Abstract
Despite accumulating evidence for individual variation in behavioural plasticity, there is currently little understanding of the causes and consequences of this variation. An outstanding question is whether individual reaction norm (RN) slopes are consistent across different environmental variables—that is, whether an individual that is highly responsive to one environmental variable will be equally responsive to a second variable. Another important and related question is whether RNs are themselves consistently expressed through time or whether they are simply state dependent. Here, we quantified individual activity rates of zebrafish in response to independent manipulations of temperature and food availability that were repeated in discrete ‘bursts’ of sampling through time. Individuals that were thermally responsive were not more responsive to food deprivation, but they did exhibit greater unexplained variation. Individual RN slopes were consistent (repeatable) over time for both temperature (Rslope = 0.92) and food deprivation responses (Rslope = 0.4), as were mean activity rates in the standard environment (Rintercept = 0.83). Despite the high potential lability of behaviour, we have demonstrated consistency of behavioural RN components and identified potential energetic constraints leading to high consistency of thermal RNs and low consistency of food deprivation RNs.
Keywords: animal personality, behavioural predictability, residual model, state-behaviour feedback, temporal plasticity, endogenous plasticity
1. Introduction
Many studies have now explored the proximate and ultimate factors thought to be important to the maintenance of individual differences in behaviour [1–5]. More recently, there is increasing focus on individual differences in behavioural plasticity (e.g. [6–9], reviewed in [10]). Individual variation in plasticity is readily quantified using a reaction norm (RN) approach, which gives the change in predicted phenotype of individuals as a function of the environment or time [6,11,12]. Plasticity may also be captured as residual intra-individual variability (rIIV), behavioural variation unexplained by our statistical models, which may represent responsiveness to unobserved endogenous or external stimuli [10,13–16].
Despite the present interest in behavioural plasticity, there is still relatively little understanding of the causes and constraints which may produce individual differences in behavioural plasticity (but see [17,18]). An important and outstanding question is whether behavioural plasticity is domain general, whereby some individuals may generally show greater responsiveness in a given trait to different environmental variables (given by RN slopes) and/or show greater rIIV [10]. One reason to expect among-individual correlations of RN slopes estimated in response to different environmental variables is because of common proximate factors underlying plasticity, such as individual differences in cognition [19].
Energetics has been a focus for theory development as it may provide a general proximate explanation for understanding individual differences in behaviour, because behaviour can affect the rate at which energy is spent and acquired [1,20,21]. Consequently, we may also predict energetics to underlie how individuals respond to changes in environmental conditions that affect either energy intake or expenditure rates. Thus, when food abundance is low we could expect activity rates to decrease to reduce energy expenditure. Similarly, changes in temperature have strong and direct positive effects on ectotherm metabolism [22,23] and, therefore, a decrease in temperature typically decreases activity rates as individuals generate less energy [7,24,25].
In light of these observations of the effects of changing food and temperature on individual state, we predict energetics to act as a proximate cause for individual differences in RN slopes of food deprivation and temperature, and for the responsiveness of individuals to the two gradients to covary. These two environmental gradients are major factors affecting energy budgets of any animal, but especially ectotherms. Indeed, activity rates form a substantial portion of the energy budget of individuals [21,26,27] and consequently, activity often decreases with time of food deprivation in fishes [21,27]. Therefore, individuals with higher activity rates should expend energy more rapidly and thus exhibit a greater rate of decline in activity as time of food deprivation progresses. We already know that individuals commonly differ in their responsiveness to environmental temperature in both activity rates [7,24,25] and metabolic rate [23,28]. Further, individuals which are highly active and with higher metabolic rates tend to be more responsive to temperature [23,24]. For these reasons, we therefore predicted an among-individual covariance between RN slopes of temperature and food deprivation gradients.
Researchers have previously quantified among-individual covariances between RN slopes in response to different environmental gradients, but have found limited support for this idea. For instance, there was no correlation between RN slopes of parental provisioning in response to nestling age and partner visitation rate in house sparrows (Passer domesticus [9]), nor a correlation of RN slopes in response to ontogenetic and circadian effects on boldness and activity rates in yabbies (Cherax destructor [29]). However, domain generality of plasticity was secondary to the focus of those studies, and as such the different environmental gradients varied simultaneously. Consequently, effects can be lost or confounded as multiple environmental variables may interact to affect the expressed trait within individuals. Ideally, a strong test of whether plasticity is domain general would require independent manipulations of two or more environmental variables. Therefore, the question of whether behavioural plasticity is domain general remains an open question, as it speaks to individual differences in flexibility which may be carried over across different contexts or traits from underlying proximate constraints.
Related to the question of domain generality of behavioural plasticity is the question of whether the RNs are expressed consistently through time. Plasticity in response to one environmental variable can interact with plasticity in response to another environmental variable [9,30], or potentially with temporal or endogenous state variables [31,32]. Therefore, individuals may express different RNs though time [31,32]. However, RNs are typically recorded just once per individual, meaning among-individual variation in RNs may commonly be inflated by, or an artefact of, temporary variation in state-dependent variables [31,32]. Consistency of individual differences in RN slopes over time is an underlying assumption for the evolution of individual differences in behavioural plasticity as it is a prerequisite for heritable variation [10,32], yet there has not been due consideration of the validity of this assumption and only recently has any evidence emerged for consistency in behavioural RNs [31]. By contrast, the consistency and heritability of plasticity for traits that are less labile than behaviour have been better studied, most notably in laying date plasticity [33,34].
