Abstract
This first comprehensive analysis of the global biogeography of marine protistan plankton with acquired phototrophy shows these mixotrophic organisms to be ubiquitous and abundant; however, their biogeography differs markedly between different functional groups. These mixotrophs, lacking a constitutive capacity for photosynthesis (i.e. non-constitutive mixotrophs, NCMs), acquire their phototrophic potential through either integration of prey-plastids or through endosymbiotic associations with photosynthetic microbes. Analysis of field data reveals that 40–60% of plankton traditionally labelled as (non-phototrophic) microzooplankton are actually NCMs, employing acquired phototrophy in addition to phagotrophy. Specialist NCMs acquire chloroplasts or endosymbionts from specific prey, while generalist NCMs obtain chloroplasts from a variety of prey. These contrasting functional types of NCMs exhibit distinct seasonal and spatial global distribution patterns. Mixotrophs reliant on ‘stolen’ chloroplasts, controlled by prey diversity and abundance, dominate in high-biomass areas. Mixotrophs harbouring intact symbionts are present in all waters and dominate particularly in oligotrophic open ocean systems. The contrasting temporal and spatial patterns of distribution of different mixotroph functional types across the oceanic provinces, as revealed in this study, challenges traditional interpretations of marine food web structures. Mixotrophs with acquired phototrophy (NCMs) warrant greater recognition in marine research.
Keywords: biogeography, mixotrophy, acquired phototrophy, kleptoplasty, photosymbiosis, marine protists
1. Background
Primary production in the oceans is critical for life on the Earth, with almost half of global photosynthesis undertaken by marine plankton [1]. This production is traditionally viewed as mediated by ‘plant-like’ phytoplankton, which are in turn grazed by ‘animal-like’ zooplankton. However, it is now recognized that a large proportion of marine plankton do not follow this traditional ‘plant–animal’ dichotomy but are actually mixotrophic protists—single-celled organisms that can perform both photosynthesis and phagocytosis simultaneously [2–5]. In reflection of this shift in our understanding of the marine trophic paradigm, a new functional group classification of marine planktonic protists has been proposed [6] in which mixotrophic protists are broadly divided into constitutive mixotrophs (i.e. those that have a constitutive ability to photosynthesize; CMs), and non-constitutive mixotrophs (i.e. those that do not possess an innate ability to photosynthesize; NCMs).
The CM group conforms to the popular perception of a planktonic mixotroph as a ‘plant that eats’ [7–9]. This group includes many harmful algal bloom (HAB) species, which have traditionally been considered strictly phototrophic [9]. NCMs, on the other hand, acquire the ability to photosynthesize either by ‘stealing’ and using plastids from a variety of prey (generalists; GNCMs) or acquiring plastids from specific prey (plastidic specialists; pSNCMs); in some instances, they harbour intact photosynthetic prey as symbionts (endosymbiotic specialists; eSNCMs) [10–12]. However, the presence and importance of NCMs is often overlooked, except for some notable cases such as the ciliate Mesodinium rubrum and the HAB dinoflagellates ‘green Noctiluca’ and Dinophysis [13–16].
Here, we report the first analysis of the global biogeography of non-constitutive mixotrophs—marine predatory plankton that exploit/use/recycle the photosynthetic machinery of their prey. Our analysis shows that these organisms are not only ubiquitous and abundant, but their biogeography differs markedly between the different groups. Our study establishes NCMs as important members of marine planktonic food webs across different biogeographic provinces at large spatial and temporal scales.
2. Methods
We conducted a global analysis of field data for the different groups of non-constitutive mixotrophs (NCMs); see the electronic supplementary material for detailed methods. Species were classified a priori to the GNCM, pSNCM and eSNCM groups according to their physiology. We adapted the Longhurst biogeographic classification system for oceanic provinces (electronic supplementary material, figure S1 and table S1) [17]; these provinces were grouped into seven principal biomes according to primary production and physical forcing. Coordinates corresponding to the locations where mixotrophic species had been recorded were aligned with these biogeographic provinces.
