Abstract
Inbreeding depression refers to the reduction of fitness that results from matings between relatives. Evidence for reduced fitness in inbred individuals is widespread, but the strength of inbreeding depression varies widely both within and among taxa. Environmental conditions can mediate this variation in the strength of inbreeding depression, with environmental stress exacerbating the negative consequences of inbreeding. Parents can modify the environment experienced by offspring, and have thus the potential to mitigate the negative consequences of inbreeding. While such parental effects have recently been demonstrated during the postnatal period, the role of prenatal parental effects in influencing the expression of inbreeding depression remains unexplored. To address this gap, we performed matings between full-sibs or unrelated individuals in replicated lines of Japanese quail (Coturnix japonica) experimentally selected for high and low maternal egg provisioning. We show that in the low maternal investment lines hatching success was strongly reduced when parents were related. In the high maternal investment lines, however, this negative effect of inbreeding on hatching success was absent, demonstrating that prenatal maternal provisioning can alleviate the negative fitness consequences of inbreeding.
Keywords: inbreeding depression, maternal effects, parental care, maternal rescue, prenatal maternal investment, environmental stress
1. Introduction
Inbreeding depression occurs when matings between relatives result in decreased offspring fitness. This reduction in fitness is likely due to an increase in homozygosity that exposes deleterious recessive alleles to selection [1]. This phenomenon has been observed across many taxa [2,3], but the degree to which an individual experiences decreased fitness at a given level of inbreeding varies between species and populations.
Some of this variation is explained by differences in genetic load, the reduction in the mean fitness of a population from that of a theoretically optimal genotype [1,3]. However, there is increasing evidence that environmental conditions can also influence the degree of inbreeding depression experienced by an individual [4–6]. In a benign environment, the deleterious effects of inbreeding may not be expressed, but when exposed to environmental stressors such as heat, drought or food limitation inbreeding depression can increase with the magnitude of the stressor [2,7–9].
The environment an individual experiences during the first stages of life is provided by the parents in most taxa, and this early life environment can have long-lasting effects on offspring phenotype and fitness [10,11]. At the same time, inbreeding depression is particularly strong during early life stages [12]. Parents thus have the potential to mitigate the negative consequences of inbreeding by increasing their investment in parental care, and thereby providing a more favourable early life environment for the offspring [13,14]. In line with this idea, a recent study in burying beetles (Nicrophorus vespilloides) showed that postnatal parental care can buffer the negative effects of inbreeding [15].
However, parents influence not only the offspring's postnatal environment but also the conditions experienced before birth. This prenatal environment is provided by the mother in most taxa. While it is well documented that inbreeding negatively affects early development and hatching success [16–19], the role of the prenatal environment in influencing the expression of inbreeding depression has not been experimentally tested.
To address this gap, we performed experimental matings between full-sibs and unrelated individuals in replicated lines of Japanese quail (Coturnix japonica) experimentally selected for high and low maternal egg provisioning (high and low maternal investment lines). This 2 × 2 design allowed us to test experimentally if prenatal maternal provisioning can buffer the negative effects of inbreeding on hatching success. We predict that if mothers can mitigate the negative consequences of inbreeding by providing a favourable prenatal environment for their offspring, inbreeding depression will be pronounced in the low maternal investment lines but absent, or strongly reduced, in the maternal high investment lines.
2. Material and methods
(a). Artificial selection lines for divergent maternal egg provisioning
We established replicated selection lines for high and low maternal egg provisioning in a population of Japanese quail (Coturnix japonica) maintained at the University of Zurich, Switzerland [20]. The founder population for this study consisted of 91 females and 98 males. It was obtained from a commercial quail egg farm located in the southeast of Switzerland, where birds from two different origins were maintained in two separate populations. These populations had been maintained since 1998 at the farm before our selection experiment began in 2012, and no (intentional) artificial selection had been imposed on the birds during this time. Although no pedigree was available for the founders, large populations were maintained on the farm and efforts were made to avoid inbreeding. To further increase genetic diversity in our study population, we crossed birds from the two origins and used these crosses as the starting population for the selection experiment (see [20] for more details).
