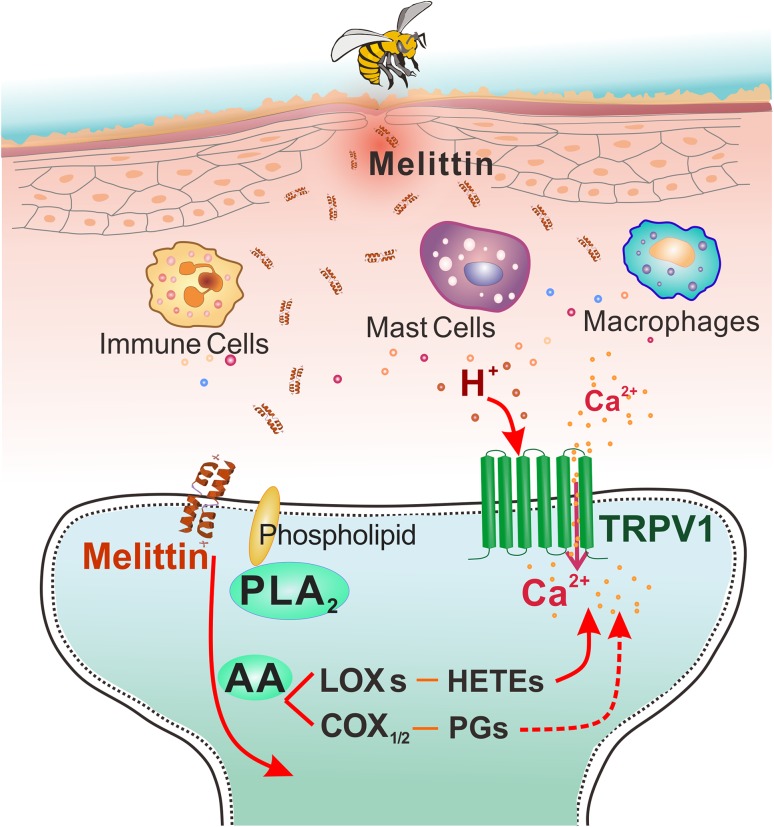
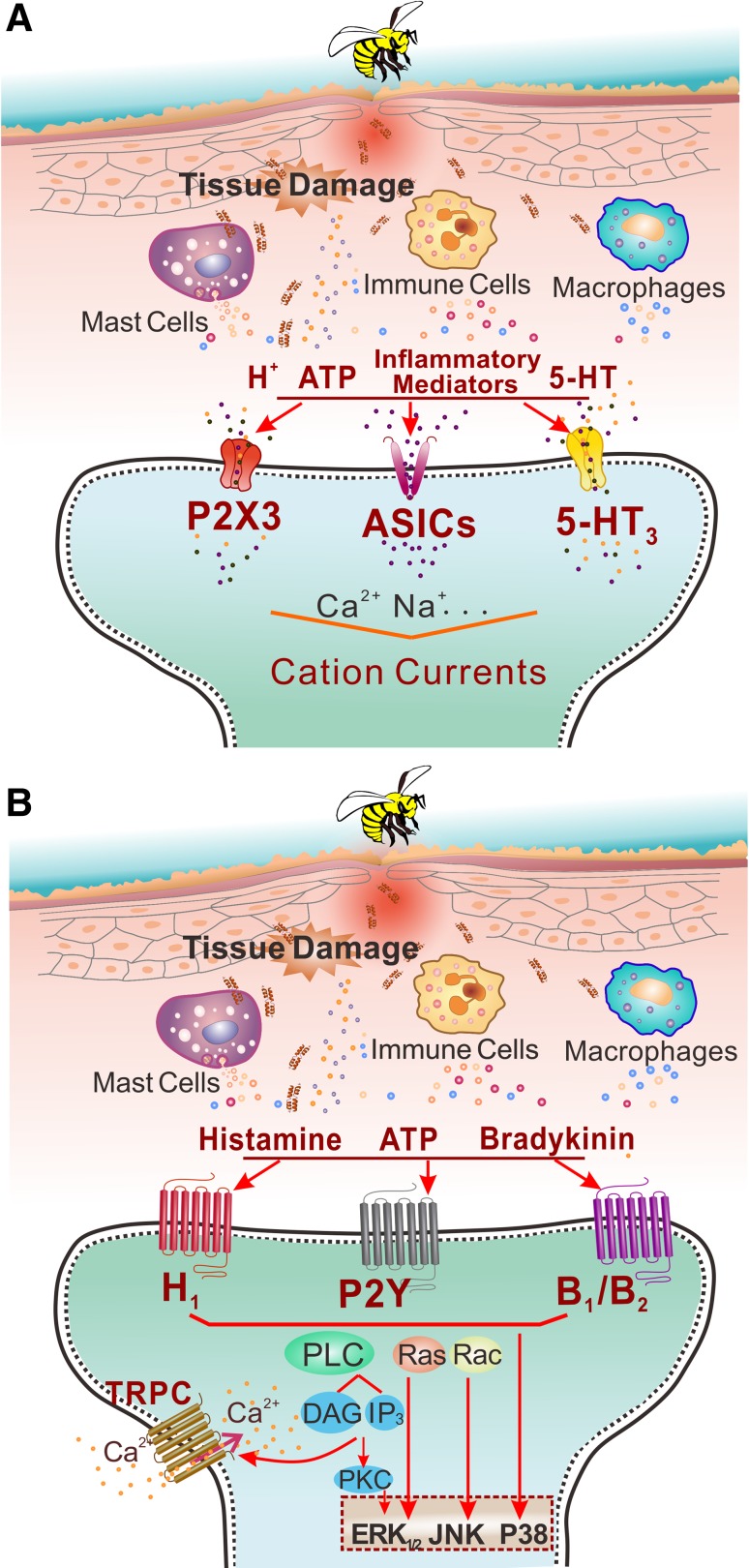
To unravel the molecular and cellular mechanisms of melittin-induced pain (nociception), molecular targets have been searched for pharmacologically using either whole-cell recordings from DRG cells or behavioral tests. First, an experimental cellular model was designed to evoke inward currents in the DRG cells by topical application of melittin (2–10 μmol/L for 200–300 s, 20-s intervals) and then blockers or inhibitors of the candidate target molecules were applied together with melittin to reveal which are involved in the genesis of melittin-evoked inward currents [23]. For instance, voltage-clamp recordings at a holding potential of −70 mV have shown that melittin can evoke large inward currents in ~50% of DRG neurons in a concentration-dependent manner [23]. In addition, repeated application of the lowest concentration of melittin results in an increased amplitude of the inward currents, suggesting that melittin induces sensitization in the primary nociceptors [23]. On the other hand, molecular targets can also be studied pharmacologically using animal behavioral tests in which pre- or post-administration of a blocker or inhibitor of the candidate molecular target can be performed. In general, two sets of mechanisms