Abstract
In this study, we investigated the role of structural asymmetry of the dorsolateral prefrontal cortex (DLPFC) in the continuum of depression from healthy individuals to patients. Structural magnetic resonance imaging was performed in 70 patients with major depressive disorder (MDD), 49 matched controls, and 349 healthy university students to calculate structural asymmetry indexes of the DLPFC. First-episode, treatment-naive MDD patients showed a relatively lower asymmetry index than healthy controls, and their asymmetry index was negatively correlated with the depressive symptoms. This abnormality was normalized by antidepressants in medicated MDD patients. Furthermore, the asymmetry index was negatively correlated with the depressive symptoms in university students; this was replicated at two time points in a subgroup of students, suggesting good test–retest reliability. Our findings are consistent with previous studies that support the imbalance hypothesis of MDD and suggest a potential structural basis underlying the functional asymmetry of the DLPFC in depression. In future, the structural index of the DLPFC may become a potential biomarker to evaluate individuals’ risk for the onset of MDD.
Keywords: Major depressive disorder, Structural magnetic resonance imaging, Dorsolateral prefrontal cortex, Structural asymmetry
Introduction
Functional and structural asymmetry have been reported in the brains of animals and humans [1] and have been linked to language [2], motor control [3], cognitive performance [4], and psychiatric disorders including schizophrenia [5, 6] and depression [7–9].
One of the interesting aspects of brain asymmetry is its association with emotion-related psychological processes [10]. Using electroencephalography (EEG), previous researchers have linked greater left than right frontal activity to positive characteristics including more positive affect [11], approach motivation [12], a high level of well-being [13], and a strong ability to regulate negative emotions [14]. In contrast, decreased left (versus right) hemispheric activation has been reported in patients with ongoing major depressive disorder (MDD) [15] and even those in remission [16] as well as in individuals with cognitive [17] or genetic [18] vulnerability for the onset of MDD. Evidence from positron emission tomography, functional magnetic resonance imaging (fMRI) and noninvasive brain stimulation including repetitive transcranial magnetic stimulation and transcranial direct-current stimulation has provided further support for a role of functional asymmetry in prefrontal brain regions, especially the right and left dorsolateral prefrontal cortex (DLPFC), in MDD [7–9, 19–23]. Taken together, these functional studies support the imbalance hypothesis of MDD [24, 25], which postulates prefrontal asymmetry with relative hypoactivity in the left and hyperactivity in the right DLPFC. To the best of our knowledge, although functional imbalance of the DLPFC has been observed using functional brain imaging, the structural basis underlying this functional asymmetry and its relationship with individuals’ symptoms of depression remain largely unexplored. In this study, we investigated the association between structural asymmetry of the DLPFC and depression in healthy participants and MDD patients by analyzing high-resolution T1-weighted anatomical images.
Typical psychiatric studies usually explore the structural abnormalities of MDD just by comparing a group of individuals diagnosed with MDD with another group who do not meet the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria. Our analysis of healthy individuals is important for two reasons: (1) subthreshold depressive levels are associated with a variety of negative outcomes [26, 27] and even a significantly enhanced risk of developing MDD [28, 29]; and (2) studies only concentrating on patients with MDD have difficulty in providing comprehensive insights into the mechanism of MDD because depression occurs along a continuum [30, 31]. Our dimensional, rather than categorical, approach reflects a growing trend in depression research [32].
Materials and Methods
Participants
Patients with MDD were recruited from the Outpatient Department at the First Affiliated Hospital of Chongqing Medical School in Chongqing, China. All were independently diagnosed by two psychiatrists according to the Structured Clinical Interview of DSM-IV. First-episode, drug-naive MDD patients (n = 39, 24 females) who were experiencing the first episode of depression, had never received treatment and had no comorbidities. Medicated MDD patients (n = 31, 21 females) were receiving antidepressant drugs and had responded well to the treatment (mean Beck Depression Inventory (BDI) score = 12.03). Twenty-one of them were taking selective serotonin reuptake inhibitors such as citalopram, fluoxetine, paroxetine, and sertraline; 9 used serotonin-norepinephrine reuptake inhibitors such as venlafaxine, mirtazapine, and trazodone; and one patient used a tricyclic antidepressant (amitriptyline). Matched healthy controls (n = 49; 24 females) were recruited from the local community by advertisement. The shared exclusion criteria for patients and controls were: meeting the DSM-IV criteria for any psychiatric disorders other than MDD, neurological diseases, conditions that are not suitable for scanning, medication that may change brain function, a history of loss of consciousness, head trauma, pregnancy, or breast-feeding. All participants were right-handed according to self-reported questionnaires.
Right-handed healthy young adults (n = 349; 191 females; 19.9 ± 1.32 years old, age range: 17–27 years) were also included in this study. Notably, the PFC of these participants, especially those are younger than 25 would not yet be mature. This point may affect the brain structure analysis in this study. In this sample, individuals who scored ≥15 in the BDI, but did not meet the diagnostic criteria of MDD or any other psychiatric illness were assigned to a subclinical depression group (n = 33) according to recommendations regarding the use of the BDI [33] and a brain structure study of subclinical depression [34]. Then the remaining participants were divided into two subgroups, a 5 ≤ BDI < 15 group (n = 156) and a BDI < 5 group (n = 160). Sixty-nine students (mean age at the first scan, 19.82 ± 1.18 years, 36 females) completed the first and second scans along with psychological measurements (mean interval between first and second scans, 319.15 days). We used this sample to replicate the correlation at two separate time points. The exclusion criteria were the same as for the control group. Subsequently, the Structured Clinical Interview for DSM-IV was performed by two well-trained and experienced graduate students in the Department of Psychology. None of the 349 students met the DSM-IV criteria for any psychiatric disorder and none used drugs that could affect brain function (including antidepressants). None of the 69 students noted above developed any psychiatry illness between the two scans. This study was approved by the Research Ethics Committees of the Brain Imaging Center of Southwest University and the First Affiliated Hospital of Chongqing Medical School. Informed written consent was given by each participant and by the guardians (college instructors) of the two youngest participants (17 years old). This study was conducted in accordance with the Helsinki Declaration as revised in 1989 [35].