Qualitative data were obtained mainly from the Ocean Biogeographic Information System (OBIS) database; over 110 000 distribution records of greater than 60 species were obtained (electronic supplementary material, table S2). Quantitative data were obtained through a bibliographic survey of the published literature (1970 to present) using the ISI Web of Science database on 13 March 2017. We targeted works from which the quantitative contribution of mixotrophs to the microzooplankton assemblage could be estimated. Over 180 articles were examined (electronic supplementary material, table S3), of which approximately 45 articles provided quantitative data for mixotrophic oligotrich ciliates (GNCMs) and the mixotrophic Mesodinium spp. (pSNCM), hereafter referred to as Mesodinium (electronic supplementary material, table S4). The relative contribution of mixotrophic Rhizaria (eSNCMs) to the planktonic assemblage within the topmost 100 m was estimated from recent research that used a non-destructive in situ imaging system [18].
Non-metric multidimensional scaling (NMDS) was used to explore dissimilarities between non-constitutive mixotrophic species according to their spatial distributions. For this analysis, we used the qualitative data (electronic supplementary material, table S2) to build a presence–absence matrix of species occurrences across the different provinces and biomes (electronic supplementary material, table S1). The quantitative distribution of the NCMs across the seven biomes was also analysed, as was the seasonal progression of biomass for mixotrophic ciliates (both GNCMs and pSNCMs) and Rhizaria (eSNCMs). Two-way ANOVAs were conducted to compare mixotrophic biomass (relative and absolute values) across time and space. All analyses were carried out using R software [19].
3. Results
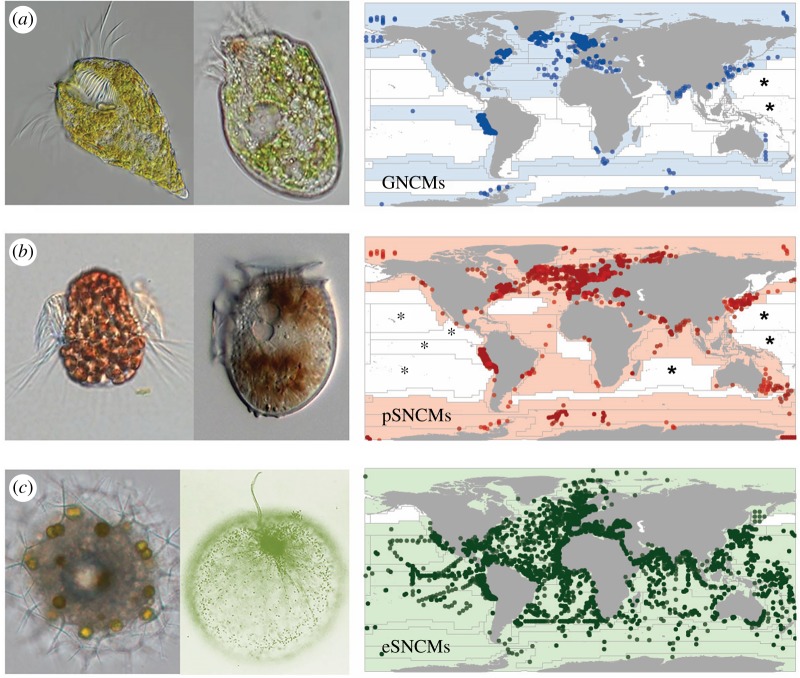
Our analysis revealed that acquired phototrophy is ubiquitous in the global oceans; however, the biogeography of the three functional groups differed markedly (figure 1). While the eSNCMs were observed to be widely distributed, GNCMs and pSNCMs were more restricted spatially (figure 1a,b versus figure 1c). Indeed, each of the three major plankton taxa within the eSNCM functional grouping (dinoflagellates, Radiolaria and Foraminifera) has a wider distribution than the GNCMs and the pSNCMs (electronic supplementary material, figure S2).
Figure 1.