In this study, we explored among-individual variation in spontaneous activity RNs of zebrafish (Danio rerio) in response to temperature and food deprivation, while also quantifying individual differences in rIIV. We focus on activity because it is readily quantified and is an important trait that affects encounter rates with food, predators and potential mates. We independently manipulated these environmental variables and replicated these manipulations four times each in discrete intervals (bursts) of sampling over the course of four weeks. This permitted us to test two common predictions in behavioural ecology; that plasticity is domain general, and that plasticity is consistently expressed through time.
2. Material and methods
(a). Study population
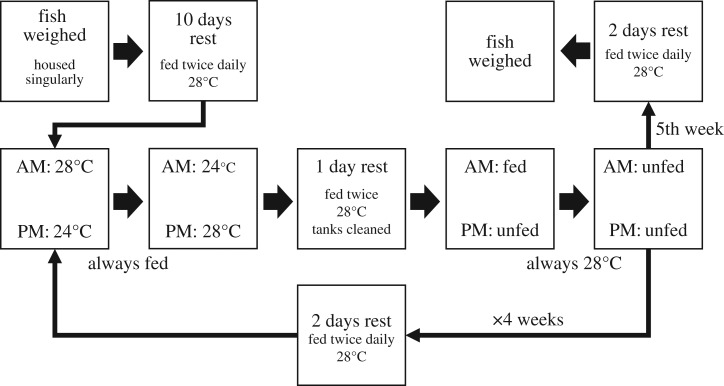
We randomly selected 57 one-year old female wild-type zebrafish (Danio rerio) from a stock bred population of unrelated individuals, originally derived from the aquarium trade, at Deakin University. Prior to the experiment, fish were kept in small groups on a recirculation system, in 3 l tanks (25 × 15 × 12 cm). At the beginning of the experiment, fish were anaesthetized with MS-222 (Sigma E10521, 0.2 g l−1), gently dabbed dry with a Kimwipe and weighed to the nearest 0.001 g; mass measurements were repeated at the end of all behavioural trials (figure 1). Fish were then placed individually into a 1.5 l tank (25 × 7.5 × 12 cm), put on a recirculation system and left to acclimate for 10 days. The recirculation system was held at 28°C (range ± 0.3 units), with a pH of 7.2 (range 6.9–7.3) and kept in a 12 L : 12 D photoperiod, identical to the conditions they were previously raised and held in. One side of the tank was covered with opaque white plastic to prevent visual stimuli of other fish. Fish were fed twice daily a pinch (approx. 0.01 g) of NRD (0.5/0.8 mm) fish feed.
Figure 1.
The time sequence of the experiment is given as a flow chart. Behavioural measures are given in the middle row and the mass measurements of fish were taken at the prior to and after the completion of the behavioural experiment.
(b). Quantifying activity
Assays of spontaneous activity rates were conducted using EthoVision XT 9.0 tracking software, which recorded the cumulative distance moved during a 30 min trial. Nine tanks each containing a focal individual were slowly removed from the recirculation system and carefully placed on a nine-arena filming stage located within the same 3 m× 3 m constant temperature room, thus minimizing disturbance to the fish. When all fish were in place and the tracking software had a fix on individuals, the trial commenced. The arena within the stage was randomized between each trial (arena location was statistically controlled for, see equation (2.1)). The camera recorded the tanks from a side view, and given the narrow width of the tanks, fish swam predominantly in two dimensions. The stage was backlit with infrared lighting to aid contrast and tracking of the focal fish. EthoVision was controlled and monitored remotely from an adjacent room so fish were not disturbed for the duration of a trial.
We collected data on a 7 day schedule (figure 1), starting with 2 days of observations while manipulating temperature (days 1–2), followed by 1 day of rest (day 3), then 2 days of observations while manipulating levels of food deprivation (days 4–5), then 2 days rest (days 6–7). We then repeated this 7 day sampling schedule for four consecutive weeks (four ‘bursts’ of sampling). We aimed to collect data from 54 individuals, though there was a small amount of mortality and fish were replaced. In total, we recorded activity rates of 57 individuals, with typically four bursts of observations per fish (NID*Burst = 217) and eight observations per burst (Nobs = 1726).
(c). Manipulation of environmental gradients
(i). Temperature
Activity was measured at 24°C and 28°C, for two assays at each temperature and with alternating treatment orders and trial orders of fish that were randomized each day. On the morning of the first day of observations (08.00–11.00) activity assays were taken at 28°C. After the six rounds of trials (54 fish total), the temperature of the recirculation system and animal holding room was reduced to 24°C, which required about 1 h. Trials were then repeated in the afternoon in the same order (13.00–16.30). The temperature was then left overnight at 24°C and in the morning another assay was taken at 24°C, before the temperature was raised for afternoon assays at 28°C. Fish to be assayed were fed half an hour before each trial to standardize hunger levels. Temperature of the tanks were checked immediately prior to the trial and was always within 0.3°C of the target temperature.
(ii). Food deprivation
Activity was measured for four assays across 30 h of food deprivation, at their normal 28°C holding temperature. Following the temperature manipulations (days 1–2), fish were then rested for 1 day and the tanks were cleaned of any uneaten food (figure 1). In the morning of the following day (day 4), each fish was fed half an hour before its morning trial (i.e. 0 h of food deprivation). After the trial, any excess food was skimmed from the surface of the water. The next trials occurred that afternoon (5 h of food deprivation), the following morning (24 h of food deprivation) and the following afternoon (30 h of food deprivation), before being fed at the conclusion of all trials. Therefore, there were four assays across 30 h of food deprivation. Trial orders were kept constant across the two days to maintain spacing along the food deprivation gradient.
