Abstract
Phosphofructokinase-1 (PFK-1), a major regulatory glycolytic enzyme, has been implicated in the functions of astrocytes and neurons. Here, we report that PFK-1 negatively regulates neurogenesis from neural stem cells (NSCs) by targeting pro-neural transcriptional factors. Using in vitro assays, we found that PFK-1 knockdown enhanced, and PFK-1 overexpression inhibited the neuronal differentiation of NSCs, which was consistent with the findings from NSCs subjected to 5 h of hypoxia. Meanwhile, the neurogenesis induced by PFK-1 knockdown was attributed to the increased proliferation of neural progenitors and the commitment of NSCs to the neuronal lineage. Similarly, in vivo knockdown of PFK-1 also increased neurogenesis in the dentate gyrus of the hippocampus. Finally, we demonstrated that the neurogenesis mediated by PFK-1 was likely achieved by targeting mammalian achaete-scute homologue-1 (Mash 1), neuronal differentiation factor (NeuroD), and sex-determining region Y (SRY)-related HMG box 2 (Sox2). All together, our results reveal PFK-1 as an important regulator of neurogenesis.
Keywords: Phosphofructokinase-1, Neural stem cell, Neurogenesis, Neuronal differentiation, Proliferation, Hypoxia
Introduction
Neural stem cells (NSCs), present in both the developing and adult nervous system, can not only self-renew by mitotic cell division, but can also differentiate into a diverse range of specialized cell types, such as neurons, astrocytes, and oligodendrocytes [1, 2]. In adult mammalian brain, two principal neurogenic regions are the subventricular zone, which lines the border between the lateral ventricle and the striatum, and the subgranular zone of the dentate gyrus (DG) in the hippocampus [3–5]. NSCs in these neurogenic niches undergo proliferation, migration, differentiation, and eventual integration into the existing network circuitry, contributing to normal functioning of the nervous system [6, 7]. Due to their proliferative capacity and multi-directional differentiation potential, NSCs provide an attractive candidate for the reconstruction of damaged neural circuitry [7–11]. More importantly, if they differentiate into the optimal types and numbers of neurons, NSCs could be an ideal therapeutic tool for neurodegenerative disorders as well as stroke. Thus, it is critical to illuminate the mechanisms of NSC maintenance and fate specification, especially under pathological conditions such as hypoxia, which is generally caused by ischemic stroke and plays an important role in the development of NSCs [12].
Phosphofructokinase-1 (PFK-1) is a rate-limiting enzyme in glycolysis by phosphorylating fructose-6-phosphate to form fructose-1, 6-bisphosphate, and is involved in the regulation of peripheral muscle fatigue by interaction with neuronal nitric oxide synthase [13, 14]. As a pacemaker of glycolysis, PFK-1 is intricately regulated by various signals, including its reaction products, substrates, and other cellular metabolites [13, 15]. For example, PFK-1 can be inhibited by ATP, citrate, lactate, and long-chain fatty acids at different levels [15–17]. The most potent allosteric activator of PFK-1 is fructose-2, 6-bisphosphate whose biosynthesis is almost exclusively dependent on 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase-3 (PFKFB3) [18]. In addition, PFK-1 is tightly regulated by changes in cellular metabolism and physiology, such as signals from cell proliferation and tumor growth [19, 20].
PFK-1 is also involved in mediating the functions of the central nervous system [21–24]. Increasing evidence indicates that nitric oxide-mediated inhibition of mitochondrial respiration is anti-apoptotic in astrocytes but pro-apoptotic in neurons, and this has been ascribed to the different glycolytic capacity of the cells [23]. More specifically, the inhibition of respiration redirects glucose flux to the glycolytic pathway to prevent ATP depletion in astrocytes, and confers an anti-apoptotic effect. Unlike astrocytes, neurons do not invoke glycolysis due to the absence of PFKFB3, thereby failing to maintain ATP production [21–24]. In contrast to the detailed reports on astrocytes and neurons, little is known about the effects of PFK-1 on NSCs. Here, using loss- and gain-of-function approaches, we showed that PFK-1 negatively regulates neurogenesis by mediating the proliferation of neural progenitors and the neuronal fate commitment of NSCs, and that this effect is probably achieved by modulating mammalian achaete-scute homologue-1 (Mash 1), neuronal differentiation factor (NeuroD), and sex-determining region Y (SRY)-related HMG box 2 (Sox2).
Materials and Methods
Animals
The experimental protocol was approved by the Animal Care and Use Committee of Nanjing Medical University. C57/BL/6 mice (6–7 weeks old) were maintained at a controlled temperature (20 ± 2 °C) and group-housed (12-h light/dark cycle) with access to food and water ad libitum.
Cell Culture
Embryonic NSCs were isolated from embryonic day 14 (E14) mouse cortex as we previously described [25]. Cells were floating cultured in proliferation medium, DMEM/F12 (1:1; Invitrogen, Carlsbad, CA) containing 20 ng/mL basic fibroblast growth factor (Sigma-Aldrich, St Louis, MO), 20 ng/mL epidermal growth factor (Sigma-Aldrich) and 2% B27 supplements (Invitrogen), and passaged every 4–6 days when neurospheres with a diameter of 100 μm were formed. These embryonic NSCs were still able to proliferate and self-renew, and to generate differentiated progeny until passage 10 [25]. Embryonic NSCs from passages 2–5 were used in this study.
Cultures were maintained in an incubator (HERAcell 150, Thermo Fisher Scientific, Waltham, MA) with a humidified atmosphere of 95% air and 5% CO2 at 37 °C, except for the hypoxia experiments.
NSC Differentiation
Monolayer-cultured NSCs were allowed to differentiate in growth factor-free DMEM/F12 containing 2% B27 and 0.5% fetal bovine serum. After the indicated times of differentiation, cells were fixed for immunohistochemistry or collected for western blot analysis.
Cell Proliferation Assays
Cell proliferation was assessed by bromodeoxyuridine (BrdU) incorporation in monolayer-cultured NSCs. NSCs infected with LV-PFK-1-shRNA or LV-Control-shRNA were plated on polyornithine/laminin-coated coverslips and cultured for 4 days. During the last 24 h, cultures were incubated with 10 μmol/L BrdU (Sigma-Aldrich) and then fixed for BrdU staining.