Global distribution of protists with acquired phototrophy (non-constitutive mixotrophs, NCMs). Functional groups identify protists which acquire plastids from a variety of prey (generalist NCMs, GNCMs; blue (a)), from specific prey (plastidic specialist NCMs, pSNCMs; red (b)), or enslave entire specific autotrophic prey as symbionts (endosymbiotic SNCMs, eSNCMs; green (c)). Images next to each map provide protist genus examples within each functional group. From left to right (size as length): (a) GNCMs Laboea (100 µm) and Strombidium (50 µm); (b) pSNCMs, Mesodinium (60 µm) and Dinophysis (40 µm); (c) eSNCMs, Sphaerozoum (200 µm) and Noctiluca (500 µm). On maps, symbols correspond to the exact location where mixotrophic species/taxa were found (from more than 110 000 records); the grid indicates biogeographic provinces. Colour-cast provinces indicate the presence of NCMs and white provinces correspond to the absence. Provinces marked with asterisks indicate that studies conducted in these areas did not record the presence of mixotrophic species; unmarked white provinces indicate a lack of field studies providing information on acquired phototrophy among microzooplankton.
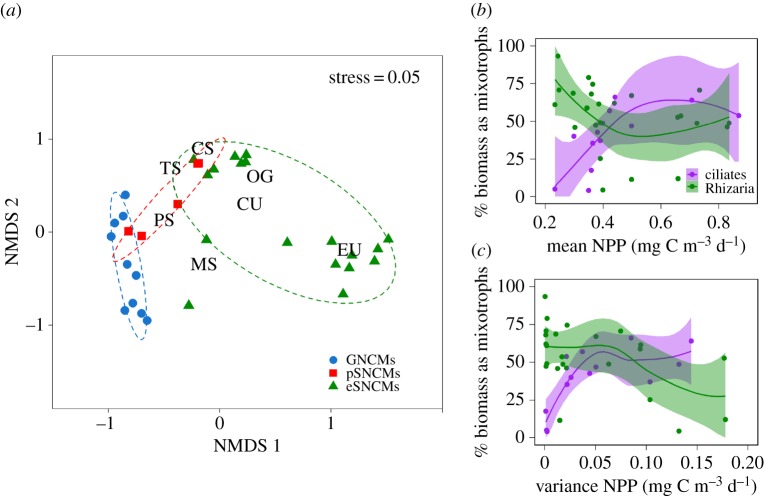
The NMDS analysis based on the dissimilarities among species distributions (derived from their presence or absence within the biogeographic provinces) revealed species clustered together according to the NCM functional groups (figure 2a). Notably, pSNCMs were positioned between the GNCMs and eSNCMs; certain pSNCM species (e.g. Amylax triacantha and Dinophysis mitra) were closer to the GNCMs, while others (e.g. Mesodinium rubrum and Dinophysis acuminata) were closer to the eSNCMs. Spatially, while GNCMs were mainly associated with the Temperate Seas, Polar Seas and Mediterranean Sea biomes, eSNCMs were primarily associated with the Oligotrophic Gyres, Coastal Upwelling and Equatorial Upwelling biomes (figure 2a). The eSNCM distribution was observed to follow two distinctly diverse patterns; one group was closely related to the Equatorial Upwelling biome, while a second cosmopolitan group occurred in the intersection between most biomes (figure 2a). The Coastal Sea biome was not associated with any single functional group; all the NCM functional groups occurred at least in one biogeographic province within this biome. Our results suggest that the contribution of mixotrophic ciliates increases towards more productive and less variable systems, while the opposite pattern is expected for mixotrophic Rhizaria (figure 2b,c; see also the electronic supplementary material).
Figure 2.
Differences in the biogeography of non-constitutive mixotrophs (NCMs). (a) Results from the NMDS analysis showing the ordination of species and biomes in a two-dimensional space. Species were classified according to the non-constitutive mixotroph functional groups (GNCMs, pSNCMs and eSNCMs). Each symbol represents an NCM species; different symbols and dashed ellipses (at 80% CI) represent different functional groups. The different biomes are: MS, Mediterranean Sea; PS, Polar Seas; TS, Temperate Seas; CS, Coastal Seas; OG, Oligotrophic Gyres; CU, Coastal Upwelling; EU, Equatorial Upwelling (see also the electronic supplementary material, figure S3). (b,c) Relative contribution of mixotrophs (% biomass) as a function of (b) nutrient load and (c) system variability. Contribution of mixotrophic ciliate biomass (GNCMs + the pSNCM Mesodinium; purple) is plotted relative to total ciliate biomass while contribution of mixotrophic Rhizarian biomass (green) is presented relative to total Rhizarian biomass. Annual average and variance of net primary productivity (NPP) were used as a proxy for nutrient load and system variability, respectively (data obtained from [17]); each symbol represents a biogeographic province (see also the electronic supplementary material). Loess regressions were fitted to data using R package ggplot2; 95% CIs shown.
