Abstract
Neurosteroids are synthesized in the nervous system from cholesterol or steroidal precursors imported from peripheral sources. These compounds are important allosteric modulators of γ-aminobutyric acid A receptors (GABAARs), which play a vital role in pain modulation in the lateral thalamus, a main gate where somatosensory information enters the cerebral cortex. Using high-performance liquid chromatography/tandem mass spectrometry, we found increased levels of neurosteroids (pregnenolone, progesterone, deoxycorticosterone, allopregnanolone, and tetrahydrodeoxycorticosterone) in the chronic stage of neuropathic pain (28 days after spared nerve injury) in rats. The expression of the translocator protein TSPO, the upstream steroidogenesis rate-limiting enzyme, increased at the same time. In vivo stereotaxic microinjection of neurosteroids or the TSPO activator AC-5216 into the lateral thalamus (AP −3.0 mm, ML ±3.0 mm, DV 6.0 mm) alleviated the mechanical allodynia in neuropathic pain, while the TSPO inhibitor PK 11195 exacerbated it. The analgesic effects of AC-5216 and neurosteroids were significantly attenuated by the GABAAR antagonist bicuculline. These results suggested that elevated neurosteroids in the lateral thalamus play a protective role in the chronic stage of neuropathic pain.
Keywords: Neurosteroids, Translocator protein, Spared nerve injury, Thalamus, GABAA receptors
Introduction
Steroids were considered to be hormones produced only in endocrine glands such as the adrenals, gonads, and placenta until the 1980s, when Baulieu and colleagues reported higher concentrations of pregnenolone, dehydroepiandrosterone, and their sulfate and lipoid esters in the nervous system than in plasma [1, 2]. Furthermore, steroids remain in the brain long after gonadectomy or adrenalectomy [1, 2]. A series of enzymes for steroidogenesis have been detected in the nervous system [3–5], indicating local synthesis of steroids. These “neurosteroids” include pregnenolone (PREG) and dehydroepiandrosterone as well as their sulfates, progesterone (PROG), deoxycorticosterone (DOC), and reduced metabolites such as allopregnanolone (AP) and tetrahydrodeoxycorticosterone (THDOC).
Fluctuations in neurosteroid concentrations regulate a variety of physiological responses including anxiety, stress, reproduction, and sexual behaviors [3–5]. In general, neurosteroids execute their functions through not only classic steroid hormone nuclear receptors, but also ion-gated neurotransmitter receptors such as γ-aminobutyric acid A receptors (GABAARs) [6–8]. Direct binding of neurosteroids to GABAARs, extrasynaptic receptors containing the δ subunit in particular, results in the potentiation of GABAAR-mediated inhibitory currents [9, 10].
Translocator protein (TSPO, 18 kDa), previously named peripheral benzodiazepine receptor, is a five transmembrane domain protein located predominantly in the outer mitochondrial membrane [11]. It is particularly abundant at the contact sites of outer and inner mitochondrial membranes in steroid-synthesizing tissues, including the brain [11, 12]. TSPO mediates the rate-limiting step of steroidogenesis by translocating the substrate cholesterol from the outer to the inner mitochondrial membrane [13, 14], where it is metabolized to PREG by the mitochondrial cholesterol side-chain cleavage enzyme P450scc [3, 4].
Systemic or intrathecal injection of neurosteroids has strong analgesic effects in animals [15–17]. The lateral thalamus, including the ventral posterior thalamus (VP) and the reticular thalamic nucleus, is a key relay station for the transmission of peripheral somatosensory and nociceptive information to the neocortex [18, 19]. It belongs to the lateral pain pathway [20–22], and processes the sensory-discriminative dimension of pain. Key enzymes in steroidogenesis, as well as synaptic and extrasynaptic GABAARs, are widely expressed in the thalamus [4, 23]. But whether and how neurosteroids in the thalamus participate in pain modulation remains unclear. In the present study, we investigated the levels of five neurosteroids (PREG, PROG, DOC, AP, and THDOC) and the steroidogenesis enzyme TSPO in the lateral thalamus of normal and neuropathic rats, and further investigated their role in pain modulation.
Materials and Methods
Animals
Adult male Sprague-Dawley rats (240 ± 30 g) were provided by the Department of Experimental Animal Sciences, Peking University Health Science Center (Beijing, China). Animals were housed in standard cages with food and water ad libitum under a 12-h light-dark cycle. All experimental procedures were approved by the Animal Care and Use Committee of the University, according to the guidelines of the International Association for the Study of Pain.
Chemicals and Drugs
PREG (purity 98%), PROG (purity 99%), DOC (purity 97%), AP (purity 98%), and THDOC (purity 95%) were from Sigma-Aldrich (St. Louis, MO). 17-alpha-Methyltestosterone (MT, purity 99%) was purchased from Dr. Ehrenstorfer (Augsburg, Germany). AC-5216 (purity 99%) was a gift from Professor Yun-Feng Li in the Beijing Institute of Pharmacology and Toxicology. PK 11195 (purity 98%) was from Cayman (Ann Arbor, MI). Bicuculline (purity 98%) was from Tocris Bioscience (Avonmouth, Bristol, UK).
Spared Nerve Injury (SNI) Model of Neuropathic Pain
The SNI model was established in rats as previously described [24, 25]. In brief, the left common peroneal and tibial nerves were tightly ligated with 5.0 silk sutures, and sectioned distal to the ligation with removal of 2–4 mm of the nerve stump, leaving the sural nerve intact. Sham-surgery rats experienced all surgical procedures except for nerve injury.