In the first generation of the selection experiment, eggs from the 25% of females producing the largest and smallest eggs relative to their body size were incubated to create the high and low investment lines, respectively. In subsequent generations, we selected the most extreme 50% of females within each line. We repeated this procedure with two independent starting populations to create two independent replicates per line [20]. During the selection procedure, matings between relatives were prevented and as a result the inbreeding coefficient (f) of the parental generation used in this experiment (see below) was low (less than 0.058, based on six generations of complete pedigree data).
We observed a strong response to selection on egg size, as well as a positively correlated response in dried egg components (i.e. fat and protein), but not in the number of eggs laid [20]. The lack of an egg size/number trade-off was surprising, but appears to be not uncommon (reviewed and discussed in [20]), and we are currently exploring alternative costs associated with increased maternal offspring provisioning in our population.
Forty males and 40 females from the sixth generation of these divergently selected lines were used for this experiment (mean egg mass (mean ± s.d.) of females from the high investment lines: 12.391 ± 0.892 g; mean egg mass of females from the low investment lines: 11.390 ± 0.698 g (line: F1,37 = 15.473, p < 0.001; inbreeding status: F1,37 = 0.599, p = 0.444; line × inbreeding status: F1,36 = 0.156, p = 0.695; N = 40)). Females were kept separately from males before the experiment to ensure that they had not mated before.
(b). Experimental inbreeding
Individuals from the high and low investment lines were assigned to breed either with a full sibling (inbreeding) or an unrelated partner from the same line replicate (outbreeding), resulting in 40 breeding pairs that were paired up simultaneously: 10 high investment line inbreeding (HI) pairs, 10 high investment line outbreeding (HO) pairs, 10 low investment line inbreeding (LI) pairs and 10 low investment line outbreeding (LO) pairs. We measured the birds' body size (i.e. tarsus length) at the beginning of the breeding experiment to the nearest 0.1 mm. There was a significant difference in body size between females from the H and L lines (F1,37 = 10.997, p = 0.002; see also [20]), but not between females that were paired to a related or unrelated partner (F1,37 = 0.002, p = 0.968; interaction line × inbreeding status: F1,36 = 3.070, p = 0.088). To control for these line differences in body size, female tarsus length was included as a covariate in the statistical analyses (see below).
All birds received ad libitum food, water and grit. Breeding cages (122 × 50 × 50 cm) were lined with sawdust, and contained a house and a sand bath. The facility was maintained on a 16 L : 8 D cycle and at a temperature of approximately 20°C. Eggs were collected over a period of 15 days. During this entire period, breeding pairs were housed together in the breeding cages. Males and females were in breeding condition when entering the cages and all couples copulated immediately after being released into the cages.
We calculated the inbreeding coefficient (f) for the offspring of all these pairings: offspring produced by outbreeding pairs had an inbreeding coefficient 0.002 < f < 0.02, while those produced by inbreeding pairs had an f ≥ 0.25.
(c). Hatching success
Eggs were collected daily between 08.00 and 11.00 h, weighed to the nearest 0.01 g and stored for up to 5 days at 12°C until incubation. Incubation occurred in three batches (batch 1: eggs from day 1 to 5, batch 2: eggs from day 6 to 10, batch 3: eggs from day 11 to 15) at 37.8°C and 55% humidity for 14 days (Favorit, HEKA Brutgeräte, Rietberg). Eggs were then transferred to individual compartments in a hatcher (Favorit, HEKA Brutgeräte, Rietberg), and kept at 37.6°C and 80% humidity until hatching [20]. Eggs that did not hatch after 18 days of incubation were classified as ‘did not hatch’ [20]. Eggs of all treatment groups were treated in the same way and there was no significant effect of inbreeding status (χ2 = 0.030, p = 0.862), line (χ2 = 0.190, p = 0.663) or their interaction (χ2 = 1.958, p = 0.162) on the number of eggs laid (i.e. incubated) (number of eggs incubated per breeding pair: 1–16; total number of eggs incubated: N = 526).