Measures
We used the BDI-I [36] score and the state anxiety score derived from the State Anxiety Inventory [37] to measure the symptoms of depression and anxiety in both the university students and the MDD patients. The BDI-I is a 21-item self-report questionnaire measuring the severity of depressive symptoms within the past week. Participants who score higher in the BDI have more depressive symptoms [36]. The BDI-I is a reliable and widely-used measure that assesses the severity of depressive symptoms from non-clinical to clinical samples [38]. The State Anxiety Inventory (A-State) is a subscale of the State-Trait Anxiety Inventory [37]. The latter is a self-report questionnaire to evaluate the severity of anxiety, using two scores to clearly distinguish between the state anxiety (a temporary condition) and trait anxiety (a long-standing quality). We used the A-State score here as a controlling variable to explore the specificity of the results to depressive symptoms.
MRI Data Acquisition
All MR images were acquired on a 3.0-T Siemens Trio MRI scanner (Siemens Medical, Erlangen, Germany). High-resolution T1-weighted anatomical images were acquired using a magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR/TE/TI = 1900 ms/2.52 ms/900 ms; flip angle = 9°; slices = 176; slice thickness = 1.0 mm; resolution matrix = 256 × 256; voxel size = 1 × 1 × 1 mm3).
Voxel-Based Morphometry
MR images were processed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Each image was first displayed in SPM8 to screen for artifacts or gross anatomical abnormalities. For better registration, the reorientation of images was manually set to the anterior commissure. Segmentation of T1-weighted anatomical images into gray matter (GM) and white matter (WM) was done using the new segmentation in SPM8. Subsequently, we performed Diffeomorphic Anatomical Registration through Exponentiated Lie (DARTEL) algebra in SPM8 for registration, normalization, and modulation [39]. To ensure that regional differences in the absolute amount of GM or WM were conserved, the image intensity of each voxel was modulated by Jacobian determinants. Then, registered images were transformed into Montreal Neurological Institute (MNI) space. Finally, normalized modulated images (GM and WM images) were smoothed with an 8-mm full-width at half-maximum Gaussian kernel to increase the signal-to-noise ratio.
ROI Definition and Signal Extraction
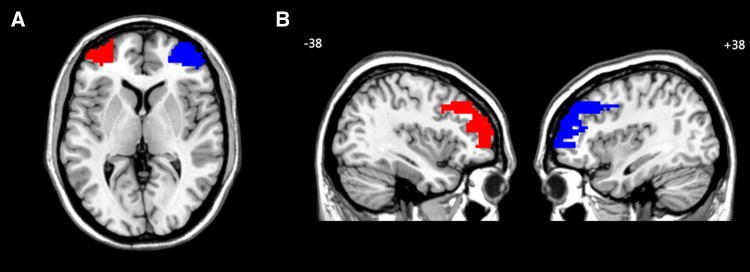
Using Wake Forest University PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas), the DLPFC was defined using a mask of the middle frontal gyrus, restricted to Brodmann areas 9, 10, and 46 (using a dilation of 3 to ensure coverage of all voxels in the area) (Fig. 1). This method has been used in previous research focusing on cognitive regulation [40]. It is of note that this region encompasses the peak voxels reported in a study that indicates an imbalance of activity in the left and right DLPFC in emotional processing by MDD patients (coordinates: left: −42, 10, 30; right: 40, 24, 42) [9]. Other ROIs were also created using PickAtlas. Left and right masks of the hippocampus, amygdala, and insula were each extracted on an Automated Anatomical Labeling (AAL) template. The bilateral prefrontal cortex was defined by combing the areas of Frontal_Sup, Frontal_Sup_Orb, Frontal_Mid, Frontal_Mid_Orb, Frontal_Inf_Oper, Frontal_Inf_Tri, and Frontal_Inf_Orb in each hemisphere based on the AAL template. The left and right hemisphere masks included all of the areas in the left or right hemisphere. The ROIs generated were resliced to the same dimensions as those of tissue segmented images obtained from the voxel-based morphometry preprocessing step and were subsequently used to mask the individual modulated, normalized GM images and extract the average volume within each ROI using the REX toolbox (http://web.mit.edu/swg/software.htm).
Fig. 1.
Visual display of regions of interest. A Visual display of the left DLPFC (red) and right DLPFC (blue) on the axial MRI image; B Visual display of the left DLPFC (red) and right DLPFC (blue) on the sagittal MRI images. DLPFC dorsolateral prefrontal cortex.
Statistical Analysis
The GM volumes (GMVs) of the bilateral DLPFC were used to calculate the asymmetry index by subtracting the volume in the right hemisphere from that in the left hemisphere. The asymmetry indexes of the hippocampus, amygdala, insula, PFC and the whole brain were computed using the same method. This method has been used in research examining the relationship between individual differences in structural asymmetry and overt aggression [41], and a study that reported increased asymmetry of the internal capsule in patients with schizophrenia [6]. Partial correlation was performed between the asymmetry index and depressive symptoms controlling for sex, age, and whole-brain GMV. The state-anxiety score was further treated to be a variable of no interest to test whether the correlation was influenced by anxiety level. Group differences in the asymmetry indexes were tested by one-way analysis of variance, followed by the Least Significant Difference (LSD) test. The difference between left and right DLPFC volumes was assessed using the paired two-sample t test.