are likely to be involved in the melittin-induced pain and hypersensitivity [3, 4]: (1) direct action on the primary nociceptors (Fig. 1), and (2) indirect action on the primary nociceptors through other algogens, pro-inflammatory and inflammatory mediators released by mast cell-degranulation, tissue damage, and immune responses (Fig. 2). As a consequence, melittin activates cation channel-carrying nociceptor cells leading to pain sensation. It can also enhance the activity of nociceptor cells by regulating or modifying various ligand-gated and G-protein-coupled receptors (GPCRs) via intracellular cascades, leading to pain hypersensitivity (hyperalgesia and/or allodynia).
Fig. 1.
Direct actions of melittin on primary nociceptor cells. Following subcutaneous injection of a low concentration of melittin, phospholipase A2 (PLA2) is activated and catalyzes the production of arachidonic acid (AA) from phospholipids. AA is further catalyzed by lipoxygenases (LOXs) to produce hydroperoxyeicosatetraenoic acids (HETEs) that serve as endogenous ligands to open TRPV1 receptor channels, leading to Ca2+ influx and membrane depolarization. Meanwhile, AA is also catalyzed by cyclooxygenases (COXs) to produce PGs that enhance the activity of TRPV1, leading to sensitization of the primary nociceptors.
Fig. 2.