(d). Statistical analysis
We aimed to evaluate the consistency in behavioural plasticity through time and across the two environmental variables. We were also interested in individual differences in rIIV and whether this was related to other aspects of plasticity. To address these questions we used a doubly hierarchical generalized linear model which allows for iterations between two linear mixed-effect models: one explaining the mean and the other explaining residual dispersion [16,35]. We further extended these models to include covariances between the random effects in each of the mean and residual model, to quantify any correlations between individual intercepts (means), slopes of the RNs and rIIV [36,37].
We fitted a mean model (equation (2.1)) with the fixed effects of temperature (β1), food deprivation (β2) and mass (β3), with random intercepts, and random slopes for temperature and food deprivation at the individual level (ID, where ‘j’ = 1 : NID) and the level of burst nested within-individual (hereafter ID*Burst, created as a unique identifier for the interaction between ID and week as a categorical variable, where ‘k’ = 1 : NID*Burst = 217). We also fitted a random intercept of arena identity to control for position effects, giving a predicted value for each of the nine arenas (l) as a deviation from the fixed effects. The residual model (equation (2.2)) had the same fixed effects as the mean model (with ‘γ’ representing fixed effect coefficients), and also a random intercept of ID, that modelled individual differences in rIIV. As there were only eight observations per ID*Burst, each with a random intercept and two random slopes, we did not attempt to fit a random intercept of ID*Burst in the residual model. Thus, each observation (i) has a predicted value given by the mean model and predicted deviance from the residual model:
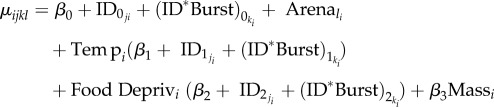 |
2.1 |
and
| 2.2 |
The random effects representing among-individual variance in intercepts (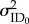 ) and slopes for each the environmental variable of temperature (
) and slopes for each the environmental variable of temperature (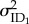 ) and food deprivation (
) and food deprivation (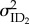 ), as well as among-individual variance in rIIV (
), as well as among-individual variance in rIIV (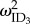 ) were fitted with an unstructured variance–covariance matrix, which evaluates all possible covariances between the random effects (equation (2.3)). We also fitted an unstructured covariance matrix at the ID*Burst level (equation (2.4)). The model was specified to explicitly test the a priori aims and hypotheses, so we did not attempt to cull any terms from the model. Repeatability of the intercept and slopes of the RNs (RRN) can then be calculated by substituting in the relevant variance parameter of each ID and ID*Burst into equation (2.5) [32]:
) were fitted with an unstructured variance–covariance matrix, which evaluates all possible covariances between the random effects (equation (2.3)). We also fitted an unstructured covariance matrix at the ID*Burst level (equation (2.4)). The model was specified to explicitly test the a priori aims and hypotheses, so we did not attempt to cull any terms from the model. Repeatability of the intercept and slopes of the RNs (RRN) can then be calculated by substituting in the relevant variance parameter of each ID and ID*Burst into equation (2.5) [32]:
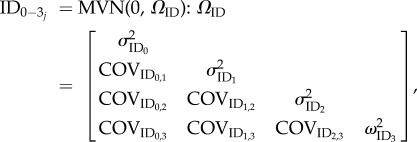 |
2.3 |
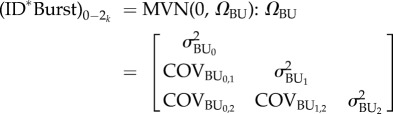 |
2.4 |
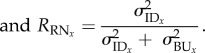 |
2.5 |
Raw activity rates were Z-transformed (set to a mean of 0 and variance of 1) to aid model fitting. A log-transformation followed by Z-transform was attempted first, but this resulted in pronounced negative skewing of the residuals and was therefore discarded. Temperature was right-centred on 28°C and set to vary between −1 and 0 (representing 24°C and 28°C, respectively). Food deprivation was left-centred on the first trial post feeding and set to vary continuously between 0 and 1, where 1 equals 30 h. This created a common intercept at 28°C and just fed represented a standard environment, from which deviations across the two gradients could be independently assessed; these conditions also represented the conditions which the fish were raised in and held during our experiment. The common scaling of the two environmental variables allows easy comparison of the magnitude of the slope variances and the gradient of the fixed effects. Mass was averaged across the two repeated measures, then also Z-transformed.
The model was run in the Bayesian software Stan [38], through the RStan interface [39]. All parameters were given uninformative priors (electronic supplementary material), and followed the methods described in [37] and details specific to this analysis and model code is given in the electronic supplementary material. Normality of random effects and the residual variation were checked visually in plots of predicted random effect values and fitted versus residual values. Model code and a table of model output are available in the electronic supplementary material.