Hypoxia
Six hours after plating, NSCs were exposed to hypoxia. Briefly, cells were rinsed twice with growth factor-free DMEM/F12 containing 2% B27 and 0.5% fetal bovine serum and incubated in a hypoxia chamber (MACS MICS Jar Gassing System, Don Whitley Scientific, Shipley, UK). The chamber was flushed with a mixture of 95% N2 and 5% CO2 through two anaerobic cycles, and then sealed and maintained at 37 °C for the indicated times. After hypoxia, the cultures were immediately collected for western blot analysis or returned to a normoxic incubator, maintained for 4 days, and then fixed for immunofluorescence studies.
Lentivirus Production and Infection
PFK-1 shRNA lentiviral particles expressing green fluorescent protein (GFP) were constructed for the inhibition of PFK-1 expression in mouse cells (LV-PFK-1-shRNA, GeneChem, Shanghai, China). The target sequence used against mouse PFK-1 was 5′-CTAGTGCTACGGCTAGATT-3′. LV-PFK-1-shRNA or LV-Control-shRNA was added into single-cell suspensions of NSCs when passaged [multiplicity of infection (MOI) = 10]. Twenty-four hours later, the medium was completely replaced with fresh proliferation medium. Five days after infection with the lentivirus, NSCs were passaged for experiments.
The lentiviral vector, expressing full-length PFK-1 and GFP, was designed to overexpress PFK-1 (LV-PFK-1-GFP, GeneChem). The experimental procedures of LV-PFK-1-GFP and LV-GFP infection were performed as described above (MOI = 8).
For lentivirus infection in vivo, as we previously reported, stereotaxic surgery was used to deliver 2 μL of virus suspension (LV-PFK-1-shRNA or LV-Control-shRNA) to the right DG of the hippocampus at coordinates 2.3 mm posterior to bregma, 1.3 mm lateral to the midline, and 2.0 mm below the dura (an intermediate region that has partly overlapping characteristics with dorsal and ventral posterior) [26, 27]. The mice were treated with BrdU (50 mg/kg, i.p., four times at 12-h intervals) on days 4 and 5, and sacrificed on days 7 and 14, for immunofluorescence assessment.
Immunofluorescence
Procedures for immunofluorescence in brain sections and cultured cells were performed as we previously reported [25]. The primary antibodies used were as follows: mouse anti-β-III-tubulin (1:300; MAB1637 Millipore, Darmstadt, Germany), mouse anti-nestin (1:100; sc-33677 Santa Cruz, CA, USA), rabbit anti-GFP (1:1000; ab290 Abcam, Cambridge, UK), rabbit anti-doublecortin (DCX, 1:1000; ab18723 Abcam), chicken anti-GFP (1:400; AB16901, Millipore), and mouse anti-BrdU (1:1000; MAB4072 Millipore). The secondary antibodies were goat anti-rabbit Cy3 (1:200; 111-165-003 Chemicon, CA, USA), goat anti-mouse Cy3 (1:200; 115-165-003 Jackson ImmunoResearch, West Grove, PA), goat anti-rabbit Dylight 488 (1:400; 111-485-003 Jackson ImmunoResearch), goat anti-chicken Dylight 488 (1:400; 103-485-155 Jackson ImmunoResearch), and goat anti-mouse 647 (1:300; 115-605-003 Jackson ImmunoResearch). Finally, cell cultures were counterstained with Hoechst 33258 (Sigma-Aldrich) to label nuclei. The percentage of NSCs (neurons) was calculated by the ratio of nestin+ cells (β-III-tubulin+ cells) to Hoechst+ cells, in which Hoechst+ cells was used instead of GFP+ cells due to the high infection efficiency of LV-PFK-1-shRNA and LV-PFK-1-GFP (96% and 94%, respectively). Images were captured with a fluorescence microscope (Axio Imager, Zeiss, Oberkochen, Germany) or a confocal laser-scanning microscope (LSM700, Carl Zeiss) and analyzed with Image-Pro Plus software (Media Cybernetics, Silver Spring, MD).
Western Blot Analysis
Western blot analysis was carried out as described in our previous study [28]. The primary antibodies were as follows: rabbit anti-PFK-1 (1:1000; sc-67028 Santa Cruz), rabbit anti-GFP (1:3000; ab290 Abcam), rabbit anti-PFKFB3 (1:500; #9645, CST, Boston, MA), rabbit anti-Sox2 (1:400; sc-20088 Santa Cruz), rabbit anti-NeuroD (1:1000; ab109224 Abcam), and rabbit anti-Mash 1 (1:2000; ab74065 Abcam). The internal control was mouse anti-GAPDH (1:4000; KC-5G4 KangChen Bio-tech, Shanghai, China) or mouse anti-β-actin (1:1000; ab8226, Abcam). Appropriate horseradish peroxidase-linked secondary antibodies were used for detection by enhanced chemiluminescence (Pierce, Rockford, IL).
Time-Lapse Recordings
NSCs infected with LV-Control-shRNA or LV-PFK-1-shRNA were seeded onto polyornithine/laminin-coated 6-well plates and allowed to differentiate for 4 days. During the last 24 h, the cultures were transferred onto a microscope stage, incubated with 5% CO2 and 95% air at 37 °C, and imaged at the 0th hour and 24th hour for analysis of cell death and total cell numbers.
Quantitative Analysis of Neurogenesis from NSCs
The mathematical description of neurogenesis was as described previously [29]. Briefly, Nj+1 is defined as the total cell number present at the beginning of the (j + 1)th day; nj+1 as the total number of neurons (β-III-tubulin+ cells) present at that time; β j as the rate of conversion from progenitors to neurons on that day, and δ j as the death rate of all cells. Thus, the number of neurons present at the beginning of the (j + 1)th day is obtained by the equation nj+1 = nj + β j (Nj − nj) − δ nj nj; in which δ nj refers to the death rate of neurons and the quantity δ nj nj presents the number of neurons dying during the jth day. This equation reveals that the neuron number at the beginning of the (j + 1)th day is composed of the neurons present at the start of the jth day (nj), plus those converted from progenitors, β j (Nj − nj), minus the number of dead neurons. This death number is unknown, but the inequality δ j Nj ≥ δ nj nj ≥ 0 sets limits on neuronal death. These equations, together with the measured numbers of total cells, neurons, and cell death enable us to obtain the maximum and minimum β on each day.
Statistical Analysis
Comparisons among multiple groups were made with one-way ANOVA followed by Scheffe’s post hoc test. Comparisons between two groups were made with a two-tailed Student’s t test. Data are presented as the mean ± SEM, and P < 0.05 was considered statistically significant.
