Abstract
Plastic pollution is an anthropogenic stressor in marine ecosystems globally. Many species of marine fish (more than 50) ingest plastic debris. Ingested plastic has a variety of lethal and sublethal impacts and can be a route for bioaccumulation of toxic compounds throughout the food web. Despite its pervasiveness and severity, our mechanistic understanding of this maladaptive foraging behaviour is incomplete. Recent evidence suggests that the chemical signature of plastic debris may explain why certain species are predisposed to mistaking plastic for food. Anchovy (Engraulis sp.) are abundant forage fish in coastal upwelling systems and a critical prey resource for top predators. Anchovy ingest plastic in natural conditions, though the mechanism they use to misidentify plastic as prey is unknown. Here, we presented wild-caught schools of northern anchovy (Engraulis mordax) with odour solutions made of plastic debris and clean plastic to compare school-wide aggregation and rheotactic responses relative to food and food odour presentations. Anchovy schools responded to plastic debris odour with increased aggregation and reduced rheotaxis. These results were similar to the effects food and food odour presentations had on schools. Conversely, these behavioural responses were absent in clean plastic and control treatments. To our knowledge, this is the first experimental evidence that adult anchovy use odours to forage. We conclude that the chemical signature plastic debris acquires in the photic zone can induce foraging behaviours in anchovy schools. These findings provide further support for a chemosensory mechanism underlying plastic consumption by marine wildlife. Given the trophic position of forage fish, these findings have considerable implications for aquatic food webs and possibly human health.
Keywords: plastic pollution, forage fish, Engraulis mordax, olfaction, foraging
1. Introduction
Chemoreception (olfaction and gustation) is the most ancient and ubiquitous sensory modality [1,2]. In marine fish, olfactory-mediated behaviours have been well studied in the contexts of predator avoidance [3], homing [4,5] and foraging [6,7]. Recently, the intersection of foraging, olfaction and conservation has been a major focus of research on fish populations especially in the face of rapid anthropogenic change [3,7,8]. One pervasive example of anthropogenic change in the marine environment is plastic debris. There are more than 5 trillion pieces of plastic in the global oceans; the vast majority consisting of small fragments less than 10 mm in length [9,10]. Within weeks these small plastic fragments biofoul by algae and bacteria [11,12] and may be ingested by hundreds of marine species [13]. An explanation for why marine wildlife misidentify plastic as prey remains elusive, but olfaction has been suggested as a possible mechanism [12].
Olfaction is a common mechanism of food localization in many species of fish. Specifically, fish species specializing on primary consumers (e.g. zooplankton, including krill) display an attraction to algal-derived infochemicals, such as dimethyl sulfide (DMS) and its chemical precursor, dimethylsulfoniopropionate (DMSP) [6,14,15]. Forage fish, such as anchovy (Engraulis sp.), are zooplanktivorous as adults, live in large schools and represent a critical link in marine food webs [16]. It is not well known how forage fish find their planktonic prey, though olfaction has been implicated [17]. The behavioural contexts in which adult anchovy use odours are not well known; however, they do have highly developed chemosensory systems [18].
In the California current ecosystem, the northern anchovy (Engraulis mordax) is an abundant forage fish and critical prey source for coastal and pelagic seabirds, predatory fish, pinnipeds and cetaceans [19,20]. The life history, large-scale movements, and population dynamics of northern anchovy are well studied, but recent population fluctuations, including regional stock collapses, have put the future of this vital marine resource in doubt [21,22]. Climate forcing and fishing pressure are known threats [22], while other risks, such as plastic ingestion, have not been extensively studied; however, multiple species of anchovy have been documented to ingest plastic in the wild [23,24]. Fish that ingest plastic exhibit a variety of sublethal effects including compromised liver function, reduced activity rates, and weakened schooling behaviour [25,26].
To mitigate the consumption of plastic debris by marine fish, it is essential to understand the sensory mechanism(s) underpinning this maladaptive foraging behaviour. Recent work focusing on tube-nosed seabirds (order: Procellariiformes) suggests that the chemical signature that marine plastic debris acquires and emits via biofouling may explain why plastic debris is frequently mistaken for food [12]. Here, we present the first behavioural assay testing the hypothesis that the odour profile plastic acquires at sea influences the behaviour of a marine consumer, the northern anchovy (E. mordax). To do so, we examined aggregation and rheotactic responses of wild-caught anchovy schools to presentations of krill (Euphausia pacifica; hereafter ‘food’), as well as odour solutions made from krill, clean plastic and biofouled plastic (i.e. algae-coated polypropylene beads 4–6 mm in diameter) at three environmentally relevant concentrations. We compared these school-wide responses to control (dyed seawater) treatments to determine if the odour signature of biofouled plastic can elicit olfactory search behaviours in anchovy schools. Our results elucidate both if anchovy use odours to forage and if the odour signature of plastic debris alone can induce behaviours consistent with foraging in anchovy schools.
2. Material and methods
(a). Anchovy acquisition and husbandry
This experiment was conducted at the Aquarium of the Bay in San Francisco, CA, USA, from May to August 2015. Adult anchovy were caught in San Francisco Bay, and in the Pacific Ocean off the coast of Marin and Sonoma Counties of California during the spring and summer of 2015. Anchovy were donated from J&P Bait in San Francisco, CA, USA, in schools of 200–400 individuals. Each school was held in a live bait pen for 4–14 days prior to transportation to the aquarium by Aquarium of the Bay staff in a 120 l cooler filled with aerated seawater. This process was repeated six times for the different schools of anchovy used in this experiment. Upon arrival at the aquarium, anchovy were immediately placed in a circular flow-through tank (0.7 m water height × 1.8 m diameter, approx. 1325 l water), with a flow rate of approximately 1500 l h−1. Flow in the tank was circular and counterclockwise. Water was maintained at 16–18°C, 92–98% dissolved oxygen, 7.7–8.1 pH and 32–36‰ salinity, mimicking the conditions of the marine environments where the anchovy were collected. During the 3- to 5-day acclimation period, each anchovy school was kept on a diet of 250 g thawed whole krill (Eu. pacifica) per day.