The quantitative data analysis revealed that the biomass contribution of acquired phototrophy across biomes varied according to the functional groups in a similar fashion as did their presence/absence (figure 3; electronic supplementary material, S4–S6 and tables S5–S6). The absolute biomass of GNCMs was highest during summer within the Mediterranean Sea biome (figure 3a), making up 70% of total ciliate biomass (electronic supplementary material, figure S6). The absolute pSNCM biomass (represented by Mesodinium) was highest during spring in the Coastal Seas biome (figure 3b), encompassing up to 80% of total ciliate biomass (electronic supplementary material, figure S6). The highest absolute biomass values of mixotrophic Rhizaria (eSNCMs) were observed during autumn within the Equatorial Upwelling biome (figure 3c). Lower absolute biomass values were observed within the Coastal Seas and Mediterranean Sea biomes (figure 3c); the mixotrophic Rhizaria contributed up to 65% of total Rhizaria biomass within these regions (electronic supplementary material, figure S6).
Figure 3.
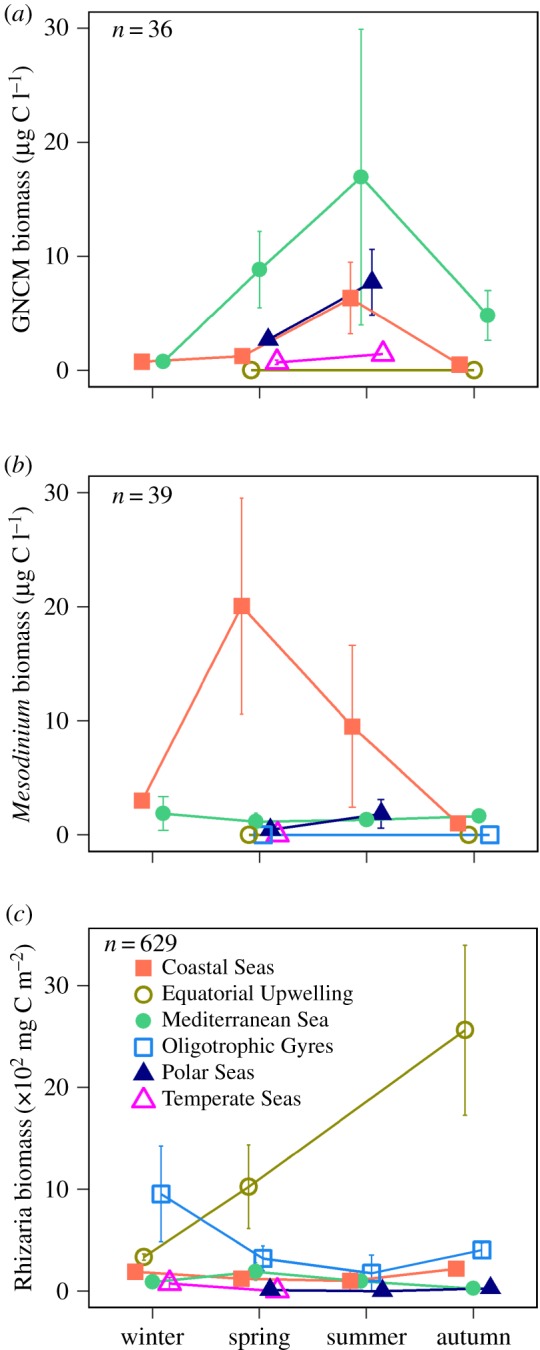
Spatial and temporal distribution of protists with acquired phototrophy. (a) GNCM ciliates, (b) pSNCM Mesodinium spp., (c) eSNCM Rhizaria. Seasonal biomass abundance for each group is shown across different biomes (note that biomass units in (a,b) are different from (c)); n indicates the total number of observations used. No published data were available for the Coastal Upwelling biome.
