Abstract
Major depression during pregnancy is a common psychiatric disorder that arises from a complex and multifactorial etiology. Psychosocial stress, sex, hormones, and genetic vulnerability increase the risk for triggering mood disorders. Microglia and toll-like receptor 4 play a crucial role in triggering wide and varied stress-induced responses mediated through activation of the inflammasome; this leads to the secretion of inflammatory cytokines, increased serotonin metabolism, and reduction of neurotransmitter availability along with hypothalamic–pituitary–adrenal axis hyperactivity. Dysregulation of this intricate neuroimmune communication network during pregnancy modifies the maternal milieu, enhancing the emergence of depressive symptoms and negative obstetric and neuropsychiatric outcomes. Although several studies have clearly demonstrated the role of the innate immune system in major depression, it is still unclear how the placenta, the brain, and the monoaminergic and neuroendocrine systems interact during perinatal depression. Thus, in the present review we describe the cellular and molecular interactions between these systems in major depression during pregnancy, proposing that the same stress-related mechanisms involved in the activation of the NLRP3 inflammasome in microglia and peripheral myeloid cells in depressed patients operate in a similar fashion in the neuroimmune placenta during perinatal depression. Thus, activation of Toll-like receptor 2 and 4 signaling and the NLRP3 inflammasome in placental immune cells may promote a shift of the Th1/Th2 bias towards a predominant Th1/Th17 inflammatory response, associated with increased secretion of pro-inflammatory cytokines, among other secreted autocrine and paracrine mediators, which play a crucial role in triggering and/or exacerbating depressive symptoms during pregnancy.
Keywords: Depression; Pregnancy; Immune system; Inflammation; Cytokine; Serotonin; Indoleamine 2,3 dioxygenase; Glucocorticoid; Brain; Placenta
Introduction
Major depression (MD) is one of the most common psychiatric disorders in the western world and has been predicted to be the leading cause of burden of disease by 2030 (http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_part4.pdf). MD has a complex and multifactorial etiology that arises from interactions between genetic, developmental, and environmental factors, reflecting the heterogeneity of the disorder [1]. Such heterogeneity is reflected in the estimates of the number of MD individuals who receive antidepressant treatment. Only a third of patients receive adequate treatment and up to half of these relapse despite the increasing number of antidepressant drugs available [1, 2]. However, psychosocial stress and systemic disease can affect the onset of depression. For example, the comorbidity of depression in patients with diabetes, cancer, or cardiac disease is 17–29%, much higher than that in the general population (10.3%) [3].
Depression during pregnancy is an emerging field in terms of understanding the pathophysiology of the disease and determining adequate treatment. Women are more than twice as susceptible to depression as men, and despite the popular notion that pregnancy protects against depression [4] perinatal depression is highly prevalent, with point prevalence estimates commonly exceeding 10% in most high-income countries [5]. The prevalence of perinatal depression differs substantially among studies, differing from 7.4% in the first, 12.8% in the second, and 12.0% in the third trimester, [6–8] to 20.4% in the entire pregnancy [6, 8]. Nonetheless, other reports have shown that ~9% of women suffer major or minor depression during each of the trimesters, while postpartum depression has a prevalence of 13% at 3 months postpartum [9, 10].
Depression in pregnancy is linked to poor maternal self-care, inadequate nutrition [11, 12], premature labor, and adverse obstetric outcomes [13]. Epidemiological studies have revealed that nearly 40% of pregnant Afro-American women who are underdiagnosed with depression during the antenatal period [14] exhibit increased depressive symptomology during the postpartum period [14–16]. Antenatal depression has been associated with poorer birth outcomes, reduced neonatal neurological development [17], and a high risk of postpartum depression [18]. Furthermore, pregnant women experience more stressful events than non-pregnant women [13, 19] and commonly report tiredness or fatigue (87.2–96.5%) [20], symptoms commonly associated with depression and anxiety [21]. Interestingly, pregnant women with antenatal depression have a higher rate of preeclampsia and preterm birth associated with poor fetal and infant outcomes (motor and mental development) [22].
It is well known that a healthy immune system is needed to maintain a successful pregnancy, besides the finely-regulated immunomodulation to maintain immune balance (Th2 > Th1 response) during gestation [4]. Immunomodulation during pregnancy may determine several of the neuropsychological components of maternal well-being (such as stress and fatigue) associated with abnormal function of the stress-response system (the hypothalamic–pituitary–adrenal (HPA) axis) that may trigger or exacerbate depressive symptoms in women with major depressive disorder (MDD) [23]. HPA axis hyperactivity in individuals with MDD is largely thought to result from corticotropin-releasing hormone (CRH) hypersecretion [23]. Animals exposed to CRH show behavioral changes that occur in human depression, such as mood, appetite, sleep, locomotor activity, and cognition [24]. However, recent studies have shown an intricate interaction between inflammation, the innate immune system, Toll-like receptor (TLR) activity, and the production and secretion of pro-inflammatory cytokines in MDD [25]. For instance, novel aspects of the neuroinflammatory process in response to stressful challenges and depression have recently been documented where glucocorticoids (GCs) and interkeukin-1β (IL-1β) and its regulator, “the Nod-like receptor (NLR) family, the pyrin domain-containing 3 (NLRP3) inflammasome” appear to bridge the gap between psychological stress and depression [25, 26].
Although a role of inflammation and its associated cytokines in MD was first suggested in the 1980s, evidence has been accumulating since then, showing a role of the innate immune system and inflammation in support of the inflammatory hypothesis of depression. Past and recent studies have shown an increase of immune markers in psychiatric patients, suggesting that the immune system is increasingly associated with various psychosomatic illnesses [25, 26]. Patients with MDD have increased circulating inflammatory cytokines and immune signaling levels [27, 28], while treatment that reduces or remits depressive symptomology is correlated with normalization of immune signaling levels [29]. These data provide strong evidence implicating the immune system in MDD.
Thus, in order to understand the role of the immune system in perinatal depression, one needs to focus on both the placental and maternal immune systems in normal and pathological conditions. In this context, we describe the roles of the innate immune system and its pro-inflammatory mediators during pregnancy and perinatal depression, emphasizing the role of endogenous stressors in activating TRLs and their signaling pathways, including the NLRP3 inflammasome in the brain, the periphery, and the placental immune system, which contribute to triggering inflammatory activity in mood-related disorders during pregnancy.
Puberty and Mood Disorders
Puberty is a major life transition from a non-reproductive juvenile to a reproductively competent adult; great developmental plasticity occurs during this time window and adolescence [30–32].
The neuroplasticity of puberty may also contribute to vulnerability to the development of mental diseases [32, 33]. The National Comorbidity Survey Replication study reported that affective disorders such as bipolar disorder and MDD emerge during adolescence with a peak age of onset at 14 years [32, 34]. One important predictor of an individual’s susceptibility to developing neuropsychiatric disorders is sex. For instance, males have a higher risk of the early onset of neurological disorders, showing higher rates of autism spectrum disorder, attention deficit hyperactivity disorder, and psychopathologies of brain organization such as schizophrenia and Tourette syndrome [32, 35], while women are more vulnerable and prone to develop anxiety, depression, and eating disorders that emerge during puberty and adolescence [32]. Females are about twice as likely as males to have experienced an episode of depression, and this gender gap persists for the next 35–40 years [30, 32]. Moreover, pubertal status (Tanner stage III) predicts sex difference better than age [36, 37] suggesting that ovarian hormones play a role in the etiology of affective disorders [36].
Recent studies have provided clues about how stressors experienced in puberty alter steroid hormone-influenced behaviors in adulthood and how these behavioral changes are mediated through alterations in the ongoing processes of brain development [38]. It seems that the organizational actions of hormones are limited to critical windows of enhanced brain plasticity occurring during both gestation and puberty [36, 39, 40]. Prior to and after such a critical window, hormones may no longer affect brain development, suggesting that puberty is an organizational period mediated by steroid hormones [36, 41].
Both pubertal status and the underlying hormonal milieu are critical factors in an individual’s susceptibility to the experiences occurring in adolescence [36]. Indeed, stress during pubertal development and/or adolescence results in alterations in physiology and behavior as indicated by increases in anxiety and depressive disorders [33], risk-taking novelty-seeking behaviors [42], changes in learning and cognition [43], and drug and alcohol use and abuse [44].
Sex and Brain Immune Cells
Microglia are the primary immune cells in the brain; they constantly scan the microenvironment in the healthy adult brain, surveying for pathogens, monitoring the status of local synapses [36, 45–47], and regulating synaptic maturation or elimination via axonal guidance [36, 48]. Synaptic pruning by microglia is necessary for normal brain development in the neonatal period. Neurons and glia born during puberty are functionally incorporated in neural circuits and contribute to the regulation of adult behaviors [36, 49]. Microglia are in an active state during early postnatal life, and this is correlated with increased levels of circulatory cytokines. Moreover, environmental factors such as stressors can increase the rate of colonization and density of microglia [50], altering both the adult and the developing brain with effects on behavior. Interestingly, peri-pubertal female (postnatal day 30) and adult (postnatal day 60) rats have more activated microglia than males of the same age in the hippocampus, parietal cortex, and amygdala, suggesting that females are more sensitive to immune dysregulation during puberty/adolescence and early adulthood. Interestingly, primed microglia do not chronically produce cytokines or other pro-inflammatory mediators [51].
Chronic activation of microglia and astrocytes (referred to as neuroinflammation) [36, 52] leads to the exaggerated expression of pro-inflammatory mediators such as cytokines and reactive oxygen species that can damage cellular structures [53]. For instance, increased pro-inflammatory cytokines have been reported to lead to impaired hippocampal structure and function [1, 54, 55] (Fig. 1).
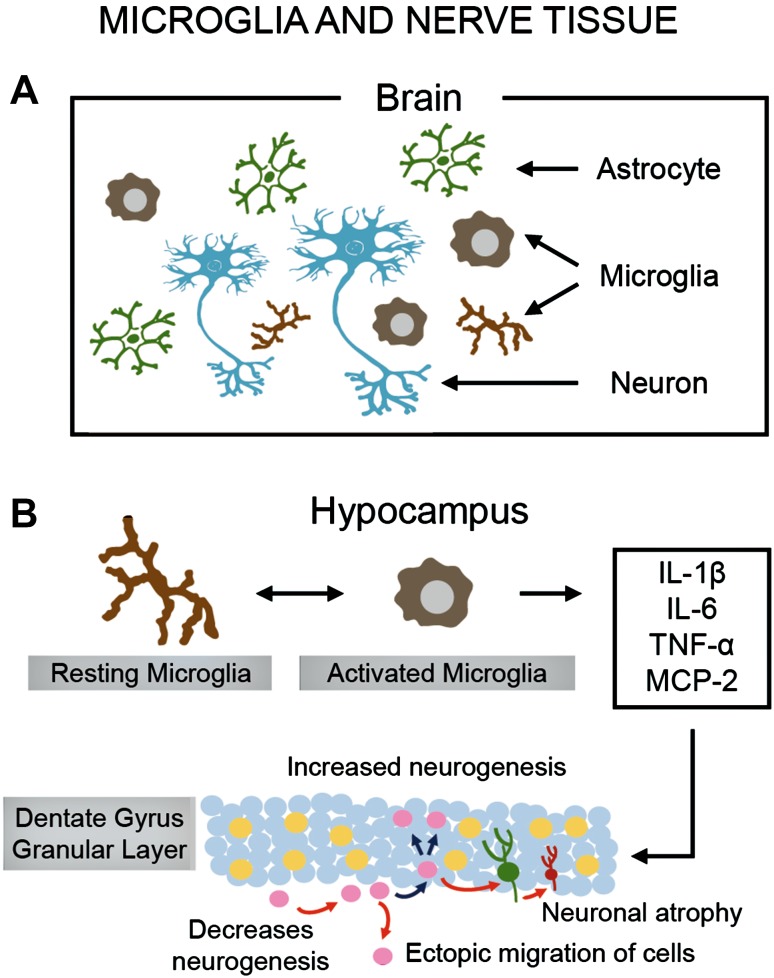
Fig. 1.
Microglia and nerve tissue. A Schematic of the arrangement of interacting neurons, microglia, and astrocytes in the human brain, highlighting two morphological conformations adopted by microglia (resting and active states) in response to incoming insults. Microglia are the primary immune cells of the CNS and, like peripheral macrophages, they act as the major inflammatory cell type (scavengers) that responds to external or internal “stressors” (pathogens, injuries, and life experiences) by becoming “activated” (a process that changes cell morphology and function) and enhancing its own proliferation and migration to the infection or injury site. B Activated microglia secrete pro-inflammatory cytokines and chemokines (MCP-1), together with prostaglandins, nitric oxide, and reactive oxygen species (see Fig. 2C) which regulate and increase the local immune response. Inflammatory cytokines are triggering factors involved in the reduced neurogenesis and neuronal atrophy of granule cells in the dentate gyrus of the hippocampus. This leads to a decrease in hippocampal volume and impairment of hippocampal-related functions, as shown in MDD.
A growing body of experimental and clinical research has revealed a pivotal role of neuroinflammation in the etiology of MD [56], anxiety [57, 58], and post-traumatic stress disorder [57] in addition to neurodegenerative diseases associated with cognitive dysfunction, such as Alzheimer’s disease and Parkinson’s disease [36, 53].
Chronic stress activates microglia in adulthood, leading to increased cytokine levels [59], and similarly, stressors experienced in childhood or adolescence induce short- and long-term alterations in immune function [60, 61] with higher levels of expression of the inflammatory biomarker C-reactive protein (CRP) [61]. Thus, vulnerability to developing mood-related disorders during puberty and adolescence may be due in part to long-term activity of the neuroimmune system, which leads to an enduring neuroinflammatory response or neuroinflammation.
Thus, neuroinflammation could be a common pathway by which pubertal stressors may alter the adult brain and behavior, particularly when it is associated with mental disorders, such as anxiety and depression along with learning, memory, and cognitive deficits [36, 62–64]. Chronic activation of microglia and neuroinflammation associated with increased levels of brain circulating cytokines might be relevant to perinatal depression, a condition that requires further elucidation as demonstrated in stress-induced major depression in pubertal and adolescent girls [36].
Inflammation and Depression
The role of inflammation in major depression has been extensively documented. Patients with MDD exhibit the cardinal features of an inflammatory response, including increased expression of pro-inflammatory cytokines and their receptors, and increased levels of acute-phase reactants, chemokines, and soluble adhesion molecules in peripheral blood and cerebrospinal fluid (CSF) [65, 66].