Mechanical Allodynia Measured as 50% Paw Withdrawal Threshold (PWT)
Rats were habituated to a transparent plastic box on a metal mesh floor before testing. Von Frey filaments (0.41–15.1 g; North Coast, Gilroy, CA) were applied to the lateral plantar surface of the hind paws (i.e., to the receptive field of the sural nerve). The 50% PWT was calculated by the “up and down” method as described by Chaplan et al. [26–29]. These behavioral tests were designed and carried out single-blindly.
Mechanical allodynia was measured 1 day before and 3, 7, 14, 21, and 28 days after SNI or sham surgery.
Measurement of Neurosteroid Levels by High Performance Liquid Chromatography/Tandem Mass Spectrometry (HPLC/MS)
After isoflurane anesthesia, rats were quickly decapitated between 16:00 and 18:00. Brains were removed rapidly and placed on ice. Bilateral cerebral cortices and hippocampi were dissected to expose the thalamus. The lateral thalamus [mainly the VP, with part of the nearby reticular nucleus (40–80 mg)] was isolated according to the atlas of Paxinos and Watson [30] and stored in liquid nitrogen. After weighing, samples were homogenized in 500 μL water with 10 μL MT as internal standard (0.3 pg/mL in methanol) on ice. The homogenates were extracted with 1,200 μL of ethyl acetate/hexane (9:1, V/V), and vortexed for 5 min. After centrifugation at 12,000 rpm for 5 min, the clear supernatant was removed to another Eppendorf tube, dried under nitrogen, and re-suspended with 100 μL of 0.1% formic acid aqueous/methanol (1:1, V/V). The samples were centrifuged at 12,000 rpm for 15 min and the supernatants were transferred to autosampler vials for HPLC/MS analysis.
PREG, PROG, DOC, AP, and THDOC levels were measured by HPLC/MS according to the protocol described by Liu [31] with minor modification. The brain extracts were analyzed on a Zorbax SB-C18 column (50 mm × 4.6 mm, 1.8 μm, Agilent, Santa Clara, CA). The mobile phase was composed of 5 mmol/L ammonium acetate in water (A) and methanol (B) using the following gradient program: 0 to 2.5 min, isocratic at 60% B; 2.5 to 5.5 min, linear gradient from 60% to 95% B; 5.5 to 7.0 min, isocratic at 95% B; 7.0 to 7.1 min, linear gradient from 95% to 60% B; 7.1 to 10.0 min, isocratic at 60% B. The flow-rate was 0.50 mL/min, and the column temperature was 45°C. The injection volume was 10 μL. MT was used as the internal standard for quantitative analysis. The chromatographic conditions were optimized by analyzing standard solutions and also spiked blank extracts.
Tandem MS detection was performed using an AB SCIEX QTRAP (6500, Applied Biosystems, Foster City, CA) triple quadrupole mass analyzer equipped with an ESI ion source operating at room temperature in the positive mode. Detection and quantitation of all analytes were accomplished using multiple reaction monitoring with two transitions monitored per analyte. Analyst Software (Applied Biosystems) was used for instrument control, data acquisition, and qualitative and quantitative data analysis. The analytical MS parameters of the target compounds are listed in Table 1.
Table 1.
Mass spectrometry parameters for target neurosteroids.
| Analytes | Parent ion (m/z) | Product ion (m/z) | Retention time (min) | Declustering potential (V) | Collision energy (eV) |
|---|---|---|---|---|---|
| Pregnenolone | 317.2 | 281.2 | 7.0 | 36 | 16.4 |
| 159.1a | 30.8 | ||||
| Progesterone | 315.5 | 109.1 | 6.7 | 76 | 34.0 |
| 97.0a | 26.6 | ||||
| 11-Deoxycorticosterone | 331.5 | 109.0 | 5.6 | 79 | 26.0 |
| 97.0a | 27.0 | ||||
| Allopregnanolone | 319.2 | 301.2a | 7.4 | 25 | 13.5 |
| 213.2 | 32.8 | ||||
| Tetrahydrodeoxycorticosterone | 352.2 | 317.2a | 6.7 | 40 | 21.2 |
| 335.2 | 27.4 | ||||
| 17-alpha-Methyltestosterone | 303.5 | 109.1 | 6.2 | 66 | 37.8 |
| 97.0 | 33.9 |
aQuantitative ion.
Neurosteroid levels were measured 7, 14, and 28 days after SNI or sham surgery.
Cannula Implantation and Drug Microinjection
Rats were deeply anaesthetized with 1% pentobarbital sodium in saline (50 mg/kg, i.p.). Infusion guide cannulae (internal diameter, 0.38 mm; RWD Life Science, Shenzhen, China) were stereotaxically implanted above the VP nuclei on both sides (AP −3.0 mm, ML ±3.0 mm, and DV 1.0 mm) [30]. A sterile obturator was left inside each cannula until drug delivery. Rats were allowed to recover for at least 7 days before further experiments.
For drug delivery, the injection needle (internal diameter, 0.20 mm; 5 mm longer than the matched cannula) was introduced through the guide cannula until its lower end was 5 mm below the cannula, into the VP. The TSPO activator AC-5216 was prepared as a suspension in tragacanth gum (0.5% aqueous final solution). PK 11195, AP, DOC, and bicuculline were dissolved in 100% DMSO and stored in sterile aliquots at −40°C. Immediately prior to administration, aliquots were thawed and diluted to final concentrations of 0.4, 1.2, and 4.0 μg/μL in artificial cerebrospinal fluid (<4% DMSO in final solution). Drugs or vehicle solutions (1 μL) were microinjected into both VP nuclei using a polyethylene catheter (PE-10) connected to a 2-μL syringe needle (Gaoge, Shanghai, China) over 2 min. The needle was left in situ for 1 min to maximize diffusion. The 50% PWTs before and 1 h (AC-5216, AP, DOC) or 4 h (PK 11195) after drug delivery were measured by another experimenter who was single-blinded to drugs and animal groupings. Bicuculline (1.0 μg/μL) was injected 0.5 h prior to AC-5216, AP, or DOC injection. Vehicle injections served as controls.