(d). Statistical analysis
The probability of hatching (hereafter referred to as ‘hatching success’) was analysed on the level of the breeding pair using a generalized linear model with a binomial error structure and a logit-link function. In a first model, we included selection line, inbreeding status and their interaction as fixed effects, and maternal tarsus length as a covariate. In a second model (same as above), we replaced selection line with a female's mean egg mass (in grams) to provide further evidence that the line effects observed in the first model are mediated by differences in maternal egg provisioning. To infer significance, we compared two nested models, with and without the variable of interest, using likelihood ratio tests (all d.f. = 1; N = 40 breeding pairs). Data were analysed using the lme4 [21] and multcomp [22] packages in R v. 3.21 (R Development Core Team 2015).
3. Results
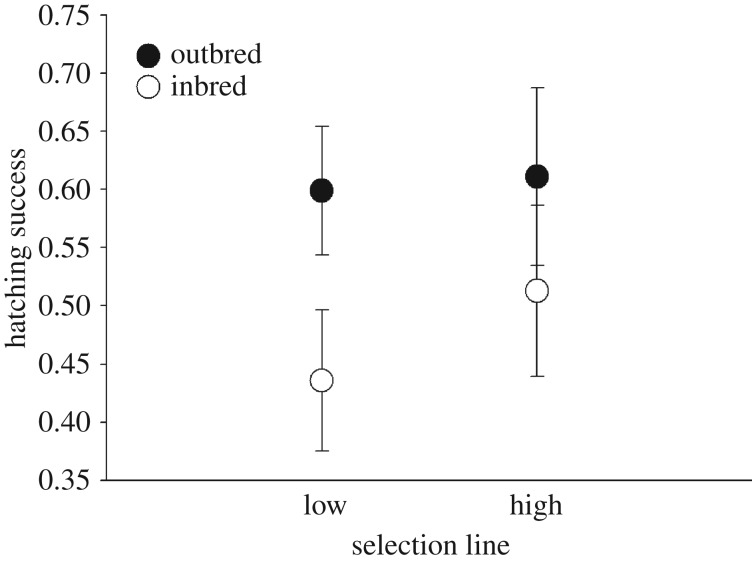
Hatching success was influenced by a significant interaction effect between selection line and inbreeding treatment (χ2 = 5.355, p = 0.021; figure 1, table 1a for full model output). Post hoc contrasts revealed that in the low maternal investment lines, hatching success was significantly lower when parents were related (Tukey's HSD test; LO versus LI: z = 4.237, p < 0.001, figure 1). By contrast, in the high investment lines the hatching success of eggs from related parents was not significantly different from the hatching success of eggs from unrelated parents (HO versus HI: z = 1.041, p = 0.724, figure 1). Furthermore, the hatching success of eggs from related or unrelated parents from the high investment lines did not differ significantly from hatching success of eggs from unrelated parents from the low investment lines (LO versus HI: z = 1.297, p = 0.564; LO versus HO: z = 0.357, p = 0.984, figure 1).
Figure 1.
Hatching success of eggs from inbreeding and outbreeding parents in the high and low maternal investment lines. Plotted values are means ± s.e. of the proportion of eggs hatched per breeding pair. Inbreeding significantly reduces hatching success in the low investment lines but not in the high investment lines.
Table 1.
Effects of the inbreeding status of the parents (inbreeding versus outbreeding) and prenatal maternal provisioning on hatching success: (a) including selection line as a measure of prenatal maternal provisioning, (b) including egg mass (grams) as a measure of prenatal maternal provisioning.