Results
PFK-1 is Involved in Regulating the Neuronal Fate of Embryonic NSCs
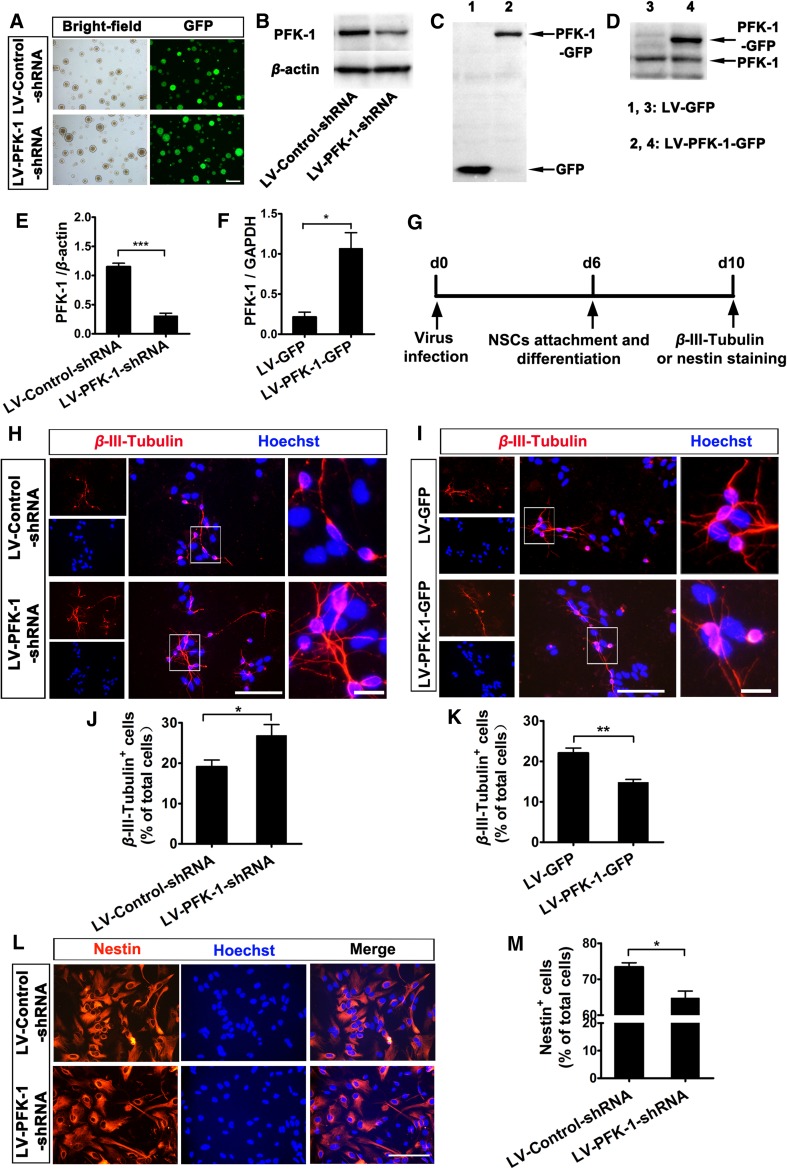
PFK-1, an important control point in the glycolytic pathway, is involved in the development of tumors and is becoming a potential therapeutic target [19, 30]. In the central nervous system, it plays different roles in astrocytes and neurons [21–24], so we investigated whether PFK-1 has an impact on neurogenesis from NSCs. To address this question, we analyzed the effect of PFK-1 knockdown and overexpression on neurogenesis in vitro by generating a lentiviral vector containing shRNA of PFK-1 (LV-PFK-1-shRNA) (Fig. 1A, B, E) and another expressing full-length PFK-1 (LV-PFK-1-GFP) (Fig. 1C, D, F). After 4 days of differentiation, we assessed the expression of β-III-tubulin, a cell-type-specific marker for neurons (Fig. 1G). LV-PFK-1-shRNA-infected NSCs displayed markedly increased neuronal differentiation (Fig. 1H, J), while LV-PFK-1-GFP-infected NSCs showed substantially decreased neuronal differentiation (Fig. 1I, K), suggesting a negative role of PFK-1 in neurogenesis.
Fig. 1.
Phosphofructokinase-1 (PFK-1) is involved in regulating neurogenesis from embryonic neural stem cells (NSCs). A Representative images showing GFP expression in neurospheres infected with LV-Control-shRNA or LV-PFK-1-shRNA (scale bar 200 μm). B, E Immunoblots (B) and statistics (E) showing PFK-1 levels in NSCs infected by LV-Control-shRNA or LV-PFK-1-shRNA. C, D Immunoblots showing the expression of PFK-1-GFP in NSCs infected with LV-GFP or LV-PFK-1-GFP using GFP antibody (C) and PFK-1 antibody (D). F PFK-1 levels in NSCs infected by LV-GFP or LV-PFK-1-GFP. G Schematic of the experimental design for (H–M). Images (H) and statistics (J) of the effect of PFK-1 knockdown on neurogenesis. β-III-tubulin (red), neuronal marker; nuclei were counterstained with Hoechst (blue) (scale bar 100 μm). Images (I) and statistics (K) of the effect of PFK-1 overexpression on neurogenesis. β-III-tubulin (red); Hoechst (blue) (scale bar 100 μm). Images (L) and statistics (M) of the effect of PFK-1 knockdown on the stem-cell-like properties of NSCs. Nestin (red), neural stem/progenitor cell marker; Hoechst (blue) (scale bar 100 μm). Data are mean ± SEM, n = 4, *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed t test).
To further ascertain the effect of PFK-1 on the cell-fate choice of NSCs, we next investigated its role in maintaining stem-cell-like properties by staining for nestin, a neural stem/progenitor cell marker, after 4 days of differentiation (Fig. 1G). As expected, knockdown of PFK-1 resulted in a significant reduction in the percentage of nestin+ cells (Fig. 1L, M), indicating a weakened capacity for NSCs to maintain self-renewal, or an enhanced tendency to differentiate. Therefore, all these results confirmed that PFK-1 negatively regulates neurogenesis from NSCs.