Furthermore, several reports have described the blood gene expression profiles of the pro-inflammatory ‘M1’ macrophage phenotype with over-representation of IL-6, IL-8, and type I IFN-induced signaling pathways [64, 67], in addition to the increased expression of a wide variety of innate immune genes and proteins such as IL-1β, IL-6, TNF-α, TLR3, and TLR4, found in post-mortem brain samples from depressed suicide victims [68–70]. Meta-analyses showed that circulating cytokines, such as IL-1β, IL-6, and TNF, as well as CRP, are the most reliable biomarkers of inflammation in patients with depression, and the expression of polymorphisms of these inflammatory cytokine genes correlate with depression and its response to antidepressant treatment [64, 71, 72].
Further studies linking inflammation with MDD have revealed that administration of inflammatory cytokines such as IFN-α or their inducers (endotoxin or typhoid vaccination) to non-depressed individuals causes depressive symptoms [73–75], while blockade of cytokines such as TNF-α or their inflammatory signaling pathways and components, such as cyclooxygenase 2 (COX-2) reduces the mood-related symptomology in patients with MDD and other illnesses such as rheumatoid arthritis, psoriasis, and cancer [76, 77].
It is notable that clinical findings supporting the inflammatory theory of the pathogenesis of MDD have revealed increases of IL-6 levels in the CSF in individuals who had attempted suicide [78], and these levels are correlated with the severity of depression [79, 80]. However, the most relevant finding is that increased inflammatory cytokines in response to peripheral infections inducing the ‘sickness behavior syndrome’ [54], a syndrome whose symptoms overlap considerably with those of depression and are ameliorated with antidepressant treatment [64, 74]. Moreover, it has been reported that the onset of depression is often mistaken for the development of sickness behavior, and conversely, symptoms associated with infections are often mistaken for the onset of depression [65].
Most adaptive theories of depression have looked at the potential benefits of how depressive symptoms and treatments can enhance the relationships with other humans [81]. However, recent models have shifted the focus away from relationships with people to relationships with pathogens [82, 83]. This model postulates that modern humans have inherited a genomic bias towards inflammation, enhancing host survival and reproduction in the highly pathogenic environment in which humans evolved [83]. Moreover, this hypothesis suggests that vulnerability to depression most probably evolved from a behavioral repertoire—referred to as ‘sickness behavior’—which led humans to survive and adapt to their environment at the expense of the costs and benefits caused by pathogens and infections [65].
Psychosocial stress is the most significant and reproducible predictor of developing depression in humans and the primary experimental pathway for investigating depressive-like behaviors in animals. Over the years, questions of how stress is translated into an inflammatory response may have been explained through the immune activation and expression of both the peripheral and central cellular inflammasomes [65]. Inflammasomes are cytosolic protein complexes formed in myeloid cells in response to pathogenic microorganisms and non-pathogenic or ‘sterile’ stressors. Conceptualizing the sterile nature of psychosocial stress, researchers have sought the mechanisms of how inflammasome activation may induce depression, when triggered by pathogens or damaging endogenous molecules [damage-associated molecular patterns referred to as DAMPs] in addition of a wide variety of molecules linked with oxidative stress.
Recent evidence has shown that such endogenous molecules may be induced by psychological or physical stressors in animals through the release of catecholamines [65, 84]. In spite of the major focus on the stress-response system (HPA axis and sympathetic nervous system) suggesting that its dysfunction is a major pathophysiological mechanism driving psychosocial stressors to cause depressive symptomology, recent evidence has shown that the inflammasome appears to be the crucial immune interface between stress and neuroendocrine and inflammatory responses in MDD [65].
Furthermore, assembly of the inflammasome leads to the activation of crucial enzymes enhancing the synthesis and secretion of inflammatory cytokines (IL-1β and IL-18). The increased levels of inflammatory cytokines in blood, CSF, and brain tissue converge to elicit a wide spectrum of functional and morphological changes in the brain, immune, and neuroendocrine systems in individuals with MDD that parallel the stress-induced depressive-like behaviors in animals [25, 65] (Figs. 1, 2).
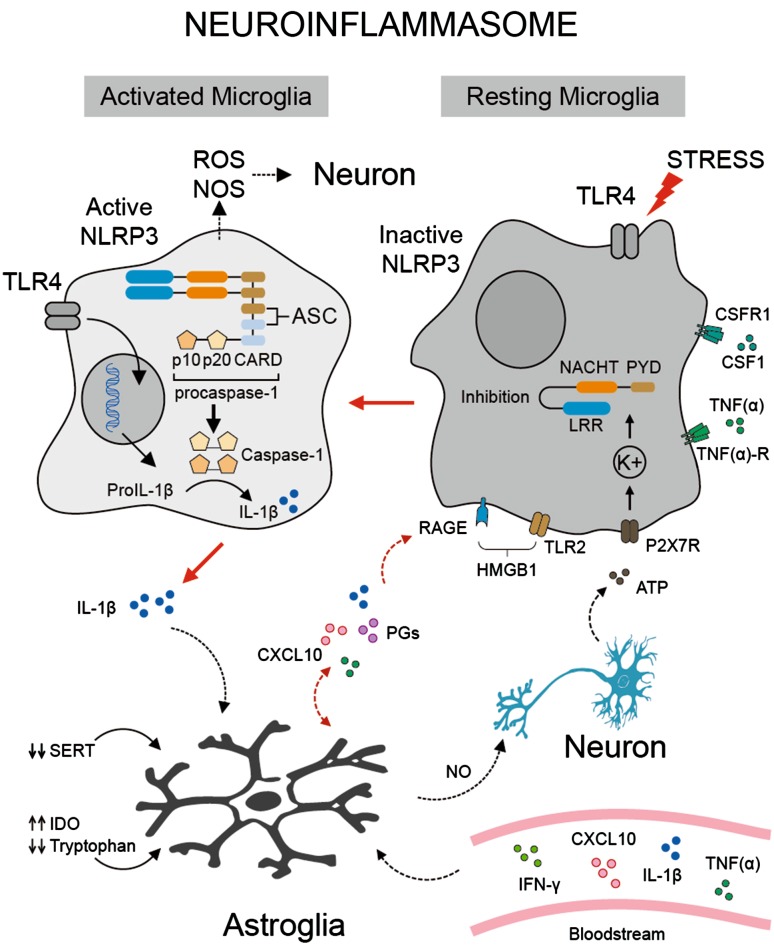
Fig. 2.
Schematic of the NLRP3 inflammasome in microglia. Microglia (resting state) may become active through the binding of ligand agonists (LPS and HMGB1) to Toll-like receptors (TLR4 and TLR2) and/or by psychosocial stressors. HMGB1 is an intracellular DNA-binding protein involved in chromatin remodeling. This protein is released by necrotic cells (apoptotic neurons) or immune cells (macrophages, natural killer cells, and dendritic cells) acting as cytokine mediators of inflammation. Interaction of HMGB1 with the RAGE receptor (a 35-kDa transmembrane receptor of the immunoglobulin superfamily) and TLR2 leads to the expression of inflammatory genes (not shown) that activate the NLRP3 inflammasome. Activation of the NLRP3 inflammasome in microglia requires extracellular ATP, whose binding to the P2X7 receptor ionophore, causes K+ efflux which leads to NLRP3 oligomerization. In the resting state, the NLRP3 inflammasome adopts an autoinhibitory conformation by binding of the LRR domain to the NACHT domain, producing an inhibitory oligomerization response as well as ASC binding. However, stimulation removes the former inhibition and enables NLRP3 to bind with procaspase-1 through the adaptor protein ASC. This leads to the cleavage of procaspase-1 into the activated caspase-1, cleaving pro-IL-1β into IL-1β. IL-1β released from microglia activates inflammatory signaling pathways (STAT1, IRF-1, NF-kB, and p38 MAPK) in astrocytes (not shown), increasing serotonin (5-HT) metabolism via activation of indoleamine 2,3 dioxygenase (IDO). This causes a depletion of tryptophan and reduction of serotonin availability. Activated microglia release both NO and ROS targeting damaged neurons or cells undergoing apoptosis. Astrocytes contribute to the neuroinflammatory process by expressing cytokine receptors and releasing an extensive repertoire of interleukins (IL-1β, TNFα, IL6, IL-10, IL-15, INFβ, and TGFβ), chemokines (CXCL10), prostaglandins (PGD2 and PGE2), and nitric oxide (NO) which promote the inflammatory responses on adjacent or same cells via autocrine and paracrine mechanisms. Inflammatory signals may be responsible for the reduced expression of the serotonin transporter (5-HTT) in these cells. Furthermore, immune mediators from maternal blood may increase the neuroinflammatory process and exacerbate depressive symptoms during pregnancy (see text for details). Abbreviations: ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; CARD, caspase-recruitment domain; HMGB1, high mobility group protein B1; MCP-1, monocyte chemoattractant protein-1 chemokine; LRRs, leucine-rich repeats; NACHT, nucleotide-binding oligomerization; NLRP3, NLR family; NO, nitric oxide; PYD, Pyrin domain; RAGE, advanced glycation end product receptor.
The Inflammasome
Both physical and psychological stressors activate immune cells in the periphery and the central nervous system (CNS) to induce the release of inflammatory cytokines that lead to neurotransmitter changes and altered behaviors [54, 85]. Recent studies have provided important clues of how psychological stress can affect the immune system and how stressors activate both the innate and adaptive immune systems [25]. Stressors and inflammation are tightly linked through regulation by two complementary subsystems, the innate and the adaptive immune systems, each of which is stimulated by different signals or stress stimuli. The innate immune system is activated by invasive pathogens that target pattern-recognition receptors or TLRs (which detect different kinds of stressors) and cytosolic NOD-like receptors (NLRs), which comprise a wide and varied set of proteins that respond to cytosolic agonists. Members of the NLR family act as scaffolds that can oligomerize into larger protein complexes, thereby forming a molecular platform called the inflammasome [25]. These multi-protein complexes contribute to the activation of inflammatory caspases (caspase-1) that result in the proteolytic processing and secretion of cytokines, including IL-1β and IL-18 [86]. Thus, the inflammasome includes distinct members of the NLR family (NLRP1, NLRP3, and IPAF) as well as the PYHIN family member AIM2, and is a critical mediator of cellular stress in the innate and adaptive immune systems [25].
The immune system is able to detect damage signals in the absence of any pathogen through the release of DAMPs such as heat-shock protein (HSP)-72, uric acid, and ATP through a process termed ‘‘sterile inflammation’’ [87, 88]. DAMPs released by pathogens such as lipopolysaccharide (LPS) stimulate the innate immune system by activating what is known as the “NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome” (a cytosolic multiprotein complex involved in the processing of interleukins). DAMP-induced stimulation of the NLRP3 inflammasome leads to activation of the immune cell caspase-1 enzyme, cleaving the pro-peptide protein precursor of IL-1β and IL-18 into their mature releasable forms [88, 89]. Cellular release of IL-1β enhances the production of other inflammatory cytokines that are released during stress (Fig. 2).
Studies in animals indicate that chronic mild stress activates the NLRP3 inflammasome, showing a response to DAMPs. Interestingly, blockade of NLRP3 reverses the stress-induced increases of IL-1β in the blood and brain, abolishing the stress-induced depressive-like behavior in mice [90, 91]. Furthermore, glucocorticoids arising from chronic stress or prolonged exposure to inflammatory cytokines can lead to an increased predisposition for the release of IL-1β and other cytokines [54, 92, 93]. However, recent evidence has shown that upregulation of the NLRP3 inflammasome and caspase activation mediating the cleavage of the GC receptor, can cause a resistance to the systemic effects induced by GCs (the most prominent anti-inflammatory hormones) [94, 95]. Interesting studies showed that stressors inducing GC resistance (a well-characterized abnormality in MDD patients) increase inflammatory activity with high levels of pro-inflammatory cytokines in blood [93, 96].
In addition, recent findings supporting the role of the NLRP3 inflammasome in depression have shown that the increased expression of NLRP3 and caspase 1 activity in peripheral blood mononuclear cells (PBMCs) of patients with depression is associated with increased blood concentrations of IL-1β and IL-18, which are correlated with the severity of depression [68, 97]. Thus, both DAMPs and the NLRP3 inflammasome [25, 86] appear to reflect the primary link by which stressors are translated into signals of damage that promote inflammatory activity, and contribute to depression as well as co-morbid illnesses associated with chronic stress [89].
In support of the latter, animal studies showed that non-pathogenic (commensal) bacteria and derived microbial-associated molecular patterns (MAMPs) in the gut can leak into the peripheral circulation during psychosocial stress, activating both peripheral and brain NLRP3 inflammasomes [98] via activation of the sympathetic nervous system and the release of catecholamines [99]. Moreover, increases of both IL-1β and IL-18 levels in blood are attenuated by antibiotic treatment or LPS neutralization, thus supporting a role of bacterial composition in stress-induced inflammatory responses. These data led the authors to propose that the inflammasome may represent a crucial immunological key mediating the integration of stress-induced endogenous danger signals and inflammatory responses in major depression [65].
NLRP3 Inflammasome and Depression
Environmental stimuli propitiate inflammatory responses via activation of the NLRP3 inflammasome and cytokine secretion in MD. Stressors leading to the activation of inflammatory processes and cytokine release have been correlated with changes in brain function and depression onset; it has been postulated that IL-1β secretion is the initial step in the inflammatory cascade linking psychosocial stress to the development of MDD [89]. In agreement with this proposal, IL-1β has been shown to elicit depressive-like behaviors in animals exposed to either acute or chronic unpredictable stressors [85]. Furthermore, clinical findings have shown that patients with MDD have increased blood levels of IL-1β and IL-18, and this is associated with an increased activity of the NLRP3 inflammasome in PBMCs [97]. These data are correlated with findings showing that activation of the NLRP3 inflammasome leads to increased accumulation of myeloid-derived suppressor cells (a heterogeneous population of immature myeloid cells that suppress innate and adaptive immunity by inhibiting T cell proliferation; for review see [100]), which are associated with decreased numbers of peripheral blood T-reg cells and reduced concentrations of anti-inflammatory cytokines, including TGFβ and IL-10 in MD [101]. Thus, it appears that patients with depression have neuroprotective impairments and altered anti-inflammatory T-cell responses, which argues in favor of the hypothesis that activation of the NLRP3 inflammasome, pro-inflammatory signals, and altered immune cell activity concur with MDD [65].