Drugs were microinjected into rats 28 days after SNI.
Immunofluorescent Detection of TSPO Expression in the Lateral Thalamus
Rats were deeply anesthetized with 1% sodium pentobarbital in saline (50 mg/kg, i.p.) and perfused intracardially with 400 mL of 0.9% saline, followed by 400 mL of 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.2–7.4, 4°C). The brains were removed, post-fixed overnight at 4°C, and kept in 30% sucrose in 0.1 mol/L phosphate-buffered saline (PBS) for dehydration. Coronal sections (30 μm) were cut on a cryostat and kept in anti-freezing fluid (30% ethanediol, 20% propanetriol, and 50% 0.01 mol/L PBS) at −20°C. Sections were washed twice in 0.01 mol/L PBS for 10 min each and incubated in 0.01 mol/L citrate buffer (pH 6.0) for 30 min at 37°C for antigen retrieval. After washing, sections were incubated in 0.3% Triton X-100 for 30 min at room temperature. They were next blocked for 1 h in 3% bovine serum albumin (0.01 mol/L PBS with 0.3% Triton X-100). All cryostat sections were incubated overnight at 4°C with monoclonal rabbit anti-TSPO antibody (1:200, Abcam, Cambridge, UK). After washing, sections were incubated for 1 h at room temperature with the Cy3- or FITC-conjugated secondary antibody (1:500, Abcam). For double immunofluorescence staining, sections were incubated with a mixture of rabbit anti-TSPO antibody and mouse monoclonal anti-neuronal specific nuclear protein (NeuN, neuronal marker, 1:200, Millipore, Temecula, CA), mouse monoclonal anti-glial fibrillary acidic protein (GFAP, astrocyte marker, 1:500, Cell Signaling Technology, Danvers, MA), or a goat polyclonal anti-ionized calcium-binding adaptor molecule 1 (Iba1, microglia marker, 1:500, Abcam) overnight at 4°C. The sections were then incubated with a mixture of FITC- and Cy3-conjugated secondary antibody for 1 h at room temperature. The stained sections were examined under a fluorescence microscope (×200 magnification; Leica DMI 4000B, Wetzlar, Germany), and images were captured with a CCD spot camera. The fluorescence density was analyzed using Image-Pro Plus Version 6 (Media Cybernetics, Rockville, MD).
TSPO expression was detected in sham-operated and SNI rats at 7, 14, and 28 days.
Microinjection Site Control
After behavioral experiments, 2.5% Evans Blue in saline (0.5 μL) was injected to verify the location of the needle tip. Each rat was deeply anesthetized and perfused with 4% paraformaldehyde in phosphate buffer. The brain was removed and stored in paraformaldehyde solution. Sections were cut coronally through the VP nucleus at 30 μm on a cryostat microtome and then mounted on gelatin-coated glass slides. After drying, the sections were stained with 1% cresyl violet for 10 min and then washed in a gradient of alcohols. The location of the needle tip on the slide was inspected and photographed under a light microscope (Leica DMI 4000B).
Statistics
Data are presented as mean ± SEM. The 50% PWTs among groups were analyzed by two-way repeated measures analysis of variance (ANOVA) with groups and time points as independent factors, followed by Bonferroni post hoc comparisons. Immunofluorescence data were analyzed by Student’s t-test for independent-samples or one-way ANOVA according to group conditions. Data from drug injections were analyzed by two-way repeated measures ANOVA, followed by Bonferroni post hoc comparisons. The level of statistical significance was set at 5% (P < 0.05) in all analyses.
Results
Elevated Neurosteroids in the Lateral Thalamus of Rats with Neuropathic Pain
To verify the establishment of the neuropathic pain model, 50% PWTs were measured 1 day before and 3, 7, 14, 21, and 28 days after SNI or sham surgery. Rats in the SNI group showed significantly lower 50% PWTs in the injured hindpaw from days 3 to 28 than those in the sham-surgery group (Fig. 1A), indicating stable mechanical allodynia after SNI surgery.
Fig. 1.
Elevated neurosteroids in the lateral thalamus in rats with neuropathic pain after SNI. A Mechanical allodynia developed after SNI surgery (***P < 0.001 compared with sham-surgery group, two-way repeated measures ANOVA followed by Bonferroni post hoc test, n = 16 animals/group). B Schematic of the biosynthetic steps for major neurosteroids. C–H Concentrations of five neurosteroids in the ipsilateral thalamus on days 7 (C), 14 (E), and 28 (G), and in the contralateral thalamus on days 7 (D), 14 (F), and 28 (H) after surgery (*P < 0.05, **P < 0.01, ***P < 0.001 compared with sham-surgery group, two-way repeated measures ANOVA followed by Bonferroni post hoc test, n = 15–21 animals/group).
The levels of five representative neurosteroids (PREG, PROG, DOC, AP, and THDOC) in the ipsilateral and contralateral lateral thalamus were measured with HPLC-MS on days 7, 14, and 28 after surgery (Fig. 1B). The levels were comparable in the SNI and sham-surgery groups (Fig. 1C–F) until 28 days, when the neurosteroids showed a significant increase in the bilateral thalamus (Fig. 1G, H). Neurosteroid levels in the thalamus on both sides were comparable at all time points (Fig. 1C–H).