To determine whether the structural index of the DLPFC is specific and sensitive enough to serve as a potential biomarker for differentiating patients from controls or high-risk from low-risk individuals, we used the Receiver Operating Characteristic (ROC) curve. ROC curve analyses were performed using the Statistical Package for the Social Sciences (SPSS). The area under the ROC curve (AUC), and the cut-off with high and balanced sensitivity and specificity were reported.
Results
Demographic, Clinical, and Psychological Characteristics
The demographic and clinical characteristics of the first-episode, treatment-naive MDD patients, the medicated MDD patients, and the healthy controls are shown in Table 1. The three groups did not differ significantly in age, years of education, or gender.
Table 1.
Demographic and clinical characteristics of the first-episode, treatment-naive MDD, medicated MDD and control groups.
| Characteristics | First-episode, drug-naive MDD patients (n = 39) | Medicated MDD patients (n = 31) | Controls (n = 49) | F/t/χ2 | P |
|---|---|---|---|---|---|
| Age (years) | 38.18 (13.83) | 35.52 (9.65) | 39.00 (10.78) | 0.88 | 0.41 |
| Female/male | 24/15 | 21/10 | 24/25 | 3.04 | 0.21 |
| Education (years) | 11.97 (3.32) | 11.39 (3.46) | 11.12 (3.69) | 0.64 | 0.52 |
| BDI score | 23.62 (7.26) | 12.03 (6.23) | 3.16 (2.91) | 147.14 | <0.001 |
| State-anxiety scorea | 50.91 (15.58) | 46.30 (13.43) | 28.82 (9.38) | 35.96 | <0.001 |
| Onset (years) | 36.90 (14.42) | 34.08 (9.47) | NA | 0.94 | 0.32 |
| Illness duration (months) | 15.35 (15.70) | 17.23 (16.47) | NA | −0.48 | 0.62 |
MDD major depressive disorder, BDI Beck depression inventory.
aState-anxiety score was available for 35 first-episode, drug-naive MDD patients and 31 medicated MDD patients.
The demographic and symptomatic information on the university students is shown in Table 2. The three groups (high-risk, middle level, and low level) did not differ in age (P > 0.05) or sex (P > 0.05).
Table 2.
Demographic and psychological characteristics of the university students.
| Characteristics | High risk (n = 33) | Middle level (n = 156) | Low level (n = 160) | F/χ 2 | P |
|---|---|---|---|---|---|
| Age (years) | 19.96 (1.46) | 19.90 (1.29) | 20.08 (1.33) | 0.70 | 0.49 |
| Female/male | 17/16 | 84/72 | 90/70 | 0.33 | 0.84 |
| BDI score | 19.75 (3.74) | 9.28 (2.55) | 2.42 (1.72) | 852.906 | <0.001 |
| A-State scorea | 44.27 (9.75) | 37.15 (7.32) | 31.51 (6.75) | 48.630 | <0.001 |
SD standard deviation, BDI Beck depression inventory.
aState-anxiety score was available for 30 individuals in the high-risk group and 157 in the low-level group. High risk, BDI ≥ 15; middle level, 5 ≤ BDI < 15; low level, BDI < 5.
MDD Patients and Controls
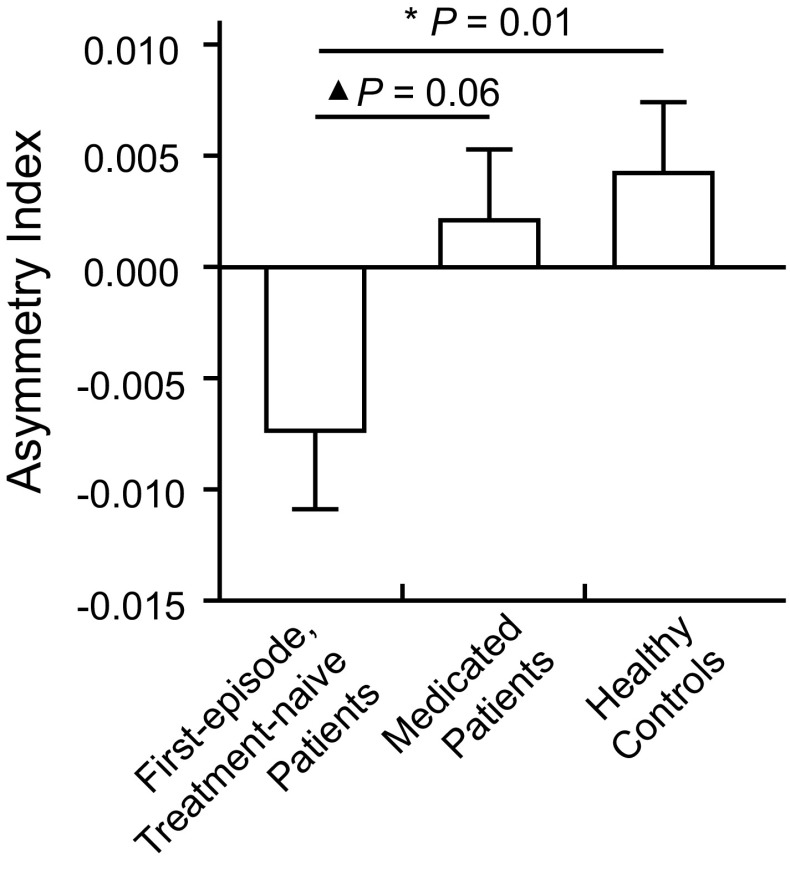
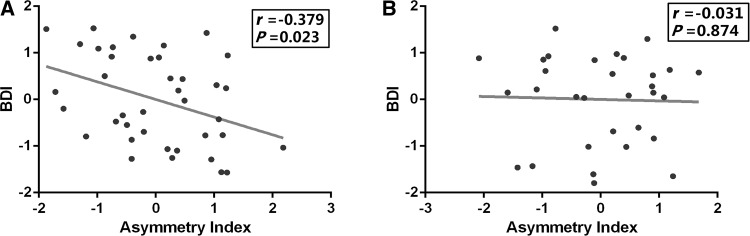