Similar findings have shown increased expression of both IL-1β and NLRP3-inflammasome mRNA in the brains of mice exhibiting depressive-like behaviors after LPS administration. These data suggest that inflammatory components and immune mediators are involved in the connection linking psychosocial stress to MDD [102]. Although some studies have proposed a critical role for mitochondrial rupture during NLRP3 activation [103, 104], recent studies showed that the inflammasome pathway in MDD does not depend on mitochondrial dysfunction [97]. While the mechanisms underlying the effect of psychosocial stress on inflammasome formation are still a matter of dispute, clinical studies have shown how antidepressant medications can regulate inflammasome activation. For example, amitriptyline (a common tricyclic antidepressant) inhibits IL-1β and IL-18 production, as well as NLRP3 and caspase-1 gene expression in patients with MDD [97]. Similar findings have been reported for glyburide, an anti-diabetic drug that inhibits caspase-1 activation and IL-1β secretion, and it has been proposed to be effective as an inhibitor of NLRP3 formation following stress-induced depression [105]. Furthermore, selective serotonin reuptake inhibitors (SSRIs) (excluding tricyclic antidepressants) increase TNF-α and IFN-gamma (IFN-χ) levels in the frontal cortex, which is inhibited by anti-inflammatory agents [106]. These findings suggest that SSRIs and tricyclic antidepressants rely on different mechanisms when interacting with anti-inflammatory drugs. Moreover, antidepressants reduce LPS-induced peripheral IL-6 and TNF-α production [107]. Chronic SSRI administration also attenuates CRH, TNF-α, and IL-1β mRNA expression in the hypothalamus [108]. Thus, it will be interesting to explore in clinical trials the pharmacobiological effects of anti-inflammatory agents on the efficacy of antidepressants in patients with MDD [108] (Fig. 2).
Glucocorticoids and the NLRP3 Inflammasome
Chronic stress and GCs have been reported to modulate the microglia immunophenotype [26, 85, 97] as demonstrated by the effects of GCs in upregulating the expression and activation of MHCII and Iba-1 antigens in CNS macrophages (perivascular macrophages) and microglia, suggesting that stress and GCs can alter the immunophenotype of myeloid cells. For instance, different studies have shown that GCs may prime a distinct set of macrophage populations, regardless of their micro-environmental milieu [26, 102]. Thus, GCs appear to play a pivotal role in chronic stress-induced neuroinflammatory priming [26, 85, 97, 103] and are sufficient to prime neuroinflammatory responses to subsequent pro-inflammatory insults. [26, 104]. The NLRP3 inflammasome [multi-protein complex associated with the adaptor protein-ASC and pro-caspase-1] is the only inflammasome known to be primed by GCs [26, 105].
Furthermore, formation and activation of the NLRP3 inflammasome lead to the formation and release of active, mature IL-1β [106] as recently demonstrated in THP-1 cells, bone marrow-derived macrophages, and primary human monocytes in vitro. GCs are able to induce NLRP3 at both the mRNA and protein levels, priming NLRP3-inflammasome formation to a subsequent stimulus such as ATP, and potentiating the pro-inflammatory responses of cytokines (IL-1β) [26, 102, 107]. In the same way, high levels of GCs increase NF-κBIα expression and p65-NF-κB transcriptional activity [26, 104], increasing the expression of pro- and anti-inflammatory cytokines. Similarly, cortisol increases the gene expression of NLRP3, Iba-1, MHCII, and NF-κBIα in a concentration-dependent manner, potentiating the microglial pro-inflammatory responses of TNFα, IL-1β, IL-6, and NLRP3 to LPS [26]. These results suggest that GCs prime the neuroinflammatory processes through distinct signaling pathways, such as NF-κB, leading to the activation of both pro- and anti-inflammatory processes. In other words, GCs appear to set in motion opponent processes, which summate to form either an anti- or a pro-inflammatory response to a subsequent challenge, depending on the severity of the GC-inducing stressor and timing of the immunological threat in relation to the stress experience [26, 108].
Toll-Like Receptors
The identification of TLRs in several pathologies have led to a better understanding of the mechanisms by which the innate immune system recognizes non-self-molecules and how TLRs promote the detection of invading pathogens [154]. TLRs recognize endogenous DAMPs, including HSPs and high-mobility group box 1 (HMGB1), in addition to exogenous pathogen-associated molecular patterns, such as LPS and MAMPs [109, 110]. Since DAMPs can activate TLR signaling and produce inflammatory responses, TLRs have been thought to discriminate dangerous from non-dangerous stressors [111].
The first recognized mammalian TLR homologue of the Drosophila Toll [112] was TLR4, which was identified a year after elucidating the role of the Drosophila Toll in fighting fungal infection [113]. Like the Drosophila Toll, human TLRs are characterized as type I transmembrane proteins with an extracellular leucine-rich repeat (LRR) domain and a cytoplasmic C-terminal Toll-interleukin-1 receptor (TIR) domain. Based on the chromosomal localization, genomic structure, and amino-acid sequences, human TLRs are classified into five subfamilies: TLR2, TLR3, TLR4, TLR5, and TLR9. The TLR2 subfamily consists of TLR1, TLR2, TLR6, and TLR10, while the TLR9 subfamily is composed of TLR7, TLR8, and TLR9. TLR3, TLR4, and TLR5 each represent a family with only one member [154]. TLR4 recognizes the LPS motif in the cell membrane of all gram-negative bacteria, while TLR2 recognizes lipoteichoic acid expressed in gram-positive bacteria [114]. Furthermore, recent evidence has shown that mutants of human TLR4 transfected into human cell lines activate the NF-κB transcription factor and NF-κB-controlled genes involved in the expression of IL-1, TNF, IL-6, and IL-8, in addition to the co-stimulatory molecules CD40, CD80, and CD86 [113].
Although a major role of TLR4 is the recognition of LPS, TLR4 by itself does not sense LPS directly, but requires the LPS binding protein (LBP), which is an acute-phase protein found in the plasma that binds the lipid A component of the LPS molecule [115]. The LBP-LPS complexes together with functional membrane-bound components [CD14 antigen (a glycosylphosphatidylinositol-anchored molecule) and the MD-2 molecule] play an important role during LPS signaling by TLR4.
In this context, both TLR2 and TLR4 signals that have been co-opted by endogenous danger signals (psychosocial stress) or DAMPs are thought to alert microglia, as well as peripheral myeloid cells, of a variety of internal conditions such as cellular stress, damage, or death [116] by stimulating the release of pro-inflammatory cytokines, chemokines, and active reactants [nitric oxide (NO) and prostaglandins (PGE2 and PGD2)] implicated in pro-inflammatory responses (Fig. 2).
Most of the TLRs expressed in cells are pre-assembled into heterodimeric complexes on the cell surface or in the cytosol, in order to activate the cell-signaling pathway and transcription factors regulating the expression of genes responsible for promoting host-defense mechanisms, such as, the mammalian antimicrobial peptide (defensins), the antiviral response (type I interferons), pro-inflammatory cytokine secretion, co-stimulation of membrane-bound antigens, and dendritic cell maturation after activation of inflammatory responses (for extensive review, see [154, 155]).
Thus, the innate immune system senses invading microorganisms by a phylogenetically-conserved family of pattern-recognition receptors of which TLRs are the most important; although this system provides a less specific response, it is nevertheless critical for the prevention of microbial invasion. Activation of TLRs results in the induction of innate immunity mechanisms, including the development of antigen-specific adaptive immune responses bridging innate and adaptive immunity in mammalian and other vertebrate species [154].
TLRs have been implicated in the pathophysiology of affective disorders, as shown by the correlation of genetic variants of TLR2 and TLR4 with the early onset of bipolar depression and childhood sexual abuse [117] as well as in MD [118].
Thus, given the nature of the expression of TLRs in the peripheral and central immune systems, including placental immune cells, it could be that these stress-related components may be relevant to both immune activation and inflammatory responses in affective disorders during pregnancy, in a fashion similar to their involvement in MDD.
Toll-Like Receptor 4 and the HPA Axis
TLR4 belongs to the interleukin-1 receptor/TLR superfamily containing a TIR domain and an LRR motif in the extracellular domain [110]. TLRs recognize endogenous DAMPs, including HSPs and HMGB1, exogenous pathogen-associated molecular patterns such as LPS, and MAMPs [109, 110] (Fig. 2).
Given the nature of TLR4 as a crucial component of the stress-related immune system, it has been proposed that it might interact with the HPA axis in stress-induced mood-related disorders [110]. TLR4 signaling is capable of stimulating the HPA axis upon LPS administration [110, 119]. Interestingly, different studies showed that TLR4 activation not only causes GC release from adrenal cells [120, 121] but also induces upregulation of the CRH gene in paraventricular (PVN) neurons in the hypothalamus [122], enhancing the increased levels of this peptide hormone in blood [123]. Although pituitary cells stimulated by LPS also stimulate the release of ACTH, it is uncertain whether this effect is CRH-dependent [93, 110, 124].
Recent studies have shown that cytokines secreted following immune stimulation upregulate HPA axis signaling through two main pathways: (1) by reducing negative feedback on HPA signaling, and (2) by directly stimulating HPA activation. Through the first pathway, pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) reduce the efficacy of the GC receptor (GR), promoting disinhibition of the GR-induced negative feedback on HPA activity [93, 125] . This mechanism has been suggested to rely on protein-protein interactions with GRs, enabling cytokines to modulate GR-dependent ligand binding affinity, cytosol-dependent GR translocation, and GR binding to the GC response element within the nucleus [93].
Furthermore, TLR4 activation may not only lead to short-term stimulation of the HPA axis, but also appear to influence HPA activity long after the stressor has resolved. For instance, animal studies showed that a single LPS challenge during early life is sufficient to hypersensitize both the CRH and ACTH responses upon subsequent LPS exposure or restraint stress in adulthood [126]. These findings suggest that TLR4 activation during early life increases anxiety behaviors in adulthood [127], besides leading eventually to changes in the stress-response system during life.
Thus, TLR4 activity during sensitive periods of development may shape the response of the HPA axis to incoming danger signals, priming the system towards abnormal hyperactivity, enhancing the predisposition toward stress-related disorders [110]. This hyperactivity is produced by increased activity of IL-1β, IL-6, and COX-2 as well as PGE2 on PVN neurons [128], pituitary ACTH, and the adrenals (this latter drive the steroidogenesis pathway, regulating GC synthesis) [121, 126, 129]. Thus, the TLR4 pathway appears to have an important influence on HPA activity, rather than a direct impact on adrenal function; the HPA responses are driven by mechanisms operating within the CNS through neuroimmune signaling pathways [110].
Toll-Like Receptor-4 and Depression
MD is recognized as neuroimmune disorder and TLR4 has been implicated [130, 131]. Molecular studies have revealed that TLR4 activation by DAMPs, LPS, or MAMPs [109, 110] triggers transcription via two adaptor proteins; the myeloid differentiation primary response 88 (MyD88) and the TIR domain-containing the adapter inducing IFN-β (TRIF), which induce the transcription factors NF-κB, AP-1, and IRF3 [132, 133]. Activation of these factors leads to the production and release of pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) and chemokines (CXCL10) among several other proteins, such as COX-2, which in turn activate pro-inflammatory signaling [109] in central and peripheral immune cells. In the CNS, TLR4 is predominantly expressed in microglia and to a lesser extent in neurons [134]. TLR4 has recently been implicated in several neuropathological conditions of the CNS, for instance, neuropathic pain [135] and neurodegenerative disorders [136].
Furthermore, high TLR4 levels have been reported in the PBMCs of patients with MDD, and antidepressant treatment significantly reduces the high TLR expression along with reduced depressive symptomatology [131]. This responsiveness clearly suggests a direct role of TLR4 activity in the pathogenesis of MDD. As stated above, microglia play a crucial role in modulating several neuroinflammatory activities.
Microglia expressing TLR4 and TLR-dependent cell signaling systems [130] have been correlated with changes in microglial reactivity states, commonly referred to as M1 and M2, the pro- and anti-inflammatory phenotypes [137] and with the expression of depressive-like behavior in animal models of stress [195]. TLR4 activation promotes a shift of microglia towards the M1 phenotype (exhibiting an amoeboid morphology) enhancing their pro-inflammatory activity and the secretion of immune mediators that affect local and peripheral immune cells [138] (Figs. 1, 2).
Microglia also function as antigen-presenting cells through MHC-II expression, to trigger adaptive immune responses [139]. It is notable that minocycline, an antibiotic that suppresses central immune signaling, abolishes the development of stress-induced depressive-like behaviors in mice [140], and reduces microglial reactivity and LPS-induced effects on microglial morphology [141, 142] and cell proliferation [110]. However, chronic inhibition of microglia is not a viable treatment option, since central immune suppression exacerbates depressive-like behavior in animal models of inflammation. Therefore, it has been proposed that treatment should be aimed toward a balance between high- or low-reactivity states of these cells [110] during MDD. Furthermore, glia appear to respond to antidepressants such as SSRIs, which decrease gliotransmission [143], thereby attenuating the secretion of inflammatory cytokines in response to LPS. Recent evidence has shown that antidepressants are protective against microglial [144] and MPTP-induced neurotoxicity [145]. These reports argue that microglia are crucial elements in central immune signaling, facilitating communication between the immune system and the brain in mood-related disorders. However, this relationship is not unidirectional, and appears to operate in a time-dependent fashion [110].
Immune System During Pregnancy
Placental Immune Cells
During pregnancy, the innate immune system provides a less specific response which nevertheless is critical for the prevention of microbial invasion. However, evidence has been accumulating over the years about how the immune system is finely tuned at the maternal-fetal interface to allow the recognition and development of the fetal “allograft” [23]. During early pregnancy, different subsets of natural immune cells [natural killer (NK) cells, dendritic cells (DCs), and macrophages (Mφs), including T-reg cells] infiltrate the decidua around the invading trophoblast cell-layer [23, 146], enhancing the recruitment and migration of immune cells via secretion of inflammatory cytokines (IL-6) [23]. During the first trimester, NK cells, DCs, and Mφs infiltrate the decidua and accumulate around the invading trophoblast. Interestingly, the absence of these cells does not favor pregnancy, but instead enhances its termination [23, 146]. For instance, depletion of decidual NK cells has deleterious effects on placental development and blastocyst implantation, suggesting their critical role in trophoblast invasion in the uterus, in contrast to decidual DCs, whose depletion affects blood vessel maturation and decidua formation [24, 46, 47]. Different studies have shown that the vast majority of decidual NKs appear to localize at the site of implantation, meditated by the released cytokines (IL-6, IL-8, and TNF-α), chemokines (CX3CL1, CCL7, CCL14, and CCL4) and growth factors (h-EGF and VEGF) from decidual cells and trophoblasts [23, 147, 148]. Furthermore, interesting studies have shown that NK cells differ in both phenotype and function. Peripheral NK cells exhibiting the CD56dim/CD16+ antigen promote cell lysis, while decidual NKs expressing the CD56bright/CD16-antigen have reduced cytotoxicity, with a phenotype of cells producing cytokines [149, 150].