Indirect actions of melittin on primary nociceptor cells. A Following subcutaneous injection of a high concentration of melittin, the tissue matrix and mast cells are damaged by its pore-forming effect, resulting in the release of algogens such as H+, ATP, and 5-HT. These pain-inducing substances open the ligand-gated receptor channels such as the purinergic P2X3 receptor, acid-sensing ion channels, and 5-HT3, leading to cation influx. B On the other hand, histamine, bradykinin, and ATP released by the pore-forming effect on the tissue matrix also activate GPCRs such as H1, P2Y, and B1/B2 that result in the phosphorylation of PLC. PLC cleaves phosphatidylinositol 4,5-bisphosphate into diacyl glycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG is an endogenous activator of TRPC. When TRPC is activated by DAG, a subpopulation of primary nociceptor cells that do not express TRPV1 are depolarized. Mitogen-activated protein kinases, including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 MAPK are also involved in the process of melittin-induced pain and hypersensitivity, possibly through activation of PLC as well.
Direct Actions on Primary Nociceptor Cells: Activation and Sensitization of TRPV1 Receptors via PLA2 Cascade Pathways
It has been demonstrated that melittin activates TRPV1 receptors via PLA2 cascade pathways. TRPV1, a non-selective cation channel with high permeability to Ca2+, belongs to a class of structurally- and functionally-identified molecular sensors mediating thermal and chemical nociception [24, 26]. In the cellular model, the melittin-induced inward currents and [Ca2+]i rise are completely blocked by co-application of CPZ, a selective TRPV1 antagonist, in a reversible manner, suggesting the existence of a selective action of melittin on the TRPV1 cation channels of primary nociceptive neurons [23]. Moreover, because the average response latency of melittin-sensitive DRG cells is long-term delayed up to 124 ± 57 s, the inward current is likely to be mediated by slow intracellular cascades rather than single fast transmembrane effect. Further experiments have shown that: (1) inhibitors of PLA2, but not phospholipase C (PLC), suppress the melittin-induced inward currents; (2) inhibitors of cyclooxygenases (COXs) and lipoxygenases (LOXs), two key components of the arachidonic acid (AA) metabolism pathway, each partially suppress the inward current evoked by melittin; and (3) inhibitors of protein kinase A, but not of PKC, also abolish the melittin-induced inward currents. Because melittin is a powerful PLA2 activator and releases AA from plasma membrane phospholipids [16–20], it is likely that topical application of melittin can cause the release of COX/LOX metabolites such as PGs and hydroperoxyeicosatetraenoic acids (HETEs) by activating the PLA2-COX/LOX pathways. As reported previously, HETEs, as LOXs products, are endogenous ligands of TRPV1 receptors [24]. Together, these results indicate that melittin can excite primary nociceptive neurons at least in part by activating TRPV1 receptors via PLA2-LOXs-HETEs, leading to pain, or sensitize primary nociceptive neurons in part by enhancing TRPV1 receptors via PLA2-COX-PGs, leading to pain hypersensitivity (Fig. 1) [4, 23].
Indirect Actions on Primary Nociceptor Cells: Activation of Ligand-Gated Receptor Channels via Tissue-Derived Algogens
Based on the pore-forming actions of melittin, it is possible that pain-producing substances such as H+, ATP, and 5-HT are released by mast-cell degranulation, tissue damage, and immune responses (Fig. 2A). It is well known that protons directly activate TRPV1 and acid-sensitive ion channels (Figs 1 and 2A), while ATP and 5-HT activate ATP P2X3 receptors and 5-HT3 receptors, respectively (Fig. 2A), leading to opening of these ion channels and an influx of cations (Na+ and Ca2+) and depolarization of free nerve-endings in and surrounding the injection site. One important line of evidence has shown that, in the melittin-induced inflammatory pain state, post-treatment of the primary injury site with s.c. injection of A-317491, a potent P2X3/P2X2/3 receptor antagonist, significantly suppresses the development of melittin-evoked persistent nociceptive paw flinches and primary mechanical and thermal pain hypersensitivity [55].