While there is a paucity of data for GNCMs and Mesodinium within certain oceanic regions and/or periods of the year (electronic supplementary material, table S5), we carried out the ANOVAs to gain a basic understanding of the impact of seasonality and/or biomes on their biogeographies. These ANOVAs (electronic supplementary material, table S7) suggest that the combined effect of biome and season on Mesodinium (absolute biomass) and mixotrophic Rhizaria (absolute and relative biomass) was significant. The ANOVA of relative biomass of GNCMs showed a significant effect only when considering seasonality.
4. Discussion
From our analysis, we conclude that mixotrophic plankton with acquired phototrophy are prevalent across all ocean biomes, from polar to tropical regions, and from coastal to oceanic environments, in both hemispheres (figure 1). However, the distribution patterns of the three functional types (i.e. GNCMs, pSNCMs and eSNCMs) differed markedly among provinces and also displayed seasonal variation. There was no obvious latitudinal constraint on the occurrence of GNCMs and pSNCMs (figure 1a,b). However, most records for these functional groups were from studies of coastal environments; there are very few reports of these groups within oceanic systems, particularly from within oligotrophic gyres [5,20,21]. By contrast, eSNCMs were present across all latitudinal and coastal-oceanic gradients [5,18,22], with Radiolaria and Foraminifera dominating oceanic waters at low latitudes (0°–30° N and S; figure 1c). Quantitatively, the relative contribution of GNCMs to total ciliate assemblage (abundance and biomass) was lower in low latitudes (electronic supplementary material, figure S7), while the contribution of mixotrophic Rhizaria to total Rhizaria assemblage has been observed to decrease towards the higher latitudes [18].
Protists with acquired phototrophy occur in an ecophysiological continuum from species that have low or moderate control over the acquired phototrophic machinery (GNCMs and pSNCMs, respectively) to those that can strongly regulate phototrophy by control of their endosymbiont populations (eSNCMs) [5,6,11]. Our results suggest that these differences are reflected in their biogeography (figure 2a). For example, pSNCM species with lower physiological control over their acquired plastids (e.g. A. triacantha and D. mitra) were closer in the NMDS analysis to the GNCMs, while those with a higher level of control (e.g. M. rubrum and D. acuminata) were closer to the eSNCMs from coastal regions (figure 2a) [23,24].
While GNCMs and pSNCMs share biogeographies that are similar and somewhat restricted to neritic regions, temporal and spatial differences can be drawn between them. Within temperate seas, GNCMs tend to dominate after the phytoplankton bloom, particularly in summer, under stratified water column conditions, while pSNCMs are more commonly encountered during spring, in nutrient-replete conditions (figure 3a,b). Mesodinium also thrive in upwelling zones, largely due to their high rates of phototrophic growth in nutrient-rich waters [12,23]. By contrast, GNCMs are usually outnumbered by heterotrophic ciliates (mainly tintinnids) in upwelling regions [25]. The striking dominance of eSNCMs among mixotrophs in less productive ecosystems with low abiotic variability (figure 2b versus figure 2c), such as within the oligotrophic open ocean, underscores the importance of resource partitioning and symbiosis in these low nutrient environments, and further helps to explain the anomalous preponderance of large cells there [26]. The limited success of GNCMs in these conditions could be attributed to the low availability of prey, which is detrimental to growth of the GNCMs as they rely on a near-constant supply of prey for acquired phototrophy as well as for essential nutrients [5,11] (figure 2b,c).