(b). Preparation of odour solutions
Using the same methods as [12], polypropylene plastic (PP) beads (size and weight range: 4–6 mm in diameter, 0.02–0.03 g each) were left to biofoul near an oceanographic monitoring buoy located approximately 1 km offshore of the University of California's Bodega Marine Laboratory (BML) (38°18′58″ N 123°04′29″ W). Plastics were left to float for three weeks during the upwelling season (May–July) in the summers of 2014 and 2015. After retrieval from the ocean, the plastic bags were immediately placed in a −80°C freezer.
To prepare the biofouled plastic solution, we first removed 15 g biofouled PP beads from the Nitex bags collected from the BML buoy. Then, we added 500 ml filtered seawater from the aquarium to the 15 g biofouled plastic in a 1 l glass beaker. This mixture was left to sit in a refrigerator (8–10°C) for 6 h, stirring occasionally. After 6 h, the mixture in the beaker was strained into a 1 l glass Erlenmeyer flask through Whatman filter paper (Grade 4, GE Healthcare, Chicago, IL, USA) to remove particles greater than or equal to 25 µm. The minimum observed food size for adult anchovy in experimental feeding studies is 150 µm [27–29]. Therefore, the particulate matter (less than 25 µm) left in the solution after filtration should not have been able to provide a food source to the anchovy. After filtration, the solution was homogenized with a magnetic stirbar for 5 min. Next, we separated 500 ml of this biofouled plastic solution into ten 50 ml aliquots in sterilized glass jars. Each glass jar was wrapped externally in Parafilm M (Bemis Co. Inc., Oshkosh, WI, USA) to prevent gas exchange and stored frozen (−20°C). This procedure was repeated twice for each school of anchovy tested to ensure adequate stock solution for the experimental treatments. To prepare the virgin (clean) plastic solution, we added 15 g of virgin PP beads—4–6 mm in diameter, 0.02–0.03 g each—to 500 ml filtered seawater in a 1 l beaker. The process of stirring, straining, mixing, separating and storing this solution was identical to that described for the biofouled plastic solution.
We made food odour solutions by thawing 15 g of whole Pacific krill (Eu. pacifica) with 500 ml seawater in a 1 l beaker under refrigeration (8–10°C) for 6 h, stirring occasionally. Afterwards, the procedure of straining, mixing, separating and storing this solution was identical to that described for the plastic solutions. To create our control seawater solution, 1 l of filtered seawater was placed in a glass beaker and left to sit in a refrigerator for 6 h, stirring occasionally. Afterwards, the procedure of straining, mixing, separating and storing this solution was identical to that described for all other solutions.
All glassware and stirbars used to prepare and store solutions were unique to each solution type to prevent chemical cross-contamination between solutions. After solution preparation, all equipment was triple washed with 70% ethanol and rinsed thoroughly with distilled water.
(c). Experimental methods
On the day prior to treatment presentation, we removed the aliquots of each solution needed for the following day's treatments from the freezer and left them in the refrigerator overnight to thaw. Thirty minutes before treatment presentation, we removed the solution from the refrigerator to allow the solution to reach the same temperature of the water in the experimental tank (16–20°C). We injected 100 ml of the solution to the tank with a 100 ml PP syringe (VvW Scientific) via 1.25 m of Tygon tubing. One drop (0.05 ml) of red propylene glycol food dye was added to all treatments except food presentations to control for visual cues of the solution injection and to blind video scorers to the treatment type.
For the ‘high’ concentration of each solution, the stock solution was injected into the experimental tank without dilution at a source concentration of 30 g l−1. The ‘medium’ concentrations used the same stock solutions described as above, but were diluted 1 : 1 with seawater 5 min prior to injection, for a source concentration of 15 g l−1. The ‘low’ concentrations also used the same stock solution, but were diluted 9 : 1 with seawater 5 min prior to injection for a source concentration of 3 g l−1. Once these solutions were injected into the tank, the resulting dilution would yield concentrations similar to recorded accumulations of plastic in the open ocean [9,10,30].
For feeding trials, 15 g of whole krill was thawed in 250 ml−1 of plain seawater 30 min before filming began. The food was introduced into the tank with a 400 ml Europlex Plexiglas syringe (Primis Medical, Kaysville, UT, USA) via 1 m of Tygon tubing. Immediately after injection, the syringe was drawn back with 250 ml water from the tank and flushed back into the tank three times. To prevent fish from associating treatments with feeding, fish were fasted randomly during experimental periods. The fish were never fasted or fed more than 3 days in a row. Preliminary observations showed that once the fish fed their motivation to respond to odours—including odours of their food—was dramatically reduced. As a result, on days when fish were fed, the food presentation was always the last treatment recorded.
The video of each trial lasted 12 min. Filming began 2 min before the treatment was injected to record behaviour prior to treatment addition, and continued 10 min after treatment addition. We spaced treatments apart by a minimum of 1 h to allow the tank's water to cycle and anchovy behaviour to return to a pre-foraging state. Each treatment and concentration was replicated five to seven times on four different schools (n ≥ 20 trials per treatment, n = 289 trials total). For more details on the experimental methods, see the electronic supplementary material.