3. Results
(a). Population effects
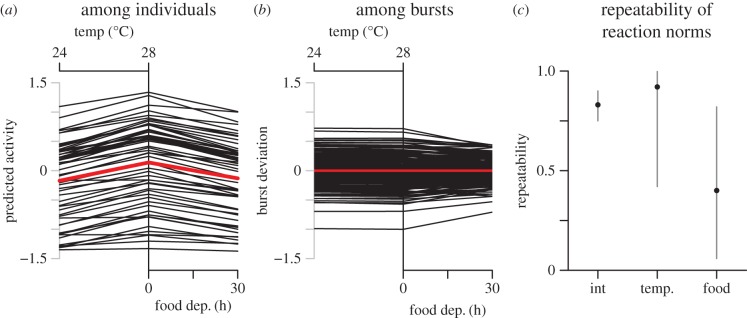
Spontaneous activity rates in female zebrafish were lower at 24°C than 28°C (β1 = 0.31 [0.24, 0.39]), and decreased with time of food deprivation (β2 = −0.27 [−0.35, −0.19]; these two mean level effects are shown as the thick red trend line in figure 2a). Larger females were less active than smaller females (β3 = −0.3 [−0.49, −0.11]). The intercept in the mean model (β0) was 0.14 [−0.068, 0.35]. Importantly, the sign of the fixed effects of temperature and food deprivation give the directionality for the interpretation of the RNs. Given the mean level slopes, plasticity is said to be greatest in individuals with the largest positive RN of temperature and the largest negative RN of food deprivation, and therefore, the two are predicted to negatively correlate if plasticity is domain general.
Figure 2.
Displayed are behavioural RNs of individuals centred on the fixed effect (red line) across each environmental variable (a), and the RNs of ID*Burst as residual deviation from the predicted value of ID, centred on 0 (b). The repeatability of RN components with 95% CI is also given (c). (Online version in colour.)
After accounting for fixed and random effects on the mean model, the mean residual standard deviation was small in the standard environment (exp(γ0) = 0.44 [0.38, 0.5]). The amount of rIIV was lower at 28°C (γ1 = −0.15 [−0.24, −0.053]), which represented the familiar environment, and decreased with increasing time deprived of food (γ2 = −0.18 [−0.31, −0.058]). Mass had no effect on rIIV (γ3 = −0.038 [−0.16, 0.085]).
(b). Variability of plasticity
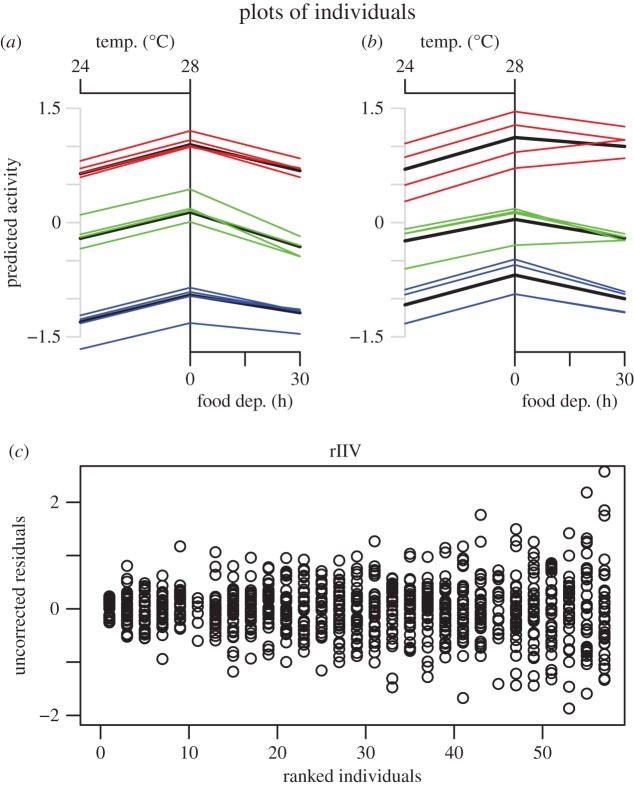
Activity rates varied substantially among individuals in the standard environment of 28°C and just fed (ID: σ0 (s.d.) = 0.74 [0.61, 0.91]; figure 2a). Individuals also varied in their responses to each of the temperature (ID: σ1 = 0.16 [0.11, 0.23]) and food deprivation gradients (ID: σ2 = 0.16 [0.065, 0.26]; figure 2a). There was some among-burst (within-individual) variation in mean activity rates in the standard environment (ID*Burst: σ0 = 0.33 [0.28, 0.39]) and the response of individuals to food deprivation also varied among-bursts (ID*Burst: σ2 = 0.2 [0.093, 0.31]; figures 2b and 3a,b). Conversely, there was little evidence for time-related change in the RNs of individuals responding to the temperature manipulation (ID*Burst: σ1 = 0.05 [0.004, 0.16]; figures 2b and 3a,b). There was also pronounced among-individual variation in rIIV, indicating some individuals were highly predictable in a given situation and time, and others were not (ID: ω3 = 0.44 [0.36, 0.56]; figure 3c).
Figure 3.
Plots of six sample individuals (a,b) are shown with the black line representing the mean individual RN and coloured lines being the reaction norms of individuals in a given burst. Plots of uncorrected residuals for every second individual ranked by predicted rIIV are given in (c).