Indirect Actions on Primary Nociceptor Cells: Activation of TRPC Receptors via GPCR-Mediated Pathways
Similar to TRPV1, the TRPC family also contains non-selective cation channels that are highly permeable to Ca2+ when activated [24]. It is known that TRPC1, TRPC3, and TRPC6 are the major subunits localized in rat DRG cells [56]. Unlike TRPV1 channels that use HETEs as endogenous activators, TRPC3/6/7 channels use DAG as the endogenous activator. It is well known that PLC cleaves phosphatidylinositol 4,5-bisphosphate into DAG and IP3 that depends upon the activation of GPCRs. As ATP, histamine, 5-HT, bradykinin, and even glutamate can be released by mast-cell degranulation and the tissue damage caused by melittin’s pore-forming effect, they can serve as endogenous ligands of GPCRs in the free nerve-endings of primary nociceptor cells. As a line of supporting evidence, treatment of the primary injury site with Reactive Blue 2, an antagonist of ATP P2Y receptors, significantly attenuates nociceptive paw flinches as well as primary thermal and mechanical hypersensitivity, implying the involvement of ATP GPCRs in the melittin-produced pain processes [55]. Also because PLC can be activated by mitogen-activated protein kinases (MAPKs), the involvement of ERKs, p38 MAPK, and JNKs in the induction and maintenance of melittin-induced persistent nociception and pain hypersensitivity can serve as another line of indirect evidence for the production of DAG [57]. Finally, blocking TRPC with SKF-96365, a potent antagonist of TRPC3/6/7, inhibits the melittin-induced inward current and the [Ca2+]i rise [56] as well as melittin-induced pain-related behaviors [58]. Because inhibition of PLC fails to suppress the melittin-evoked inward current in CPZ-sensitive DRG cells [23], it is possible that the SKF-96365-sensitive DRG cells belong to another subpopulation of nociceptor cells containing IB4, but not TRPV1 [56]. Based on these data, it is proposed that melittin indirectly excites primary nociceptive neurons in part by opening TRPC receptor channels by DAG, which is produced through activation of the GPCR-mediated PLC signaling pathway.
Up-regulation of Nav1.8 and Nav1.9 Subunits Contributes to Melittin-Induced Enhancement of Sodium Currents in DRG Cells and Pain-Related Behaviors
In a previous study [see Supplemental Fig. 1 of reference 23], melittin was shown to induce tonic AP firing in isolated DRG neurons even in the presence of 500 nmol/L TTX. Moreover, electrical stimulus-evoked AP firing in small-to-medium-sized DRG neurons was resistant to TTX. These data strongly suggest that the TTX-resistant Na+ channels in primary sensory neurons contribute to the melittin-induced effects. To label the DRG cells, the retrograde tracer 1, 10-dioctadecyl-3, 3, 30, 30-tetramethylindocarbocyanine perchlorate (DiI) was injected at the melittin injection site two weeks in advance to allow full labeling of the cell bodies of the DRG cells innervating the glabrous skin of the rat hind paw [59]. Then, TTX-sensitive and TTX-resistant voltage-gated Na+ currents were recorded in the DiI-labeled DRG neurons. Following s.c. injection of melittin, significant increases in the Na+ current densities mediated by Nav1.8 and Nav1.9, but not the TTX-sensitive current, were recorded [59].
Moreover, real-time PCR showed that the relative mRNA levels of both Nav1.8 and Nav1.9 were significantly increased by s.c. injection of melittin, so were their protein levels as demonstrated by Western blot. Furthermore, the number of Nav1.8- and Nav1.9-positive neurons also increased in small-to-medium-sized DRG neurons, but not in large NF-200 positive neurons, suggesting that the up-regulation of Nav1.8 and Nav1.9 subunits in primary nociceptor cells contributes to the melittin-induced enhancement of Na+ currents [59]. However, antisense-mediated knockdown of Nav1.9, but not Nav1.8, in the DRG resulted in the inhibition of melittin-induced pain-related behaviors [59]. In contrast, knockdown of Nav1.8, but not Nav1.9, relieved the inflammatory pain induced by complete Freund’s adjuvant [60]. Patch-clamp recordings from DRG cells dissociated from rats 2 h after s.c. melittin injection revealed that the melittin-responsive cells were the tonic type, but not the phasic type, in terms of electrophysiological characteristics [61]. This suggests that the tonic subpopulation of DRG cells is likely to be the key primary nociceptor cellular type in the production and conduction of AP firing induced by melittin and is involved in the driving of melittin-induced spontaneous pain and pain hypersensitivity.