(d). Video and image analysis
We recorded all videos above the experimental tank using a GoPro Hero4 in wide mode (focal length: 17.2 mm; field of view: 118.2° by 69.5°) at 24 frames s−1 and 1080p resolution. For analyses, we used VLC (v. 2.2, VideoLAN Organization) to take screenshots of each video at 5-s intervals (see, e.g. electronic supplementary material, figures S1 and S2). For the aggregation (clustering) analysis, we used ImageJ (v. 1.49; https://imagej.nih.gov/) to turn each screenshot into a black and white image, where the fish were black and the background white (electronic supplementary material, figure S2).
We also measured rheotactic behaviour for 3 min (75 s before through 105 s after treatment addition). Fish displaying positive rheotaxis swam clockwise around the tank because the flow in the tank was counterclockwise. A fish was considered to be positively rheotactic in an individual screenshot if its main body axis was facing ±45° of the clockwise direction; this value has been used as a cut-off in other studies measuring rheotactic behaviour in fish [31,32]. We determined rheotactic angle by drawing a line from each fish's dorsal fin to the nearest wall of the tank and measuring the angle of the fish relative to the wall. To analyse each image, we selected a quadrant at random using a random number generator (https://www.random.org/integers/) and assigned scorers to count the total number of fish in that quadrant, denoting the number of fish that were not positively rheotactic. Observers were blinded to which treatments they were scoring.
(e). Statistical analyses
Aggregation data were generated from black and white images by calculating Moran's I values [33] using the ‘raster’ package in R (v. 3.2, R Development Core Team 2015). For the purposes of our analyses, Moran's I values were used as descriptive statistics to measure the amount of clustering in images relative to one another. There was slight variability in the pre-treatment clustering values; to control for this variation we standardized pre-treatment images with a z-score transformation. For analysis, images were assigned to one of three time-bins: pre-treatment (first 2 min of each video), post-treatment (the next 2 min, directly following treatment presentation) or greater than 2 min post-treatment (the last 8 min of each video). We used a generalized linear mixed model (GLMM) fit with the package ‘lme4’ in R to test for differences between treatment, time bin, and the interactive effect of treatment and time bin. This multilevel model also contained random effects to control for the influence of school number and day on the outcome variable, clustering intensity. To account for our outcome variable being non-normal, heteroscedastic and autocorrelated, we conducted a permutation test (a non-parametric resampling technique) with 10 000 simulations to determine significance. Finally, to test for differences in the time to peak aggregation response by treatment, we used a GLMM fit with the package ‘lme4’ in R. This multilevel model contained random effects to control for the influence of school and day on the outcome variable, time to peak response.
For the rheotactic analysis, the scored images were assigned as either pre- or post-treatment label. We used a GLMM fit with the package ‘lme4’ in R to test for differences between treatment, time bin and the interactive effect of treatment type and time bin. This multilevel model also contained random effects to control for the influence of video scorer, school and day on the outcome variable, proportion of fish that were not rheotactic. This outcome variable is also heteroscedastic and autocorrelated. Here again, we conducted a permutation test with 10 000 simulations to determine significance. Permutation tests for both the aggregation and rheotactic analyses were done with the R package ‘predictmeans’. Significance was defined at α = 0.05. Results are presented ±s.e. unless otherwise indicated.
3. Results
(a). Aggregation behaviour
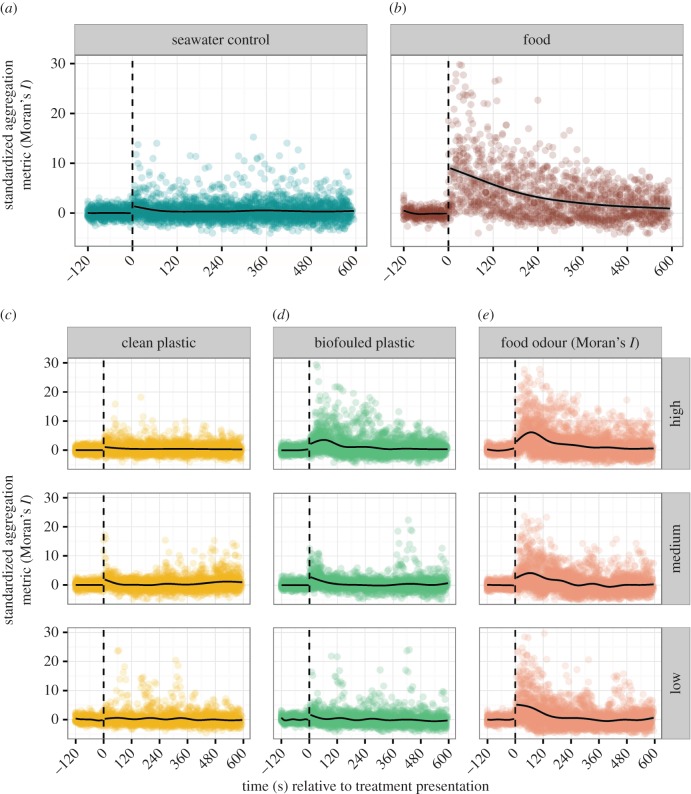
School-wide aggregation responses varied considerably between treatments (figures 1 and 2). In relation to the seawater control treatment (figure 1a), there was no difference in aggregation to the clean plastic solution at any concentration (low concentration: t = −1.12, permutation test: p = 0.28; medium concentration: t = −0.05, permutation test: p = 0.96; high concentration: t = 0.16, permutation test: p = 0.87; figure 1c). In addition, the maximum intensity of the clustering response for the clean plastic treatments was not significantly different than the control (low concentration: t10,272 = −0.27, p = 0.79; medium concentration: t10,272 = 0.69, p = 0.49; high concentration: t10,272 = −0.13, p = 0.90).