(c). Correlations across forms of behavioural plasticity
When individuals maintained relatively high activity rates in the standard environment in a given week, they tended to then reduce activity more strongly with increasing time of food deprivation, evidenced by a negative correlation between the intercept and slope of food deprivation within individuals (ID*Burst: r0,2 = −0.56 [−0.79, −0.21]; figure 2b). A similar pattern also existed among individuals, where individuals with higher activity rates in the standard environment tended to reduce activity relatively more with increasing food deprivation (ID: r0,2 = −0.37 [−0.68, 0.026]; figure 2a). However, while the credible distribution overlapped 0 at the ID level, the result appeared congruent with the effect across bursts, indicating most likely a similar pattern is present at each level of analysis. The negative covariance of bursts shows the RNs to be converging through time post-feed (figure 2b), though the weaker effect at the ID level meant no meaningful decrease in among-individual variation occurred over the time-frame of food deprivation in this experiment (figure 2a). Consequently, variation in activity decreased with food deprivation each in residual variation and among-bursts. There was no correlation between predicted values in the standard environment and thermal RN slopes at either the among-individual (ID: r0,1 = 0.31 [−0.047, 0.62] or within-individual levels (ID*Burst: r0,1 = 0.07 [−0.61, 0.68]; figure 2a,b).
There was no evidence for a correlation between RN slopes of temperature and food deprivation gradients, at either the among individual level (ID: r1,2 =−0.22 [−0.63, 0.25]) or the within-individual level (ID*Burst: r1,2 = 0.07 [−0.61, 0.68]), counter to our prediction. However, the relatively low variation in RN slopes among individuals in response to the two gradients, and the high degree of variation in response to food deprivation within individuals (the ID*Burst variance) yielded little precision in testing for this correlation among individuals. While the effect very weakly trended in the expected direction, the credible distribution clearly overlapped with each 0 and what would be a biologically important correlation. The lack of a correlation at the within-individual level was expected given there was almost no variation in temperature responses over time (i.e. individuals had highly consistent responses to temperature over time, see below).
Individuals that were more responsive to a change in temperature also had higher rIIV (ID: r1,3 = 0.54 [0.2, 0.8]), supporting the hypothesis of domain generality of plasticity. However, individuals with high rIIV were not more responsive to food deprivation (ID: r2,3 = −0.26 [−0.61, 0.13]), but again there was low precision in the estimate of the correlation. There was a weak trend indicating that individuals with higher rIIV also tended to be more active on average (r0,3 = 0.29 [0.026, 0.51]).
(d). Consistency of behavioural reaction norm components
Individual predicted mean values in the standard environment (intercept: 28°C, just fed) were highly consistent over the four weeks of observations (Rint = 0.83 [0.75, 0.9]; figure 2c), providing evidence for maintenance of individual differences in mean values through time, a key aspect of animal personality. RN slopes across the temperature and food deprivation gradients were also both shown to be consistent through time; individual variation in responses to temperature were highly repeatable through time (RTemp = 0.92 [0.42, 1]), whereas individuals showed relatively low repeatability in their responses to food deprivation (RFood Dep. = 0.4 [0.059, 0.82]; figure 2c).
4. Discussion
Overall, we found limited evidence for domain generality in behavioural plasticity; while individuals that were more responsive to temperature displayed higher rIIV, they were not also more responsive to food deprivation. While the correlation between RNs of food deprivation and temperature very weakly trended in the predicted direction, there was little precision in the estimate of the correlation coefficient, and thus any firm inference on this question is inappropriate. Consistency in responsiveness across different environmental variables is often assumed, particularly when speculating on the causes and consequences of plasticity [19,40]. However, there is currently an absence of empirical evidence for such correlations in labile behavioural traits [10]. While the degree of sampling in this study was extensive (Nobs = 1726), the magnitude of slope variances in this study was relatively low. We, therefore, had limited power to rigorously quantify the correlation between RN slopes.
Conversely, the greater among-individual variation in rIIV relative to the slope variances yielded greater power in testing for a correlation between rIIV and the slope terms. Individuals that were more responsive to the temperature gradient also showed greater rIIV, although there was no such significant correlation between rIIV and food deprivation slopes. Such effects could represent common proximate causes, such as constraints to the range of behavioural scores an individual is capable of [40]. Higher residual variability of phenotypes in more plastic individuals (or genotypes) is also hypothesized in developmental plasticity, with higher plasticity predicted to increase ‘organismal error’ [41], whereby seemingly random variance is created by uncertainty in biological processes of evaluating the environment and the pathways leading to the plastic response [13,40]. Alternatively, rIIV may represent a response to an unobserved endogenous factor [10,15]. The manipulation of temperature in the external environment has a direct passive effect on the endogenous metabolic physiology of ectotherms [22] and thus the correlation between rIIV and the slope of temperature could each represent high sensitivity to the endogenous state.
Behaviour and the state of an individual can create feedback effects, suggested as a potential mechanism to create and maintain individual differences in behaviour [42]. In the context of our study, high activity rates are energetically costly [21,26,27] and more active individuals should more rapidly deplete their energy reserves, if not replenished. Indeed, activity rates decreased with increasing time of food deprivation, reflecting this decreasing energy budget and apparent limitation in the ability to maintain high activity rates. The magnitude of this decrease in activity rates was greatest when a given individual was more active than its average activity in that week (figure 3a,b). In addition, this pattern was also equivocally observed at the among-individual level, as predicted by energetic models of behaviour [1,20,21,43] (figure 2a). This demonstrates energetic trade-offs between activity rates and resource acquisition as being important in maintaining among-individual differences, while constraining within-individual variation in behavioural traits. Food deprivation, while reducing variation among individuals and bursts, also reduced average levels of rIIV, making individuals more predictable in their behaviour at a given point in time. Reduced rIIV with increasing hunger may reflect a reduction in the upper limit of possible trait scores an individual could maintain for the duration of the 30 min trial. Together, the results at these three levels of analysis appear congruent in demonstrating how the interaction between the internal state and the external environment can be mediated by behaviour, to inform later behavioural variation at multiple levels.