Summary
In both humans and rats, melittin has been demonstrated to be the unique pain-producing substance of honeybee venom. It activates primary nociceptor cells directly and indirectly due to its ability to activate plasma membrane PLA2 and its pore-forming activity. The melittin-triggered PLA2-LOX/COX down-stream metabolite pathways are involved in activating or enhancing TRPV1 receptor activity via HETEs or PGs, leading to the depolarization of primary nociceptor cells. Meanwhile, the algogens and inflammatory/pro-inflammatory mediators released from the tissue matrix due to the membrane pore-forming effect of melittin activate primary nociceptor cells through both ligand-gated receptor channels and the GPCR-mediated opening of TRPC channels. The activation of tonic-type primary nociceptor cells may be maintained by up-regulation of the Nav1.8 and Nav1.9 subunits of voltage-gated Na+ channels that facilitate the generation of long-term AP firing, causing plastic changes in spinal WDR neurons, and subsequently resulting in the occurrence of spontaneous nociceptive paw flinches and pain hypersensitivity to thermal and mechanical stimuli.
Acknowledgments
This review was supported by grants from the National Basic Research Development Program of China (2013CB835100), the National Natural Science Foundation of China (81171049, 31300919, and 31400948), the National Key Technology R&D Program, China (2013BAI04B04), and the Twelfth Five-Year Project of China (AWS12J004).
References
- 1.Habermann E. Bee and wasp venoms. Science. 1972;177:314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 2.Son DJ, Lee JW, Lee YH, Song HS, Lee CK, Hong JT. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol Ther. 2007;115:246–270. doi: 10.1016/j.pharmthera.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Lariviere WR. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: A double-edged sword. Prog Neurobiol. 2010;92:151–183. doi: 10.1016/j.pneurobio.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Guan SM. Bee venom and pain. In Toxinology: Toxins and Drug Discovery. Edited by Gopalakrishnakone P. New York: Springer; in press.
- 5.Williams JC, Bell RM. Membrane matrix disruption by melittin. Biochim Biophys Acta. 1972;288:255–262. doi: 10.1016/0005-2736(72)90246-5. [DOI] [PubMed] [Google Scholar]
- 6.Tosteson MT, Tosteson DC. The sting. Melittin forms channels in lipid bilayers. Biophys J. 1981;36:109–116. doi: 10.1016/S0006-3495(81)84719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosteson MT, Alvarez O, Hubbell W, Bieganski RM, Attenbach C, Caporales LH, Levy JJ, Nutt RF, Rosenblatt M, Tosteson DC. Primary structure of peptides and ion channels. Role of amino acid side chains in voltage gating of melittin channels. Biophys J. 1990;58:1367–1375. doi: 10.1016/S0006-3495(90)82483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarz G, Zong RT, Popescu T. Kinetics of melittin induced pore formation in the membrane of lipid vesicles. Biochim Biophys Acta. 1992;1110:97–104. doi: 10.1016/0005-2736(92)90299-2. [DOI] [PubMed] [Google Scholar]
- 9.Fattal E, Nir S, Parente RA, Jr, Szoka FC. Pore-forming peptides induce rapid phospholipid flip-flop in membranes. Biochemistry-US. 1994;33:6721–6731. doi: 10.1021/bi00187a044. [DOI] [PubMed] [Google Scholar]
- 10.Smith R, Separovic F, Milne TJ, Whittaker A, Bennett FM, Cornell BA, Makriyannis A. Structure and orientation of the pore-forming peptide, melittin, in lipid bilayers. J Mol Biol. 1994;241:456–466. doi: 10.1006/jmbi.1994.1520. [DOI] [PubMed] [Google Scholar]