Figure 1.
Coloured dots show the raw aggregation data (standardized Moran's I) generated from black and white images taken at 5-s intervals of each video treatment (see, e.g. electronic supplementary material, figure S1). The solid black line represents the local polynomial regression (LOESS) line of best fit; the shaded regions show the 95% CI. The vertical dashed line indicates when the treatment was injected into the tank. Each treatment was replicated at least 20 times. (a) Control treatment, (b) food treatment, (c) clean plastic treatment at three source concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (d) biofouled plastic odour treatment at three source concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (e) food odour treatment at three source concentrations, high: 30 g krill l−1, medium: 15 g krill l−1 and low: 3 g krill l−1. (Online version in colour.)
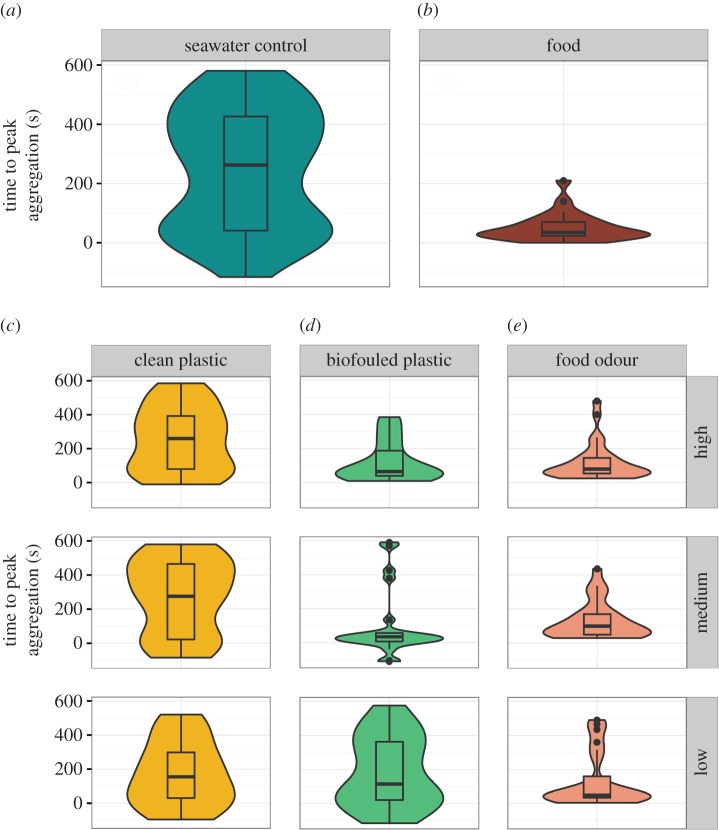
Figure 2.
Violin and boxplots of time after treatment presentation until the schools reached their peak aggregation, demonstrating a temporally constrained response in food, food odour, as well as medium and high concentration of biofouled plastic treatments that occurred earlier than in any of the clean plastic treatments or the control treatment. (a) Control treatment, (b) food treatment, (c) clean plastic treatment at three source concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (d) biofouled plastic odour treatment at three source concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (e) food odour treatment at three source concentrations, high: 30 g krill l−1, medium: 15 g krill l−1 and low: 3 g krill l−1. (Online version in colour.)
By comparison, anchovy schools significantly increased aggregation in the 2-min interval following the introduction of the biofouled plastic solution in all but the lowest concentration (low concentration: t = 0.06, permutation test: p = 0.95; medium concentration: t = 0.62, permutation test: p = 0.003; high concentration: t = 10.48, permutation test: p < 0.001; figure 1d). The maximum intensity of the clustering response from the biofouled plastic was significantly greater than the control in the highest concentration only (low concentration: t10,272 = 0.49, p = 0.62; medium concentration: t10,272 = 0.23, p = 0.82; high concentration: t10,272 = 2.82, p = 0.005).
The responses to food odour were similar to those found for the biofouled plastic solution. There were marked increases in aggregation in the 2-min interval following the food odour introduction at all concentrations (low concentration: t = 15.42, permutation test: p < 0.001; medium concentration: t = 12.76, permutation test: p < 0.001; high concentration: t = 21.41, permutation test: p < 0.001; figure 1e). Additionally, for the high concentration this clustering signal persisted for the subsequent 8-min interval (t = 5.03, permutation test: p < 0.001; figure 1e). The peak intensity of the clustering signal was greater than the control for all food odour concentrations tested (low concentration: t10,272 = 4.76, p < 0.001; medium concentration: t10,272 = 4.42, p < 0.001; high concentration: t10,272 = 5.56, p < 0.001).
Behavioural responses to the food presentations revealed a similar pattern. There was a sharp increase in aggregation immediately following food presentation (t10,272 = 32.99, permutation test: p < 0.001); clustering remained significantly elevated in the final 8-min period (t = 11.65, permutation test: p < 0.001; figure 1b). The peak intensity of the clustering signal resulting from food presentations was greater than the control (t10,272 = 6.54, p < 0.001).
(b). Time to peak aggregation
We also examined how long it took for the maximum (i.e. peak) aggregation response to occur for each treatment. In the control trials, the median time to peak response occurred the latest of any treatment with a large variance (263 ± 31 s after injection; figure 2a). Similarly, all clean plastic treatments illustrate a time to reach their peak response that did not differ from the control treatment; the associated variances were also large (low concentration: 175 ± 40 s after injection, t10,272 = −1.24, p = 0.22; medium concentration: 275 ± 47 s after injection, t10,272 = 0.63, p = 0.53; high concentration: 260 ± 36 s after injection, t10,272 = 0.26, p = 0.80; figure 2c).