| χ2 | p-value | |
|---|---|---|
| (a) hatching success | ||
| inbreeding status | 14.976 | <0.001 |
| selection line | 2.125 | 0.145 |
| selection line × inbreeding status | 5.355 | 0.021 |
| maternal tarsus length | 3.395 | 0.065 |
| (b) hatching success | ||
| inbreeding status | 13.681 | <0.001 |
| egg mass | 2.439 | 0.118 |
| egg mass × inbreeding status | 15.539 | <0.001 |
| maternal tarsus length | 3.681 | 0.055 |
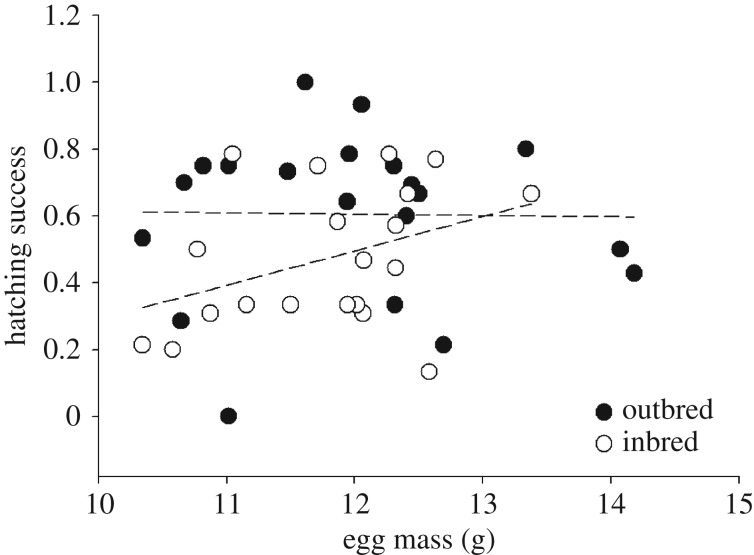
To confirm that these line-specific effects of inbreeding on hatching success are mediated by egg size, we ran a second model in which we replaced selection line with mean maternal egg mass as a predictor. Again, we found that the interaction effect between inbreeding treatment and egg mass significantly affected hatching success (χ2 = 15.539, p < 0.001; figure 2; table 1b for full model output). Larger eggs from an inbreeding pair were more likely to hatch than smaller eggs, whereas no relationship between egg size and hatching success was found in outbreeding pairs (figure 2). In both models, there was a trend for a negative relationship between a female's body size and the hatching success of her eggs (table 1a,b).
Figure 2.
Relationship between hatching success and egg mass in inbreeding and outbreeding pairs. The proportion of eggs hatched per breeding pair are plotted. When parents are related large eggs are more likely to hatch than small eggs (open dots), but when parents are unrelated egg size does not impact hatching success (filled dots).
4. Discussion
We show that favourable prenatal conditions can buffer the negative effects of inbreeding on hatching success. Inbreeding strongly reduced hatching success when offspring developed in a small, nutrient poor egg (i.e. under harsh prenatal conditions), but this inbreeding effect was absent when offspring developed in a large, nutrient rich egg (i.e. under benign prenatal conditions). This demonstrates that the prenatal environment affects the expression of inbreeding depression, and that mothers can mitigate the negative consequences of inbreeding by increasing their prenatal provisioning.
There is widespread and increasing evidence for environmental mediation of inbreeding depression [5,7,9,23,24]. However, despite the importance of parents in shaping the early environment experienced by an individual, the role of parental care in modulating the expression of inbreeding depression has received little attention to date. An exception is a pair of recent studies in burying beetles that provide support for ‘parental rescue’ from inbreeding depression during the postnatal period [15,25]. Burying beetle parents provide food to the larvae, but this parental provisioning is facultative. Pilakouta et al. [15] set up experimental matings between siblings and unrelated individuals, and removed the care-providing mother before larval hatching from half of the broods. They found that inbred offspring without a mother present suffered a greater decline in fitness-related traits than did those with an attendant mother [15]. A subsequent study revealed that maternal quality can also impact the expression of inbreeding depression, with offspring of large mothers experiencing less inbreeding depression than offspring of small mothers [25]. However, a similar study in another care-giving insect, the European earwig (Forficula auricularia), failed to find evidence that postnatal parental care alleviates the negative consequences of inbreeding [26].
While there is mixed empirical evidence for a role of parental care during the postnatal period in shaping the consequences of inbreeding (see above), the role of care provided before birth, and in particular of prenatal maternal resource provisioning, has not been experimentally tested.