We also demonstrated that behavioural RNs are themselves consistent through time, an outstanding assumption which currently has little empirical evidence (but see [31,33,34]). In the case of the temperature gradient, individual differences in responsiveness to temperature was almost perfectly consistent across four weeks of repeated testing (Rslope = 0.92; figure 3). Conversely, individual responsiveness to food deprivation was not very consistent through time (Rslope= 0.4). This appeared to be owing to internal state, as activity declined with food deprivation was greater in weeks when individual activity was higher than average for that individual. In a typical ‘single burst’ study design, the among-individual variation in slopes would be confounded by variation created by internal state-dependence and thus, temporary within-individual variance would inflate among-individual variance. Quantifying the consistency of RN components is an important step in understanding how selection may act on behavioural plasticity and is a prerequisite for heritable variation [44].
Our results also provide strict and robust evidence for ‘animal personality’. Personality is usually defined as individual differences in behaviour that are maintained through time and across contexts—that is, individual predicted mean values should be consistent over time within individuals. Indeed, individuals differed substantially in predicted mean values in the standard environment (σInt = 0.74), and these differences in mean activity were highly consistent through time (Rint = 0.84). By contrast, the majority of studies infer personality from the repeatability of observations (i.e. consistency of scores) within individuals and not the consistency of individual means [37]. Individual differences in mean behaviour were also largely stable across the two environmental variables (figure 2a), further demonstrating robust and stable personality differences.
The high level of rank order consistency across the temperature manipulation contrasts with other studies looking at thermal RNs of behaviour in ectotherms ([7,25,45], but see [24]). The trial order of manipulations can interact with temperature effects [45] and RNs across temperature gradients may be confounded by temporal change among and within individuals, inflating the estimated variation among individuals in thermal plasticity. In this study, we controlled for temporal effects by both changing the direction of the manipulation within bursts, and replicating the manipulation using the multiple burst sampling design. While it was not possible to control for temporal and trial order effects on the food deprivation gradient within bursts, the replicate bursts allow us to statistically control for individual differences in time-related change. The results of the two environmental variables together highlight the importance of considering temporal effects when studying behavioural plasticity.
By creating a standard environment (intercept) at 28°C and just fed, we were able to independently explore deviations from this environment across the two gradients. We suggest that such methodology will again be useful in future laboratory experiments or in other studies where the gradients can be controlled to address the outstanding question of whether plasticity is domain general. It is important to note that while this design is effective in independently exploring plasticity in response to different environmental variables, this sampling design will generally create data which is better ‘anchored’ (more observations) at the intercept for each slope effect, with comparatively greater leverage of data along those gradients (i.e. Temp. ≠ 28°C, Food Depriv. ≠ 0 h). That is, in this study, the two observations of activity at 24°C and the observations nearer 30 h of food deprivation within each ID*Burst were more influential on the results and conclusions of the experiment. This increased leverage will limit the precision in the predicted slopes when compared with more evenly structured designs and this consideration should be factored in when planning future studies.
In conclusion, we did not observe a covariance between the different RN slopes, though we did observe a covariance between responsiveness to a temperature gradient and rIIV. Whether individuals consistently differ in their responsiveness to a suite of environmental variables remains unanswered and a valuable direction for future work. Understanding and evaluating such correlations will aid in forming a broad understanding in the costs and benefits of phenotypic plasticity. We observed very high consistency of responsiveness to temperature, while the response to food deprivation was less consistent, perhaps owing to an interaction between the internal and external environment affecting state, mediated by past behaviour. This is an important step in understanding behavioural plasticity, as repeatability is a prerequisite for heritable variation, and exploring the sources of within-individual variation in plasticity will help inform on the proximate constraints and limitations to the heritability or evolvability of behavioural plasticity.
Supplementary Material
Acknowledgements
We wish to thank Ben Fanson for advice on statistics, Judy Stamps and two anonymous reviewers for comments on a previous version of the manuscript, Michelle Green for assistance with animal husbandry and Christa Beckmann for discussion and support.
Ethics
All work was approved by Deakin University Animal Ethics Committee (Permit Number: B39-2014).
Data accessibility
Raw data are available at Dryad (http://dx.doi.org/10.5061/dryad.hn48m) [46].
Authors' contributions
D.J.M. collected, analysed the data and wrote the first draft. Both authors conceived of ideas, experimental design and edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Equipment used to house fish and quantify behaviour was provided by an ARC Discovery grant to P.A.B.