Autocrine mediators released by trophoblast cells (TGF-β, IL-1β, TNF-α, IL-6, IL-8, IL-10, and decorin) and paracrine factors secreted by decidual cells [leukemia inhibitory factor (LIF, a member of the IL-6 protein family); IL-11, granulocyte macrophage colony-stimulating factor (GM-CSF); and macrophage colony-stimulating factor (M-CSF)], in addition to proteins such as prokineticin 1 and heparin-binding epidermal growth factor (hb-EGF), upregulate the expression and synthesis of soluble mediators, such as LIF/IL-6 [151, 152], chemokines (CCL7/MIP3 and CCL4/ MIP1β), COX-2, and prostanoids (prostaglandins, prostacyclin, and thromboxane) [153], as well as serotonin (5-HT) and histamine [154–156], which stimulate and drive placental processes (blastocyst implantation, trophoblast proliferation, decidualization of endometrial tissue, implantation, and blood flow regulation) in a time- and space-dependent manner, facilitating the tolerance and development of the fetal “allograft” [157]. In addition, LIF, IL-6, and IL-11 upregulate the expression of adhesion molecules in human endometrial epithelial cells, facilitating implantation and trophoblast invasion during early gestation [152] (Fig. 3).
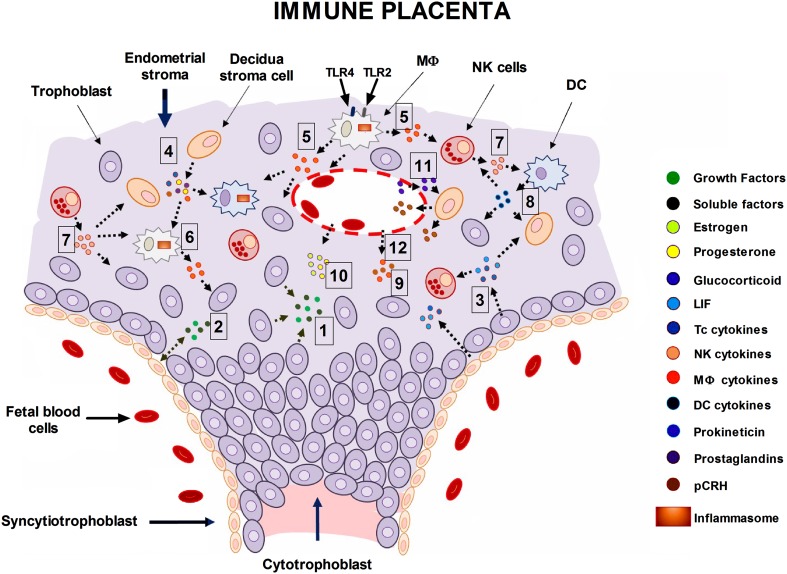
Fig. 3.
Schematic of the neuroendocrine and immune placenta. The interaction between the maternal innate immune system, neurosecretory cells, and trophoblastic tissue at the trophoblast-decidua interface is shown. A wide variety of active components are secreted by immune and non-immune cells at the fetoplacental unit and play crucial roles during blastocyst implantation, trophoblast invasion, placentation, and decidualization. From early to late pregnancy, trophoblasts (TCs) from the blastocyst differentiate into invasive trophoblasts (cytotrophoblasts) that penetrate the endometrium along with syncytiotrophoblasts. Trophoblasts release growth factors (hb-EGF, IGF1, VEGF, and TGF-β) and proteins that regulate cell growth and invasion into the endometrial epithelium [1, 2]. TCs secrete autocrine mediators (TNF-α, IL-1β, IL-8, IL-10, and IL-11) as well as leukemia inhibitory factor (LIF) [3] and soluble factors (MCP-1 and GRO-α) that allow trophoblast development and function during blastocyst implantation and placentation. Decidual cells involved in remodeling the endometrium secrete LIF and stimulating factors (GM-CSF and M-CSF) that mediate trophoblast proliferation, migration, and invasiveness into the decidua in a time- and space-dependent manner [4]. Furthermore, these cells secrete the pleiotropic protein prokineticin 1 whose binding to its cognate receptor (PROKR1) is responsible for the inflammatory responses mediated through the release of interleukins from adjacent cells [4, 5]. Activation of TLR4 and TLR2 by specific ligands (LPS and MD2) on Mφs and/or DCs may lead to the activation of transcription factors (AP1 and IRF3) and gene enhancers (NF-κB) that orchestrate the synthesis and release of interleukins (IL-6 and IL-8) and chemokines (CCXL10) as well as COX-2 and prostaglandins (PGE2) [5, 6]. These immune mediators may influence the functional activity of immune and non-immune cells in the decidual stroma in a reciprocal fashion [7, 8]. However, cytokines from the maternal circulation [9] may increase the inflammatory signals at the maternal-fetal interface, enhancing the establishment of a preferential Th1 immune response during perinatal depression. Moreover, sex hormones (estrogen and progestins) play a crucial role in remodeling the endometrium, in addition of their regulatory hormone-related activity induced on immune and decidual cells during pregnancy, including TCs, as well [10]. Glucocorticoids and cytokines (IL-1β) among other factors (stress and anoxia) upregulate the expression and secretion of pCRH from decidual cells [11], while progestin and NO have opposite effects (data not shown). pCRH reaching the maternal circulation may increase the HPA axis activity [12], leading to the exacerbation of depressive symptoms during this period. Moreover, pCRH binding to its cognate receptor (CRHR1) mediates the expression of the apoptotic Fas ligand by both cytotrophoblasts and decidual cells, suggesting that pCRH regulates the invasiveness of trophoblasts into the decidua [12]. Similar to the inflammasome expressed by microglia in the brain, activation of TLR4 in APC cells (Mφs and DCs) may lead to activation of a proposed “placental inflammasome” [5, 6] precipitating the release of local inflammatory cytokines (IL-1β and TNF-α) [5–8], and leading to wide and varied effects on neurotransmitter and glucocorticoid metabolism (data not shown). Overall, this suggests that same cellular mechanisms that operate in the brain and the immune system during MDD, operate in the “neuroimmune placenta” during perinatal depression (see text for details). Abbreviations: AP1, activator protein 1; APC, antigen-presenting cell; IRF3, interferon regulatory factor 3; LIF, leukemia inhibitory factor; NF-κB, nuclear factor of kappa light polypeptide gene enhancer; NO, nitric oxide; pCRH, placental corticotropin-releasing hormone.
These findings support the hypothesis that pregnancy needs a reduced activity of NK cells and inflammatory Mφs (these cells are primed through the activation of the Th1 immune response and Th17-cells producing inflammatory cytokines), in addition to high T-reg cell activity and the production of anti-inflammatory cytokines (IL-4 and IL-10), as previously reported [158]. This activity sets the immune balance in a state of self-tolerance. However, disruption of this fine balance during pregnancy may promote an overwhelming Th17 response, shifting the system towards an increased inflammatory profile that may lead either to autoimmune disease (by attacking the feto-placental tissue) or to neuropsychiatric disorders, when increases in Th17 responses and T-reg activity work simultaneously to sustain placental function and fetal viability [158].
Th1/Th2 Balance
One crucial aspect of pregnancy is the Th1/Th2 bias, which under proper conditions may facilitate the maintenance or rejection of the conceptus. It has been associated with recurrent spontaneous miscarriages or preterm birth [159]. Over the years, it has become clear that the Th1 and Th2 subsets originate from undifferentiated Th0 cells under the influence of several factors, such as IFN-γ and IL-4 [159, 160]. Soluble mediators, such as progesterone [159], LIF [161], estradiol [162], and PGD2 [163] promote the Th2 profile and are likely to be partially responsible for the Th2 bias associated with pregnancy [164]. Thus, the original hypothesis of the Th2 predominance and downregulation of the Th1 response in pregnancy, formulated decades ago, has been supported by several animal (mouse) and human studies showing both Th2 and Th1 bias during pregnancy [159, 164, 165].
Pro- and anti-inflammatory cytokines in peripheral blood [164, 166] have been extensively studied during pregnancy [164, 165]. In line with this, placental immune mediators such as T cell-derived IL-4, IL-10, and M-CSF are associated with a successful pregnancy [164], suggesting that such mediators promote Th2 predominance and full-term pregnancy. Furthermore, trophoblast, decidual, and amniotic cells also appear to contribute to the Th2 cytokine environment by enhancing the local release of IL-13 [167], IL-10 [168], IL-4, and IL-6 [169, 170], while pro-inflammatory cytokines such as IL-2, IFN-γ, and TNF-α produce miscarriages in mice and treatment with Th1-cytokine inhibitors or anti-inflammatory Th2 cytokines abolishes this effect [164, 171]. This immunomodulation promotes the expression of the Th2 immune response during pregnancy, which is regulated by the expression of GATA-3 and STAT-6 transcription factors that leads to the production of Th2 cytokines by trophoblasts and T cells. Such response enhances the inhibition of the Th1 transcription factor STAT-4, and thereby, the reduction of IFN-γ and TNF-α [170].
In addition, Th2 cytokines (IL-4 and IL-10) potently inhibit Th1 cell and macrophage-related inflammatory activity, preventing rejection of the fetal allograft [172], besides upregulating the synthesis of COX-2 and PGE2 in amniotic cells [173], a mechanism thought to inhibit the onset of labor. These data parallel those showing that placental PGD2 released from the invading trophoblast cell layer enhances the expression and activation of the Th2-chemoattractant receptor-homologous molecule (CRTH2), facilitating the PGD2-chemoattractant activity of Th2 cells [174].
Interestingly, women suffering from recurrent or unexplained failure of pregnancy have reduced placental CRTH2+ cells [175] and reduced IL-4 and IL-10 production by decidual CD4+ cells [176], compared to women undergoing elective termination. These data suggest that the immune processes promoting a Th2 bias over a Th1 immune response are crucial for fetal development. In addition, both progestins and estradiol influence the shift of the Th1/Th2 bias towards a type 2 immune response [177], while cortisol at the maternal-fetal interface modulates and maintains the type 2 cell response throughout pregnancy [178, 179].
Interestingly, the Th2 bias during pregnancy decreases cell-mediated immunity [180], thus increasing the susceptibility to infections by intracellular pathogens such as influenza, leprosy, and Listeria monocytogenes [164, 165, 181, 182]. However, Th1 cytokines maintain the ability to mount defensive responses to infections, as shown by in vivo and in vitro studies that demonstrate an increased percentage of cells secreting IFN-γ in neonates exposed to intrauterine infections [183], and increased IFN-γ and reduced IL-4 secretion in cultured cord-blood mononuclear cells exposed to LPS [184].
Estimates have shown that only 30% of preterm births [164, 185], in contrast to the high rate of 80–85% of early preterm births (<28 weeks), are associated with infections [164, 185]. Immune and non-immune cells contribute to a cytokine-rich environment in the presence of infection and inflammation. Pro-inflammatory cytokines (TNF-α and IL-1β) activating the NF-κB signaling system evoke the release of PGE2, PGD2, and MMPs [164, 186] from local immune, trophoblast, and decidual cells, respectively, which if activated during early pregnancy, may trigger a cascade of pro-labor events leading to preterm labor and birth [164, 186].
Thus, women with MDD during late pregnancy may show a shift in Th1/Th2 bias, exhibiting a preferential Th1 immune response with higher inflammatory cytokines (IL-1β, LIF/IL-6, IL-8, and TNF-α) [165, 187–189] and higher levels of circulating steroids than healthy pregnant women [187, 190]. These findings suggest that the innate immune system together with both the hypothalamic-pituitary/gonadal (HPG) and HPA axes are highly active and engaged in MDD during pregnancy [187].
Toll-Like Receptors at the Maternal-Fetal Interface
TLRs are expressed by both immune (Mφ) and non-immune cells (trophoblasts and decidual cells); their co-receptors and accessory proteins (CD14) are also expressed in the placenta [191, 192]. TLRs vary with the stage of pregnancy [191]. During early pregnancy, at least seven TLRs (TLRs 2–6 and TLR 9) are expressed by fetal membrane cells in vitro (cyto- and syncytiotrophoblast-rich cells) [191–193], while choriocarcinoma cell lines (i.e., JAR and BeWo) express ten distinct TLRs, in addition to their co-receptors and accessory proteins (CD14, CD36, MyD88, MD-2, TIRP, TRAP, and TRIF) [194].
Similarly, molecular studies have revealed that trophoblastic cell lines (Swan 71, 3A, and HTR8) from fetal membranes express TLRs (TLR1-4 but not TLR6) during early gestation [195–197], while third trimester trophoblasts express high levels of placental TLR4 [197] and TLR6 [195, 196]. These studies led the authors to posit that trophoblasts during early pregnancy are less responsive to pathogens than the same placental cells at term [198]. Although the mechanisms that regulate the temporal expression of TLRs are still unclear, their spatial regulation has been investigated in different trophoblast layers. For instance, early expression of TLR2 and TLR4 occurs in villous cytotrophoblasts and extravillous trophoblasts (inner trophoblast cell layer) but not within the syncytiotrophoblast layer (the outer trophoblast cell layer) in the first trimester [195–197]. The latter, TLR-negative cell-layer represents the ‘second battlefront” or barrier that reacts to pathogens that have breached the villous trophoblast and decidua (endometrium) [192, 193].
Other macrophage-like cells expressing TLRs are the Hofbauer cells in the placental villi, which express high TLR4 levels in the term placenta [195, 196]. In contrast, TLR2 expression has been found in endothelial cells and peripheral macrophages, in addition to syncytiotrophoblasts and fibroblasts, but is weakly expressed in the term placenta [197].
Thus, these findings suggest that local immune cells, trophoblasts, and peripheral macrophages invading the early and term placenta appear to respond to invading pathogens, providing a crucial protective immune barrier to the conceptus during development [192, 198]. In contrast, the expression of TLRs in the decidua has not been extensively investigated. Nonetheless, two studies have shown the expression of ten TLR-mRNAs in the first trimester and term decidua, and the expression of TLR2, TLR4, and TLR6 proteins in first trimester decidua [193, 195, 199] (Fig. 3).
Along the same line, related studies have demonstrated the expression of both TLR2 and TLR4 in the amnion, suggesting their role in monitoring either pathogens in the amniotic fluid and/or interfering with the recognition of TLR2 ligands by TLR2 [196, 200]. These studies suggest that TLRs play a crucial role in monitoring intra-amniotic inflammatory responses to pathogens, as shown by the moderate levels of soluble TLR2 [200].