There was no significant difference in the left (F = 0.80, P = 0.448) or the right DLPFC volume (F = 0.56, P = 0.568) among the first-episode, treatment-naive MDD, medicated MDD, and control groups (Table 3). In the first-episode, treatment-naive MDD patients, the GMV of the left DLPFC was marginally lower than that of the right DLPFC (t = −2.01, P = 0.052). This difference did not exist in the control group (P > 0.05) or the medicated MDD group (P > 0.05). Then, one-way ANOVA analysis of the DLPFC asymmetry index showed a significant group difference among the three groups (F = 3.39, P = 0.037) (Fig. 2, Table 3). Post-hoc analysis revealed that the first-episode, treatment-naive MDD patients had a lower DLPFC asymmetry index than the controls (P = 0.013, LSD test) and that the medicated patients had a marginally higher DLPFC asymmetry index (P = 0.068, LSD test) than the first-episode, treatment-naive MDD patients. Furthermore, partial correlation was performed between the DLPFC asymmetry index and the BDI-I score controlling for sex, age, and whole-brain GMV. We found a significant negative correlation between the asymmetry index and depressive symptoms in the first-episode, treatment-naive MDD patients, but not in the medicated MDD patients (Fig. 3).
Table 3.
Gray matter volume (GMV) and DLPFC asymmetry index in patients and controls.
| Brain region or index | First-episode, drug-naive MDD patients (n = 39) | Medicated MDD patients (n = 31) | Controls (n = 49) | F | P |
|---|---|---|---|---|---|
| Left DLPFC GMV | 0.363 (0.05) | 0.362 (0.05) | 0.375 (0.04) | 0.80 | 0.448 |
| Right DLPFC GMV | 0.370 (0.05) | 0.360 (0.04) | 0.370 (0.04) | 0.56 | 0.568 |
| Asymmetry index | −0.007 (0.02) | 0.002 (0.01) | 0.004 (0.02) | 3.39* | 0.037 |
Mean (SD).
* P < 0.05.
Fig. 2.
Group differences in the DLPFC asymmetry index between first-episode, treatment-naive MDD patients, medicated MDD patients, and healthy controls. First-episode, treatment-naive MDD patients showed a significantly lower DLPFC asymmetry index (relatively lower GMV in the left than the right DLPFC) compared to controls. However, the medicated MDD patients showed no difference from controls. *P < 0.05. ▲ P < 0.1.
Fig. 3.
Relationship between depressive symptoms and DLPFC asymmetry index in treatment-naïve MDD patients and medicated patients. Scatter-plots showing the pattern of correlation between the BDI-I score and the DLPFC asymmetry index. The DLPFC asymmetry index is the Z-transformed difference of GM volume between the left and right DLPFC adjusted for age, gender, and total GMV. This association was significant for first-episode, treatment-naive MDD patients (A) but not for medicated MDD patients (B).
Healthy University Students
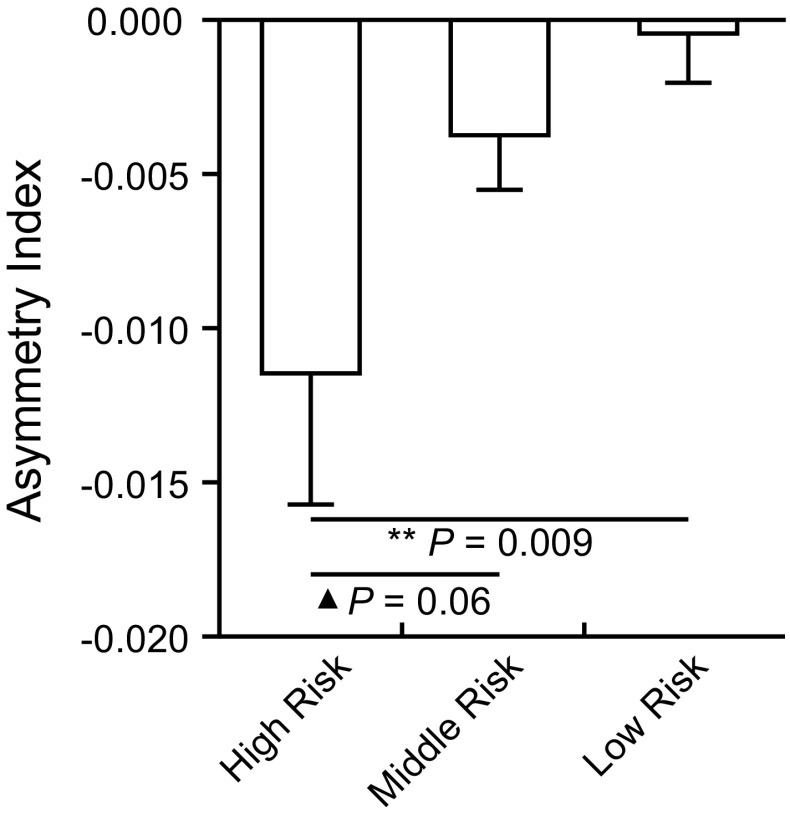
The three groups of university students did not differ in the left (F = 0.90, P = 0.405) or the right DLPFC volume (F = 0.11, P = 0.894) (Table 4). The GMV of the left DLPFC was significantly lower than that of the right DLPFC in the high-risk group (t = −2.69, P = 0.011) and the middle-level group (t = −2.00, P = 0.047). No significant difference was found in the low-level group (t = −0.21, P = 0.832). There was a significant group difference in asymmetry index among the three groups (F = 3.60, P = 0.028) (Fig. 4; Table 4). Post-hoc tests showed that the asymmetry index of the high-risk group was smaller than that of the low-level group (P = 0.009), and was marginally lower than that of the middle-level group (P = 0.069).