It is well documented that prenatal care has positive effects on offspring fitness [27–29]. Chicks developing in larger, more nutrient rich eggs are, for example, heavier, grow faster and are more likely to survive [20,29,30]. Prenatal parental provisioning is also known to mitigate the negative effects of a harsh postnatal environment on offspring fitness. For example, large amphibian eggs increase juvenile survival in harsh environments [31], and nestlings raised under limited food conditions reach a similar fledging mass as food-supplemented nestlings if their mother had received extra food during egg laying [32]. Finally, prenatal maternal provisioning has been hypothesized to alleviate genetic disadvantages, as when female house finches (Haemorhous mexicanus) paired with low quality mates increase the deposition of androgens to their eggs [33]. Our results are in line with these previous findings and provide the first experimental evidence that mothers can reduce the negative fitness consequences of inbreeding for offspring by increasing their resource provisioning before birth. It implies that population structure, and thus the likelihood of mating with a relative, may shape the evolution of parental care, in general, and the evolution of prenatal maternal provisioning in particular (see also [34]). Selection for increased parental provisioning might be particularly strong in small and isolated populations, in which inbreeding is common [34], but weaker in large populations where outbreeding is the norm. Population structure might, therefore, contribute to the maintenance of variation in parental provisioning observed across populations [35,36].
Egg size has a strong heritable component and has been shown to respond rapidly to selection [20,37]. In addition, there is evidence for a substantial non-genetic effect of maternal egg size on the egg size of the next generation (i.e. a cascading maternal effect, JL Pick, E Postma, B Tschirren 2017, unpublished data) that further accelerates the response to selection on prenatal maternal provisioning. This positive feedback loop will allow for a fast response in prenatal provisioning to changing environmental conditions, which may buffer the next generation from the negative impact of environmental or genetic stressors [38].
In addition, our results suggest that plastic changes in prenatal maternal provisioning in response to the relatedness of the partner may be adaptive. On the one hand, we may predict increased prenatal maternal provisioning when a female is breeding with a relative in order to alleviate the negative consequences of inbreeding for the offspring. On the other hand, reduced prenatal maternal provisioning may be predicted when the risk of inbreeding is high. Indeed, the higher susceptibility of inbred offspring to harsh prenatal conditions may provide females (which mate with multiple partners) with a post-zygotic inbreeding avoidance opportunity and prevent females from wasting postnatal investment in unfit offspring. To our knowledge, no data on the plastic change of egg size in response to the relatedness of the partner are currently available from natural populations, but testing for evidence for these different scenarios would clearly be a fruitful next step.
In conclusion, we provide the first experimental evidence that prenatal maternal provisioning can alleviate the negative consequences of inbreeding. Our results, along with those of Pilakouta and co-workers [15,25], demonstrate that parental buffering of inbreeding depression may be widespread and suggest that the risk of inbreeding may shape the evolution of parental care.
Acknowledgements
We thank the quail team for help with animal husbandry and two anonymous reviewers for constructive comments on the manuscript.
Ethics
All procedures conform to the relevant regulatory standards and were conducted under licences provided by the Veterinary Office of the Canton of Zurich, Zurich, Switzerland (195/2010; 14/2014; 156).
Data accessibility
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.kk4qn [39].
Authors' contributions
B.T. designed the study. K.E.I., P.H. and B.T. collected data. K.E.I. and B.T. performed statistical analyses and drafted the manuscript. All authors commented on the manuscript.
Competing interests
We have no competing interests.
Funding
The study was financially supported by Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (PP00P3_128386 and PP00P3_157455 to B.T.) and the Faculty of Science of the University of Zurich.
