Abstract
Mechanosensitive channels mediate touch, hearing, proprioception, and blood pressure regulation. Piezo proteins, including Piezo1 and Piezo2, represent a new class of mechanosensitive channels that have been reported to play key roles in most, if not all, of these modalities. The structural architecture and molecular mechanisms by which Piezos act as mechanosensitive channels, however, remain mysterious. Two new studies have now provided critical insights into the atomic structure and molecular basis of the ion permeation and mechano-gating properties of the Piezo1 channel.
Keywords: Mechanosensation, Mechanosensory, Mechanotransduction, Mechanical, Cryo-EM, Piezo
Mechanosensitive cation channels respond to mechanical forces and allow ions to enter or exit the cell. They have been postulated to serve as a key set of mechanotransducers that convert mechanical stimuli into biological activities such as touch, hearing, proprioception, and blood-pressure regulation [1]. However, the molecular identities of mechanosensitive cation channels in mammals remained mysterious until the identification of the evolutionarily-conserved Piezo family of proteins, including Piezo1 and Piezo2, by Patapoutian and colleagues in 2010 [2]. This landmark discovery has heralded a new era for exploring the physiological significance and molecular mechanisms of mechanosensitive cation channels in mammals [3].
The physiological importance of Piezo proteins in various mechanotransduction processes in mammals has recently been highlighted by knockout studies in mice [1]. Specifically, Piezo1 expressed in endothelia cells plays an essential role in sensing the shear stress caused by blood flow, which is important for proper blood vessel development [1], while Piezo2 in primary sensory neurons and specialized touch receptors located in the skin mediates gentle touch sensation and proprioception [1]. Furthermore, mutations in the human Piezo1 and Piezo2 genes have been linked to various genetic diseases due to alterations in their channel properties [1].
Despite these advances, some major fundamental questions about Piezos remain unsolved. In particular, it remains a mystery as to how Piezo proteins function as mechanosensitive cation channels at the molecular level. Piezos are large transmembrane proteins with >2500 amino-acids and 30–40 putative transmembrane segments [2, 4]. They do not possess notable sequence homology with any known class of ion channels. Patapoutian and colleagues have shown that purified glutathione-S-transferase-tagged mouse Piezo1 proteins form homo-oligomers and are sufficient to mediate spontaneous ruthenium red-sensitive cation currents when reconstituted into lipid bilayers, suggesting that Piezo proteins may form the largest known ion channel complex in the plasma membrane [5]. These unique features make structure–function studies of Piezo proteins highly challenging, partly explaining the relatively slow progress in our understanding of the molecular basis of the ion permeation and mechanno-gating properties of this novel class of ion channels.
Two new studies from Bailong Xiao and colleagues at Tsinghua University have now made major breakthroughs in demystifying these channels. They first reported in Nature a cryo-electron microscopy (cryo-EM) structure of the full-length mouse Piezo1 protein (2547 residues per monomer), revealing its overall architecture and distinct domains [6]. In another study published recently in Neuron, on the basis of the Piezo1 structure they carried out a comprehensive structure-guided functional analysis, leading to the identification of the ion-conducting pore and key pore determinants, as well as mechanotransduction components, which define the ion-conducting properties and gating by mechanical stimuli, respectively [7].
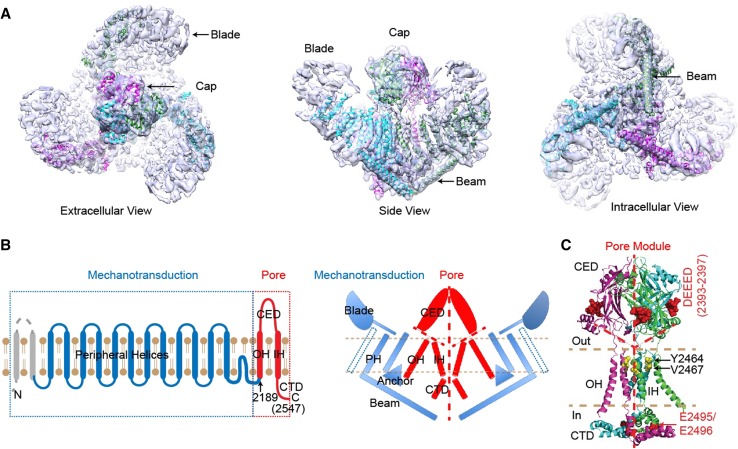
In the first study, by taking advantage of the recent advances in cryo-EM techniques, and in combination with their expertise in protein engineering, expression, and purification in mammalian expression systems, Ge et al. [6] successfully determined a medium-resolution cryo-EM structure of the Piezo1 channel. Strikingly, the Piezo1 protein assembles as a ~0.9 megaDalton trimeric three-bladed, propeller-like architecture comprising a central pore module and three extended peripheral wings (Fig. 1A). The central pore module, which consists of an outer helix, C-terminal extracellular domain, inner helix, and intracellular C-terminal domain (Fig. 1B, C), displays a remarkable architectural similarity to acid-sensing ion channel 1 (ASIC1) and ATP-gated P2X4 receptors, despite their lack of sequence homology. In contrast, the three peripheral wings, which contain the extracellular ‘blades’ of the peripheral transmembrane helix skeleton, ‘anchors’, and intracellular ‘beams’ (see Fig. 1A, B), are absent from other trimeric ion channels, defining the unique structural architecture of Piezo channels. Based on these structural features, the authors speculated that Piezo1 channels sense and transduce forces to control their ion conductance in a way similar to propellers. For instance, the peripheral regions may function as force sensors and transducers to gate the central pore. In line with this idea, comparing the different classes of cryo-EM structures of Piezo1 reveals that the peripheral regions, particularly the ‘blade’ domains, are highly flexible [6].