Table 4.
Gray matter volume (GMV) and DLPFC asymmetry index in the university students.
| Brain region or index | High risk (n = 33) | Middle level (n = 156) | Low level (n = 160) | F | P |
|---|---|---|---|---|---|
| Left DLPFC GMV | 0.377 (0.05) | 0.383 (0.06) | 0.389 (0.04) | 0.90 | 0.405 |
| Right DLPFC GMV | 0.389 (0.05) | 0.387 (0.05) | 0.389 (0.05) | 0.11 | 0.894 |
| Asymmetry index | −0.011 (0.02) | −0.003 (0.02) | −0.0003 (0.02) | 3.60* | 0.028 |
Mean (SD).
* P < 0.05.
Fig. 4.
Group difference of DLPFC asymmetry index in the three university student groups. The asymmetry index of the high-risk group was lower than low-risk group and tended to be lower than the middle-risk group. **P < 0.01. ▲ P < 0.1.
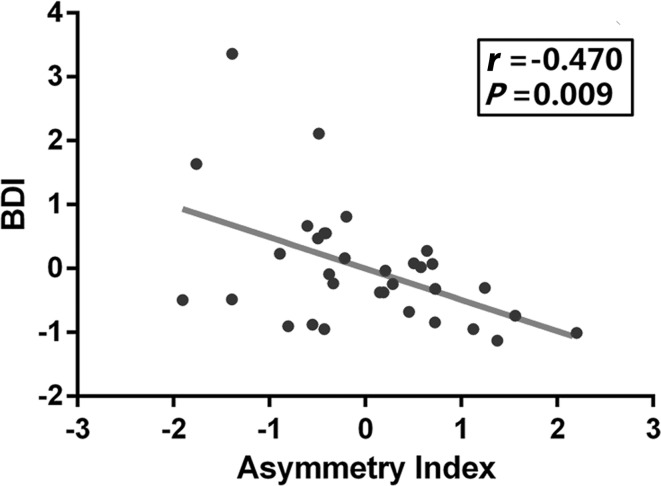
We also found that the DLPFC asymmetry index was negatively correlated with the depressive symptoms (r = −0.134, P = 0.012) in the whole sample of university students (n = 349). The DLPFC asymmetry index also correlated with BDI-I score in the high-risk group (r = −0.470, P = 0.009) (Fig. 5).
Fig. 5.
Association between depressive symptoms and DLPFC asymmetry index in the high-risk group. Scatter-plot showing the pattern of correlation between DLPFC asymmetry index and BDI score in the high-risk group. The asymmetry index is the Z-transformed difference of GMV between the left and right DLPFC adjusted for age, gender, and total GMV.
To test the robustness of the relationship between the asymmetry index and the depressive symptoms, we conducted a test–retest validation in a subgroup of university students with the brain structure data and depression symptoms at two time points. The DLPFC asymmetry index calculated from the first scans correlated with the depressive symptoms measured at the same time (r = −0.277, P = 0.026). After nearly one year (mean interval, 319.15 days), the asymmetry index derived from the second scans also correlated with the depressive symptoms measured immediately after the scanning (r = −0.245, P = 0.049). These results indicated good test–retest reliability of the correlation between asymmetry index and depressive symptoms.
No Correlation between Structural Asymmetry of Other Brain Regions and Depressive Symptoms
Correlations were also tested for other brain regions. First, the hippocampus, amygdala, and insula were selected since they are involved in emotional processing. No correlation was found between the structural asymmetry index of the hippocampus, amygdala, or insula and the depressive symptoms (P > 0.05 for all) in the first-episode, treatment-naive MDD patients or the university students. To further test the hypothesis that our findings were specific to the structural asymmetry of the DLPFC rather than asymmetry of a larger area (prefrontal cortex (PFC) or the whole brain), we calculated the asymmetry index of the PFC and the whole brain and performed correlational analysis. We found no significant correlation between the asymmetry index of the PFC or the whole brain and the depressive symptoms in the first-episode, treatment-naive MDD patients or in the university students (P > 0.05 for both).
Controlling for the Influence of State-Anxiety
To explore the effects of state-anxiety on the correlation, we further included the state-anxiety score as a covariate in the correlational analysis. The negative correlation between the DLPFC asymmetry index and depressive symptoms remained in the university students (r = –0.103, P = 0.059) and the first-episode, treatment-naive MDD patients (r = –0.470, P = 0.007).