- 11.Bechinger B. Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 12.Chen LY, Cheng CW, Lin JJ, Chen WY. Exploring the effect of cholesterol in lipid bilayer membrane on the melittin penetration mechanism. Anal Biochem. 2007;367:49–55. doi: 10.1016/j.ab.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Wang J, Kristalyn CB, Chen Z. Real-time structural investigation of a lipid bilayer during its interaction with melittin using sum frequency generation vibrational spectroscopy. Biophys J. 2007;93:866–875. doi: 10.1529/biophysj.106.099739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzaki K, Yoneyama S, Miyajima K. Pore formation and translocation of melittin. Biophys J. 1997;73:831–838. doi: 10.1016/S0006-3495(97)78115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klocek G, Schulthess T, Shai Y, Seelig J. Thermodynamics of melittin binding to lipid bilayers: Aggregation and pore formation. Biochemistry. 2009;48:2586–2596. doi: 10.1021/bi802127h. [DOI] [PubMed] [Google Scholar]
- 16.Mollay C, Kreil G. Enhancement of bee venom phospholipase A2 activity by melittin, direct lytic factor from cobra venom and polymyxin B. Febs Lett. 1974;46:141–144. doi: 10.1016/0014-5793(74)80354-6. [DOI] [PubMed] [Google Scholar]
- 17.Hassid A, Levine L. Stimulation of phospholipase activity and prostaglandin biosynthesis by melittin in cell culture and in vivo. Res Commun Chem Pathol Pharmacol. 1977;18:507–517. [PubMed] [Google Scholar]
- 18.Nishiya T. Interaction of melittin and phospholipase A2 with a zobenzene containing phospholipid. J Biochem. 1991;109:383–388. doi: 10.1093/oxfordjournals.jbchem.a123390. [DOI] [PubMed] [Google Scholar]
- 19.Vernon LP, Bell JD. Membrane structure, toxins and phospholipase A2 activity. Pharmacol Ther. 1992;54:269–295. doi: 10.1016/0163-7258(92)90003-I. [DOI] [PubMed] [Google Scholar]
- 20.Sharma SV. Melittin-induced hyperactivation of phospholipase A2 activity and calcium influx in ras-transformed cells. Oncogene. 1993;8:939–947. [PubMed] [Google Scholar]
- 21.Li KC, Chen J. Altered pain-related behaviors and spinal neuronal responses produced by s.c. injection of melittin in rats. Neuroscience. 2004;126:753–762. doi: 10.1016/j.neuroscience.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 22.Chen YN, Li KC, Li Z, Zhang ZW, Ji YH, Gao GD, Chen J. Effects of bee venom peptidergic components on rat behaviors related to pain and inflammation. Neuroscience. 2006;138:631–640. doi: 10.1016/j.neuroscience.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 23.Du YR, Xiao Y, Lu ZM, Ding J, Xie F, Fu H, Wang Y, Strong JA, Zhang JM, Chen J. Melittin activates TRPV1 receptors in primary nociceptive sensory neurons via the phospholipase A2 cascade pathways. Biochem Biophys Res Commun. 2011;408:32–37. doi: 10.1016/j.bbrc.2011.03.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon SB, Koltzenburg M, Tracey I, Turk DC. Wall and Melzack’s Textbook of Pain. Philadelphia: Elsevier; 2006. [Google Scholar]
- 25.Schmidt RF, Willis WD. Encyclopedia of Pain. Berlin: Springer; 2007. [Google Scholar]
- 26.Chen J, Han JS, Zhao ZQ, Wei F, Hsieh JC, Bao L, Chen ACN, Dai Y, Fan BF, Gu JG, Hao SL, Hu SJ, Ji YH, Li YJ, Li YQ, Lin Q, Liu XG, Liu YQ, Lu Y, Luo F, Ma C, Qui YH, Rao ZR, Shi L, Shyu BC, Song XJ, Tang JS, Tao YX, Wan Y, Wang JS, et al. Pain. In Neuroscience in the 21stCentury: From Basic to Clinical. Edited by Pfaff DW. New York; Springer; 2013: 965–1023.