For the medium and high concentrations of the biofouled plastic treatment, however, the anchovy schools' peak responses were temporally constrained and occurred significantly earlier than in the control treatment (low concentration: 115 ± 44 s after injection, t10,272 = −1.30, p = 0.20; medium concentration: 35 ± 41 s after injection, t10,272 = −3.39, p < 0.001; high concentration: 65 ± 23 s after injection, t10,272 = −2.93, p = 0.004; figure 2d). Similarly, for food odour treatments, the peak responses were temporally constrained and occurred significantly earlier than in the control treatment (low concentration: 50 ± 32 s after injection, t10,272 = −2.65, p = 0.008; medium concentration: 100 ± 22 s after injection, t10,272 = −2.75, p = 0.006; high concentration: 65 ± 23 s after injection, t10,272 = −3.06, p = 0.002; figure 2e). The anchovy schools reached their peak response most rapidly in the food treatment (figures 1, 2 and 3). When compared with the control treatment, peak aggregation occurred significantly earlier and was temporally constrained (i.e. lower variance) in the food treatment (35 ± 10 s after injection, t10,272 = −4.95, p < 0.001; figure 2b). These results support the findings in the aggregation analysis (figure 1).
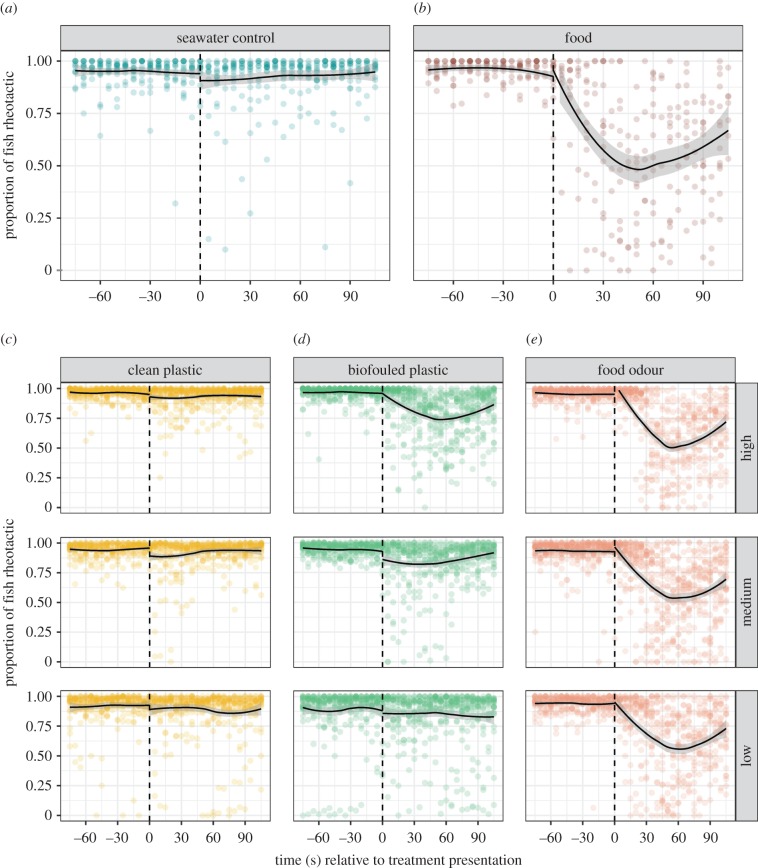
Figure 3.
Coloured dots show the raw rheotactic data (as percentage of fish that were positively rheotactic) generated from colour images taken at 5-s intervals. The solid black line represents the local polynomial regression (LOESS) line of best fit; the shaded regions show the 95% confidence intervals. The vertical dashed line indicates when the treatment was injected into the tank. Each treatment was replicated at least 20 times. (a) Control treatment, (b) food treatment, (c) clean plastic treatment at three source concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (d) biofouled plastic odour treatment at three concentrations, high: 30 g plastic l−1, medium: 15 g plastic l−1 and low: 3 g plastic l−1, (e) food odour treatment at three source concentrations, high: 30 g krill l−1, medium: 15 g krill l−1 and low: 3 g krill l−1. (Online version in colour.)
(c). Rheotactic behaviour
Rheotactic responses also differed between treatments (figure 3). Anchovy typically display positive rheotaxis (i.e. rheotaxis), orienting themselves into oncoming flow, except while foraging when their rheotactic positioning decays as a consequence of food search [34]. Our results were consistent with these previous findings. Prior to treatment presentation, most anchovy swam clockwise around the outer rim of the tank where water flow was greatest, displaying a high degree of positive rheotaxis (93.99 ± 0.16% pooled across all treatments). However, there was a sharp decline in rheotaxis after food was introduced as compared to the control treatment (control: 92.44 ± 0.53% positively rheotactic after treatment presentation; food: 58.34 ± 1.46% positively rheotactic after treatment, t = 14.84, p < 0.001; figure 3a,b).
As with the aggregation analyses, there was no significant change in rheotactic behaviour following the introduction of the clean plastic solution at any concentration (low concentration: 88.06 ± 0.92% positively rheotactic after treatment, t = 1.18, p = 0.25; medium concentration: 91.37 ± 0.64% positively rheotactic after treatment, t = 0.31, permutation test: p = 0.75; high concentration: 92.95 ± 0.45% positively rheotactic after treatment, t = 0.57, permutation test: p = 0.64; figure 3c). By contrast, there was a significant loss of rheotaxis after the introduction of the biofouled plastic solution in all but the lowest concentration (low concentration: 89.14 ± 1.06% positively rheotactic after treatment, t = 0.69, permutation test: p = 0.52; medium concentration: 84.80 ± 0.89% positively rheotactic after treatment, t = 2.60, permutation test: p = 0.03; high concentration: 80.58 ± 0.88% positively rheotactic after treatment, t = 9.67, permutation test: p < 0.001; figure 3d).