References
- 1.Charlesworth D, Willis JH. 2009. The genetics of inbreeding depression. Nat. Rev. Genet. 10, 783–796. ( 10.1038/nrg2664) [DOI] [PubMed] [Google Scholar]
- 2.Fox CW, Reed DH. 2011. Inbreeding depression increases with environmental stress: an experimental study and meta-analysis. Evolution 65, 246–258. ( 10.1111/j.1558-5646.2010.01108.x) [DOI] [PubMed] [Google Scholar]
- 3.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 4.Crnokrak P, Roff DA. 1999. Inbreeding depression in the wild. Heredity 83, 260–270. ( 10.1038/sj.hdy.6885530) [DOI] [PubMed] [Google Scholar]
- 5.Armbruster P, Reed DH. 2005. Inbreeding depression in benign and stressful environments. Heredity 95, 235–242. ( 10.1038/sj.hdy.6800721) [DOI] [PubMed] [Google Scholar]
- 6.Fox CW, Stillwell RC, Wallin WG, Curtis CL, Reed DH. 2011. Inbreeding–environment interactions for fitness: complex relationships between inbreeding depression and temperature stress in a seed-feeding beetle. Evol. Ecol. 25, 25–43. ( 10.1007/s10682-010-9376-3) [DOI] [Google Scholar]
- 7.Marr AB, Arcese P, Hochachka WM, Reid JM, Keller LF. 2006. Interactive effects of environmental stress and inbreeding on reproductive traits in a wild bird population. J. Anim. Ecol. 75, 1406–1415. ( 10.1111/j.1365-2656.2006.01165.x) [DOI] [PubMed] [Google Scholar]
- 8.Szulkin M, Sheldon BC. 2007. The environmental dependence of inbreeding depression in a wild bird population. PLoS ONE 2, e1027 ( 10.1371/journal.pone.0001027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Boer RA, Eens M, Fransen E, Müller W. 2015. Hatching asynchrony aggravates inbreeding depression in a songbird (Serinus canaria): an inbreeding–environment interaction. Evolution 69, 1063–1068. ( 10.1111/evo.12625) [DOI] [PubMed] [Google Scholar]
- 10.Williams TD. 1994. Intraspecific variation in egg size and egg composition in birds—effects on offspring fitness. Biol. Rev. 69, 35–59. ( 10.1111/j.1469-185X.1994.tb01485.x) [DOI] [PubMed] [Google Scholar]
- 11.Lindström J. 1999. Early development and fitness in birds and mammals. Trends Ecol. Evol. 14, 343–348. ( 10.1016/S0169-5347(99)01639-0) [DOI] [PubMed] [Google Scholar]
- 12.Hemmings NL, Slate J, Birkhead TR. 2012. Inbreeding causes early death in a passerine bird. Nat. Comm. 3, 1770 ( 10.1038/ncomms1870) [DOI] [PubMed] [Google Scholar]
- 13.Aviles L, Bukowski TC. 2006. Group living and inbreeding depression in a subsocial spider. Proc. R. Soc. B 273, 157–163. ( 10.1098/rspb.2005.3308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alonso-Alvarez C, Velando A. 2012. Benefits and costs of parental care. In The evolution of parental care (eds Royle NJ, Smiseth PT, Kölliker M), pp. 40–61. Oxford, UK: Oxford University Press. [Google Scholar]
- 15.Pilakouta N, Jamieson S, Moorad JA, Smiseth PT. 2015. Parental care buffers against inbreeding depression in burying beetles. Proc. Natl Acad. Sci. USA 112, 8031–8035. ( 10.1073/pnas.1500658112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spottiswoode C, Møller AP. 2004. Genetic similarity and hatching success in birds. Proc. R. Soc. Lond. B 271, 267–272. ( 10.1098/rspb.2003.2605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller LF. 1998. Inbreeding and its fitness effects in an insular population of song sparrows (Melospiza melodia). Evolution 52, 240–250. ( 10.1111/j.1558-5646.1998.tb05157.x) [DOI] [PubMed] [Google Scholar]
- 18.Reid JM, Arcese P, Keller LF. 2003. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. R. Soc. Lond. B 270, 2151–2157. ( 10.1098/rspb.2003.2480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson DS, Komdeur J, Burke T. 2004. Inbreeding in the Seychelles warbler: environment-dependent maternal effects. Evolution 58, 2037–2048. ( 10.1111/j.0014-3820.2004.tb00488.x) [DOI] [PubMed] [Google Scholar]