Inflammatory Signals and Perinatal Depression
Evidence for a role of inflammation and its associated cytokines in depression has accumulated since its initial description [26]. Clear evidence implicates innate immune genes and proteins, such as IL-1β, IL-6, TNF-α, TLR2-TLR4, and its regulator the NLR family and the NLRP3 inflammasome in MDD [89], providing a bidirectional pathway between endogenous danger signals or psychosocial stress and depression, including other co-morbid illnesses associated with chronic stress [89].
Perinatal depression is associated with increases in brain and peripheral inflammatory cytokines (IL-1β, IL-6, and TNF-α) and acute biomarkers similar to MDD [201–204]. These appear to be correlated with depression onset [89] and with changes in brain function (i.e., reduced hippocampal activity associated with cognitive deficits, reduced memory, and poor behavioral performance) [1, 54, 205] as well as neuroendocrine responses and reduced neurotransmitter function [1, 54, 206–208], suggesting that the brain translates immune signals into a “mood-related disorder linked to an inflammatory process” in MDD during pregnancy [1, 54, 206–208].
Thus, based on multiple lines of evidence showing an active role of the immune system in MDD [130, 131], it may be feasible to assume that TLR signaling in response to endogenous danger signals (psychological stress), activation of the NLPR3 inflammasome, and increased secretion of IL-1β and IL-18 from microglia (M1-active state phenotype) [138] or PBMCs [97] [97], in addition to the release of placental cytokines (IL-1β, TNF-α, LIF/IL-6, and IFN-γ) from immune (Mφs, NKs, and DCs) and non-immune cells (decidual cells and trophoblasts) [157] during the third trimester, may contribute to triggering the changes in the prefrontal cortex, anterior cingulate, and hippocampus as well as neuroendocrine (hypothalamus, pituitary, and adrenal) and placental processes, which elicit the hallmark symptoms of depression during pregnancy [157].
In line with this, the major features of hypothalamic, adrenal, and placental dysfunction in MDD during pregnancy appear to be linked to stress-induced neuroimmune responses via endogenous danger signals that promote the activation of TLR4 signaling (NF-κB, AP-1, and IRF3) [132, 133] and increase the secretion of pro-inflammatory cytokines [185, 188], based on the following findings:
In the hypothalamus: (1) TLR4 activation and upregulation of the CRH gene in hypothalamic PVN neurons [122] with increased peptide levels in blood [123]; (2) potent stimulatory effects of IL-1β inducing increased expression of CRH mRNA and CRH protein in the hypothalamus and amygdala (regions implicated in fear and anxiety responses in MMD) [209, 210]; (3) increased IL-1β-induced expression of c-fos, CRH, and AVP (arginine vasopressin) in PVN/CRH cells [211, 212] associated with increased excitability of rat hypothalamic parvocellular neurons [213], secretion of CRH and AVP, and overstimulation of the HPA axis [211]; (4) increased activity of IL-1β, IL-6, COX-2, and PGE2 on PVN-CRH neurons [128], pituitary ACTH, and adrenals [126, 129]; (5) synergistic effect of endogenous danger signals (LPS) and IL-1β on hypothalamic CRH secretion [214], in contrast to the potentiating effect of IL-6, eliciting CRH-dependent activation of pituitary ACTH [215]; (6) endogenous danger signals upregulate the NLRP3 inflammasome leading to caspase-dependent GR cleavage (an effect enhancing potential resistance to the effects of GCs) [94]; and (7) pro-inflammatory cytokines (IL-1 α-β, TNF-α, and IFN-α) inhibit GR function [216–218].
In adrenals: (1) TLR4-dependent upregulation of COX-2 activity, steroidogenesis, and GC release from adrenal cells [120, 121], leading to the activation and potentiation of pro-inflammatory signals in brain and peripheral tissues [109, 185]; and (2) direct GC-priming effect of the NLRP3 inflammasome response to a subsequent stimulus (ATP), enhancing the amplification of pro-inflammatory signals to NF-κB, AP-1, and STAT3 transcription factors [219], and potentiation of pro-inflammatory responses to IL-1β [26, 102, 107], TNFα, IL-6, and LIF in both brain and neurosecretory tissues [220].
In placenta: (1) expression of high levels of placental TLR4 [197] and TLR6 [195, 196] in third trimester trophoblasts; (2) expression of TLR4 in macrophage-like cells (Hofbauer cells) in the term placenta [195, 196]; (3) increased secretion of placental (IL-1β, TNF-α, and LIF, IL-6) and peripheral cytokines (IL-2, IFN-γ, and TNF-α) facilitating the release of endometrial prostaglandins (PGE2 and PGD2), chemokines (CCL7 and CCL4), and protein factors (MMPs), which promote dysregulation of the Th1/Th2 balance towards a predominant Th1 over Th2 immune response [165, 187–189]; and (4) effects of GCs, pro-inflammatory cytokines, anoxic conditions, and stress conditions of pregnancy such as preeclampsia on the increased CRH secretion and CRH blood levels in women delivering preterm, compared to the low hormone levels in women delivering at term [157].
These findings support a role of TLR4 in activating the NLRP3 inflammasome and the release of pro-inflammatory cytokines, enhancing the altered neuroendocrine responses in women exhibiting MDD during pregnancy [157], which include increased secretion and high levels of circulating placental and hypothalamic CRH and AVP, leading to disproportionate hyperactivity of the HPA axis and high baseline levels of cortisol (hypercortisolemia); an exaggerated response to the dexamethasone/CRH test and GR insensitivity; and GC- and cytokine-induced reduction of placental (syncytiotrophoblast) 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) activity (enhancing maternal-fetal transfer of active GCs and fetal corticoid toxicity) [157].
These altered neuroendocrine responses during pregnancy play a pivotal role in triggering the hallmark symptoms of perinatal depression associated with predominant Th1 inflammatory responses over Th2 anti-inflammatory activity, which may enhance preterm labor and birth with negative obstetric and perinatal outcomes for both mother and offspring, as previously described [157] (Fig. 3).
In offspring: Prenatal exposure to stress, excess GCs, and reduced fetoplacental 11β-HSD2 expression and activity may be crucial factors by which fetal growth and development lead to adult pathophysiology. In line with this, it has been reported that increases of fetal GCs may lead to fetal growth retardation associated with the development of neuropsychiatric disorders [218].
Prenatal stress-induced increases in fetal GCs, together with the increase in the Th1/Th17 pro-inflammatory activity, may be responsible for the changes in early-life programming of the brain, immune, neuroendocrine, and placental systems in the offspring, triggering neuropsychiatric disorders [218] and/or autoimmune disorders such as lupus erythematosus [158].
Thus, it may be feasible to assume that increases in placental GCs and the inhibition of 11β-HSD2 activity during late pregnancy [218] may lead to GC priming of the NLRP3 inflammasome, enhancing the amplification and potentiation of pro-inflammatory signals [219] and cytokine responses [26, 102, 107] in offspring exposed to prenatal stressors. The offspring of pregnant women with MDD have high basal levels of inflammatory cytokines and cortisol in the blood, associated with reduced GR activity [157, 217], which makes them a highly vulnerable risk group for developing adult affective behaviors.
Brain and Placental Neurotransmission
Perinatal depression has the same features of inflammatory signals inducing the dysfunction of monoaminergic transmission systems as shown in MDD, based on the following findings. In the brain and placenta: (1) direct effect of cytokines (IFN-γ, IFN-α, TNF-α, and IL-6) on activation of the indoleamine 2,3 dioxygenase (IDO) and kynurenine (KYN) pathways in astrocytes, microglia, and/or adjacent peripheral macrophages, leading to the depletion of brain 5-HT and increases of KYN, quinolinic acid (QUIN) and kynurenic acid levels in CSF [221]; (2) cytokines (IFN-γ, IFN-α, TNF-α, and IL-6) increase CSF-IL-6 and QUIN and decrease CSF-kynurenic acid levels, and these are correlated with the worsening of depressive and suicidal symptoms [222]; (3) IL-1β-induced downregulation of 5HT1A receptors [223] in regions essential to the regulation of emotion, psychomotor function, and reward [224–226]; (4) association with the downregulation of astrocyte glutamate (GLU) transporters, leading to GLU neurotoxicity and subsequent neuronal death in cortex and hippocampus [227–229]; (5) cytokine inducers (LPS and BCG) increase the turnover of 5-HT in regions [206, 224, 225] that normalize with antidepressant treatment (SSRIs and paroxetine) [56, 64, 226, 230]; (6) IL-1β increases the expression of 5HT (SERT) and norepinephrine membrane transporters [56] in cortex and hippocampus [231], enhancing the extracellular clearance of these neurotransmitters [232]; with similar findings in placental SERT, showing IL-1β-dependent upregulation of SERT mRNA and protein expression in endometrial cells [233]; (7) increased expression of microglial QUIN in the cingulate cortex, in contrast to decreased microglial QUIN (immunoreactivity) in the hippocampus of acutely depressed patients [234, 235]; (8) increased circulating KYN : Tryptophan ratio in response to LPS-induced overstimulation of IDO activity [206]; and (9) conversely, reduced pro-inflammatory signaling, along with depressive-like behaviors and circulating KYN concentrations upon pharmacological inhibition of IDO activity [206].
These data support the idea that inflammatory signals and inflammatory cytokines mediate major changes in the 5-HT and GLU transmission systems in the CNS and placenta during perinatal depression, enhancing the depletion of both brain and placental 5-HT, and thereby, for the appearance and/or exacerbation of depressive symptomology during pregnancy in addition to the neurotoxic effects of the high GLU levels in forebrain structures (cortex and hippocampus; Fig. 1).
Although the role of placental IDO activity in mood-related disorders is still elusive, recent evidence has revealed that the human placenta expresses IDO from early stages of pregnancy (depleted L-tryptophan and increased KYN concentrations), by which IDO and KYN appear to mediate the suppression of maternal T cells and PBMC proliferation that are implicated in immune tolerance and self-recognition of the fetal allograft [236, 237]. Interestingly, the increased activity of pro-inflammatory cytokines (IFN-γ) in response to infections appears to decrease the expression of placental IDO and thereby, stimulates T helper lymphocyte proliferation, leading to obstetric pathologies (pre-eclampsia) associated with an increased Th1 inflammatory response [236]. As pregnancy is associated with a less severe inflammatory activity [238], it may be argued that changes in placental IDO activity could contribute to the pathogenesis of perinatal depression, among other mental disorders.
Concluding Remarks
The role of the immune system in stress-inducing mood-related disorders and behaviors has been extensively documented. MDD during pregnancy is a common psychiatric disorder that arises from a complex and multifactorial etiology. The immune system plays a crucial role in MDD, mostly as a major triggering factor. Researchers have established much of the basis and understanding of the nature of this illness in highly vulnerable women. During pregnancy, both placental and immune responses are modified to allow the tolerance and development of the semi-allogeneic fetus to full term.
Pregnant women with MDD have increased levels of circulating pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and IFN-α) and their soluble receptors as well as CRP, suggesting that modification of the Th1/Th2 bias towards a predominant Th1/Th17 pro-inflammatory activity may be responsible for the wide spectrum of functional and morphological changes in brain structures [73]—monoaminergic systems, immune function, neurosecretory activity, and placental function—leading to the hallmark symptoms and behavioral repertoire of MDD during pregnancy and associated with preterm labor.
Both TLR4 and the inflammasome are crucial links between stress, neuroendocrine and inflammatory responses in MDD, by which endogenous stressors promote a pro-inflammatory activity, mediated through the increased secretion of IL-1β and IL-18, among other cytokines (IL-6, TNF-α, IFN-γ, and IFN-α), prostanoids, and active reactants (NO, ROS, PGE2, and PGD2) in response to DAMPs, MAMPs, and/or psychosocial stress (the latter suggested to be mediated by MAMPs leaking from the gut microbiome into the peripheral circulation, inducing the TLR4-dependent activation of the NLRP3 inflammasome) [26, 130] (Fig. 2).
Thus, DAMPs, MAMPs, and the NLRP3 inflammasome illustrate the link by which stressors are translated into damage signals, leading to an inflammatory process and the risk of developing depression and chronic stress-related co-morbid illnesses [89]. Stimulation of the innate immune system leading to an enhanced inflammatory activity suggests that both the inflammatory theory [64] and the most recently posited “pathogen host defense hypothesis of depression” [65] may well explain the pathogenesis of depression during pregnancy, assuming that the same mechanisms by which the innate and adaptive immune systems interact with neurocircuits, neurotransmitters, and neurosecretory systems in MDD, operate in a similar fashion in the same structures and with the neuroimmune placenta (Fig. 3) during perinatal depression, as previously discussed.
In this context, it might be feasible to propose that chronic stressors (DAMPs, MAMPs, and psychological stimuli) activating the NLRP3-inflammasome may play a crucial role in translating damage signals into an inflammatory process in the placenta, establishing a novel link in the inflammatory circuit between the brain, immune system, and immune placenta in MDD during pregnancy (Fig. 4).
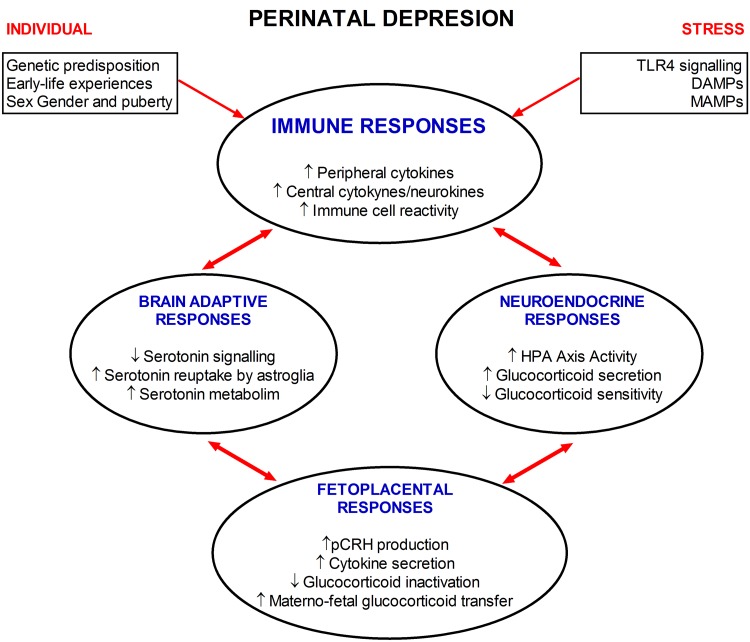
Fig. 4.