References
- 1.Careau V, Thomas D, Humphries M, Réale D. 2008. Energy metabolism and animal personality. Oikos 117, 641–653. ( 10.1111/j.0030-1299.2008.16513.x) [DOI] [Google Scholar]
- 2.Smith BR, Blumstein DT. 2008. Fitness consequences of personality: a meta-analysis. Behav. Ecol. 19, 448–455. ( 10.1093/beheco/arm144) [DOI] [Google Scholar]
- 3.Wolf M, Van Doorn GS, Leimar O, Weissing FJ. 2007. Life-history trade-offs favour the evolution of animal personalities. Nature 447, 581–584. ( 10.1038/nature05835) [DOI] [PubMed] [Google Scholar]
- 4.Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil. Trans. R. Soc. B 365, 4051–4063. ( 10.1098/rstb.2010.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruitt JN, Stachowicz JJ, Sih A. 2012. Behavioral types of predator and prey jointly determine prey survival: potential implications for the maintenance of within-species behavioral variation. Am. Nat. 179, 217–227. ( 10.1086/663680) [DOI] [PubMed] [Google Scholar]
- 6.Martin JG, Réale D. 2008. Temperament, risk assessment and habituation to novelty in eastern chipmunks, Tamias striatus. Anim. Behav. 75, 309–318. ( 10.1016/j.anbehav.2007.05.026) [DOI] [Google Scholar]
- 7.Biro PA, Beckmann C, Stamps JA. 2010. Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc. R. Soc. B 277, 71–77. ( 10.1098/rspb.2009.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingemanse NJ, Bouwman KM, van de Pol M, van Overveld T, Patrick SC, Matthysen E, Quinn JL. 2012. Variation in personality and behavioural plasticity across four populations of the great tit Parus major. J. Anim. Ecol. 81, 116–126. ( 10.1111/j.1365-2656.2011.01877.x) [DOI] [PubMed] [Google Scholar]
- 9.Westneat DF, Hatch MI, Wetzel DP, Ensminger AL. 2011. Individual variation in parental care reaction norms: integration of personality and plasticity. Am. Nat. 178, 652–667. ( 10.1086/662173) [DOI] [PubMed] [Google Scholar]
- 10.Stamps JA. 2016. Individual differences in behavioural plasticities. Biol. Rev. 91, 534–567. ( 10.1111/brv.12186) [DOI] [PubMed] [Google Scholar]
- 11.Dingemanse NJ, Kazem AJ, Réale D, Wright J. 2010. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol. Evol. 25, 81–89. ( 10.1016/j.tree.2009.07.013) [DOI] [PubMed] [Google Scholar]
- 12.Fuller T, Sarkar S, Crews D. 2005. The use of norms of reaction to analyze genotypic and environmental influences on behavior in mice and rats. Neurosci. Biobehav. Rev. 29, 445–456. ( 10.1016/j.neubiorev.2004.12.005) [DOI] [PubMed] [Google Scholar]
- 13.Westneat DF, Wright J, Dingemanse NJ. 2014. The biology hidden inside residual within-individual phenotypic variation. Biol. Rev. 90, 729–743. ( 10.1111/brv.12131) [DOI] [PubMed] [Google Scholar]
- 14.Biro PA, Adriaenssens B. 2013. Predictability as a personality trait: consistent differences in intraindividual behavioural variation. Am. Nat. 182, 621–629. ( 10.1086/673213) [DOI] [PubMed] [Google Scholar]
- 15.Stamps JA, Briffa M, Biro PA. 2012. Unpredictable animals: individual differences in intraindividual variability (IIV). Anim. Behav. 83, 1325–1334. ( 10.1016/j.anbehav.2012.02.017) [DOI] [Google Scholar]
- 16.Westneat DF, Schofield M, Wright J. 2013. Parental behavior exhibits among-individual variance, plasticity, and heterogeneous residual variance. Behav. Ecol. 24, 598–604. ( 10.1093/beheco/ars207) [DOI] [Google Scholar]
- 17.Carere C, Drent P, Privitera L, Koolhaas J, Groothuis T.. 2005. Personalities in great tits, Parus major: stability and consistency. Anim. Behav. 70, 795–805. ( 10.1016/j.anbehav.2005.01.003) [DOI] [Google Scholar]
- 18.Benus R, Koolhaas J, Van Oortmerssen G. 1987. Individual differences in behavioural reaction to a changing environment in mice and rats. Behaviour 100, 105–122. ( 10.1163/156853987X00099) [DOI] [Google Scholar]
- 19.Sih A, Del Giudice M. 2012. Linking behavioural syndromes and cognition: a behavioural ecology perspective. Phil. Trans. R. Soc. B 367, 2762–2772. ( 10.1098/rstb.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biro PA, Stamps JA. 2010. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 25, 653–659. ( 10.1016/j.tree.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 21.Van Dijk P, Staaks G, Hardewig I. 2002. The effect of fasting and refeeding on temperature preference, activity and growth of roach, Rutilus rutilus. Oecologia 130, 496–504. ( 10.1007/s00442-001-0830-3) [DOI] [PubMed] [Google Scholar]
- 22.Clarke A, Johnston NM. 1999. Scaling of metabolic rate with body mass and temperature in teleost fish. J. Anim. Ecol. 68, 893–905. ( 10.1046/j.1365-2656.1999.00337.x) [DOI] [PubMed] [Google Scholar]
- 23.Careau V, Gifford ME, Biro PA. 2014. Individual (co) variation in thermal reaction norms of standard and maximal metabolic rates in wild-caught slimy salamanders. Funct. Ecol. 28, 1175–1186. ( 10.1111/1365-2435.12259) [DOI] [Google Scholar]
- 24.Nakayama S, Laskowski KL, Klefoth T, Arlinghaus R. 2016. Between- and within-individual variation in activity increases with water temperature in wild perch. Behav. Ecol. 27, 1676–1683. ( 10.1093/beheco/arw090) [DOI] [Google Scholar]