- 27.Koyama N, Hirata K, Hori K, Dan K, Yokota T. Computer-assisted infrared thermographic study of axon reflex induced by intradermal melittin. Pain. 2000;84:133–139. doi: 10.1016/S0304-3959(99)00192-X. [DOI] [PubMed] [Google Scholar]
- 28.Koyama N, Hirata K, Hori K, Dan K, Yokota T. Biphasic vasomotor reflex responses of the hand skin following intradermal injection of melittin into the forearm skin. Eur J Pain. 2002;6:447–453. doi: 10.1016/S1090-3801(02)00029-0. [DOI] [PubMed] [Google Scholar]
- 29.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. A comparison of hyperalgesia and neurogenic inflammation induced by melittin and capsaicin in humans. Neurosci Lett. 2003;337:147–150. doi: 10.1016/S0304-3940(02)01325-3. [DOI] [PubMed] [Google Scholar]
- 30.Sumikura H, Andersen OK, Drewes AM, Arendt-Nielsen L. Secondary heat hyperalgesia induced by melittin in humans. Eur J Pain. 2006;10:121–125. doi: 10.1016/j.ejpain.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher MJ, Schmidt JO, Egen NB, Dillon KA. Biochemical variability of venoms from individual European and Africanized honeybees (Apis mellifera) J Allergy Clin Immunol. 1992;90:59–65. doi: 10.1016/S0091-6749(06)80011-4. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher MJ, Tveten MS, Egen NB. Rate and quantity of delivery of venom from honeybee stings. J Allergy Clin Immunol. 1994;93:831–835. doi: 10.1016/0091-6749(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 33.Armstrong D, Dry RM, Keele CA, Markham JW. Method for studying chemical excitants of cutaneous pain in man. J Physiol. 1951;115:59–60. doi: 10.1113/jphysiol.1951.sp004686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armstrong D, Dry RM, Keele CA, Markham JW. Pain-producing substances in blister fluid and in serum. J Physiol. 1952;117:4p–5p. [PubMed] [Google Scholar]
- 35.Armstrong D, Dry RM, Keele CA, Markham JW. Pain-producing actions of tryptamine and 5-hydroxytryptamine. J Physiol. 1952;117:70P–71P. [PubMed] [Google Scholar]
- 36.Armstrong D, Dry RM, Keele CA, Markham JW. Observations on chemical excitants of cutaneous pain in man. J Physiol. 1953;120:326–351. doi: 10.1113/jphysiol.1953.sp004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Armstrong D, Keele CA, Jepson JB, Stewart JW. Development of pain producing substance in human plasma. Nature. 1954;174:791–792. doi: 10.1038/174791a0. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong D, Jepson JB, Keele CA, Stewart JW. Activation by glass of pharmacologically active agents in blood of various species. J Physiol. 1955;129:80–81. doi: 10.1113/jphysiol.1955.sp005387. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong D, Jepson JB, Keele CA, Stewart JW. Pain-producing substance in human inflammatory exudates and plasma. J Physiol. 1957;135:350–370. doi: 10.1113/jphysiol.1957.sp005715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keele CA. Chemical causes of pain & itch. Proc R Soc Med. 1957;50:477–484. [PMC free article] [PubMed] [Google Scholar]
- 41.Keele CA. The chemistry of pain production. Proc R Soc Med. 1967;60:419–422. [PMC free article] [PubMed] [Google Scholar]
- 42.Bleehen T, Hobbiger F, Keele CA. Identification of algogenic substances in human erythrocytes. J Physiol. 1976;262:131–149. doi: 10.1113/jphysiol.1976.sp011589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lariviere WR, Melzack R. The bee venom test: a new tonic-pain test. Pain. 1996;66:271–277. doi: 10.1016/0304-3959(96)03075-8. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Luo C, Li HL, Chen HS. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: A comparative study with the formalin test. Pain. 1999;83:67–76. doi: 10.1016/S0304-3959(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 45.Chen HS, Chen J. Secondary heat, but not mechanical, hyperalgesia induced by subcutaneous injection of bee venom in the conscious rat: effect of systemic MK-801, a non-competitive NMDA receptor antagonist. Eur J Pain. 2000;4:389–401. doi: 10.1053/eujp.2000.0197. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Chen HS. Pivotal role of capsaicin-sensitive primary afferents in development of both heat and mechanical hyperalgesia induced by intraplantar bee venom injection. Pain. 2001;91:367–376. doi: 10.1016/S0304-3959(00)00458-9. [DOI] [PubMed] [Google Scholar]