Immune, placental, and neuroendocrine network in perinatal depression. The interactions between different systems during perinatal depression suggest the complexity of this mood disorder. Individual predisposition and stress factors combine to cause adaptive immune, brain, neuroendocrine, and placental changes involved in MDD during pregnancy. Adaptive immune changes include activation of the TLR4 signaling system by specific ligands (DAMPs and MAMPs) or by stressors. However, both genetic and early-life experiences may predispose an individual to respond to stressors, in addition to sex and the hormonal environment during puberty. Chronic stress in susceptible individuals may lead to neuroadaptive changes in monoaminergic systems, increased HPA axis activity and high levels of circulatory GCs, due to impaired GR function. Increased GCs interact with TLR4 and GR-activating mechanisms, enhancing functional changes in systems such as the neuroendocrine placenta. These changes may be responsible for the exacerbation of depressive symptoms during pregnancy. Thus, the neuroadaptive changes in maternal systems during perinatal depression lead to dysfunction in the multi-pathway communicating network between the brain, immune system and placenta, enhancing deleterious effects on both mother and offspring during and after gestation (see text for details). DAMPs, damage-associated molecular pattern molecules; MAMPs, microbe-associated molecular pattern.
Furthermore, endogenous damage signals priming TLR signaling and the NLRP3 inflammasome in placental macrophages may be a crucial mechanism by which the placenta-immune system becomes alerted by wide and varied endogenous conditions [116], enhancing the secretion of pro-inflammatory cytokines and the expression of overstimulated Th1/Th17 pro-inflammatory activity, implicated in triggering affective disorders during pregnancy. Interestingly, direct priming of TLRs by GCs may lead to the early onset of MD during pregnancy, similar to the roles of TLRs and GCs in the onset of MDD [118], bipolar depression, and childhood sexual abuse [117].
The question of why some women develop depressive symptoms during pregnancy while others do not requires deeper exploration. Puberty and steroid hormones appear to be critical in organizing brain plasticity during a critical window of vulnerability, influencing long-term neuroinflammatory activity [50] in vulnerable individuals who appear to be more likely to display immune dysregulation leading to mental disorders in adulthood [36].
Current epidemiological studies report that 27% of pregnant women show depressive symptoms and anxiety before pregnancy, whereas 33% show affective symptoms during pregnancy and 40% during the postpartum period [239]. These data suggest that factors priming immune cells in the maternal brain, periphery, and placenta, and TLR signaling for pro-inflammatory activity, may set the mechanisms underlying mood disorders from early postnatal life, while puberty, sex hormones, and cortisol may awaken the quiescent but primed immune system into active inflammatory processes (NLRP3 inflammasome) and pro-inflammatory responses leading to MDD in vulnerable individuals.
Moreover, the role of epigenetics in shaping and orchestrating the structural anatomy and distribution of neurotransmitters in the brain in response to stressors has been widely documented [240, 241], and epigenetic modification of functional receptors and molecules enrolled in TLR signaling and activation of the NLRP3 inflammasome may help to elucidate the link between inflammation and the risk of developing psychiatric disorders in susceptible women during pregnancy [240, 241].
Perinatal depression is a complex and serious psychiatric disorder with negative obstetric and perinatal outcomes for both mother and baby. Hypotheses and theories explaining the etiopathogenesis of MDD may also apply to perinatal depression and therefore it should be viewed as an immune-related disorder impinging the brain, neuroendocrine system, and placental structures, caused by maladaptive host-parasite defensive mechanisms, producing lifelong mood-related disorders and behaviors in highly vulnerable individuals.
Acknowledgements
This review was supported by the National Institute of Perinatology, Mexico City (234560) and FONSEC SSA/IMSS/ISSSTE 2015-1 (261435).
References
- 1.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;35:722–729. doi: 10.1016/j.pnpbp.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thase ME. Preventing relapse and recurrence of depression: a brief review of therapeutic options. CNS Spectr. 2006;11:12–21. doi: 10.1017/S1092852900010622. [DOI] [PubMed] [Google Scholar]
- 3.Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58:175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Galindo-Sevilla N, Leff-Gelman P, Cruz Fuentes C, Cordova Barrios A, Mancilla-Ramirez J, Ramírez-Ramírez A, et al. Immune Function in Pregnant Women with Affective Disorders. Current Psychiatry Reviews. 2014;10:258–273. doi: 10.2174/1573400509666131217010344. [DOI] [Google Scholar]
- 5.Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric symptoms and proinflammatory cytokines in pregnancy. Psychosom Med. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CY, Pickler RH. Perinatal stress, fatigue, depressive symptoms, and immune modulation in late pregnancy and one month postpartum. ScientificWorldJournal. 2014;2014:652630. doi: 10.1155/2014/652630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- 8.Marcus SM, Flynn HA, Blow FC, Barry KL. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt) 2003;12:373–380. doi: 10.1089/154099903765448880. [DOI] [PubMed] [Google Scholar]
- 9.Bittner A, Peukert J, Zimmermann C, Junge-Hoffmeister J, Parker LS, Stobel-Richter Y, et al. Early intervention in pregnant women with elevated anxiety and depressive symptoms: efficacy of a cognitive-behavioral group program. J Perinat Neonatal Nurs. 2014;28:185–195. doi: 10.1097/JPN.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 10.Stuart-Parrigon K, Stuart S. Perinatal depression: an update and overview. Curr Psychiatry Rep. 2014;16:468. doi: 10.1007/s11920-014-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milgrom J, Gemmill AW. Screening for perinatal depression. Best Pract Res Clin Obstet Gynaecol. 2014;28:13–23. doi: 10.1016/j.bpobgyn.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerman B, Amaro H, Bauchner H, Cabral H. Depressive symptoms during pregnancy: relationship to poor health behaviors. Am J Obstet Gynecol. 1989;160:1107–1111. doi: 10.1016/0002-9378(89)90170-1. [DOI] [PubMed] [Google Scholar]
- 13.Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68:938–946. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 14.Canady RB, Bullen BL, Holzman C, Broman C, Tian Y. Discrimination and symptoms of depression in pregnancy among African American and White women. Womens Health Issues. 2008;18:292–300. doi: 10.1016/j.whi.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol. 2012;94:202–209. doi: 10.1016/j.jri.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Orr ST, Blazer DG, James SA. Racial disparities in elevated prenatal depressive symptoms among black and white women in eastern north Carolina. Ann Epidemiol. 2006;16:463–468. doi: 10.1016/j.annepidem.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Marcus S, Lopez JF, McDonough S, Mackenzie MJ, Flynn H, Neal CR, Jr, et al. Depressive symptoms during pregnancy: impact on neuroendocrine and neonatal outcomes. Infant Behav Dev. 2010;34:26–34. doi: 10.1016/j.infbeh.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Pakenham KI, Smith A, Rattan SL. Application of a stress and coping model to antenatal depressive symptomatology. Psychol Health Med. 2007;12:266–277. doi: 10.1080/13548500600871702. [DOI] [PubMed] [Google Scholar]
- 20.Chien LY, Ko YL. Fatigue during pregnancy predicts caesarean deliveries. J Adv Nurs. 2004;45:487–494. doi: 10.1046/j.1365-2648.2003.02931.x. [DOI] [PubMed] [Google Scholar]
- 21.Fairbrother N, Hutton EK, Stoll K, Hall W, Kluka S. Psychometric evaluation of the Multidimensional Assessment of Fatigue scale for use with pregnant and postpartum women. Psychol Assess. 2008;20:150–158. doi: 10.1037/1040-3590.20.2.150. [DOI] [PubMed] [Google Scholar]
- 22.Field T, Diego M, Hernandez-Reif M. Prenatal depression effects on the fetus and newborn: a review. Infant Behav Dev. 2006;29:445–455. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Veenstra van Nieuwenhoven AL Heineman MJ, Faas MM. The immunology of successful pregnancy. Hum Reprod Update. 2003;9:347–357. doi: 10.1093/humupd/dmg026. [DOI] [PubMed] [Google Scholar]
- 24.Nemeroff CB. The corticotropin-releasing factor (CRF) hypothesis of depression: new findings and new directions. Mol Psychiatry. 1996;1:336–342. [PubMed] [Google Scholar]
- 25.Choi AJ, Ryter SW. Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Molecules and cells. 2014;37:441–448. doi: 10.14348/molcells.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain, behavior, and immunity. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtblau N, Schmidt FM, Schumann R, Kirkby KC, Himmerich H. Cytokines as biomarkers in depressive disorder: current standing and prospects. International review of psychiatry. 2013;25:592–603. doi: 10.3109/09540261.2013.813442. [DOI] [PubMed] [Google Scholar]
- 28.Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Science signaling 2012, 5: pe45. [DOI] [PubMed]
- 29.Gazal M, Souza LD, Fucolo BA, Wiener CD, Silva RA, Pinheiro RT, et al. The impact of cognitive behavioral therapy on IL-6 levels in unmedicated women experiencing the first episode of depression: a pilot study. Psychiatry Res. 2013;209:742–745. doi: 10.1016/j.psychres.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 30.McGuinness TM, Dyer JG, Wade EH. Gender differences in adolescent depression. Journal of psychosocial nursing and mental health services. 2012;50:17–20. doi: 10.3928/02793695-20121107-04. [DOI] [PubMed] [Google Scholar]
- 31.Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: implications for psychopathology. Development and psychopathology. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- 32.Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Archives of general psychiatry. 2000;57:21–27. doi: 10.1001/archpsyc.57.1.21. [DOI] [PubMed] [Google Scholar]
- 33.Patton GC, Viner R. Pubertal transitions in health. Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- 34.Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, et al. Prevalence and treatment of mental disorders, 1990 to 2003. The New England journal of medicine. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE, et al. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. Journal of abnormal psychology. 2012;121:282–288. doi: 10.1037/a0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holder MK, Blaustein JD. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front Neuroendocrinol. 2014;35:89–110. doi: 10.1016/j.yfrne.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. The Journal of adolescent health: official publication of the Society for Adolescent Medicine. 2002;30:49–58. doi: 10.1016/S1054-139X(02)00336-1. [DOI] [PubMed] [Google Scholar]
- 38.Scott JP, Stewart JM, De Ghett VJ. Critical periods in the organization of systems. Developmental psychobiology. 1974;7:489–513. doi: 10.1002/dev.420070602. [DOI] [PubMed] [Google Scholar]
- 39.Lenz KM, Nugent BM, McCarthy MM. Sexual differentiation of the rodent brain: dogma and beyond. Frontiers in neuroscience. 2012;6:26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy MM. How it’s made: organisational effects of hormones on the developing brain. J Neuroendocrinol. 2010;22:736–742. doi: 10.1111/j.1365-2826.2010.02021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Roberti J. A review of behavioral and biological correlates of sensation seeking. Journal of Research in Personality. 2004;38:256–279. doi: 10.1016/S0092-6566(03)00067-9. [DOI] [Google Scholar]
- 43.Im-Bolter N, Cohen NJ, Farnia F. I thought we were good: social cognition, figurative language, and adolescent psychopathology. Journal of child psychology and psychiatry, and allied disciplines. 2013;54:724–732. doi: 10.1111/jcpp.12067. [DOI] [PubMed] [Google Scholar]
- 44.MacPherson L, Magidson JF, Reynolds EK, Kahler CW, Lejuez CW. Changes in sensation seeking and risk-taking propensity predict increases in alcohol use among early adolescents. Alcoholism, clinical and experimental research. 2010;34:1400–1408. doi: 10.1111/j.1530-0277.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 46.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verney C, Monier A, Fallet-Bianco C, Gressens P. Early microglial colonization of the human forebrain and possible involvement in periventricular white-matter injury of preterm infants. J Anat. 2011;217:436–448. doi: 10.1111/j.1469-7580.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohr MA, Sisk CL. Pubertally born neurons and glia are functionally integrated into limbic and hypothalamic circuits of the male Syrian hamster. Proc Natl Acad Sci U S A. 2013;110:4792–4797. doi: 10.1073/pnas.1219443110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez-Gonzalez B, Escobar A. Prenatal stress alters microglial development and distribution in postnatal rat brain. Acta Neuropathol. 2010;119:303–315. doi: 10.1007/s00401-009-0590-4. [DOI] [PubMed] [Google Scholar]
- 51.Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- 52.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 53.Streit WJ. Microglial activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Frontiers in aging neuroscience. 2010;2:22. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2010;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 56.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones KA, Thomsen C. The role of the innate immune system in psychiatric disorders. Molecular and cellular neurosciences. 2013;53:52–62. doi: 10.1016/j.mcn.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 58.Hou R, Baldwin DS. A neuroimmunological perspective on anxiety disorders. Human psychopharmacology. 2012;27:6–14. doi: 10.1002/hup.1259. [DOI] [PubMed] [Google Scholar]
- 59.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Frontiers in behavioral neuroscience. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Archives of pediatrics & adolescent medicine. 2009;163:1135–1143. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salim S, Chugh G, Asghar M. Inflammation in anxiety. Advances in protein chemistry and structural biology. 2012;88:1–25. doi: 10.1016/B978-0-12-398314-5.00001-5. [DOI] [PubMed] [Google Scholar]
- 63.Bilbo SD, Smith SH, Schwarz JM. A lifespan approach to neuroinflammatory and cognitive disorders: a critical role for glia. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7:24–41. doi: 10.1007/s11481-011-9299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature reviews. Immunology. 2015;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Daghri NM, Al-Ajlan AS, Alfawaz H, Yakout SM, Aljohani N, Kumar S, et al. Serum cytokine, chemokine and hormone levels in Saudi adults with pre-diabetes: a one-year prospective study. Int J Clin Exp Pathol. 2015;8:11587–11593. [PMC free article] [PubMed] [Google Scholar]