The response to food odour was identical to the clustering analysis of the food odour solutions; there was a significant change in rheotactic behaviour after the introduction of the food odour treatment at all concentrations (low concentration: 66.99 ± 1.34% positively rheotactic after treatment, t = 11.49, permutation test: p < 0.001; medium concentration: 64.71 ± 1.25% positively rheotactic after treatment, t = 11.63, permutation test: p < 0.001; high concentration: 66.13 ± 1.29% positively rheotactic after treatment, t = 16.35, permutation test: p < 0.001; figure 3e).
4. Discussion
These results demonstrate that odours associated with plastic debris stimulate a behavioural response consistent with foraging in captive anchovy schools. As when foraging, the anchovy responded to the medium and high concentrations of biofouled plastic odour with a temporally constrained spike in aggregation coupled with a significant reduction in rheotaxis (figures 1–3). Similar results were seen in response to all concentrations of food odour, thus indicating anchovy use odours to initiate foraging behaviours. To our knowledge, this is the first experimental demonstration that adult anchovy use odours in foraging contexts.
By contrast, anchovy schools did not change their aggregation or rheotactic behaviour after addition of the seawater control or clean plastic treatments at any concentration (figures 1–3). Furthermore, the lowest concentration of biofouled plastic odour had no effect on school clustering or rheotactic positioning. This likely indicates that the biofouled plastic odours were below the detection threshold at this concentration, but further tests are required to confirm this. Taken together, these findings suggest that chemical cues associated with biofouled plastic debris can trigger olfactory search in a marine forage fish. This is the first behavioural evidence that plastic debris may be chemically attractive to marine consumers. These chemical cues may lure consumers, such as anchovy, into regions of high plastic density and activate foraging behaviours, thus making it difficult to ignore or reject plastic items as potential prey.
DMS and DMSP are keystone infochemicals in marine ecosystems [35,36] and many organisms including seabirds and fish use these compounds to locate productive areas to forage [6,15,37]. Plastic debris acquires an ecologically relevant DMS signature after less than a month of exposure to the marine environment [12]. The methods and location used to biofoul plastic in this study were identical to those in [12]; as a result, it is plausible that detection of DMS/P was key in triggering the behavioural responses recorded in the biofouled plastic treatments. Further behavioural studies using isolates of DMS/P are needed to confirm this.
Results from this and previous work on olfactory foraging and dietary specialization [38] help explain why species dependent on primary consumers (e.g. krill) as a food source, are unable to reliably and consistently distinguish between plastic debris and food. It is therefore not surprising that species groups including zooplankton-reliant procellariiform seabirds, fish and sea turtles are both DMS/P-responsive [6,37,39] and frequent consumers of plastic debris. In addition, plastic debris has visual cues (e.g. its colour and shape) that may resemble prey [40]; visual and chemical cues associated with plastic debris likely interact synergistically, exacerbating this evolutionary trap. This multimodal sensory explanation elucidates why certain marine consumers repeatedly confuse plastic for food.
Plastic ingestion has been demonstrated to retard activity levels and negatively influence schooling behaviour in captive fish [26]. This reduced activity and schooling could cause fish that have ingested plastic to be more likely to be predated, thus catalysing the bioaccumulation of contaminants associated with plastic debris through the food web [41–43]. This presumption should be tested on forage fish, because they represent a critical trophic link in marine and coastal ecosystems. Humans are at the top of these food chains; therefore, results of such future studies may have important consequences for human health.
Despite a recent surge of legislative initiatives aimed at reducing plastic use and improper disposal, global plastic production has risen over 600% since 1975 and plastic waste entering the oceans from terrestrial sources is on pace to increase by an order of magnitude—to greater than 100 million tonnes annually—by 2025 [44]. Nearly 700 species have been found to consume plastic at sea [45]. Uncovering the mechanisms driving plastic consumption by marine fauna is urgently needed. Our study confirms that olfactory cues associated with plastic debris may lead marine consumers to mistake plastic for food. Species such as forage fish that specialize on primary consumers are especially at risk due, in part, to their possible behavioural attraction to DMS/P. The previously overlooked chemical signature of marine plastic debris should be considered when developing mitigation strategies to combat this global environmental issue.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We are greatly indebted to Eric Sandquist of J&P Bait in San Francisco for donating the anchovy used in this study. Also we thank the Aquarium of the Bay staff, especially Melissa Schouest and Kevin McEligot, for assistance with anchovy acquisition and husbandry. The rheotactic data were analysed by a team of dedicated video observers: Abigail Alfonso, Kerstin Ozkan, Lucia Yu, Anna Lu, Gabrielle Fuerst, Brandon Hong, Nicole Hage and Joyce Wong. We are also appreciative of Geoff Gregory, Pete Richards and Pranav Deshpande for logistical support. Finally, we are grateful to Gabrielle Nevitt for early discussions on the experimental design, Neil Willits for statistical consulting, and Rachel Anderson for editorial assistance.
Ethics
Fish were caught under commercial fishing licence from the State of California Department of Fish and Wildlife, no. L44917. Experimental procedures were approved by UC Davis’ IACUC, protocol no. 17920.
Data accessibility
The datasets and code supporting this article can be found at https://github.com/mssavoca/AnchovyBehaviorMS.