- 47.Wheeler-Aceto H, Porreca F, Cowan A. The rat paw formalin test: comparison of noxious agents. Pain. 1990;40:229–238. doi: 10.1016/0304-3959(90)90073-M. [DOI] [PubMed] [Google Scholar]
- 48.Hong Y, Abbott FV. Behavioural effects of intraplantar injection of inflammatory mediators in the rat. Neuroscience. 1994;63:827–836. doi: 10.1016/0306-4522(94)90527-4. [DOI] [PubMed] [Google Scholar]
- 49.Cooper B, Bomalaski JS. Activation of mechanonociceptors by pro-inflammatory peptides melittin and PLAP peptide. Exp Brain Res. 1994;100:18–28. doi: 10.1007/BF00227275. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Luo C, Li HL. The contribution of spinal neuronal changes to development of prolonged, tonic nociceptive responses of the cat induced by subcutaneous bee venom injection. Eur J Pain. 1998;2:359–376. doi: 10.1016/S1090-3801(98)90034-9. [DOI] [PubMed] [Google Scholar]
- 51.You HJ, Chen J. Differential effects of subcutaneous injection of formalin and bee venom on responses of wide-dynamic-range neurons in spinal dorsal horn of the rat. Eur J Pain. 1999;3:177–180. doi: 10.1053/eujp.1999.0119. [DOI] [Google Scholar]
- 52.Li MM, Yu YQ, Fu H, Xie F, Xu LX, Chen J. Extracellular signal-regulated kinases mediate melittin-induced hypersensitivity of spinal neurons to chemical and thermal but not mechanical stimuli. Brain Res Bull. 2008;77:227–232. doi: 10.1016/j.brainresbull.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Yu YQ, Chen J. Activation of spinal extracellular signaling-regulated kinases by intraplantar melittin injection. Neurosci Lett. 2005;381:194–198. doi: 10.1016/j.neulet.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Yu YQ, Zhao F, Chen J. Activation of ERK1/2 in the primary injury site is required to maintain melittin-enhanced wind-up of rat spinal wide-dynamic-range neurons. Neurosci Lett. 2009;459:137–141. doi: 10.1016/j.neulet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Lu ZM, Xie F, Fu H, Liu MG, Cao FL, Hao J, Chen J. Roles of peripheral P2X and P2Y receptors in the development of melittin-induced nociception and hypersensitivity. Neurochem Res. 2008;33:2085–2091. doi: 10.1007/s11064-008-9689-6. [DOI] [PubMed] [Google Scholar]
- 56.Ding J, Xiao Y, Lu D, Du YR, Cui XY, Chen J. Effects of SKF-96365, a TRPC inhibitor, on melittin-induced inward current and intracellular Ca2+ rise in primary sensory cells. Neurosci Bull. 2011;27:135–142. doi: 10.1007/s12264-011-1018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao J, Liu MG, Yu YQ, Cao FL, Li Z, Lu ZM, Chen J. Roles of peripheral mitogen-activated protein kinases in melittin-indced nociception and hyperalgesia. Neuroscience. 2008;152:1067–1075. doi: 10.1016/j.neuroscience.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 58.Ding J, Zhang JR, Wang Y, Li CL, Lu D, Guan SM, Chen J. Effects of a non-selective TRPC channel blocker, SKF-96365, on melittin-induced spontaneous persistent nociception and inflammatory pain hypersensitivity. Neurosci Bull. 2012;28:173–181. doi: 10.1007/s12264-012-1213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu YQ, Zhao ZY, Chen XF, Xie F, Yang Y, Chen J. Activation of tetrodotoxin-resistant sodium channel NaV1.9 in rat primary sensory neurons contributes to melittin-induced pain behavior. NeuroMol Med 2013, 15: 209–217. [DOI] [PubMed]
- 60.Yu YQ, Zhao F, Guan SM, Chen J. Antisense-mediated knockdown of NaV1.8, but not NaV1.9, generates inhibitory effects on complete Freund’s adjuvant-induced inflammatory pain in rat. PLoS One. 2011;6:e19865. doi: 10.1371/journal.pone.0019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu YQ, Chen XF, Yang Y, Yang F, Chen J. Electrophysiological identification of tonic and phasic neurons in sensory dorsal root ganglion and their distinct implications in inflammatory pain. Physiol Res. 2014;63:793–799. doi: 10.33549/physiolres.932708. [DOI] [PubMed] [Google Scholar]