ROC Curve
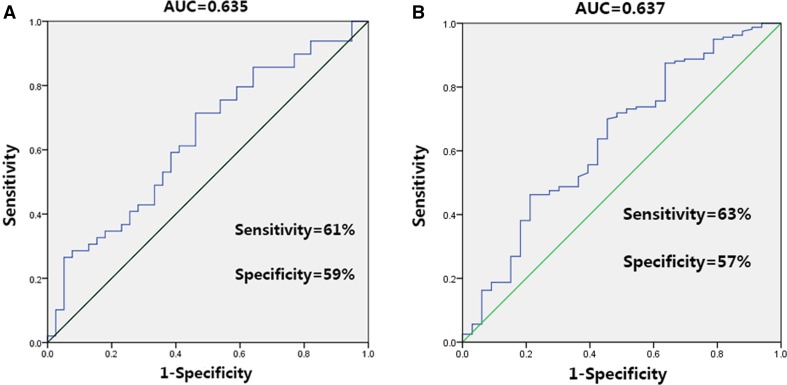
First, we tried to distinguish the first-episode, treatment-naïve MDD patients from the controls. The diagnostic power of the DLPFC asymmetry index was modest (AUC = 0.635) but significant (P = 0.03). The cut-off (asymmetry index = –0.00045) showed a sensitivity of 61% and a specificity of 59% (Fig. 6A). Then, we investigated the diagnostic power of the asymmetry index to differentiate high-risk from low-risk individuals among the university students. The ROC analysis revealed that the DLPFC asymmetry index exhibited a modest performance (AUC = 0.637, P = 0.01). The cut-off (asymmetry index = –0.0092) showed a sensitivity of 63% and a specificity of 57% (Fig. 6B).
Fig. 6.
Performance of DLPFC Asymmetry Index in ROC analysis. A Performance of DLPFC asymmetry index in differentiating MDD patients from controls. B Performance of asymmetry index in differentiating high-risk from low-risk individuals. AUC area under the curve.
Discussion
In this study, we aimed to explore the relationship between structural asymmetry of the DLPFC and depressive symptoms in MDD patients and a large sample of university students. We found that the DLPFC asymmetry existed not only in first-episode, drug-naive MDD patients but also in individuals with subclinical depression. More importantly, the individual differences in the DLPFC asymmetry index were correlated with the severity of depression in MDD patients, individuals with subclinical depression, and the large sample of students. This correlation was replicated at two separate time points in a subgroup of students. These results are consistent with previous studies linking the functional asymmetry of the DLPFC to depression and may reveal the structural basis underlying the functional asymmetry. Moreover, these findings provide a novel perspective for understanding the relationship between hemispheric asymmetry and emotional processing in the human brain.
In line with our hypotheses, the DLPFC asymmetry index was lower in first-episode, treatment-naive MDD patients, and individuals with subclinical depression than in healthy controls. The DLPFC plays important roles in working memory and episodic memory [42], executive function [43], and the regulation of emotion [44], and this region is commonly impaired in bipolar I depressive disorder and unipolar depressive disorder [45]. In this study, instead of exploring the group differences in GMV without considering brain asymmetry, we adopted a novel perspective to explore asymmetry of the DLPFC in depression. Brain asymmetry has been reported in structure and function, and this lateralization is thought to reflect pathological change [1]. Depression is associated with an inter-hemispheric imbalance [46], which has been supported by several recent studies. For instance, the prevalence of occipital bending is three times higher among patients with MDD than in healthy individuals, linking occipital lobe asymmetry (occipital bending) to depression [47]. A significant reduction in the average global functional connectivity after electroconvulsive therapy has been reported, and the reduction is restricted and lateralized, limited to an area within the left DLPFC [48]. More directly, EEG studies have indicated that depressed patients have a negative asymmetry index in alpha power, reflecting left frontal hypo- and right frontal hyper-activation. The more negative the asymmetry index in the frontal lobes, the more severe are the depressive symptoms [49]. The abnormal structural asymmetry of the DLPFC found in our study is consistent with the view that depression is associated with an inter-hemispheric imbalance. Although we did not record the EEG in our samples, we speculated that the structural asymmetry of the DLPFC that we found could be the structural foundation of the functional asymmetry of the DLPFC in MDD patients and individuals at high risk for MDD [9, 15–18]. Over 70 published EEG studies [50] and recent functional MRI studies [19, 51] have established a differential role of the left and right PFC for processing positive and negative emotional information, respectively. Since brain structure may be the neural substrate of brain function, the structural asymmetry of the DLPFC may be associated with an imbalance of positive and negative emotions. And depression, in fact, is an affective disorder with an imbalance of emotion. The relatively low DLPFC asymmetry index could indicate impaired and unbalanced emotional processing. Thus, MDD patients and individuals with subclinical depression had a relatively low DLPFC asymmetry index. We also found that the asymmetry index in first-episode, drug-naive MDD patients were marginally lower than in medicated patients. The reason for this phenomenon may be that the GMV of the left DLPFC increases due to the effects of antidepressants. This finding is consistent with the results of a longitudinal study, which investigated the effects of antidepressants on the GMV of MDD patients [52]. In this study, the GMV of the left rather than the right DLPFC of depressed individuals increased in a way that correlated with the decreasing self-reported severity of depression. These results suggest that antidepressants can affect and normalize the abnormal structural asymmetry index in MDD patients who respond to medications and provide further support for the association between the asymmetry index and depressive symptoms. However, the evidence from our study is preliminary and does not illustrate a causal relationship. A longitudinal design should be used to directly validate the effect of antidepressants on the asymmetry index.
We found that the DLPFC asymmetry index correlated with the depressive symptoms from healthy young adults to individuals with subclinical depression and patients with MDD. These results suggest that individual differences in the structural asymmetry of the DLPFC reflects the severity of depression across the different cohorts. The relationship between structural asymmetry and depressive symptoms may be related to the valence-lateralization theory, which proposes dominance of the left PFC in positive emotions and of the right PFC in negative emotions [25, 53]. This theory is supported by the linear dependence of positive and negative emotion judgment on activity in the left and right DLPFC, respectively, reported in an fMRI study [51] and by an association between greater left than right frontal activity and positive affect [11], approach motivation [12], well-being [13], and emotional regulation [14] reported in EEG studies. Individuals with a low asymmetry index had a relatively smaller GMV in the left than in the right DLPFC. Therefore, we speculate that the smaller GMV in left DLPFC may be associated with weak positive emotional processing while the larger GMV in the right DLPFC may be linked to stronger negative emotional processing. The imbalance of the GMV of the DLPFC may lead to stronger negative emotion and weaker positive emotion and subsequently become the root of depressive symptoms.