Knockdown of PFK-1 Promotes Neurogenesis in the Dentate Gyrus of the Hippocampus
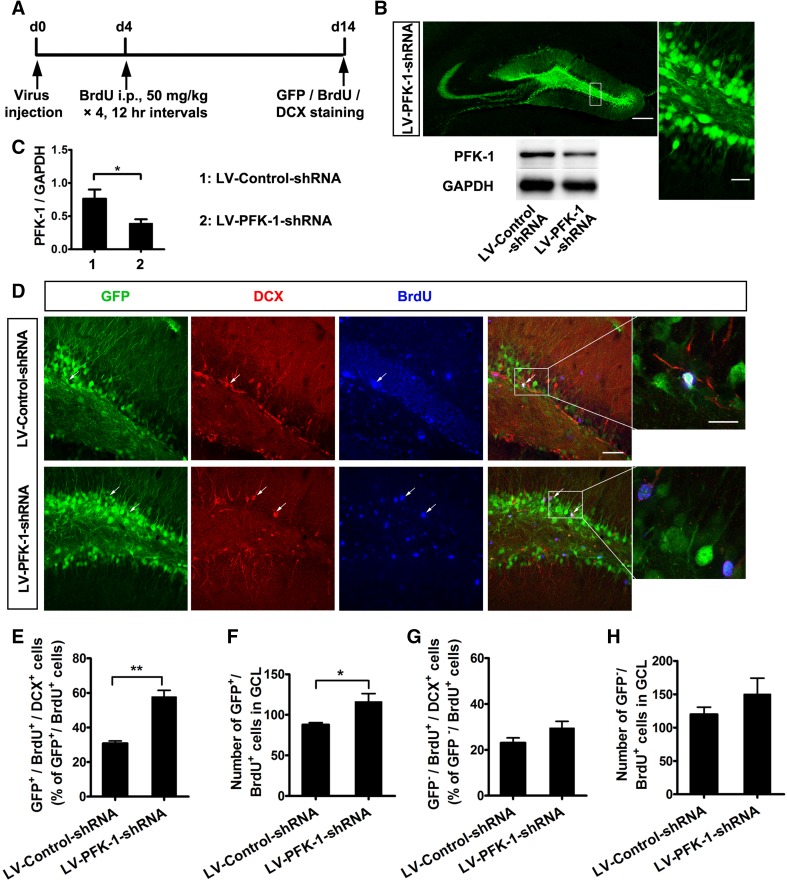
The DG is an important neurogenic niche; neurons generated from this site are responsible for higher cognitive function, most notably memory processes, as well as certain affective behaviors [4]. Thus, we next determined whether PFK-1 affects neurogenesis in the DG. To address this question, we treated mice with BrdU (50 mg/kg, i.p., four times at 12-h intervals), a marker of dividing cells, to label proliferating cells on days 4 and 5 after microinjecting LV-PFK-1-shRNA or LV-Control-shRNA into the DG (Fig. 2A). By seven days after microinjection, LV-PFK-1-shRNA treatment had effectively infected cells in the DG and inhibited PFK-1 expression (Fig. 2B, C). Mice were sacrificed at day 14 to estimate the number of GFP+/BrdU+ cells (proliferating and dividing cells infected by the lentivirus) and GFP+/BrdU+/DCX+ cells (immature neurons differentiated from infected NSCs). In line with the in vitro results, compared with LV-Control-shRNA treatment, LV-PFK-1-shRNA injection significantly raised the numbers of both GFP+/BrdU+/DCX+ newborn neurons (Fig. 2D, E) and GFP+/BrdU+ dividing cells in the granule cell layer of the DG (Fig. 2D, F), suggesting increased neurogenesis from the LV-PFK-1-shRNA-infected NSCs. However, lentiviral delivery of shRNA would result in an overall knockdown of PFK-1 in the entire population of DG cells (i.e., NSCs, neurons, and glial cells), which might interfere with the effect of PFK-1 knockdown on neurogenesis from NSCs. To solve this problem, we assessed neurogenesis, the numbers of newborn neurons (GFP−/BrdU+/DCX+) and dividing cells (GFP−/BrdU+), from NSCs that were not infected by the lentivirus. The results showed no significant difference for both between LV-Control-shRNA treatment and LV-PFK-1-shRNA treatment (Fig. 2G, H), indicating that non-cell-type-specific knockdown of PFK-1 does not play a role in neurogenesis from the uninfected NSCs. Thus, it can be deduced that non-cell-type-specific knockdown of PFK-1 does not affect neurogenesis from the lentivirus-infected NSCs, either. On the whole, these results, in combination with the in vitro findings, demonstrate that knockdown of PFK-1 enhances neurogenesis from NSCs in the DG.
Fig. 2.
Knockdown of PFK-1 promotes neurogenesis in the dentate gyrus (DG) of the hippocampus. A Schematic of experimental design for (D–H). B Left representative image of PFK-1-GFP fluorescence in the DG infected with LV-PFK-1-shRNA (scale bar 200 μm). Right, magnified image from the boxed area in the left image (scale bar 40 μm). Below immunoblots showing PFK-1 levels in NSCs infected by LV-Control-shRNA or LV-PFK-1-shRNA. C Statistics from data in (B) (n = 3). (D) Images of the DG infected with lentiviruses and stained with DCX (red), GFP (green), and BrdU (blue) (scale bar 50 μm). White boxes, regions enlarged in adjacent panels (scale bar 10 μm). E Percentages of GFP+/BrdU+/DCX+ cells in the DG (n = 6). F Numbers of GFP+/BrdU+ cells in the lentivirus-infected area of the granule cell layer (GCL) (n = 6). G Percentages of GFP−/BrdU+/DCX+ cells in the DG (n = 6). H Numbers of GFP−/BrdU+ cells in the lentivirus-infected area of the GCL (n = 6). Mean ± SEM, *P < 0.05, **P < 0.01 (two-tailed t test).