Taken together, these results demonstrated increased neurosteroid levels in the lateral thalamus in the chronic stage of neuropathic pain.
Up-regulation of TSPO Expression in the Lateral Thalamus in Rats with Neuropathic Pain
We next examined the expression of TSPO, the upstream rate-limiting enzyme for steroidogenesis [13, 14], in the lateral thalamus using immunofluorescent staining.
TSPO expression was more abundant in the thalamic reticular nucleus than in the VP (Fig. 2A–H). Compared with that in the sham-surgery group (Fig. 2A, B), TSPO expression significantly increased at day 28 in the SNI group (Fig. 2G–J). Interestingly, this time course was similar to that of the neurosteroids (Fig. 1C–H). The TSPO expression was comparable in the thalamus on both sides.
Fig. 2.
Up-regulated TSPO expression in the lateral thalamus of rats with neuropathic pain. A–H Representative images showing the TSPO expression at different time points after SNI surgery in the ipsilateral and contralateral thalamus (scale bar, 200 μm). I, J TSPO expression levels in the ipsilateral and contralateral thalamus (*P < 0.05, **P < 0.01 compared with sham-surgery group, one-way ANOVA followed by Bonferroni post hoc test, n = 5 animals/group).
To determine the types of cells expressing TSPO, double immunofluorescence staining of TSPO was performed on lateral thalamic sections from sham-surgery and SNI rats with three cell-specific markers: NeuN (marker of neurons), GFAP (marker of astrocytes), and Iba1 (marker of microglia). TSPO was expressed mainly in thalamic neurons (Fig. 3A, D, and G) and microglia (Fig. 3C, F, and I), but not in astrocytes (Fig. 3B, E, and H). Compared with the sham-surgery group, the percentage of neurons that expressed TSPO was higher on day 28 of neuropathic pain (Fig. 3G–I), suggesting a neuronal but not a glial source of increased TSPO expression. The percentages of TSPO-positive astrocytes and microglia did not differ between the two groups.
Fig. 3.
Distribution of TSPO in the lateral thalamus revealed by double immunofluorescence staining for TSPO (green) with NeuN (red, marker of neurons), GFAP (red, marker of astrocytes), and Iba1 (red, marker of microglia). A–F Images from the sham-surgery group (A–C) and the neuropathic pain group (D–F) on day 28 after SNI (scale bar, 200 μm). G–I Statistics of percentages of TSPO-positive neurons (G), astrocytes (H), and microglia (I) (**P < 0.01 compared with sham-surgery group, Student’s t-test for independent samples, n = 5 animals/group).
Taken together, these results demonstrated increased TSPO expression in thalamic neurons in the chronic stage of neuropathic pain (28 days after SNI surgery).
Activation of TSPO Increased Neurosteroids and Relieved Mechanical Allodynia, While Inhibition of TSPO had Opposite Effects
We next investigated the role of neurosteroids in pain modulation by microinjecting TSPO activators and inhibitors. An example of the site of microinjection is illustrated in Fig. 4A. Only data from rats with correct cannula localization were included.
Fig. 4.
Effects of AC-5216 (a TSPO activator) and PK 11195 (a TSPO inhibitor) on neurosteroid concentrations in the lateral thalamus. A Representative image of the location of the injection needle tip (arrow: VP, ventral posterior thalamic nucleus; scale bar, 4 mm). B, C Microinjection of AC-5216 up-regulated neurosteroid concentrations in the lateral thalamus (B), while microinjection of PK 11195 down-regulated them (C) (*P < 0.05, **P < 0.01, ***P < 0.001 compared with the vehicle group, two-way repeated measures ANOVA followed by Bonferroni post hoc test, n = 5 animals/group).
We first constructed the dose-effect curves of these drugs on steroidogenesis. AC-5216, N-benzyl-N-ethyl-2-(7-methyl-8-oxo-2-phenyl-7,8-dihydro-9H-purin-9-yl) acetamide [32], is a selective and potent activator of TSPO [33]. During the measurement of neurosteroids by HPLC/MS, we found that their levels were comparable in the left and right lateral thalamus (data not shown), making unilateral vehicle injection a rational control. We microinjected vehicle into the left VP and AC-5216 in a gradient of concentrations (0.4, 1.2, and 4 μg/μL) into the right VP, and harvested the tissues 1 h later for the measurement of neurosteroids. AC-5216 dose-dependently up-regulated all five neurosteroids (Fig. 4B), and 4.0 μg induced a maximum increase of 2-3-fold.
In contrast, PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3- isoquinoline carboxamide, is a widely used inhibitor for TSPO with high affinity [34–36]. Four hours after microinjection (Fig. 4C), but not at 1 h (data not shown), 4.0 μg PK 11195 significantly decreased the neurosteroid levels.