Fig. 1.
Cryo-EM structure and working module of the mechanosensitive Piezo1 channel (provided by Dr. Bailong Xiao, Tsinghua University). A. The three-bladed, propeller-like cryo-EM structure. B. Illustration of the separable mechanotransduction and pore modules in either a topological (left panel) or a structural model (right panel). C. A structural model of the pore module with key residues highlighted.
In the second study, Xiao’s group has gone further to functionally test this working hypothesis by using chimeras, site-directed mutagenesis, and the substituted cysteine accessibility method [7]. Indeed, they provide compelling evidence that Piezo1 proteins consist of distinct and separable modules responsible for ion conduction and mechanical force sensing/transduction to coordinately fulfill their function as sophisticated mechanosensitive channels (Fig. 1B).
On the basis of the Piezo1 structure, the pore module has been tentatively assigned to the C-terminal region (residues 2189–2547) containing the last two transmembrane segments (Fig. 1B) [6]. To functionally validate this assignment, Zhao et al. took advantage of the distinct pore properties of mouse and fly Piezos. They found that replacing the putative pore region of mouse Piezo1 with that of fly Piezo yields a chimera resembling fly Piezo, demonstrating that the putative pore region indeed determines the essential pore properties. They then systematically investigated the effects of manipulating various parts of the putative ion conduction pathway on unitary conductance, ion selectivity, and channel pharmacology (Fig. 1C). Together with the structure, their data suggest that the C-terminal extracellular domain forms an extracellular ‘cap’ with fenestrations lined by negatively-charged residues that select cation over anion entry to the pore. Using the substituted cysteine accessibility method, they identified two pore-facing residues, Y2464 and V2467, showing that the last transmembrane segment is the pore-lining inner helix. Mutations in two highly-conserved adjacent negatively-charged residues, E2495 and E2496, in the intracellular C-terminal domain affect Ca2+ selectivity, unitary conductance, and ruthenium red blockade, suggesting that these two residues may be part of the Ca2+ and ruthenium red binding site in the intracellular vestibule. Collectively, these data provide strong evidence that the very C-terminal region (residues 2189–2547) encode the pore of the Piezo1 channel.
To address whether the N-terminal peripheral region of Piezo1 is sufficient to gate the central ion-conducting pore, they took advantage of the structural similarity of the pore module between Piezo1 and other trimeric channels such as ASIC1, which itself is not mechanosensitive. Remarkably, replacing the pore region of Piezo1 with that of mouse ASIC1 resulted in a chimeric channel that not only retained its responsiveness to acid but also became mechanosensitive [7]. These data demonstrate that the bulky N-terminal non-pore-containing region is sufficient to confer mechanosensitivity on the trimeric channel pores. This also represents a remarkable example of engineering artificial ion channels with desired properties in a module-like manner.
These two studies have also raised many interesting questions. For instance, exactly how does the pore module control the ion permeation and selection properties? How do the peripheral mechanotransduction modules gate the central pore module? Where are the ‘blade’ domains located in the primary sequence, and do they indeed serve as discrete force sensors? Does Piezo2 share a similar structural architecture and working mechanism with Piezo1? The studies by Xiao and colleagues clearly represent major breakthroughs in our understanding of the structure–function relationship of this evolutionarily conserved, physiologically important class of mechanosensitive cation channels. They also highlight the prowess of structure-guided functional characterization of novel and complicated membrane protein complexes.
References
- 1.Ranade SS, Syeda R, Patapoutian A. Mechanically Activated Ion Channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao R, Xu XZ. Mechanosensitive channels: in touch with Piezo. Curr Biol. 2010;20:R936–R938. doi: 10.1016/j.cub.2010.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, et al. Piezo1 ion channel pore properties are dictated by C-terminal region. Nat Commun. 2015;6:7223. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527:64–69. doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, et al. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron. 2016;89:1248–1263. doi: 10.1016/j.neuron.2016.01.046. [DOI] [PubMed] [Google Scholar]