Authors' contribution
M.S.S. performed the study, analysed the data and drafted the manuscript. C.W.T. analysed the data and edited the manuscript. M.M. and C.J.S. acquired the study specimens, helped design the experiment and edited the manuscript.
Competing interests
We have no competing interests.
Funding
This work was funded by the National Science Foundation (GRF-1148897).
References
- 1.Strausfeld NJ, Hildebrand JG. 1999. Olfactory systems: common design, uncommon origins? Curr. Opin. Neurobiol. 9, 634–639. ( 10.1016/S0959-4388(99)00019-7) [DOI] [PubMed] [Google Scholar]
- 2.Ache BW, Young JM. 2005. Olfaction: diverse species, conserved principles. Neuron 48, 417–430. ( 10.1016/j.neuron.2005.10.022) [DOI] [PubMed] [Google Scholar]
- 3.Dixson DL, Munday PL, Jones GP. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75. ( 10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 4.Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V. 2007. Smelling home can prevent dispersal of reef fish larvae. Proc. Natl Acad. Sci. USA 104, 858–863. ( 10.1073/pnas.0606777104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munday PL, Dixson DL, Donelson JM, Jones GP, Pratchett MS, Devitsina GV, Døving KB. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852. ( 10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBose JL, Lema SC, Nevitt GA. 2008. Dimethylsulfoniopropionate as a foraging cue for reef fishes. Science 319, 1356 ( 10.1126/science.1151109) [DOI] [PubMed] [Google Scholar]
- 7.Dixson DL, Jennings AR, Atema J, Munday PL. 2014. Odor tracking in sharks is reduced under future ocean acidification conditions. Glob. Change Biol. 21, 1454–1462. ( 10.1111/gcb.12678) [DOI] [PubMed] [Google Scholar]
- 8.Ou M, et al. 2015. Responses of pink salmon to CO2-induced aquatic acidification. Nat. Clim. Change 5, 950–955. ( 10.1038/nclimate2694) [DOI] [Google Scholar]
- 9.Cózar A, et al. 2014. Plastic debris in the open ocean. Proc. Natl Acad. Sci. USA 111, 10 239–10 244. ( 10.1073/pnas.1314705111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksen M, Lebreton LCM, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J. 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9, e111913 ( 10.1371/journal.pone.0111913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobelle D, Cunliffe M. 2011. Early microbial biofilm formation on marine plastic debris. Mar. Pollut. Bull. 62, 197–200. ( 10.1016/j.marpolbul.2010.10.013) [DOI] [PubMed] [Google Scholar]
- 12.Savoca MS, Wohlfeil ME, Ebeler SE, Nevitt GA. 2016. Marine plastic debris emits a keystone infochemical for olfactory foraging seabirds. Sci. Adv. 2, e1600395 ( 10.1126/sciadv.1600395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gall SC, Thompson RC. 2015. The impact of debris on marine life. Mar. Pollut. Bull. 92, 170–179. ( 10.1016/j.marpolbul.2014.12.041) [DOI] [PubMed] [Google Scholar]
- 14.DeBose JL, Nevitt GA, Dittman AH. 2010. Rapid communication: experimental evidence that juvenile pelagic jacks (Carangidae) respond behaviorally to DMSP. J. Chem. Ecol. 36, 326–328. ( 10.1007/s10886-010-9755-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dove ADM. 2015. Foraging and ingestive behaviors of whale sharks, Rhincodon typus, in response to chemical stimulus cues. Biol. Bull. 228, 65–74. ( 10.1086/BBLv228n1p65) [DOI] [PubMed] [Google Scholar]
- 16.Ganias K. (ed.). 2014. Biology and ecology of sardines and anchovies. Boca Raton, FL: CRC Press. [Google Scholar]
- 17.Dempsey CH. 1978. Chemical stimuli as a factor in feeding and intraspecific behaviour of herring larvae. J. Mar. Biol. Assoc. UK 58, 739–747. ( 10.1017/S0025315400041400) [DOI] [Google Scholar]
- 18.Uyan S, Kawamura G, Vazquez Archdale M. 2006. Morphology of the sense organs of anchovy Engraulis japonicus. Fish. Sci. 72, 540–545. ( 10.1111/j.1444-2906.2006.01182.x) [DOI] [Google Scholar]
- 19.Ainley DG, Dugger KD, Ford RG, Pierce SD, Reese DC, Brodeur RD, Tynan CT, Barth JA. 2009. Association of predators and prey at frontal features in the California current: competition, facilitation, and co-occurrence. Mar. Ecol. Prog. Ser. 389, 271–294. ( 10.3354/meps08153) [DOI] [Google Scholar]
- 20.Szoboszlai AI, Thayer JA, Wood SA, Sydeman WJ, Koehn LE. 2015. Forage species in predator diets: synthesis of data from the California current. Ecol. Inform. 29, 45–56. ( 10.1016/j.ecoinf.2015.07.003) [DOI] [Google Scholar]
- 21.MacCall AD, Sydeman WJ, Davison PC, Thayer JA. 2016. Recent collapse of northern anchovy biomass off California. Fish. Res. 175, 87–94. ( 10.1016/j.fishres.2015.11.013) [DOI] [Google Scholar]