- 67.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 68.Drago A, Crisafulli C, Calabro M, Serretti A. Enrichment pathway analysis. The inflammatory genetic background in Bipolar Disorder. Journal of affective disorders. 2015;179:88–94. doi: 10.1016/j.jad.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 69.Mostafavi S, Battle A, Zhu X, Potash JB, Weissman MM, Shi J, et al. Type I interferon signaling genes in recurrent major depression: increased expression detected by whole-blood RNA sequencing. Molecular psychiatry. 2014;19:1267–1274. doi: 10.1038/mp.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bufalino C, Hepgul N, Aguglia E, Pariante CM. The role of immune genes in the association between depression and inflammation: a review of recent clinical studies. Brain, behavior, and immunity. 2013;31:31–47. doi: 10.1016/j.bbi.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 71.Raison CL, Lowry CA, Rook GA. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Archives of general psychiatry. 2010;67:1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Annals of the New York Academy of Sciences. 2000;917:478–487. doi: 10.1111/j.1749-6632.2000.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 73.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 75.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of general psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 76.Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, Stein K, et al. Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. Journal of psychosomatic research. 2015;79:175–184. doi: 10.1016/j.jpsychores.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA psychiatry. 2014;71:1381–1391. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 78.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 79.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 80.Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2011;29:32–38. doi: 10.1002/da.20876. [DOI] [PubMed] [Google Scholar]
- 81.Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: the social navigation hypothesis. Journal of affective disorders. 2002;72:1–14. doi: 10.1016/S0165-0327(01)00459-1. [DOI] [PubMed] [Google Scholar]
- 82.Kinney DK, Tanaka M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. The Journal of nervous and mental disease. 2009;197:561–567. doi: 10.1097/NMD.0b013e3181b05fa8. [DOI] [PubMed] [Google Scholar]
- 83.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Molecular psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cox SS, Speaker KJ, Beninson LA, Craig WC, Paton MM, Fleshner M. Adrenergic and glucocorticoid modulation of the sterile inflammatory response. Brain, behavior, and immunity. 2014;36:183–192. doi: 10.1016/j.bbi.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 85.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 87.Fleshner M. Stress-evoked sterile inflammation, danger associated molecular patterns (DAMPs), microbial associated molecular patterns (MAMPs) and the inflammasome. Brain Behav Immun. 2012;27:1–7. doi: 10.1016/j.bbi.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2012;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 89.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2012;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1beta-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 91.Wu H, Che X, Tang J, Ma F, Pan K, Zhao M, et al. The K-Cl Cotransporter KCC2 and Chloride Homeostasis: Potential Therapeutic Target in Acute Central Nervous System Injury. Molecular neurobiology 2015. [DOI] [PubMed]
- 92.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, behavior, and immunity. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, et al. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nature genetics. 2015;47:607–614. doi: 10.1038/ng.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. The New England journal of medicine. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 96.Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 97.Alcocer-Gomez E, de Miguel M, Casas-Barquero N, Nunez-Vasco J, Sanchez-Alcazar JA, Fernandez-Rodriguez A, et al. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 98.Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Beninson L, Ursell L, et al. Commensal bacteria and MAMPs are necessary for stress-induced increases in IL-1beta and IL-18 but not IL-6, IL-10 or MCP-1. PLoS One. 2012;7:e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lyte M, Vulchanova L, Brown DR. Stress at the intestinal surface: catecholamines and mucosa-bacteria interactions. Cell and tissue research. 2011;343:23–32. doi: 10.1007/s00441-010-1050-0. [DOI] [PubMed] [Google Scholar]
- 100.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-Derived Suppressor Cells: Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Advances in cancer research. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Y, Xiao B, Qiu W, Yang L, Hu B, Tian X, et al. Altered expression of CD4(+)CD25(+) regulatory T cells and its 5-HT(1a) receptor in patients with major depression disorder. Journal of affective disorders. 2010;124:68–75. doi: 10.1016/j.jad.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y, Liu L, Peng YL, Liu YZ, Wu TY, Shen XL, et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS neuroscience & therapeutics. 2014;20:119–124. doi: 10.1111/cns.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Manikowska K, Mikolajczyk M, Mikolajczak PL, Bobkiewicz-Kozlowska T. The influence of mianserin on TNF-alpha, IL-6 and IL-10 serum levels in rats under chronic mild stress. Pharmacol Rep. 2014;66:22–27. doi: 10.1016/j.pharep.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 108.Alboni S, Benatti C, Montanari C, Tascedda F, Brunello N. Chronic antidepressant treatments resulted in altered expression of genes involved in inflammation in the rat hypothalamus. European journal of pharmacology. 2013;721:158–167. doi: 10.1016/j.ejphar.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 109.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 110.Liu J, Buisman-Pijlman F, Hutchinson MR. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Frontiers in neuroscience. 2014;8:309. doi: 10.3389/fnins.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 112.Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophila toll-dorsal pathway. Annual review of cell and developmental biology. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 113.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 114.Kawai T, Akira S. TLR signaling. Seminars in immunology. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 115.Tobias PS, Soldau K, Ulevitch RJ. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. The Journal of experimental medicine. 1986;164:777–793. doi: 10.1084/jem.164.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 117.Oliveira J, Etain B, Lajnef M, Hamdani N, Bennabi M, Bengoufa D, et al. Combined effect of TLR2 gene polymorphism and early life stress on the age at onset of bipolar disorders. PloS one. 2015;10:e0119702. doi: 10.1371/journal.pone.0119702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu MK, Huang TL, Huang KW, Huang YL, Hung YY. Association between toll-like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatric disease and treatment. 2015;11:1853–1857. doi: 10.2147/NDT.S88430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mohn CE, Fernandez-Solari J, De Laurentiis A, Bornstein SR, Ehrhart-Bornstein M, Rettori V. Adrenal gland responses to lipopolysaccharide after stress and ethanol administration in male rats. Stress. 2011;14:216–226. doi: 10.3109/10253890.2010.532254. [DOI] [PubMed] [Google Scholar]
- 120.Kanczkowski W, Alexaki VI, Tran N, Grossklaus S, Zacharowski K, Martinez A, et al. Hypothalamo-pituitary and immune-dependent adrenal regulation during systemic inflammation. Proc Natl Acad Sci U S A. 2013;110:14801–14806. doi: 10.1073/pnas.1313945110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vakharia K, Hinson JP. Lipopolysaccharide directly stimulates cortisol secretion by human adrenal cells by a cyclooxygenase-dependent mechanism. Endocrinology. 2005;146:1398–1402. doi: 10.1210/en.2004-0882. [DOI] [PubMed] [Google Scholar]
- 122.Loum-Ribot E, Lafon P, Chaigniau M, Tramu G, Corio M. Glucocorticoids down-regulate lipopolysaccharide-induced de novo production of neurotensin mRNA in the rat hypothalamic, paraventricular, corticotrophin-releasing hormone neurons. Neuroimmunomodulation. 2006;13:170–178. doi: 10.1159/000098130. [DOI] [PubMed] [Google Scholar]
- 123.Goebel M, Stengel A, Wang L, Reeve J, Jr, Tache Y. Lipopolysaccharide increases plasma levels of corticotropin-releasing hormone in rats. Neuroendocrinology. 2011;93:165–173. doi: 10.1159/000322590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elenkov IJ, Kovacs K, Kiss J, Bertok L, Vizi ES. Lipopolysaccharide is able to bypass corticotrophin-releasing factor in affecting plasma ACTH and corticosterone levels: evidence from rats with lesions of the paraventricular nucleus. J Endocrinol. 1992;133:231–236. doi: 10.1677/joe.0.1330231. [DOI] [PubMed] [Google Scholar]
- 125.Van Bogaert T, De Bosscher K, Libert C. Crosstalk between TNF and glucocorticoid receptor signaling pathways. Cytokine Growth Factor Rev. 2010;21:275–286. doi: 10.1016/j.cytogfr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 126.Mouihate A, Galic MA, Ellis SL, Spencer SJ, Tsutsui S, Pittman QJ. Early life activation of toll-like receptor 4 reprograms neural anti-inflammatory pathways. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:7975–7983. doi: 10.1523/JNEUROSCI.6078-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sominsky L, Fuller EA, Bondarenko E, Ong LK, Averell L, Nalivaiko E, et al. Functional programming of the autonomic nervous system by early life immune exposure: implications for anxiety. PloS one. 2013;8:e57700. doi: 10.1371/journal.pone.0057700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamaguchi N, Ogawa S, Okada S. Cyclooxygenase and nitric oxide synthase in the presympathetic neurons in the paraventricular hypothalamic nucleus are involved in restraint stress-induced sympathetic activation in rats. Neuroscience. 2010;170:773–781. doi: 10.1016/j.neuroscience.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 129.Ma Y, Matsuwaki T, Yamanouchi K, Nishihara M. Cyclooxygenase-2-related signaling in the hypothalamus plays differential roles in response to various acute stresses. Brain Res. 2013;1508:23–33. doi: 10.1016/j.brainres.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 130.Hines DJ, Choi HB, Hines RM, Phillips AG, MacVicar BA. Prevention of LPS-induced microglia activation, cytokine production and sickness behavior with TLR4 receptor interfering peptides. PloS one. 2013;8:e60388. doi: 10.1371/journal.pone.0060388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Keri S, Szabo C, Kelemen O. Expression of Toll-Like Receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav Immun. 2014;40:235–243. doi: 10.1016/j.bbi.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 132.Watters TM, Kenny EF, O’Neill LA. Structure, function and regulation of the Toll/IL-1 receptor adaptor proteins. Immunology and cell biology. 2007;85:411–419. doi: 10.1038/sj.icb.7100095. [DOI] [PubMed] [Google Scholar]
- 133.Xu Y, Tao X, Shen B, Horng T, Medzhitov R, Manley JL, et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature. 2000;408:111–115. doi: 10.1038/35047056. [DOI] [PubMed] [Google Scholar]
- 134.Zhao M, Zhou A, Xu L, Zhang X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-kappaB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience. 2014;269:93–101. doi: 10.1016/j.neuroscience.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 135.Lewis SS, Loram LC, Hutchinson MR, Li CM, Zhang Y, Maier SF, et al. (+)-naloxone, an opioid-inactive toll-like receptor 4 signaling inhibitor, reverses multiple models of chronic neuropathic pain in rats. The journal of pain: official journal of the American Pain Society. 2012;13:498–506. doi: 10.1016/j.jpain.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14:463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 137.Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS & neurological disorders drug targets. 2011;10:108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- 138.Ajmone-Cat MA, Mancini M, De Simone R, Cilli P, Minghetti L. Microglial polarization and plasticity: evidence from organotypic hippocampal slice cultures. Glia. 2013;61:1698–1711. doi: 10.1002/glia.22550. [DOI] [PubMed] [Google Scholar]
- 139.Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, et al. MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:9592–9600. doi: 10.1523/JNEUROSCI.5610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 141.Horikawa H, Kato TA, Mizoguchi Y, Monji A, Seki Y, Ohkuri T, et al. Inhibitory effects of SSRIs on IFN-gamma induced microglial activation through the regulation of intracellular calcium. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1306–1316. doi: 10.1016/j.pnpbp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 142.Obuchowicz E, Bielecka AM, Paul-Samojedny M, Pudelko A, Kowalski J. Imipramine and fluoxetine inhibit LPS-induced activation and affect morphology of microglial cells in the rat glial culture. Pharmacol Rep. 2014;66:34–43. doi: 10.1016/j.pharep.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 143.Dhami KS, Churchward MA, Baker GB, Todd KG. Fluoxetine and citalopram decrease microglial release of glutamate and D-serine to promote cortical neuronal viability following ischemic insult. Molecular and cellular neurosciences. 2013;56:365–374. doi: 10.1016/j.mcn.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 144.Zhang F, Zhou H, Wilson BC, Shi JS, Hong JS, Gao HM. Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism & related disorders. 2012;18(Suppl 1):S213–S217. doi: 10.1016/S1353-8020(11)70066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, et al. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology. 2011;60:963–974. doi: 10.1016/j.neuropharm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 146.Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R, et al. Trophoblast-macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod Immunol. 2007;57:55–66. doi: 10.1111/j.1600-0897.2006.00446.x. [DOI] [PubMed] [Google Scholar]
- 147.Houser BL. Decidual macrophages and their roles at the maternal-fetal interface. Yale J Biol Med. 2012;85:105–118. [PMC free article] [PubMed] [Google Scholar]
- 148.Makrigiannakis A, Minas V, Kalantaridou SN, Nikas G, Chrousos GP. Hormonal and cytokine regulation of early implantation. Trends in endocrinology and metabolism: TEM. 2006;17:178–185. doi: 10.1016/j.tem.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 149.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 150.Manaster I, Mandelboim O. The unique properties of uterine NK cells. Am J Reprod Immunol. 2010;63:434–444. doi: 10.1111/j.1600-0897.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 151.Evans J, Catalano RD, Brown P, Sherwin R, Critchley HO, Fazleabas AT, et al. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Marwood M, Visser K, Salamonsen LA, Dimitriadis E. Interleukin-11 and leukemia inhibitory factor regulate the adhesion of endometrial epithelial cells: implications in fertility regulation. Endocrinology. 2009;150:2915–2923. doi: 10.1210/en.2008-1538. [DOI] [PubMed] [Google Scholar]
- 153.Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 154.Barkai U, Kraicer PF. Intrauterine signaling and embryonic implantation. Biol Signals. 1996;5:111–121. doi: 10.1159/000109180. [DOI] [PubMed] [Google Scholar]
- 155.Maekawa F, Yamanouchi K. Effect of deprivation of serotonin by p-chlorophenylalanine on induction and maintenance of pseudopregnancy in female rats. Brain Res Bull. 1996;39:317–321. doi: 10.1016/0361-9230(95)02117-5. [DOI] [PubMed] [Google Scholar]
- 156.Mitchell JA, Hammer RE, Goldman H. Serotonin-induced disruption of implantation in the rat: II. Suppression of decidualization. Biol Reprod. 1983;29:151–156. doi: 10.1095/biolreprod29.1.151. [DOI] [PubMed] [Google Scholar]
- 157.Gelman PL, Flores-Ramos M, Lopez-Martinez M, Fuentes CC, Grajeda JP. Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neuroscience bulletin. 2015;31:338–350. doi: 10.1007/s12264-014-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Figueiredo AS, Schumacher A. The Th17/Treg paradigm in pregnancy. Immunology 2016. [DOI] [PMC free article] [PubMed]
- 159.Trowsdale J, Betz AG. Mother’s little helpers: mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7:241–246. doi: 10.1038/ni1317. [DOI] [PubMed] [Google Scholar]