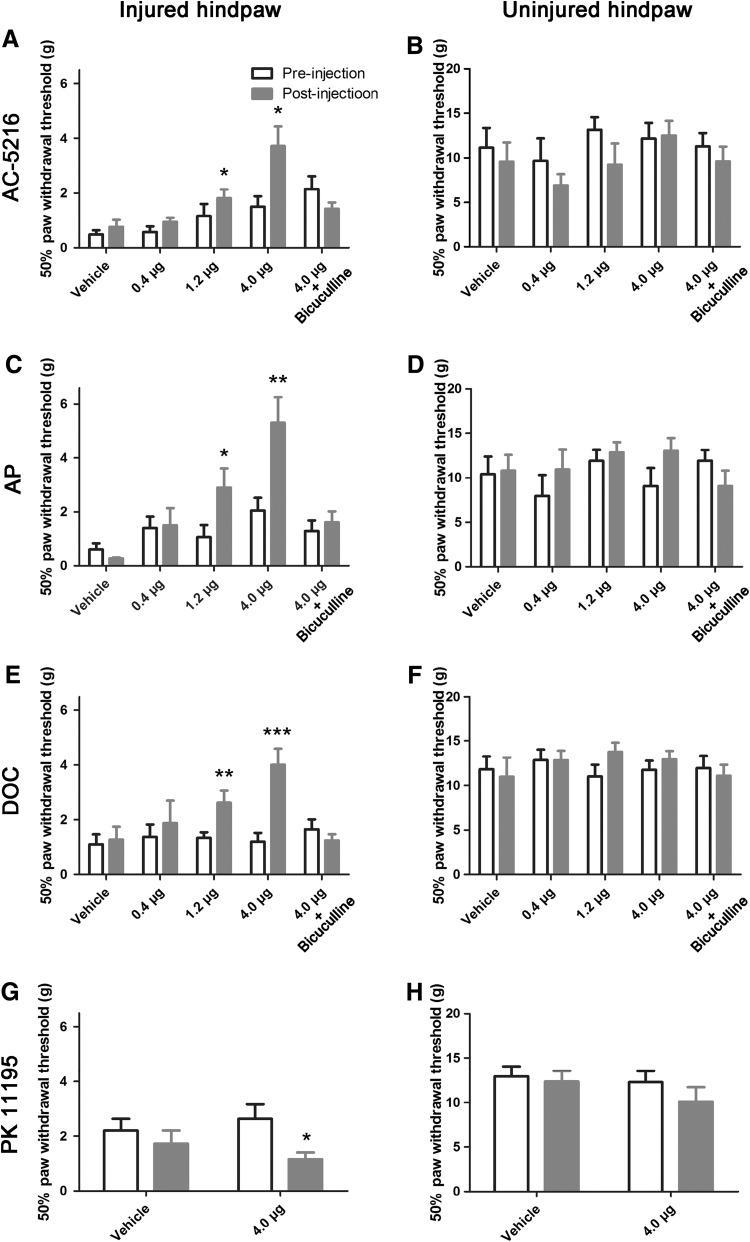
We next up- and down-regulated thalamic neurosteroids, and assessed the resulting allodynia-related behavior in the chronic stage of neuropathic pain (28 days after SNI). AC-5216 dose-dependently alleviated mechanical allodynia (Fig. 5A), with the maximum effect at 4.0 μg. Direct microinjection the neurosteroids AP (Fig. 5C) and DOC (Fig. 5E) had effects similar to AC-5216. In contrast, in vivo microinjection of PK 11195 aggravated the neuropathic pain (Fig. 5G). None of these drugs had evident effects on the mechanical pain threshold of the uninjured hindpaw (Fig. 5B, D, F, and H).
Fig. 5.
Up-regulation of neurosteroids in the lateral thalamus alleviated neuropathic pain in SNI rats. A–F Microinjection of AC-5216 (a TSPO activator) or neurosteroids (AP and DOC) into the lateral thalamus increased the 50% paw withdrawal threshold (PWT) of the injured hindpaw; this was abolished by pre-injection of bicuculline (a GABAAR antagonist). G, H PK 11195 (a TSPO inhibitor) decreased 50% PWTs. *P < 0.05, **P < 0.01, ***P < 0.001 post-injection group compared with pre-injection group, two-way repeated measures ANOVA followed by Bonferroni post hoc test, n = 7–10 animals/group.
Taken together, these results suggested that the elevated neurosteroids driven by increased expression of TSPO in the lateral thalamus in SNI rats relieves neuropathic pain.
GABAA Receptors Participated in the Analgesic Effects of Neurosteroids
Neurosteroids exert their non-genetic functions mainly by enhancing the inhibitory effect of GABAARs [6–8]. To test whether they participated in the analgesic effect of neurosteroids, we injected bicuculline, a powerful GABAAR antagonist [37, 38], into the lateral thalamus 0.5 h before the delivery of AC-5216, AP, or DOC. Pre-injection of bicuculline did not affect the mechanical pain threshold of the uninjured hindpaw (Fig. 5B, D, and F), but abolished the analgesic effects of the TSPO activator AC-5216 (Fig. 5A), and the neurosteroids AP (Fig. 5C) and DOC (Fig. 5E), indicating the involvement of GABAARs in the action of neurosteroids.
Discussion
In the present study, we showed that both the content of neurosteroids and the expression of their rate-limiting enzyme TSPO significantly increased in the lateral thalamus in the chronic stage of neuropathic pain, although the pain appeared as early as 3 days after nerve injury. AC-5216 was confirmed here to be an effective activator of TSPO, the microinjection of which in vivo up-regulated neurosteroid levels and had an analgesic effect. PK 11195, a TSPO inhibitor, had the opposite effect.
Elevated Neurosteroids in the Lateral Thalamus in the Chronic Stage of Neuropathic Pain
Neurosteroids have attracted much attention regarding their involvement in nociceptive signal processing and analgesic drug development. However, most research has focused on the spinal level [15–17, 39]. Only two studies have reported elevated neurosteroids in nociceptive supraspinal nuclei [39] and the brain [17]. The lateral thalamus plays a vital role in nociception. In the present study, we demonstrated that neurosteroids began to increase in the bilateral thalamus from day 28 after SNI. At earlier time points (days 7 and 14), their levels remained unchanged. This pattern was consistent with previous studies in the spinal cord and the brain [15, 17, 39].