- 20.Pick JL, Hutter P, Tschirren B. 2016. In search of genetic constraints limiting the evolution of egg size: direct and correlated responses to artificial selection on a prenatal maternal effector. Heredity 116, 542–549. ( 10.1038/hdy.2016.16) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bates D, Mächler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 22.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 23.Liao W, Reed DH. 2009. Inbreeding–environment interactions increase extinction risk. Anim. Cons. 12, 54–61. ( 10.1111/j.1469-1795.2008.00220.x) [DOI] [Google Scholar]
- 24.Cheptou PO, Donohue K. 2011. Environment-dependent inbreeding depression: its ecological and evolutionary significance. New Phytol. 189, 395–407. ( 10.1111/j.1469-8137.2010.03541.x) [DOI] [PubMed] [Google Scholar]
- 25.Pilakouta N, Smiseth PT. 2016. Maternal effects alter the severity of inbreeding depression in the offspring. Proc. R. Soc. B 283, 20161023 ( 10.1098/rspb.2016.1023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meunier J, Kölliker M. 2013. Inbreeding depression in an insect with maternal care: influences of family interactions, life stage and offspring sex. J. Evol. Biol. 26, 2209–2220. ( 10.1111/jeb.12217) [DOI] [PubMed] [Google Scholar]
- 27.Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352. ( 10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 28.Fleming IA. 1996. Reproductive strategies of Atlantic salmon: ecology and evolution. Rev. Fish Biol. Fish. 6, 379–416. ( 10.1007/BF00164323) [DOI] [Google Scholar]
- 29.Krist M. 2011. Egg size and offspring quality: a meta-analysis in birds. Biol. Rev. 86, 692–716. ( 10.1111/j.1469-185X.2010.00166.x) [DOI] [PubMed] [Google Scholar]
- 30.Bolton M. 1991. Determinants of chick survival in the lesser black-backed gull—relative contributions of egg size and parental quality. J. Anim. Ecol. 60, 949–960. ( 10.2307/5424) [DOI] [Google Scholar]
- 31.Räsänen K, Laurila A, Merilä J. 2005. Maternal investment in egg size: environment- and population-specific effects on offspring performance. Oecologia 142, 546–553. ( 10.1007/s00442-004-1762-5) [DOI] [PubMed] [Google Scholar]
- 32.Giordano M, Groothuis TGG, Tschirren B. 2014. Interactions between prenatal maternal effects and posthatching conditions in a wild bird population. Behav. Ecol. 25, 1459–1466. ( 10.1093/beheco/aru149) [DOI] [Google Scholar]
- 33.Navara KJ, Hill GE, Mendonça MT. 2006. Yolk androgen deposition as a compensatory strategy. Behav. Ecol. Sociobiol. 60, 392–398. ( 10.1007/s00265-006-0177-1) [DOI] [Google Scholar]
- 34.Duthie AB, Lee AM, Reid JM. 2016. Inbreeding parents should invest more resources in fewer offspring. Proc. R. Soc. B 283, 20161845 ( 10.1098/rspb.2016.184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christians JK. 2002. Avian egg size: variation within species and inflexibility within individuals. Biol. Rev. 77, 1–26. ( 10.1017/S1464793101005784) [DOI] [PubMed] [Google Scholar]
- 36.Martin TE, Bassar RD, Bassar SK, Fontaine JJ, Lloyd P, Mathewson HA, Niklison AM, Chalfoun A. 2006. Life-history and ecological correlates of geographic variation in egg and clutch mass among passerine species. Evolution 60, 390–398. ( 10.1111/j.0014-3820.2006.tb01115.x) [DOI] [PubMed] [Google Scholar]
- 37.Heath DD, Heath JW, Bryden CA, Johnson RM, Fox CW. 2003. Rapid evolution of egg size in captive salmon. Science 299, 1738–1740. ( 10.1126/science.1079707) [DOI] [PubMed] [Google Scholar]
- 38.Lehtonen J, Kokko H. 2012. Positive feedback and alternative stable states in inbreeding, cooperation, sex roles and other evolutionary processes. Phil. Trans. R. Soc. B 367, 211–221. ( 10.1098/rstb.2011.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ihle KE, Hutter P, Tschirren B. 2017. Data from: Increased prenatal maternal investment reduces inbreeding depression in offspring. Dryad Digital Repository. ( 10.5061/dryad.kk4qn) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Ihle KE, Hutter P, Tschirren B. 2017. Data from: Increased prenatal maternal investment reduces inbreeding depression in offspring. Dryad Digital Repository. ( 10.5061/dryad.kk4qn) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Data are available from Dryad: http://dx.doi.org/10.5061/dryad.kk4qn [39].