Acquired phototrophy among microzooplankton has been typically neglected in field and modelling studies; NCMs have hitherto not been considered a major component of the microzooplankton. According to our analysis, when we calculated the average contribution of mixotrophs across temporal and spatial scales, the mixotrophic ciliates (GNCMs + the pSNCM Mesodinium) contribute approximately 45% to the total ciliate numeric abundance, and approximately 40% of total ciliate biomass (electronic supplementary material, figure S4). A previous study estimated that approximately 30% of the numeric abundance of marine oligotrich ciliates globally were mixotrophic (i.e. GNCMs) [27]. Among the eSNCMs, nearly half of total Rhizaria biomass is composed of mixotrophic taxa across all temporal and spatial scales (electronic supplementary material, figure S5). Importantly, our analysis reveals great variation not only in the presence of mixotrophs temporally and spatially, but also on which mixotroph functional group dominates which biome during specific seasons. While, for simplicity, one may wish to refer to a single numeric value defining mixotroph abundance, our study indicates the danger of doing so, especially when conducting modelling studies in support of ecosystem management (e.g. HABs, fisheries and biogeochemistry).
The proposed new mixotroph-centric paradigm for marine ecology [6,28] envisions a high proportion of marine planktonic protists expressing mixotrophy, with a consequential more fully embedded, intertwined set of interactions within the microbial loop [29] than previously appreciated. There is an important contrast in physiology, and thence ecology, among protists with acquired phototrophy (NCMs; i.e. ‘animals that photosynthesize’), and also between these and the better-studied constitutive mixotrophs (i.e. ‘plants that eat’; the CMs). In the upper water column, activity by GNCMs can shorten and thus potentially increase the efficiency of energy transfer along pelagic food webs [5,6,11]. In the open ocean, eSNCMs include giant photosynthetic protists that contribute significantly to vertical carbon flux, influencing the biological carbon pump [18,28,30,31]. In eutrophic coastal regions, some pSNCMs and eSNCMs form extensive blooms. Blooms of the pSNCM ciliate Mesodinium spp. are of particularly concern, because they can act as biogeochemical hotspots and are the source of the phototrophic capability of the toxicogenic pSNCM, Dinophysis spp. [32,33]. Dinophysis, an organism colloquially considered as an alga rather than a microzooplankter with acquired phototrophy, causes diarrhetic shellfish poisoning, which can be responsible for closures of shellfish aquaculture operations [14,16]. In the Arabian Sea, shifts from diatom blooms to those of the eSNCM ‘green Noctiluca’ are circumstantially associated with hypoxia, and may adversely affect fisheries in a coastal ecosystem supporting 120 million people [15].
In conclusion, diverse physiological and ecological properties are associated with various modes of acquired phototrophy. Our biogeographic analysis of plankton communities across the world's oceans highlights the prevalence of planktonic protists with acquired phototrophy through symbiotic associations with prey or enslavement of their organelles. Thus, it is critically important that appropriate NCM functional subgroups are represented within conceptual and mathematical models supporting marine research across all spatial and temporal scales of observation. To support such action, it is necessary for surveys and monitoring studies to routinely document the presence of these organisms, to expect to find them rather than consider them a priori as novelties.
Supplementary Material
Supplementary Material
Supplementary Material
Data accessibility
The original (raw) data are available as referenced. Processed data are provided as electronic supplementary material.
Authors' contributions
The concept of this work arose from a workshop involving all authors except S.G.L. S.G.L. compiled the data and conducted the analysis guided by all authors and drafted the original manuscript under the guidance of A.M. and K.J.F. All authors contributed to building the manuscript to its final state.
Competing interests
We have no competing interests.
Funding
This work was supported by the Leverhulme Trust (International Network grant no. F00391 V) granted to K.J.F. and A.M. A.C. was funded by project FERMI (CGL2014-59227-R) from the Spanish Ministry of Economy and Competitiveness, co-funded with FEDER funds from the EU. This is contribution no. 5385 from the University of Maryland Center for Environmental Science. F.N. was funded by the ANR-15-CE02-0011-01 project IMPEKAB. S.G.L. was funded by the Brazilian government programme Science Without Borders through CNPq.