Changes in these regions have also been reported to occur earlier (on days 10 [39] or 14 [17]). This difference might result from the different animal models used [complete Freund’s adjuvant (CFA)-induced inflammatory pain and spinal nerve ligation-induced neuropathic pain] or regions investigated. Unlike the spinal cord, the thalamus receives feedback information from other brain areas, such as the prefrontal cortex, anterior cingulate cortex, hippocampus, and insular cortex [19, 40, 41]. These anatomical and functional features of the thalamus might underlie the bilateral change of neurosteroids despite unilateral nerve injury, as well as the relatively late change compared with the spinal cord. Other research regarding pain modulation has also reported a similar time course; the changes in the spinal cord occur earlier than those in the thalamus [42]. Similar mechanisms may account for the change in the steroidogenesis enzyme TSPO.
Increased TSPO Expression Mediated the Up-regulation of Neurosteroids
Three major mechanisms might underlie the increased neurosteroid levels: increased expression of steroidogenesis enzymes, increased metabolic activity of steroidogenesis enzymes, or decreased degradation of neurosteroids.
Previous studies have confirmed that TSPO is the upstream rate-limiting enzyme for steroidogenesis [13, 14]. Its expression significantly increases in the dorsal horn after neuropathic or inflammatory pain [43, 44]. Here, we found that after SNI, TSPO expression increased in the lateral thalamus at a time point similar to the change of neurosteroids. In addition, intra-thalamic microinjection of the TSPO activator AC-5216 up-regulated the five neurosteroids examined, while the TSPO inhibitor PK 11195 down-regulated them. These findings supported the possibility that the up-regulation of neurosteroids might result from increased expression of TSPO. The increase of TSPO is consistent with previous reports in the spinal cord [43, 44].
However, we found that TSPO was mainly expressed in neurons and microglia, and its up-regulation mainly occurred in neurons. This differs from the spinal cord where TSPO is expressed most extensively in astrocytes and microglia [43, 44]. This divergence might be due to the differences in regions, animal models, and stages of neuropathic pain. For example, we found elevated TSPO in the lateral thalamus in the chronic stage of the SNI model, while that in the dorsal horn occurs much earlier after spinal nerve ligation or CFA-induced inflammatory pain [43, 44]. The molecular mechanisms underlying the up-regulation of TSPO require further investigation.
Here, we found that the TSPO was extensively expressed in the thalamic reticular nucleus. Besides its role in consciousness and sleep, this nucleus serves as an “attentional gate” for sensory information [45]. The reticular nucleus is thus a promising area in which to explore analgesic effects and may be a target for novel drugs [45–47].
Up-regulation of Neurosteroids in the Lateral Thalamus Relieved Neuropathic Pain
A growing number of studies have reported the analgesic effects of neurosteroids and their analogs by enhancing spinal inhibition [15, 48]. Though a few studies have reported increased neurosteroids in the nociceptive supraspinal nuclei and the brain [17, 39], none have explored their possible contribution to chronic pain in regions other than the spinal cord.
Here, we microinjected AC-5216, an activator of the steroidogenesis rate-limiting enzyme TSPO, into the lateral thalamus and found increased neurosteroid levels and dose-dependent alleviation of mechanical allodynia. Direct microinjection of the neurosteroids AP or DOC had similar effects. These results are consistent with previous reports that systemic (intraperitoneal) or intrathecal administration of neurosteroids (PROG or AP) relieve or abolish established pain behaviors [17, 48, 49]. Intrathecal administration of Ro5-4864, another widely-used TSPO activator, also retards or prevents the development of inflammatory and neuropathic pain [43, 44].
Moreover, we used the TSPO inhibitor PK 11195 to down-regulate neurosteroids and further confirm their role in neuropathic pain. PK 11195 aggravated the mechanical allodynia in rats with neuropathic pain, opposite to the effect of the TSPO activator.
Thus, we concluded that the up-regulated neurosteroids in the chronic stage of SNI neuropathic pain protect against pain.
GABAA Receptors Were Involved in the Analgesic Effect of Neurosteroids
Neurosteroids have potent and selective effects on GABAARs [6–8]. PREG, PROG, and DOC are positive modulators in a non-genomic manner, while AP and THDOC potentiate synaptic GABAAR function and activate delta-subunit containing extrasynaptic GABAARs that mediate tonic currents [9, 10]. Synaptic and extrasynaptic GABAARs are widely distributed in the lateral thalamus [23], prompting us to ask whether the analgesic effect of neurosteroids was mediated by these receptors. Bicuculline, a powerful GABAAR antagonist [37, 38], abolished the analgesic effect of two neurosteroids (AP and DOC, Fig. 5C and E), and that of AC-5216 (Fig. 5A). Direct injection of bicuculline into the lateral thalamus does not affect the mechanical or thermal pain threshold in naïve rats or those with CFA inflammatory pain (Zhang et al, unpublished data). These results imply that GABAARs participate in the analgesic effects of neurosteroids in the lateral thalamus, consistent with previous reports [15, 48, 50, 51]. How neurosteroids modulate the function of GABAARs in the thalamus during neuropathic pain is an interesting question for further investigation.
In summary, our results showed that increased expression of TSPO in the chronic stage of SNI resulted in the up-regulation of a series of neurosteroids in the lateral thalamus, and these neurosteroids might operate as non-genetic modulators to enhance the inhibitory function of GABAARs and protect against pain. Therefore, thalamic TSPO and neurosteroids might be potential therapeutic targets for the treatment of neuropathic pain.