Knockdown of PFK-1 Promotes Neurogenesis by Increasing the Proliferation Rate and Neuronal Fate Commitment of NSCs
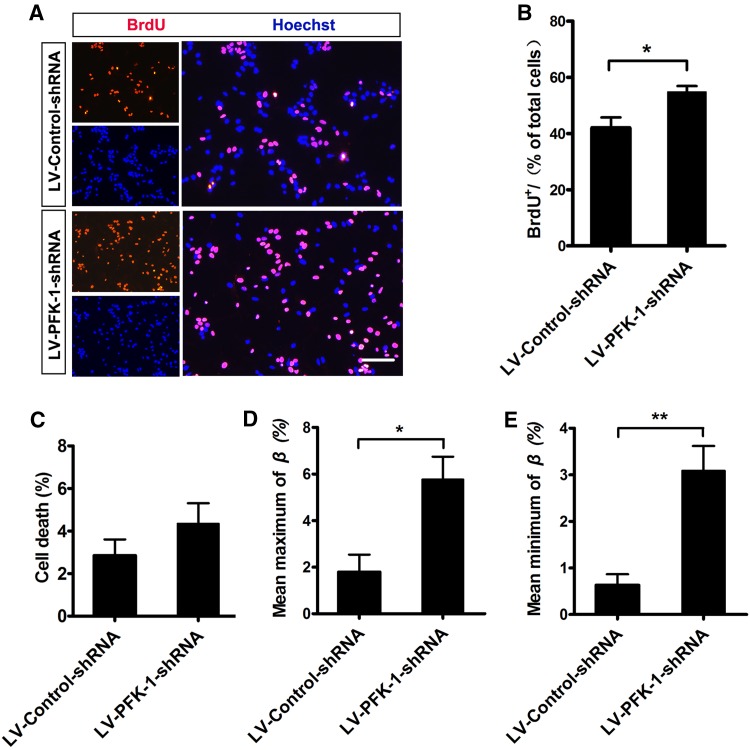
The above findings confirmed that knockdown of PFK-1 increases neurogenesis, and this increase might be attributed to better neuronal survival, enhanced proliferation of progenitors, and/or elevated neuronal cell-fate commitment. Thus, we first explored whether a decrease in neuronal progenitors or neuronal death contributes to neurogenesis. We counted the number of dead cells by Hoechst staining in live cells. The results showed a low percentage of dead cells and no significant difference in both LV-PFK-1-shRNA- and LV-Control-shRNA-infected NSCs (Fig. 3C). Therefore, increased neuronal survival does not explain the neurogenesis induced by LV-PFK-1-shRNA.
Fig. 3.
Knockdown of PFK-1 promotes neurogenesis by increasing the proliferation rate of progenitors and neuronal fate commitment of NSCs. A Images of NSCs with PFK-1 knockdown incubated with bromodeoxyuridine (BrdU) for 24 h, and stained for BrdU (red) and Hoechst (blue) (scale bar 100 μm). B Statistics of data in A (n = 4). C Percentages of cell death in NSCs with or without PFK-1 knockdown (n = 4). D, E Statistics of the maximum (D) and minimum (E) conversion rate (β) of β-III-tubulin− cells to β-III-tubulin+ cells during day 4 (n = 4). Data are mean ± SEM; *P < 0.05, **P < 0.01 (two-tailed t test).
Subsequently, to confirm whether the proliferation of neural progenitors might cause the enhanced neurogenesis, we incubated NSCs with BrdU (10 μmol/L) for 24 h and calculated the percentage of BrdU+ cells. LV-PFK-1-shRNA treatment induced a higher percentage of BrdU+ cells than LV-Control-shRNA treatment (Fig. 3A, B), suggesting that PFK-1 knockdown promotes progenitor division during NSC differentiation. Together, the PFK-1-knockdown-induced neurogenesis is mediated, at least in part, by the increased proliferation of neural progenitors.
Lastly, to illuminate whether the neurogenesis is due to increased neuronal fate commitment of NSCs, we established a mathematical description of neurogenesis, in which β j was defined as the conversion rate of progenitors (β-III-tubulin−) to neurons (β-III-tubulin+) on the jth day, and δ j was defined as the cell death rate during that day [29]. δ j was determined directly by Hoechst staining during a 24-h period. As the cell types of the dead cells remained unknown, the maximum and minimum β j were estimated by assuming that either no neurons were dead or all dead cells were neurons. The results showed that both the maximum and minimum conversion rates of progenitors were markedly higher in LV-PFK-1-shRNA-infected NSCs than in NSCs infected with non-targeting shRNA-expressing control virus (Fig. 3D, E). Taken together, PFK-1 knockdown upregulates neurogenesis by enhancing the proliferation rate of progenitors and potentiating the neuronal fate commitment of NSCs.
PFK-1 Reduces the Neuronal Differentiation of NSCs After Hypoxia
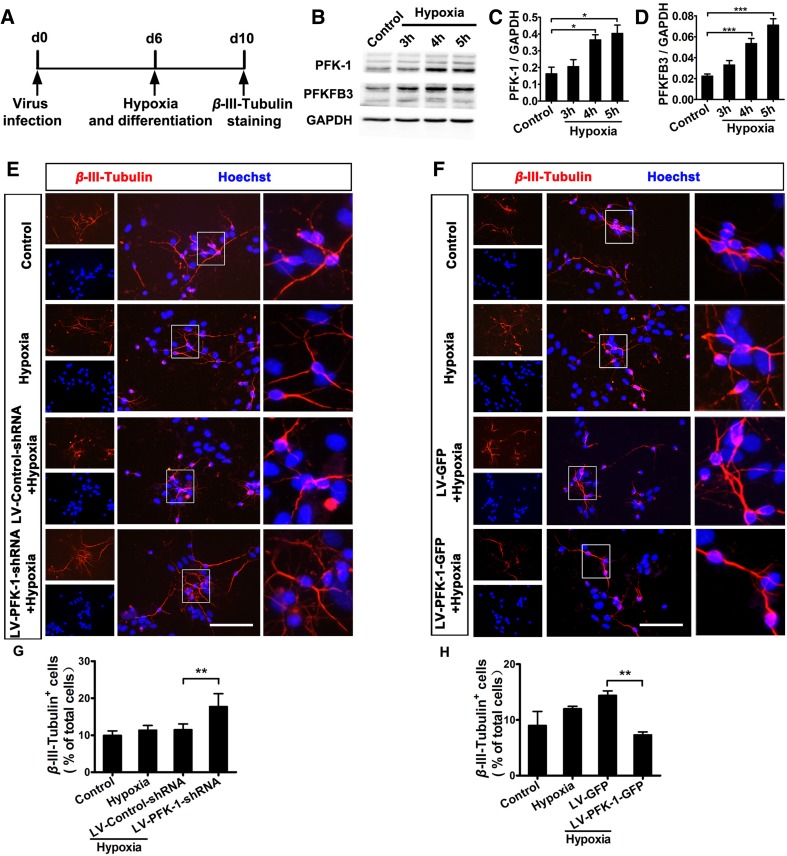
Brain injury such as ischemic stroke impairs axonal structure and function, and eventually disrupts neuronal circuitry [31, 32]. Thus, promoting neurogenesis after brain injury is extremely important for the treatment of ischemic stroke. Here, we set out to determine whether PFK-1 also mediates neurogenesis after hypoxia, which is generally caused by ischemic stroke. To answer the question, we subjected cultured NSCs with PFK-1 knockdown or overexpression to hypoxia for different times, followed by western blot analysis immediately or immunofluorescence after 4 days in normoxic culture (Fig. 4A). The expression levels of PFK-1 and PFKFB3, an enzyme responsible for synthesizing the potent allosteric activator of PFK-1, were significantly upregulated by hypoxia in a time-dependent manner (Fig. 4B–D). Meanwhile, a slight increase of neuronal differentiation of NSCs was also recorded (Fig. 4E–H), which, however, was inconsistent with the physiological finding that overexpressing PFK-1 in NSCs markedly inhibited neuronal differentiation (Fig. 1I, K). This inconsistency might be ascribed to some factors induced by hypoxia that are involved in hypoxia-induced neurogenesis such as Notch 1 signaling [33–35]. Notch 1 is known to be crucial for the self-renewal and fate-decision of NSCs and promotes ischemia-induced neurogenesis in the hippocampus by cleaving Notch 1 and increasing the level of NICD (notch intracellular domain), the active form of Notch 1 that translocates to the nucleus and regulates transcription [36, 37]. In addition, LV-PFK-1-shRNA treatment, as expected, significantly elevated the neuronal differentiation of NSCs compared with LV-Control-shRNA treatment after hypoxia (Fig. 4E, G). In contrast, overexpression of PFK-1 in NSCs markedly reversed the above phenotype (Fig. 4F, H). All together, the above results show that PFK-1 inhibits neurogenesis from NSCs after hypoxia.