References
- 1.Field CB, Behrenfeld MJ, Randerson JT, Falkowski P. 1998. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–240. ( 10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- 2.Zubkov MV, Tarran GA. 2008. High bacterivory by the smallest phytoplankton in the North Atlantic Ocean. Nature 455, 224–226. ( 10.1038/nature07236) [DOI] [PubMed] [Google Scholar]
- 3.Kirchman DL. 2012. Processes in microbial ecology. New York, NY: Oxford University Press. [Google Scholar]
- 4.Flynn KJ, Stoecker DK, Mitra A, Raven JA, Glibert PM, Hansen PJ, Granéli E, Burkholder JM. 2013. Misuse of the phytoplankton–zooplankton dichotomy: the need to assign organisms as mixotrophs within plankton functional types. J. Plankton Res. 35, 3–11. ( 10.1093/plankt/fbs062) [DOI] [Google Scholar]
- 5.Stoecker DK, Hansen PJ, Caron DA, Mitra A. 2017. Mixotrophy in the marine plankton. Annu. Rev. Mar. Sci. 9, 311–335. ( 10.1146/annurev-marine-010816-060617) [DOI] [PubMed] [Google Scholar]
- 6.Mitra A, et al. 2016. Defining planktonic protist functional groups on mechanisms for energy and nutrient acquisition: incorporation of diverse mixotrophic strategies. Protist 167, 106–120. ( 10.1016/j.protis.2016.01.003) [DOI] [PubMed] [Google Scholar]
- 7.Burkholder JM, Glibert PM, Skelton HM. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8, 77–93. ( 10.1016/j.hal.2008.08.010) [DOI] [Google Scholar]
- 8.Hartmann M, Grob C, Tarran GA, Martin AP, Burkill PH, Scanlan DJ. 2012. Mixotrophic basis of Atlantic oligotrophic ecosystems. Proc. Natl Acad. Sci. USA 109, 5756–5760. ( 10.1073/pnas.1118179109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figueiras FG, Espinoza-González O, Arbones B, Garrido JL, Teixeira IG, Castro CG. 2014. Estimating phytoplankton size-fractionated primary production in the northwestern Iberian upwelling: is mixotrophy relevant in pigmented nanoplankton? Prog. Oceanogr. 128, 88–97. ( 10.1016/j.pocean.2014.08.011) [DOI] [Google Scholar]
- 10.Okamoto N, Inouye I. 2005. A secondary symbiosis in progress? Science 310, 287 ( 10.1126/science.1116125) [DOI] [PubMed] [Google Scholar]
- 11.Stoecker DK, Johnson MD, De Vargas C, Not F. 2009. Acquired phototrophy in aquatic protists. Aquat. Microb. Ecol. 57, 279–310. ( 10.3354/ame01340) [DOI] [Google Scholar]
- 12.Johnson MD. 2011. The acquisition of phototrophy: adaptive strategies of hosting endosymbionts and organelles. Photosynth. Res. 107, 117–132. ( 10.1007/s11120-010-9546-8) [DOI] [PubMed] [Google Scholar]
- 13.Harrison PJ, et al. 2011. Geographical distribution of red and green Noctiluca scintillans. Chinese J. Oceanol. Limnol. 29, 807–831. ( 10.1007/s00343-011-0510-z) [DOI] [Google Scholar]
- 14.Reguera B, Velo-Suárez L, Raine R, Park MG. 2012. Harmful Dinophysis species: a review. Harmful Algae 14, 87–106. ( 10.1016/j.hal.2011.10.016) [DOI] [Google Scholar]
- 15.do Rosário Gomes H, Goes JI, Matondkar SGP, Buskey EJ, Basu S, Parab S, Thoppil P. 2014. Massive outbreaks of Noctiluca scintillans blooms in the Arabian Sea due to spread of hypoxia. Nat. Commun. 5, 4862 ( 10.1038/ncomms5862) [DOI] [PubMed] [Google Scholar]
- 16.Mafra LL, Tavares CP dos S, Schramm MA. 2014. Diarrheic toxins in field-sampled and cultivated Dinophysis spp. cells from southern Brazil. J. Appl. Phycol. 26, 1727–1739. ( 10.1007/s10811-013-0219-9) [DOI] [Google Scholar]
- 17.Longhurst A. 2007. Ecological geography of the sea, 2nd edn San Diego, CA: Academic Press. [Google Scholar]