- 25.Pruitt JN, Demes KW, Dittrich-Reed DR. 2011. Temperature mediates shifts in individual aggressiveness, activity level, and social behavior in a spider. Ethology 117, 318–325. ( 10.1111/j.1439-0310.2011.01877.x) [DOI] [Google Scholar]
- 26.Boisclair D, Tang M. 1993. Empirical analysis of the influence of swimming pattern on the net energetic cost of swimming in fishes. J. Fish Biol. 42, 169–183. ( 10.1111/j.1095-8649.1993.tb00319.x) [DOI] [Google Scholar]
- 27.Wang T, Hung CC, Randall DJ. 2006. The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol. 68, 223–251. ( 10.1146/annurev.physiol.68.040104.105739) [DOI] [PubMed] [Google Scholar]
- 28.Nespolo RF, Lardies MA, Bozinovic F. 2003. Intrapopulational variation in the standard metabolic rate of insects: repeatability, thermal dependence and sensitivity (Q10) of oxygen consumption in a cricket. J. Exp. Biol. 206, 4309–4315. ( 10.1242/jeb.00687) [DOI] [PubMed] [Google Scholar]
- 29.Biro PA, Adriaenssens B, Sampson P. 2014. Individual and sex-specific differences in intrinsic growth rate covary with consistent individual differences in behaviour. J. Anim. Ecol. 83, 1186–1195. ( 10.1111/1365-2656.12210) [DOI] [PubMed] [Google Scholar]
- 30.Westneat DF, Stewart IRK, Hatch MI. 2009. Complex interactions among temporal variables affect the plasticity of clutch size in a multi-brooded bird. Ecology 90, 1162–1174. ( 10.1890/08-0698.1) [DOI] [PubMed] [Google Scholar]
- 31.Araya-Ajoy YG, Dingemanse NJ. 2016. Repeatability, heritability, and age-dependence in the aggressiveness reaction norms of a wild passerine bird. J. Anim. Ecol. 86, 227–238. ( 10.1111/1365-2656.12621) [DOI] [PubMed] [Google Scholar]
- 32.Araya-Ajoy YG, Mathot KJ, Dingemanse NJ. 2015. An approach to estimate short-term, long-term, and reaction norm repeatability. Methods Ecol. Evol. 6, 1462–1473. ( 10.1111/2041-210X.12430) [DOI] [Google Scholar]
- 33.Nussey DH, Postma E, Gienapp P, Visser ME. 2005. Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306. ( 10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- 34.Brommer JE, Merilä J, Sheldon BC, Gustafsson L. 2005. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution 59, 1362–1371. ( 10.1111/j.0014-3820.2005.tb01785.x) [DOI] [PubMed] [Google Scholar]
- 35.Cleasby IR, Nakagawa S, Schielzeth H. 2014. Quantifying the predictability of behaviour: statistical approaches for the study of between-individual variation in the within-individual variance. Methods Ecol. Evol. 6, 27–37. ( 10.1111/2041-210X.12281) [DOI] [Google Scholar]
- 36.Felleki M, Lee D, Lee Y, Gilmour AR, Rönnegård L. 2012. Estimation of breeding values for mean and dispersion, their variance and correlation using double hierarchical generalized linear models. Genet. Res. 94, 307–317. ( 10.1017/S0016672312000766) [DOI] [PubMed] [Google Scholar]
- 37.Mitchell DJ, Fanson BG, Beckmann C, Biro PA. 2016. Towards powerful experimental and statistical approaches to study intraindividual variability in labile traits. R. Soc. open sci. 3, 160352 ( 10.1098/rsos.160352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stan Development Team. 2015. Stan: a C++ library for probability and sampling, version 2.8.0. See http://mc-stan.org/.
- 39.Stan Development Team. 2015. rstan: R Interface to Stan. 2.9 edn. See https://cran.r-project.org/web/packages/rstan/.
- 40.DeWitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 41.Tonsor SJ, Elnaccash TW, Scheiner SM. 2013. Developmental instability is genetically correlated with phenotypic plasticity, constraining heritability, and fitness. Evolution 67, 2923–2935. ( 10.1111/evo.12175) [DOI] [PubMed] [Google Scholar]
- 42.Sih A, Mathot KJ, Moirón M, Montiglio P-O, Wolf M, Dingemanse NJ. 2015. Animal personality and state–behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 43.Killen SS, Marras S, McKenzie DJ. 2011. Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European sea bass. J. Anim. Ecol. 80, 1024–1033. ( 10.1111/j.1365-2656.2011.01844.x) [DOI] [PubMed] [Google Scholar]
- 44.Dohm MR. 2002. Repeatability estimates do not always set an upper limit to heritability. Funct. Ecol. 16, 273–280. ( 10.1046/j.1365-2435.2002.00621.x) [DOI] [Google Scholar]
- 45.Briffa M, Bridger D, Biro PA. 2013. How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim. Behav. 86, 47–54. ( 10.1016/j.anbehav.2013.04.009) [DOI] [Google Scholar]
- 46.Mitchell DJ, Biro PA. 2017. Data from: Is behavioural plasticity consistent across different environmental gradients and through time? Dryad Digital Repository. ( 10.5061/dryad.hn48m) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mitchell DJ, Biro PA. 2017. Data from: Is behavioural plasticity consistent across different environmental gradients and through time? Dryad Digital Repository. ( 10.5061/dryad.hn48m) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data are available at Dryad (http://dx.doi.org/10.5061/dryad.hn48m) [46].