Much to our regret, the diagnostic power of the DLPFC asymmetry index was modest. This limits its potential to act as a biomarker in support of the clinical diagnosis. The reason its diagnostic power is limited may be that MDD is a complex disorder, and we only used the information from a specific brain region, the DLPFC. However, although this index is far from clinical use due to its limited diagnostic power, the findings from this study can help to understand depression from the perspective of the imbalance hypothesis of MDD. In future, the DLPFC asymmetry index may be combined with other biomarkers to achieve better performance.
There are several limitations in this study. First, the age span of the MDD patients is large, which may be a confounding variable in the structural MRI analyses. However, we included age as a covariate in the partial correlation to minimize its effect and obtained the same significant correlation in a highly homogeneous sample of university students with a narrow age span. Second, the DLPFC is not a precise anatomical structure, but rather a functional one. In this study, we defined the DLPFC based on previous research and confirmed it using the coordinates from a functional MRI study that showed an imbalance in the activity of the left and right DLPFC [9]. Third, because of the significant difference between age and years of education between the subclinical depression group and the patient group, we could not directly compare the asymmetry index between them. In future, when two comparable groups are available, this difference can be investigated. Finally, the self-rated measurement (BDI) rather than the “gold standard” of depression measurement (Hamilton Depression Scale) was used to rate the severity of symptoms in MDD patients. We did this since we intended to use data from the same measurement across different samples.
In summary, the results of this study demonstrate a relationship between structural asymmetry of the DLPFC and depressive symptoms in first-episode, treatment-naive patients with MDD and healthy young adults showing different levels of depressive tendency, including a group of individuals with subclinical depression. Our findings suggest that MDD patients and individuals with subclinical depression have a relatively low DLPFC asymmetry index and that individual differences in the structural asymmetry of the DLPFC correlate with the depressive symptoms in healthy individuals, high-risk individuals, and MDD patients.
In conclusion, the current results highlight the role of structural asymmetry of the DLPFC in the pathology of MDD. They not only provide further support for the imbalance hypothesis of MDD, but also suggest a potential structural basis underlying the functional asymmetry of the DLPFC in depression. In future, the DLPFC structural index may become a potential biomarker of individuals’ risk for the onset of MDD.
Footnotes
Wei Liu and Yu Mao have contributed equally to this work.
Contributor Information
Peng Xie, Email: xiepeng@cqmu.edu.cn.
Jiang Qiu, Email: qiuj318@swu.edu.cn.
References
- 1.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 2.Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci U S A. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zilles K, Dabringhaus A, Geyer S, Amunts K, Qü M, Schleicher A, et al. Structural asymmetries in the human forebrain and the forebrain of non-human primates and rats. Neuroscience & Biobehavioral Reviews. 1996;20:593–605. doi: 10.1016/0149-7634(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 4.Fornito A, Wood SJ, Whittle S, Fuller J, Adamson C, Saling MM, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum brain mapp. 2008;29:222–236. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yücel M, Stuart GW, Maruff P, Wood SJ, Savage GR, Smith DJ, et al. Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol psychiatry. 2002;52:15–23. doi: 10.1016/S0006-3223(02)01312-4. [DOI] [PubMed] [Google Scholar]
- 6.Zhou S-Y, Suzuki M, Hagino H, Takahashi T, Kawasaki Y, Nohara S, et al. Decreased volume and increased asymmetry of the anterior limb of the internal capsule in patients with schizophrenia. Biol psychiatry. 2003;54:427–436. doi: 10.1016/S0006-3223(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 7.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol psychiatry. 2003;54:515–528. doi: 10.1016/S0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 8.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br med bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Harmon-Jones E, Gable PA, Peterson CK. The role of asymmetric frontal cortical activity in emotion-related phenomena: a review and update. Biol Psychol. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Davidson RJ, Schwartz GE, Saron C, Bennett J, Goleman DJ. Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiol. 1979;16:202–203. [Google Scholar]
- 12.Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J abnorm psychol. 1997;106:159. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- 13.Urry HL, Nitschke JB, Dolski I, Jackson DC, Dalton KM, Mueller CJ, et al. Making a life worth living neural correlates of well-being. Psychol Sci. 2004;15:367–372. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- 14.Jackson DC, Mueller CJ, Dolski I, Dalton KM, Nitschke JB, Urry HL, et al. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol Sci. 2003;14:612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- 15.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J abnorm psychol. 1991;100:535. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- 16.Henriques J, Davidson R. Regional brain electrical asymmetries discriminate between previously depressed subjects and healthy controls. J abnorm Psychol. 1990;99:22–31. doi: 10.1037/0021-843X.99.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Nusslock R, Shackman AJ, Harmon-Jones E, Alloy LB, Coan JA, Abramson LY. Cognitive vulnerability and frontal brain asymmetry: common predictors of first prospective depressive episode. J abnorm psychol. 2011;120:497. doi: 10.1037/a0022940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, et al. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biol psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Herrington JD, Heller W, Mohanty A, Engels AS, Banich MT, Webb AG, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiol. 2010;47:442–454. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, et al. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Nuropsychopharmacol. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A, et al. The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Pychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald PB, Brown TL, Marston NA, Daskalakis ZJ, de Castella A, Kulkarni J. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60:1002–1008. doi: 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- 23.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160:835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 24.Maeda F, Keenan JP, Pascual-Leone A. Interhemispheric asymmetry of motor cortical excitability in major depression as measured by transcranial magnetic stimulation. Br J Psychiatry. 2000;177:169–173. doi: 10.1192/bjp.177.2.169. [DOI] [PubMed] [Google Scholar]