- 18.Biard T, et al. 2016. In situ imaging reveals the biomass of giant protists in the global ocean. Nature 532, 504–507. ( 10.1038/nature17652) [DOI] [PubMed] [Google Scholar]
- 19.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 20.Stoecker DK, Gustafson DE, Verity PG. 1996. Micro- and mesoprotozooplankton at 140° W in the equatorial Pacific: heterotrophs and mixotrophs. Aquat. Microb. Ecol. 10, 273–282. ( 10.3354/ame010273) [DOI] [Google Scholar]
- 21.Rychert K, Nawacka B, Majchrowski R, Zapadka T. 2014. Latitudinal pattern of abundance and composition of ciliate communities in the surface waters of the Atlantic Ocean. Oceanol. Hydrobiol. Stud. 43, 436–441. ( 10.2478/s13545-014-0161-8) [DOI] [Google Scholar]
- 22.Tarangkoon W, Hansen G, Hansen PJ. 2010. Spatial distribution of symbiont-bearing dinoflagellates in the Indian Ocean in relation to oceanographic regimes. Aquat. Microb. Ecol. 58, 197–213. ( 10.3354/ame01356) [DOI] [Google Scholar]
- 23.Hansen PJ, Nielsen LT, Johnson M, Berge T, Flynn KJ. 2013. Acquired phototrophy in Mesodinium and Dinophysis—a review of cellular organization, prey selectivity, nutrient uptake and bioenergetics. Harmful Algae 28, 126–139. ( 10.1016/j.hal.2013.06.004) [DOI] [Google Scholar]
- 24.Hattenrath-Lehmann T, Gobler CJ. 2015. The contribution of inorganic and organic nutrients to the growth of a North American isolate of the mixotrophic dinoflagellate, Dinophysis acuminata. Limnol. Oceanogr. 60, 1588–1603. ( 10.1002/lno.10119) [DOI] [Google Scholar]
- 25.Chang FH. 1990. Quantitative distribution of microzooplankton off Westland, New Zealand. New Zeal. J. Mar. Freshw. Res. 24, 187–195. ( 10.1080/00288330.1990.9516414) [DOI] [Google Scholar]
- 26.Selosse M-A, Charpin M, Not F. 2016. Mixotrophy everywhere on land and in water: the grand écart hypothesis. Ecol. Lett. 20, 246–263. ( 10.1111/ele.12714) [DOI] [PubMed] [Google Scholar]
- 27.Dolan JR, Pérez MT. 2000. Costs, benefits and characteristics of mixotrophy in marine oligotrichs. Freshw. Biol. 45, 227–238. ( 10.1046/j.1365-2427.2000.00659.x) [DOI] [Google Scholar]
- 28.Mitra A, et al. 2014. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 11, 995–1005. ( 10.5194/bg-11-995-2014) [DOI] [Google Scholar]
- 29.Jiao N, et al. 2014. Mechanisms of microbial carbon sequestration in the ocean—future research directions. Biogeosciences 11, 5285–5306. ( 10.5194/bg-11-5285-2014) [DOI] [Google Scholar]
- 30.Lampitt RS, Salter I, Johns D. 2009. Radiolaria: major exporters of organic carbon to the deep ocean. Glob. Biogeochem. Cycles 23, 1–9. ( 10.1029/2008GB003221) [DOI] [Google Scholar]
- 31.de Vargas C, et al. 2015. Eukaryotic plankton diversity in the sunlit ocean. Science 348, 1–11. ( 10.1126/science.1261605) [DOI] [PubMed] [Google Scholar]
- 32.Herfort L, Peterson TD, Prahl FG, McCue LA, Needoba JA, Crump BC, Roegner GC, Campbell V, Zuber P. 2012. Red waters of Myrionecta rubra are biogeochemical hotspots for the Columbia River Estuary with impacts on primary/secondary productions and nutrient cycles. Estuaries Coasts 35, 878–891. ( 10.1007/s12237-012-9485-z) [DOI] [Google Scholar]
- 33.Kim M, Nam SW, Shin W, Coats DW, Park MG. 2012. Dinophysis caudata (Dinophyceae) sequesters and retains plastids from the mixotrophic ciliate prey Mesodinium rubrum. J. Phycol. 48, 569–579. ( 10.1111/j.1529-8817.2012.01150.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original (raw) data are available as referenced. Processed data are provided as electronic supplementary material.