Fig. 4.
Effect of PFK-1 on neuronal differentiation of NSCs after hypoxia. A Schematic of experimental design for (B–H). B Expression levels of PFK-1 and PFKFB3 in NSCs after hypoxia. Statistics are shown in C and D, respectively (n = 4). E Images of NSCs with or without PFK-1 knockdown allowed to differentiate for 4 days after 5-h hypoxia, followed by fixation and staining for β-III-tubulin (red) and Hoechst (blue). F Images of NSCs with or without PFK-1 overexpression allowed to differentiate for 4 days after 5-h hypoxia, followed by fixation and staining for β-III-tubulin (red) and Hoechst (blue). G Statistics of data in E (n = 4). H Statistics of data in F (n = 4). Scale bars 100 μm; mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001 (ANOVA).
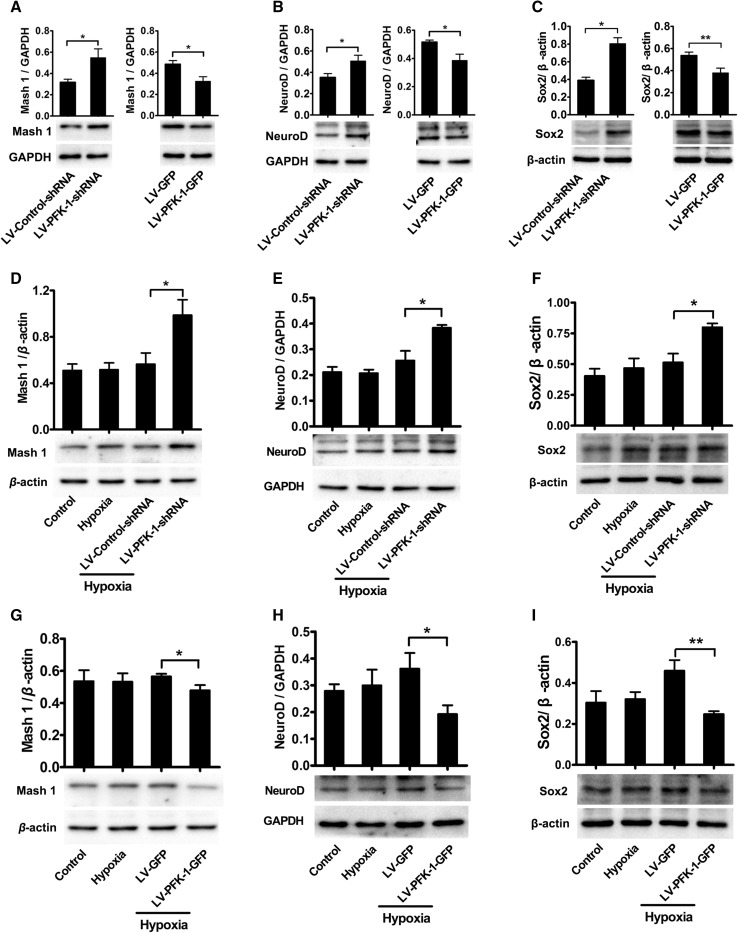
Mash 1, NeuroD, and Sox2 are Involved in the PFK-1-Mediated Neuronal Differentiation of NSCs
Mash 1 (also called Ascl1) and NeuroD, neuron-specific transcription factors, are critical for the regulation of neurogenesis [38, 39]. Accordingly, we investigated whether knockdown or overexpression of PFK-1 alters their expression in vitro. We allowed NSCs with PFK-1 knockdown or overexpression to differentiate for 6 h followed by western blot analysis, and found that PFK-1 knockdown significantly increased Mash 1 and NeuroD expression (Fig. 5A, B). In contrast, overexpression of PFK-1 decreased the expression levels of both (Fig. 5A, B), indicating that PFK-1 might attenuate neurogenesis by regulating neural transcription factors. In addition, we analyzed the expression levels of Mash 1 and NeuroD after 5 h of hypoxia. Similarly, knockdown of PFK-1 increased and its overexpression decreased the expression of both transcription factors (Fig. 5D, E, G, H). Sox2, a well-established NSC marker, has also been proposed to direct cells to the neuronal lineage by regulating neurogenic factors and plays a critical role in the maintenance of neurons in the hippocampus [40–42]. Here, consistent with the above reports, Sox2 showed an expression profile similar to NeuroD and Mash 1 (Fig. 5C, F, I). Collectively, these data suggest that PFK-1 negatively regulates neurogenesis from NSCs by regulating Sox2, Mash 1, and NeuroD.
Fig. 5.
PFK-1 negatively regulates neurogenesis via Mash 1, NeuroD, and Sox2. A–C Immunoblots (lower panels) and statistics (upper panels) of Mash 1 (A), NeuroD (B), and Sox2 (C) expression in NSCs with PFK-1 knockdown or overexpression (n = 4); the expression of Mash 1 (D), NeuroD (E), and Sox2 (F) in NSCs with PFK-1 knockdown after 5 h of hypoxia (n = 4); and the expression of Mash 1 (G), NeuroD (H), and Sox2 (I) in NSCs with PFK-1 overexpression after 5 h of hypoxia (n = 4). Data are mean ± SEM; *P < 0.05, **P < 0.01 (two-tailed t test for A–C, ANOVA for D–I).