- 160.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 161.Huber SA, Kupperman J, Newell MK. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl 2 expression. Lupus. 1999;8:384–387. doi: 10.1177/096120339900800511. [DOI] [PubMed] [Google Scholar]
- 162.Xue L, Gyles SL, Wettey FR, Gazi L, Townsend E, Hunter MG, et al. Prostaglandin D2 causes preferential induction of proinflammatory Th2 cytokine production through an action on chemoattractant receptor-like molecule expressed on Th2 cells. J Immunol. 2005;175:6531–6536. doi: 10.4049/jimmunol.175.10.6531. [DOI] [PubMed] [Google Scholar]
- 163.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 164.Sykes L, MacIntyre DA, Yap XJ, Teoh TG, Bennett PR. The Th1:th2 dichotomy of pregnancy and preterm labour. Mediators Inflamm. 2012;2012:967629. doi: 10.1155/2012/967629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63:601–610. doi: 10.1111/j.1600-0897.2010.00852.x. [DOI] [PubMed] [Google Scholar]
- 166.Piccinni MP. T cells in normal pregnancy and recurrent pregnancy loss. Reprod Biomed Online 2007, 14 Spec No 1: 95–99. [DOI] [PubMed]
- 167.Dealtry GB, Clark DE, Sharkey A, Charnock-Jones DS, Smith SK. Expression and localization of the Th2-type cytokine interleukin-13 and its receptor in the placenta during human pregnancy. Am J Reprod Immunol. 1998;40:283–290. doi: 10.1111/j.1600-0897.1998.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 168.Roth I, Corry DB, Locksley RM, Abrams JS, Litton MJ, Fisher SJ. Human placental cytotrophoblasts produce the immunosuppressive cytokine interleukin 10. J Exp Med. 1996;184:539–548. doi: 10.1084/jem.184.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bennett WA, Lagoo-Deenadayalan S, Brackin MN, Hale E, Cowan BD. Cytokine expression by models of human trophoblast as assessed by a semiquantitative reverse transcription-polymerase chain reaction technique. Am J Reprod Immunol. 1996;36:285–294. doi: 10.1111/j.1600-0897.1996.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 170.Liu F, Guo J, Tian T, Wang H, Dong F, Huang H, et al. Placental trophoblasts shifted Th1/Th2 balance toward Th2 and inhibited Th17 immunity at fetomaternal interface. APMIS. 2011;119:597–604. doi: 10.1111/j.1600-0463.2011.02774.x. [DOI] [PubMed] [Google Scholar]
- 171.Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MM, Hassan NA, Bandar A. Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol. 1999;42:273–281. doi: 10.1111/j.1600-0897.1999.tb00101.x. [DOI] [PubMed] [Google Scholar]
- 172.Piccinni MP. T cell tolerance towards the fetal allograft. J Reprod Immunol. 2010;85:71–75. doi: 10.1016/j.jri.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 173.Fortunato SJ, Menon R, Lombardi SJ. Interleukin-10 and transforming growth factor-beta inhibit amniochorion tumor necrosis factor-alpha production by contrasting mechanisms of action: therapeutic implications in prematurity. Am J Obstet Gynecol. 1997;177:803–809. doi: 10.1016/S0002-9378(97)70272-2. [DOI] [PubMed] [Google Scholar]
- 174.Michimata T, Tsuda H, Sakai M, Fujimura M, Nagata K, Nakamura M, et al. Accumulation of CRTH2-positive T-helper 2 and T-cytotoxic 2 cells at implantation sites of human decidua in a prostaglandin D(2)-mediated manner. Mol Hum Reprod. 2002;8:181–187. doi: 10.1093/molehr/8.2.181. [DOI] [PubMed] [Google Scholar]
- 175.Michimata T, Sakai M, Miyazaki S, Ogasawara MS, Suzumori K, Aoki K, et al. Decrease of T-helper 2 and T-cytotoxic 2 cells at implantation sites occurs in unexplained recurrent spontaneous abortion with normal chromosomal content. Hum Reprod. 2003;18:1523–1528. doi: 10.1093/humrep/deg280. [DOI] [PubMed] [Google Scholar]
- 176.Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- 177.Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95. doi: 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- 178.Dekel N, Gnainsky Y, Granot I, Mor G. Inflammation and implantation. Am J Reprod Immunol. 2010;63:17–21. doi: 10.1111/j.1600-0897.2009.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 180.McCracken SA, Gallery E, Morris JM. Pregnancy-specific down-regulation of NF-kappa B expression in T cells in humans is essential for the maintenance of the cytokine profile required for pregnancy success. J Immunol. 2004;172:4583–4591. doi: 10.4049/jimmunol.172.7.4583. [DOI] [PubMed] [Google Scholar]
- 181.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Piccinni MP, Romagnani S. Regulation of fetal allograft survival by a hormone-controlled Th1- and Th2-type cytokines. Immunol Res. 1996;15:141–150. doi: 10.1007/BF02918503. [DOI] [PubMed] [Google Scholar]
- 183.Matsuoka T, Matsubara T, Katayama K, Takeda K, Koga M, Furukawa S. Increase of cord blood cytokine-producing T cells in intrauterine infection. Pediatrics international: official journal of the Japan Pediatric Society. 2001;43:453–457. doi: 10.1046/j.1442-200X.2001.01445.x. [DOI] [PubMed] [Google Scholar]
- 184.Goldberg MR, Nadiv O, Luknar-Gabor N, Zadik-Mnuhin G, Tovbin J, Katz Y. Correlation of Th1-type cytokine expression and induced proliferation to lipopolysaccharide. American journal of respiratory cell and molecular biology. 2008;38:733–737. doi: 10.1165/rcmb.2007-0143OC. [DOI] [PubMed] [Google Scholar]
- 185.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutrition reviews. 2002;60:S19–S25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 186.Hollier LM, Rivera MK, Henninger E, Gilstrap LC, 3rd, Marshall GD., Jr T helper cell cytokine profiles in preterm labor. Am J Reprod Immunol. 2004;52:192–196. doi: 10.1111/j.1600-0897.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 187.Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13:89–100. doi: 10.31887/DCNS.2011.13.1/smbrody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann N Y Acad Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mastorakos G, Ilias I. Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- 191.Koga K, Izumi G, Mor G, Fujii T, Osuga Y. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol. 2014;72:192–205. doi: 10.1111/aji.12258. [DOI] [PubMed] [Google Scholar]
- 192.Mor G, Romero R, Aldo PB, Abrahams VM. Is the trophoblast an immune regulator? The role of Toll-like receptors during pregnancy. Critical reviews in immunology. 2005;25:375–388. doi: 10.1615/CritRevImmunol.v25.i5.30. [DOI] [PubMed] [Google Scholar]
- 193.Mor G. Inflammation and pregnancy: the role of toll-like receptors in trophoblast-immune interaction. Ann N Y Acad Sci. 2008;1127:121–128. doi: 10.1196/annals.1434.006. [DOI] [PubMed] [Google Scholar]
- 194.Klaffenbach D, Rascher W, Rollinghoff M, Dotsch J, Meissner U, Schnare M. Regulation and signal transduction of toll-like receptors in human chorioncarcinoma cell lines. Am J Reprod Immunol. 2005;53:77–84. doi: 10.1111/j.1600-0897.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 195.Beijar EC, Mallard C, Powell TL. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 2006;27:322–326. doi: 10.1016/j.placenta.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 196.Kumazaki K, Nakayama M, Yanagihara I, Suehara N, Wada Y. Immunohistochemical distribution of Toll-like receptor 4 in term and preterm human placentas from normal and complicated pregnancy including chorioamnionitis. Human pathology. 2004;35:47–54. doi: 10.1016/j.humpath.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 197.Mitsunari M, Yoshida S, Shoji T, Tsukihara S, Iwabe T, Harada T, et al. Macrophage-activating lipopeptide-2 induces cyclooxygenase-2 and prostaglandin E(2) via toll-like receptor 2 in human placental trophoblast cells. J Reprod Immunol. 2006;72:46–59. doi: 10.1016/j.jri.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 198.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 199.Canavan TP, Simhan HN. Innate immune function of the human decidual cell at the maternal-fetal interface. J Reprod Immunol. 2007;74:46–52. doi: 10.1016/j.jri.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 200.Dulay AT, Buhimschi CS, Zhao G, Oliver EA, Mbele A, Jing S, et al. Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J Immunol. 2009;182:7244–7253. doi: 10.4049/jimmunol.0803517. [DOI] [PubMed] [Google Scholar]
- 201.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2009;14:1095–1104. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 203.Lotrich FE, Rabinovitz M, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. J Psychosom Res. 2007;63:131–135. doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Maddock C, Landau S, Barry K, Maulayah P, Hotopf M, Cleare AJ, et al. Psychopathological symptoms during interferon-alpha and ribavirin treatment: effects on virologic response. Mol Psychiatry. 2005;10:332–333. doi: 10.1038/sj.mp.4001634. [DOI] [PubMed] [Google Scholar]
- 205.Osborne LM, Monk C. Perinatal depression–the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology. 2013;38:1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. J Psychiatry Neurosci. 2009;34:4–20. [PMC free article] [PubMed] [Google Scholar]
- 207.Maes M. Inflammatory and oxidative and nitrosative stress pathways underpinning chronic fatigue, somatization and psychosomatic symptoms. Curr Opin Psychiatry. 2009;22:75–83. doi: 10.1097/YCO.0b013e32831a4728. [DOI] [PubMed] [Google Scholar]
- 208.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24:27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 209.Gadek-Michalska A, Bugajski J. Interleukin-1 (IL-1) in stress-induced activation of limbic-hypothalamic-pituitary adrenal axis. Pharmacol Rep. 2011;62:969–982. doi: 10.1016/S1734-1140(10)70359-5. [DOI] [PubMed] [Google Scholar]
- 210.Hannibal J, Jessop DS, Fahrenkrug J, Harbuz MS, Larsen PJ. PACAP gene expression in neurons of the rat hypothalamo-pituitary-adrenocortical axis is induced by endotoxin and interleukin-1beta. Neuroendocrinology. 1999;70:73–82. doi: 10.1159/000054461. [DOI] [PubMed] [Google Scholar]
- 211.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.John CD, Buckingham JC. Cytokines: regulation of the hypothalamo-pituitary-adrenocortical axis. Curr Opin Pharmacol. 2003;3:78–84. doi: 10.1016/S1471-4892(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 213.Ferri CC, Ferguson AV. Interleukin-1 beta depolarizes paraventricular nucleus parvocellular neurones. J Neuroendocrinol. 2003;15:126–133. doi: 10.1046/j.1365-2826.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 214.Chover-Gonzalez AJ, Harbuz MS, Lightman SL. Effect of adrenalectomy and stress on interleukin-1 beta-mediated activation of hypothalamic corticotropin-releasing factor mRNA. J Neuroimmunol. 1993;42:155–160. doi: 10.1016/0165-5728(93)90005-J. [DOI] [PubMed] [Google Scholar]
- 215.Mehet DK, Philip J, Solito E, Buckingham JC, John CD. Evidence from in vitro and in vivo studies showing that nuclear factor-kappaB within the pituitary folliculostellate cells and corticotrophs regulates adrenocorticotrophic hormone secretion in experimental endotoxaemia. J Neuroendocrinol. 2012;24:862–873. doi: 10.1111/j.1365-2826.2012.02285.x. [DOI] [PubMed] [Google Scholar]
- 216.Wang X, Wu H, Miller AH. Interleukin 1alpha (IL-1alpha) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Molecular psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 217.Hu F, Pace TW, Miller AH. Interferon-alpha inhibits glucocorticoid receptor-mediated gene transcription via STAT5 activation in mouse HT22 cells. Brain, behavior, and immunity. 2009;23:455–463. doi: 10.1016/j.bbi.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Van Bogaert T, Vandevyver S, Dejager L, Van Hauwermeiren F, Pinheiro I, Petta I, et al. Tumor necrosis factor inhibits glucocorticoid receptor function in mice: a strong signal toward lethal shock. The Journal of biological chemistry. 2011;286:26555–26567. doi: 10.1074/jbc.M110.212365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends in endocrinology and metabolism: TEM. 2013;24:109–119. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Langlais D, Couture C, Balsalobre A, Drouin J. Regulatory network analyses reveal genome-wide potentiation of LIF signaling by glucocorticoids and define an innate cell defense response. PLoS genetics. 2008;4:e1000224. doi: 10.1371/journal.pgen.1000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Jo WK, Zhang Y, Emrich HM, Dietrich DE. Glia in the cytokine-mediated onset of depression: fine tuning the immune response. Frontiers in cellular neuroscience. 2015;9:268. doi: 10.3389/fncel.2015.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain, behavior, and immunity. 2015;43:110–117. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 223.Cai W, Khaoustov VI, Xie Q, Pan T, Le W, Yoffe B. Interferon-alpha-induced modulation of glucocorticoid and serotonin receptors as a mechanism of depression. J Hepatol. 2005;42:880–887. doi: 10.1016/j.jhep.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 224.Dunn AJ. Effects of cytokines and infections on brain neurochemistry. Clin Neurosci Res. 2006;6:52–68. doi: 10.1016/j.cnr.2006.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dunn AJ, Wang J, Ando T. Effects of cytokines on cerebral neurotransmission. Comparison with the effects of stress. Adv Exp Med Biol. 1999;461:117–127. doi: 10.1007/978-0-585-37970-8_8. [DOI] [PubMed] [Google Scholar]
- 226.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Fang J, Han D, Hong J, Tan Q, Tian Y. The chemokine, macrophage inflammatory protein-2gamma, reduces the expression of glutamate transporter-1 on astrocytes and increases neuronal sensitivity to glutamate excitotoxicity. Journal of neuroinflammation. 2012;9:267. doi: 10.1186/1742-2094-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Persson M, Pekna M, Hansson E, Ronnback L. The complement-derived anaphylatoxin C5a increases microglial GLT-1 expression and glutamate uptake in a TNF-alpha-independent manner. Eur J Neurosci. 2009;29:267–274. doi: 10.1111/j.1460-9568.2008.06575.x. [DOI] [PubMed] [Google Scholar]
- 229.Tilleux S, Hermans E. Down-regulation of astrocytic GLAST by microglia-related inflammation is abrogated in dibutyryl cAMP-differentiated cultures. J Neurochem. 2008;105:2224–2236. doi: 10.1111/j.1471-4159.2008.05305.x. [DOI] [PubMed] [Google Scholar]
- 230.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2011;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 232.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. 2010;35:2510–2520. doi: 10.1038/npp.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kekuda R, Leibach FH, Furesz TC, Smith CH, Ganapathy V. Polarized distribution of interleukin-1 receptors and their role in regulation of serotonin transporter in placenta. J Pharmacol Exp Ther. 2000;292:1032–1041. [PubMed] [Google Scholar]
- 234.Busse M, Busse S, Myint AM, Gos T, Dobrowolny H, Muller UJ, et al. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? European archives of psychiatry and clinical neuroscience. 2015;265:321–329. doi: 10.1007/s00406-014-0562-0. [DOI] [PubMed] [Google Scholar]
- 235.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of psychiatric research. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 236.Kudo Y. The role of placental indoleamine 2,3-dioxygenase in human pregnancy. Obstetrics & gynecology science. 2013;56:209–216. doi: 10.5468/ogs.2013.56.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. The Journal of experimental medicine. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179:80–86. doi: 10.1016/S0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 239.Howard LM, Molyneaux E, Dennis CL, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet. 2014;384:1775–1788. doi: 10.1016/S0140-6736(14)61276-9. [DOI] [PubMed] [Google Scholar]
- 240.Feng J, Fan G. The role of DNA methylation in the central nervous system and neuropsychiatric disorders. International review of neurobiology. 2009;89:67–84. doi: 10.1016/S0074-7742(09)89004-1. [DOI] [PubMed] [Google Scholar]
- 241.Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38:124–137. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]