- 22.Lindegren M, Checkley DM, Rouyer T, MacCall AD, Stenseth NC. 2013. Climate, fishing, and fluctuations of sardine and anchovy in the California current. Proc. Natl Acad. Sci. USA 110, 13 672–13 677. ( 10.1073/pnas.1305733110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochman CM, Tahir A, Williams SL, Baxa DV, Lam R, Miller JT, Teh F-C, Werorilangi S, Teh SJ. 2015. Anthropogenic debris in seafood: plastic debris and fibers from textiles in fish and bivalves sold for human consumption. Sci. Rep. 5, 14340 ( 10.1038/srep14340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Takada H. 2016. Microplastic fragments and microbeads in digestive tracts of planktivorous fish from urban coastal waters. Sci. Rep. 6, 34351 ( 10.1038/srep34351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rochman CM, Hoh E, Kurobe T, Teh SJ. 2013. Ingested plastic transfers hazardous chemicals to fish and induces hepatic stress. Sci. Rep. 3, 1–7. ( 10.1038/srep03263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattsson K, Ekvall MT, Hansson LA, Linse S, Malmendal A, Cedervall T. 2015. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 49, 553–561. ( 10.1021/es5053655) [DOI] [PubMed] [Google Scholar]
- 27.Bacha M, Amara R. 2009. Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuar. Coast. Shelf Sci. 85, 257–264. ( 10.1016/j.ecss.2009.08.009) [DOI] [Google Scholar]
- 28.James A, Findlay K. 1989. Effect of particle size and concentration on feeding behaviour, selectivity and rates of food ingestion by the Cape anchovy Engraulis capensis. Mar. Ecol. Prog. Ser. 50, 275–294. ( 10.3354/meps050275) [DOI] [Google Scholar]
- 29.Garrido S, Lingen C. 2014. Feeding biology and ecology. In Biology and ecology of sardines and anchovies (ed. Ganias K.), pp. 123–189. Boca Raton, FL: CRC Press. [Google Scholar]
- 30.Ioakeimidis C, Galgani F, Papatheodorou G. 2017. Occurrence of marine litter in the marine environment: a world panorama of floating and seafloor plastics. In The handbook of environmental chemistry (eds Takada H, Karapanagioti HK), pp. 1–28. Berlin, Germany: Springer International Publishing. [Google Scholar]
- 31.Montgomery JC, Baker CF, Carton AG. 1997. The lateral line can mediate rheotaxis in fish. Nature 389, 960–963. ( 10.1038/40135) [DOI] [Google Scholar]
- 32.Bak-Coleman J, Court A, Paley DA, Coombs S. 2013. The spatiotemporal dynamics of rheotactic behavior depends on flow speed and available sensory information. J. Exp. Biol. 216, 4011–4024. ( 10.1242/jeb.090480) [DOI] [PubMed] [Google Scholar]
- 33.Moran PAP. 1950. Notes on continuous stochastic phenomena. Biometrika 37, 17–23. ( 10.1093/biomet/37.1-2.17) [DOI] [PubMed] [Google Scholar]
- 34.Moss SA, McFarland WN. 1970. The influence of dissolved oxygen and carbon dioxide on fish schooling behavior. Mar. Biol. 5, 100–107. ( 10.1007/BF00352592) [DOI] [Google Scholar]
- 35.Ferrer RP, Zimmer RK. 2012. Community ecology and the evolution of molecules of keystone significance. Biol. Bull. 223, 167–177. ( 10.1086/BBLv223n2p167) [DOI] [PubMed] [Google Scholar]
- 36.Ferrer RP, Zimmer RK. 2013. Molecules of keystone significance: crucial agents in ecology and resource management. Bioscience 63, 428–438. ( 10.1525/bio.2013.63.6.5) [DOI] [Google Scholar]
- 37.Nevitt GA, Veit RR, Kareiva P. 1995. Dimethyl sulphide as a foraging cue for Antarctic procellariiform seabirds. Nature 376, 680–682. ( 10.1038/376680ao) [DOI] [Google Scholar]
- 38.Savoca MS, Nevitt GA. 2014. Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary producers and top predators. Proc. Natl Acad. Sci. USA 111, 4157–4161. ( 10.1073/pnas.1317120111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endres CS, Lohmann KJ. 2012. Perception of dimethyl sulfide (DMS) by loggerhead sea turtles: a possible mechanism for locating high-productivity oceanic regions for foraging. J. Exp. Biol. 215, 3535–3538. ( 10.1242/jeb.073221) [DOI] [PubMed] [Google Scholar]
- 40.Carson HS. 2013. The incidence of plastic ingestion by fishes: from the prey's perspective. Mar. Pollut. Bull. 74, 170–174. ( 10.1016/j.marpolbul.2013.07.008) [DOI] [PubMed] [Google Scholar]
- 41.Rochman CM, Hentschel BT, Teh SJ. 2014. Long-term sorption of metals is similar among plastic types: implications for plastic debris in aquatic environments. PLoS ONE 9, e85433 ( 10.1371/journal.pone.0085433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teuten EL, et al. 2009. Transport and release of chemicals from plastics to the environment and to wildlife. Phil. Trans. R. Soc. B 364, 2027–2045. ( 10.1098/rstb.2008.0284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rochman CM, Hoh E, Hentschel BT, Kaye S. 2013. Long-term field measurement of sorption of organic contaminants to five types of plastic pellets: implications for plastic marine debris. Environ. Sci. Technol. 47, 1646–1654. ( 10.1021/es303700s) [DOI] [PubMed] [Google Scholar]
- 44.Jambeck JR, Geyer R, Wilcox C, Siegler TR, Perryman M, Andrady A, Narayan R, Law KL. 2015. Plastic waste inputs from land into the ocean. Science 347, 768–771. ( 10.1126/science.1260352) [DOI] [PubMed] [Google Scholar]
- 45.Provencher J, et al. 2016. Quantifying ingested debris in marine megafauna: a review and recommendations for standardization. Anal. Methods 9, 1454–1469. ( 10.1039/C6AY02419J) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and code supporting this article can be found at https://github.com/mssavoca/AnchovyBehaviorMS.