Acknowledgements
This work was supported by grants from the National Basic Research Development Program of China (2013CB531905, 2014CB548200, and 2015CB554503), the National Natural Science Foundation of China (81230023, 81221002, 31200835, 81571067, and 21305005), a Key Project of the Ministry of Education of China (109003), and the "111" Project of the Ministry of Education of China (B07001).
Contributor Information
Ming Yi, Email: mingyi@bjmu.edu.cn.
You Wan, Email: ywan@hsc.pku.edu.cn.
References
- 1.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–4707. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, et al. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–125. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 3.Robel P, Baulieu EE. Neurosteroids: biosynthesis and function. Crit Rev Neurobiol. 1995;9:383–394. [PubMed] [Google Scholar]
- 4.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 5.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36:261–273. doi: 10.1016/S0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 6.Lambert JJ, Belelli D, Hill-Venning C, Peter JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/S0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang M. Neurosteroids and GABA-A Receptor Function. Front Endocrinol (Lausanne) 2011;2:44. doi: 10.3389/fendo.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacKenzie G, Maguire J. Neurosteroids and GABAergic signaling in health and disease. Biomol Concepts. 2013;4:29–42. doi: 10.1515/bmc-2012-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garnier M, Boujrad N, Oke BO, Brown AS, Riond J, Ferrara P, et al. Diazepam binding inhibitor is a paracrine/autocrine regulator of Leydig cell proliferation and steroidogenesis: action via peripheral-type benzodiazepine receptor and independent mechanisms. Endocrinology. 1993;132:444–458. doi: 10.1210/endo.132.1.8380386. [DOI] [PubMed] [Google Scholar]
- 12.Culty M, Li H, Boujrad N, Amri H, Vidic B, Bernassau JM, et al. In vitro studies on the role of the peripheral-type benzodiazepine receptor in steroidogenesis. J Steroid Biochem Mol Biol. 1999;69:123–130. doi: 10.1016/S0960-0760(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos V, Amri H, Boujrad N, Cascio C, Culty M, Garnier M, et al. Peripheral benzodiazepine receptor in cholesterol transport and steroidogenesis. Steroids. 1997;62:21–28. doi: 10.1016/S0039-128X(96)00154-7. [DOI] [PubMed] [Google Scholar]
- 14.Papadopoulos V, Lecanu L, Brown RC, Han Z, You ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138:749–756. doi: 10.1016/j.neuroscience.2005.05.063. [DOI] [PubMed] [Google Scholar]
- 15.Poisbeau P, Patte-Mensah C, Keller AF, Barrot M, Breton JD, Luis-Delgado OE, et al. Inflammatory pain upregulates spinal inhibition via endogenous neurosteroid production. J Neurosci. 2005;25:11768–11776. doi: 10.1523/JNEUROSCI.3841-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patte-Mensah C, Kibaly C, Boudard D, Schaeffer V, Begle A, Saredi S, et al. Neurogenic pain and steroid synthesis in the spinal cord. J Mol Neurosci. 2006;28:17–31. doi: 10.1385/JMN:28:1:17. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Soga T, Chi H, Eguchi S, Yamazaki F, Kumagai N, et al. Role of the neurosteroid allopregnanolone in the hyperalgesic behavior induced by painful nerve injury in rats. J Anesth. 2011;25:942–945. doi: 10.1007/s00540-011-1216-2. [DOI] [PubMed] [Google Scholar]
- 18.Yen CT, Lu PL. Thalamus and pain. Acta Anaesthesiol Taiwan. 2013;51:73–80. doi: 10.1016/j.aat.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Luo F, Wang JY. Neuronal nociceptive responses in thalamocortical pathways. Neurosci Bull. 2009;25:289–295. doi: 10.1007/s12264-009-0908-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnitzler A, Ploner M. Neurophysiology and functional neuroanatomy of pain perception. J Clin Neurophysiol. 2000;17:592–603. doi: 10.1097/00004691-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Wang JY, Luo F, Chang JY, Woodward DJ, Han JS. Parallel pain processing in freely moving rats revealed by distributed neuron recording. Brain Res. 2003;992:263–271. doi: 10.1016/j.brainres.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 22.Wang N, Zhang Y, Wang JY, Gao G, Luo F. Effects of pentobarbital anesthesia on nociceptive processing in the medial and lateral pain pathways in rats. Neurosci Bull. 2010;26:188–196. doi: 10.1007/s12264-010-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/S0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 24.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Wang J, Chen L, Zhang M, Wan Y. Basolateral amygdala lesion inhibits the development of pain chronicity in neuropathic pain rats. PLoS One. 2013;8:e70921. doi: 10.1371/journal.pone.0070921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 27.Sun Q, Tu H, Xing GG, Han JS, Wan Y. Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp Neurol. 2005;191:128–136. doi: 10.1016/j.expneurol.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Liu FY, Qu XX, Cai J, Wang FT, Xing GG, Wan Y. Electrophysiological properties of spinal wide dynamic range neurons in neuropathic pain rats following spinal nerve ligation. Neurosci Bull. 2011;27:1–8. doi: 10.1007/s12264-011-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Fang D, Kong LY, Jin ZR, Cai J, Kang XJ, et al. Sensitization of neurons in the central nucleus of the amygdala via the decreased GABAergic inhibition contributes to the development of neuropathic pain-related anxiety-like behaviors in rats. Mol Brain. 2014;7:72. doi: 10.1186/s13041-014-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson C, Paxinos G. The rat brain in stereotaxic coordinates. 5. Amsterdam: Elsevier Academic Press; 2005. pp. 56–60. [Google Scholar]