- 25.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/S1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 26.Cuijpers P, de Graaf R, van Dorsselaer S. Minor depression: risk profiles, functional disability, health care use and risk of developing major depression. J Affect Disord. 2004;79:71–79. doi: 10.1016/S0165-0327(02)00348-8. [DOI] [PubMed] [Google Scholar]
- 27.Fergusson DM, Horwood LJ, Ridder EM, Beautrais AL. Subthreshold depression in adolescence and mental health outcomes in adulthood. Arch Gen Psychiatry. 2005;62:66–72. doi: 10.1001/archpsyc.62.1.66. [DOI] [PubMed] [Google Scholar]
- 28.Klein DN, Glenn CR, Kosty DB, Seeley JR, Rohde P, Lewinsohn PM. Predictors of first lifetime onset of major depressive disorder in young adulthood. J Abnorm Psychology. 2013;122:1. doi: 10.1037/a0029567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatr Scand. 2004;109:325–331. doi: 10.1111/j.1600-0447.2004.00301.x. [DOI] [PubMed] [Google Scholar]
- 30.Solomon A, Haaga DA, Arnow BA. Is clinical depression distinct from subthreshold depressive symptoms? A review of the continuity issue in depression research. J Nerv Ment Dis. 2001;189:498–506. doi: 10.1097/00005053-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Lewinsohn PM, Klein DN, Durbin EC, Seeley JR, Rohde P. Family study of subthreshold depressive symptoms: risk factor for MDD? J Affect Disord. 2003;77:149–157. doi: 10.1016/S0165-0327(02)00106-4. [DOI] [PubMed] [Google Scholar]
- 32.Ayuso-Mateos JL, Nuevo R, Verdes E, Naidoo N, Chatterji S. From depressive symptoms to depressive disorders: the relevance of thresholds. Br J Psychiatry. 2010;196:365–371. doi: 10.1192/bjp.bp.109.071191. [DOI] [PubMed] [Google Scholar]
- 33.Kendall PC, Hollon SD, Beck AT, Hammen CL, Ingram RE. Issues and recommendations regarding use of the Beck Depression Inventory. Cognit Ther Res. 1987;11:289–299. doi: 10.1007/BF01186280. [DOI] [Google Scholar]
- 34.Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, et al. Cortisol awakening response and hippocampal volume: vulnerability for major depressive disorder? Biol Psychiatry. 2010;68:847–853. doi: 10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Harrison JE. Orthodontic Clinical Trials III: Reporting of ethical issues associated with clinical trials published in three orthodontic journals between 1989 and 1998. J Orthod. 2005;32:115–121. doi: 10.1179/146531205225020970. [DOI] [PubMed] [Google Scholar]
- 36.Beck AT, Ward C, Mendelson M. Beck depression inventory (BDI) Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 38.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. doi: 10.1016/0272-7358(88)90050-5. [DOI] [Google Scholar]
- 39.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci. 2012;32:13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Visser TA, Ohan JL, Whittle S, Yücel M, Simmons JG, Allen NB. Sex differences in structural brain asymmetry predict overt aggression in early adolescents. Soc Cogni Affect Neurosci 2013: nst013. [DOI] [PMC free article] [PubMed]
- 42.Balconi M. Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull. 2013;29:381–389. doi: 10.1007/s12264-013-1309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carpenter PA, Just MA, Reichle ED. Working memory and executive function: evidence from neuroimaging. Curr Opin Neurobiol. 2000;10:195–199. doi: 10.1016/S0959-4388(00)00074-X. [DOI] [PubMed] [Google Scholar]
- 44.Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacol. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai Y, Liu J, Zhang L, Liao M, Zhang Y, Wang L, et al. Grey matter volume abnormalities in patients with bipolar I depressive disorder and unipolar depressive disorder: a voxel-based morphometry study. Neurosci Bull. 2015;31:4–12. doi: 10.1007/s12264-014-1485-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res. 2010;68:77–87. doi: 10.1016/j.neures.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Maller JJ, Thomson RH, Rosenfeld JV, Anderson R, Daskalakis ZJ, Fitzgerald PB. Occipital bending in depression. Brain. 2014;137:1830–1837. doi: 10.1093/brain/awu072. [DOI] [PubMed] [Google Scholar]
- 48.Perrin JS, Merz S, Bennett DM, Currie J, Steele DJ, Reid IC, et al. Electroconvulsive therapy reduces frontal cortical connectivity in severe depressive disorder. Proc Natl Acad Sci U S A. 2012;109:5464–5468. doi: 10.1073/pnas.1117206109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saletu B, Anderer P, Saletu-Zyhlarz GM. EEG Topography and Tomography (LORETA) in Diagnosis and Pharmacotherapy of Depression. Clin EEG Neurosci. 2010;41:203–210. doi: 10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- 50.Coan JA, Allen JJ. Frontal EEG asymmetry as a moderator and mediator of emotion. Biol Psychol. 2004;67:7–50. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 51.Grimm S, Schmidt CF, Bermpohl F, Heinzel A, Dahlem Y, Wyss M, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex—an fMRI study. Neuroimage. 2006;30:325–340. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Smith R, Chen K, Baxter L, Fort C, Lane RD. Antidepressant effects of sertraline associated with volume increases in dorsolateral prefrontal cortex. J Affect Disord. 2013;146:414–419. doi: 10.1016/j.jad.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 53.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognit, Affect, Behav Neurosci. 2003;3:207–233. doi: 10.3758/CABN.3.3.207. [DOI] [PubMed] [Google Scholar]