Discussion
Neurogenesis, a dynamic, subtly-tuned process modulated by many physiological, pathological, and pharmacological stimuli, occurs extensively during embryonic and perinatal stages, but is limited in the adult central nervous system [6]. Several lines of evidence have shown that increased neurogenesis after brain injury improves cognitive outcome and promotes repair [43, 44]. Thus, if enhanced, neurogenesis may offer a better treatment alternative, and is poised to make another leap forward. Here, we showed that PFK-1 negatively controls the neuronal cell fate of NSCs, and knockdown of PFK-1 benefits neurogenesis by potentiating neuronal lineage commitment and expanding the population of progenitors.
The role of PFK-1 in proliferating cells is well established [19, 45]. Evidence shows that PFK-1 glycosylation, inhibiting the activity of PFK-1, suppresses the glycolytic pathway and promotes cancer cell proliferation by redirecting glucose flux through the pentose phosphate pathway [19]. Different roles of PFK-1 have also been reported in neurons and astrocytes [23]. In addition, a rapid loss of glycolysis has been reported in the early stages of differentiation of human embryonic stem cells [46]. In agreement with the above findings in human embryonic stem cells, we found that in vitro knockdown of PFK-1 directs NSCs to differentiate into neurons, as revealed by the increase in β-III-tubulin+ cells, and lose the stem cell-like phenotype, as shown by a decreased population of nestin+ cells. Consistently, this enhanced neurogenesis was also found in vivo by stereotaxic injection of the lentiviral-shRNA against PFK-1 into the DG. Indeed, PFK-1 knockdown in the DG not only targeted NSCs, but also the entire population of DG cells, which might have affected neurogenesis from NSCs, including lentivirus-infected and uninfected NSCs. Importantly, this non-cell-type-specific knockdown, did not affect neurogenesis from the uninfected NSCs. Thus, it can be concluded that in vivo neurogenesis from infected NSCs is mainly mediated by PFK-1-knockdown in NSCs, rather than in other cells.
A major question arising from this study is how PFK-1 knockdown affects neurogenesis. Evidence has shown that enhanced proliferation of progenitors, better neuronal survival, and increased neuronal fate commitment of NSCs are factors that can contribute to neurogenesis [29]. In the present study, by establishing a quantitative approach, we confirmed that both increased proliferation of progenitors and neuronal fate commitment contribute to the neurogenesis induced by PFK-1 knockdown.
Neurogenesis and neuroregeneration promote functional recovery after ischemic stroke, which causes local hypoxia [31, 43, 44]. Here, we showed that after hypoxia, PFK-1 knockdown increased, and PFK-1 overexpression inhibited neurogenesis from NSCs, suggesting an important role for PFK-1 in the regulation of neurogenesis under pathological conditions. Moreover, it is interesting that physiologically, the results that PFK-1 overexpression inhibited neurogenesis were inconsistent with the findings after hypoxia that displayed a significant increase in the expression of PFK-1, as well as a slight elevation in the neuronal differentiation of NSCs. There are at least two explanations for this inconsistency. One might be that some factors induced by hypoxia actively regulate neurogenesis, thereby counteracting the inhibitory effect of PFK-1. For example, Notch 1, a cell-surface transmembrane receptor, is known to control the proliferation and fate of embryonic NSCs and promotes ischemia-induced neurogenesis after activation by ischemia–reperfusion injury [36, 37]. Recently, researchers have also found that hypoxia increases the expression levels of genes downstream from NICD and Notch1, and therefore promotes neurogenesis in the hippocampus [33]. Alternatively, other molecular mechanisms underly the PFK-1-mediated neurogenesis; this needs further elucidation.
Mash 1 and NeuroD are main regulators of neuronal differentiation in the mammalian brain [38, 39]. During neurogenesis, Mash 1 activates the neurogenic differentiation program by binding to and promoting the opening of closed chromatin regions, while NeuroD facilitates the survival, differentiation, and maturation of neurons, and promotes their integration into the neuronal circuitry [38, 39]. What is more, Sox2 promotes adult hippocampal neurogenesis by activating the expression of Mash 1 and NeuroD, although its role in the maintenance of pluripotency in NSCs is well documented [40, 41, 47]. These findings raise the possibility that PFK-1 may control the fate of NSCs via regulating Sox2, as well as Mash 1 and NeuroD. Here, we showed that PFK-1 negatively regulates neurogenesis by targeting these transcriptional factors.
In summary, the data presented here show that knockdown of PFK-1 promotes neurogenesis by enhancing the proliferation of neural progenitors and instructing these progenitors to adopt a neuronal fate, and this effect is achieved by the upregulation of Mash 1, NeuroD, and Sox2. However, additional studies are necessary to further characterize the effects of PFK-1 on neurogenesis, such as the role of PFK-1 overexpression in neurogenesis in vivo, and whether the new neurons generated from the NSCs with PFK-1 knockdown are functionally integrated into existing neuronal circuits and ameliorate neurological deficits in diseases.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (91232304, 31530091, and 81571188), the National Basic Research Development Program (973 Program) of China (2011CB504404), the Natural Science Foundation of Jiangsu Province, China (BK2011029 and BK20130040), and the Collaborative Innovation Center For Cardiovascular Disease Translational Medicine.