- 31.Liu J, Zhang M, Wan Y, Wu HH, Hou YN, Zheng LM, et al. Simultaneous determination of six neurosteroids hormones in rat brain tissues by high performance liquid chromatography-tandem mass spectrometry. Chinese J Anal Chem. 2015;43:1118–1124. [Google Scholar]
- 32.Kita A, Kohayakawa H, Kinoshita T, Ochi Y, Nakamichi K, Kurumiya S, et al. Antianxiety and antidepressant-like effects of AC-5216, a novel mitochondrial benzodiazepine receptor ligand. Br J Pharmacol. 2004;142:1059–1072. doi: 10.1038/sj.bjp.0705681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Le Fur G, Vaucher N, Perrier ML, Flamier A, Benavides J, Renault C, et al. Differentiation between two ligands for peripheral benzodiazepine binding sites, [3H]RO5-4864 and [3H]PK 11195, by thermodynamic studies. Life Sci. 1983;33:449–457. doi: 10.1016/0024-3205(83)90794-4. [DOI] [PubMed] [Google Scholar]
- 35.Le Fur G, Perrier ML, Vaucher N, Imbault F, Flamier A, Benavides J, et al. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide. I. In vitro studies. Life Sci. 1983;32:1839–1847. doi: 10.1016/0024-3205(83)90062-0. [DOI] [PubMed] [Google Scholar]
- 36.Le Fur G, Guilloux F, Rufat P, Benavides J, Uzan A, Renault C, et al. Peripheral benzodiazepine binding sites: effect of PK 11195, 1-(2-chlorophenyl)-N-methyl-(1-methylpropyl)-3 isoquinolinecarboxamide. II. In vivo studies. Life Sci. 1983;32:1849–1856. doi: 10.1016/0024-3205(83)90063-2. [DOI] [PubMed] [Google Scholar]
- 37.Curtis DR. Bicuculline, GABA and central inhibition. Proc Aust Assoc Neurol. 1973;9:145–153. [PubMed] [Google Scholar]
- 38.Curtis DR, Duggan AW, Felix D, Johnston GA. GABA, bicuculline and central inhibition. Nature. 1970;226:1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- 39.Patte-Mensah C, Li S, Mensah-Nyagan AG. Impact of neuropathic pain on the gene expression and activity of cytochrome P450side-chain-cleavage in sensory neural networks. Cell Mol Life Sci. 2004;61:2274–2284. doi: 10.1007/s00018-004-4235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman SM. Thalamic relays and cortical functioning. Prog Brain Res. 2005;149:107–126. doi: 10.1016/S0079-6123(05)49009-3. [DOI] [PubMed] [Google Scholar]
- 41.Sherman SM, Guillery RW. The role of the thalamus in the flow of information to the cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1695–1708. doi: 10.1098/rstb.2002.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Modol L, Cobianchi S, Navarro X. Prevention of NKCC1 phosphorylation avoids downregulation of KCC2 in central sensory pathways and reduces neuropathic pain after peripheral nerve injury. Pain. 2014;155:1577–1590. doi: 10.1016/j.pain.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Hernstadt H, Wang S, Lim G, Mao J. Spinal translocator protein (TSPO) modulates pain behavior in rats with CFA-induced monoarthritis. Brain Res. 2009;1286:42–52. doi: 10.1016/j.brainres.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei XH, Wei X, Chen FY, Zhang Y, Xin WJ, Pang RP, et al. The upregulation of translocator protein (18 kDa) promotes recovery from neuropathic pain in rats. J Neurosci. 2013;33:1540–1551. doi: 10.1523/JNEUROSCI.0324-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAlonan K, Brown VJ. Tha thalamic reticular nucleus: more than a sensory nucleus? Neuroscientist. 2002;4:302–305. doi: 10.1177/107385840200800405. [DOI] [PubMed] [Google Scholar]
- 46.Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, et al. Promotion of non-rapid eye movement sleep and activation of reticular neurons by a novel MT2 melatonin receptor ligand. J Neurosci. 2011;31:18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez-Canul M, Palazzo E, Dominguez-Lopez S, Luongo L, Lacoste B, Comai S, et al. Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain. 2015;156:305–317. doi: 10.1097/01.j.pain.0000460311.71572.5f. [DOI] [PubMed] [Google Scholar]
- 48.Frye CA, Duncan JE. Progesterone metabolites, effective at the GABAA receptor complex, attenuate pain sensitivity in rats. Brain Res. 1994;643:194–203. doi: 10.1016/0006-8993(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, Li W, Dai L, Zhang T, Xia W, Liu H, et al. Early repeated administration of progesterone improves the recovery of neuropathic pain and modulates spinal 18kDa-translocator protein (TSPO) expression. J Steroid Biochem Mol Biol. 2014;143:130–140. doi: 10.1016/j.jsbmb.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Charlet A, Lasbennes F, Darbon P, Poisbeau P. Fast non-genomic effects of progesterone-derived neurosteroids on nociceptive thresholds and pain symptoms. Pain. 2008;139:603–609. doi: 10.1016/j.pain.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Schlichter R, Keller AF, De Roo M, Breton JD, Inquimbert P, Poisbeau P. Fast nongenomic effects of steroids on synaptic transmission and role of endogenous neurosteroids in spinal pain pathways. J Mol Neurosci. 2006;28:33–51. doi: 10.1385/JMN:28:1:33. [DOI] [PubMed] [Google Scholar]