References
- 1.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 2.Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 4.Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowther AJ, Song J. Activity-dependent signaling mechanisms regulating adult hippocampal neural stem cells and their progeny. Neurosci Bull. 2014;30:542–556. doi: 10.1007/s12264-014-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 7.Lledo PM, Alonso M, Grubb MS. Adult neurogenesis and functional plasticity in neuronal circuits. Nat Rev Neurosci. 2006;7:179–193. doi: 10.1038/nrn1867. [DOI] [PubMed] [Google Scholar]
- 8.Winner B, Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2015;7:a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chern CM, Wang YH, Liou KT, Hou YC, Chen CC, Shen YC. 2-Methoxystypandrone ameliorates brain function through preserving BBB integrity and promoting neurogenesis in mice with acute ischemic stroke. Biochem Pharmacol. 2014;87:502–514. doi: 10.1016/j.bcp.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Shen T, Pu J, Zheng T, Zhang B. Induced neural stem/precursor cells for fundamental studies and potential application in neurodegenerative diseases. Neurosci Bull. 2015;31:589–600. doi: 10.1007/s12264-015-1527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Y, Tan S. Direct lineage conversion of astrocytes to induced neural stem cells or neurons. Neurosci Bull. 2015;31:357–367. doi: 10.1007/s12264-014-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braunschweig L, Meyer AK, Wagenfuhr L, Storch A. Oxygen regulates proliferation of neural stem cells through Wnt/beta-catenin signalling. Mol Cell Neurosci. 2015;67:84–92. doi: 10.1016/j.mcn.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Mor I, Cheung EC, Vousden KH. Control of glycolysis through regulation of PFK1: old friends and recent additions. Cold Spring Harb Symp Quant Biol. 2011;76:211–216. doi: 10.1101/sqb.2011.76.010868. [DOI] [PubMed] [Google Scholar]
- 14.Wehling-Henricks M, Oltmann M, Rinaldi C, Myung KH, Tidball JG. Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphofructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy. Hum Mol Genet. 2009;18:3439–3451. doi: 10.1093/hmg/ddp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sola-Penna M, Da Silva D, Coelho WS, Marinho-Carvalho MM, Zancan P. Regulation of mammalian muscle type 6-phosphofructo-1-kinase and its implication for the control of the metabolism. IUBMB Life. 2010;62:791–796. doi: 10.1002/iub.393. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins CM, Yang J, Sims HF, Gross RW. Reversible high affinity inhibition of phosphofructokinase-1 by acyl-CoA: a mechanism integrating glycolytic flux with lipid metabolism. J Biol Chem. 2011;286:11937–11950. doi: 10.1074/jbc.M110.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa Leite T, Da Silva D, Guimaraes Coelho R, Zancan P, Sola-Penna M. Lactate favours the dissociation of skeletal muscle 6-phosphofructo-1-kinase tetramers down-regulating the enzyme and muscle glycolysis. Biochem J 2007, 408: 123–130. [DOI] [PMC free article] [PubMed]
- 18.Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. Biochem J. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, 3rd, et al. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yalcin A, Telang S, Clem B, Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Exp Mol Pathol. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Rodriguez P, Fernandez E, Almeida A, Bolanos JP. Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell Death Differ. 2012;19:1582–1589. doi: 10.1038/cdd.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci U S A. 2001;98:15294–15299. doi: 10.1073/pnas.261560998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almeida A, Moncada S, Bolanos JP. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol. 2004;6:45–51. doi: 10.1038/ncb1080. [DOI] [PubMed] [Google Scholar]
- 25.Luo CX, Jin X, Cao CC, Zhu MM, Wang B, Chang L, et al. Bidirectional regulation of neurogenesis by neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells. 2010;28:2041–2052. doi: 10.1002/stem.522. [DOI] [PubMed] [Google Scholar]
- 26.Zhu LJ, Li TY, Luo CX, Jiang N, Chang L, Lin YH, et al. CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat Med. 2014;20:1050–1054. doi: 10.1038/nm.3644. [DOI] [PubMed] [Google Scholar]
- 27.Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu M, Sun YJ, Zhou QG, Chen L, Hu Y, Luo CX, et al. Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors. J Neurochem. 2008;106:1900–1913. doi: 10.1111/j.1471-4159.2008.05554.x. [DOI] [PubMed] [Google Scholar]
- 29.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 30.Al Hasawi N, Alkandari MF, Luqmani YA. Phosphofructokinase: a mediator of glycolytic flux in cancer progression. Crit Rev Oncol Hematol 2014, 92: 312–321. [DOI] [PubMed]
- 31.Luo CX, Lin YH, Qian XD, Tang Y, Zhou HH, Jin X, et al. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J Neurosci. 2014;34:13535–13548. doi: 10.1523/JNEUROSCI.1305-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Q, Liao SJ, Yu J. Axon guidance factor netrin-1 and its receptors regulate angiogenesis after cerebral ischemia. Neurosci Bull. 2014;30:683–691. doi: 10.1007/s12264-013-1441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, Zhao T, Huang X, Wu LY, Wu K, Zhu LL, et al. Notch1 mediates postnatal neurogenesis in hippocampus enhanced by intermittent hypoxia. Neurobiol Dis. 2014;64:66–78. doi: 10.1016/j.nbd.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Gui M, Lu X, Jin D, Zhuang Z, Yan T. Electroacupuncture promotes neural stem cell proliferation and neurogenesis in the dentate gyrus of rats following stroke via upregulation of Notch1 expression. Mol Med Rep. 2015;12:6911–6917. doi: 10.3892/mmr.2015.4279. [DOI] [PubMed] [Google Scholar]
- 35.Xiong W, Morillo SA, Rebay I. The Abelson tyrosine kinase regulates Notch endocytosis and signaling to maintain neuronal cell fate in Drosophila photoreceptors. Development. 2013;140:176–184. doi: 10.1242/dev.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X, Mao X, Xie L, Greenberg DA, Jin K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009;29:1644–1654. doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 38.Raposo AA, Vasconcelos FF, Drechsel D, Marie C, Johnston C, Dolle D, et al. Ascl1 Coordinately Regulates Gene Expression and the Chromatin Landscape during Neurogenesis. Cell Rep. 2015;10:1544–1556. doi: 10.1016/j.celrep.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, et al. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2015;112:E1936–1945. doi: 10.1073/pnas.1421480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33: 1962–1974. [DOI] [PubMed]
- 42.Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- 43.Yang LC, Guo H, Zhou H, Suo DQ, Li WJ, Zhou Y, et al. Chronic oleoylethanolamide treatment improves spatial cognitive deficits through enhancing hippocampal neurogenesis after transient focal cerebral ischemia. Biochem Pharmacol. 2015;94:270–281. doi: 10.1016/j.bcp.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Blaya MO, Tsoulfas P, Bramlett HM, Dietrich WD. Neural progenitor cell transplantation promotes neuroprotection, enhances hippocampal neurogenesis, and improves cognitive outcomes after traumatic brain injury. Exp Neurol. 2015;264:67–81. doi: 10.1016/j.expneurol.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G, Xu Z, Wang C, Yao F, Li J, Chen C, et al. Differential phosphofructokinase-1 isoenzyme patterns associated with glycolytic efficiency in human breast cancer and paracancer tissues. Oncol Lett. 2013;6:1701–1706. doi: 10.3892/ol.2013.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moussaieff A, Rouleau M, Kitsberg D, Cohen M, Levy G, Barasch D, et al. Glycolysis-mediated changes in acetyl-CoA and histone acetylation control the early differentiation of embryonic stem cells. Cell Metab. 2015;21:392–402. doi: 10.1016/j.cmet.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/S0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]