Abstract
Chronic exposure to opioids induces adaptation of glutamate neurotransmission, which plays a crucial role in addiction. Our previous studies revealed that agmatine attenuates opioid addiction and prevents the adaptation of glutamate neurotransmission in the nucleus accumbens of chronic morphine-treated rats. The hippocampus is important for drug addiction; however, whether adaptation of glutamate neurotransmission is modulated by agmatine in the hippocampus remains unknown. Here, we found that continuous pretreatment of rats with ascending doses of morphine for 5 days resulted in an increase in the hippocampal extracellular glutamate level induced by naloxone (2 mg/kg, i.p.) precipitation. Agmatine (20 mg/kg, s.c.) administered concurrently with morphine for 5 days attenuated the elevation of extracellular glutamate levels induced by naloxone precipitation. Furthermore, in the hippocampal synaptosome model, agmatine decreased the release and increased the uptake of glutamate in synaptosomes from chronic morphine-treated rats, which might contribute to the reduced elevation of glutamate levels induced by agmatine. We also found that expression of the hippocampal NR2B subunit, rather than the NR1 subunit, of N-methyl-D-aspartate receptors (NMDARs) was down-regulated after chronic morphine treatment, and agmatine inhibited this reduction. Taken together, agmatine prevented the adaptation of the hippocampal glutamate system caused by chronic exposure to morphine, including modulating extracellular glutamate concentration and NMDAR expression, which might be one of the mechanisms underlying the attenuation of opioid addiction by agmatine.
Keywords: Agmatine, Opioid addiction, Hippocampus, Extracellular glutamate, NMDA receptors
Introduction
The development of a persistent addicted state is due to adaptive changes in the brain upon chronic exposure to drugs of abuse such as heroin, cocaine, methamphetamine, and alcohol. These changes can lead to altered behavior, become a driving force for continued drug-seeking [1–3], and result in a debilitating chronic relapsing disorder. Drug addiction is a serious socio-economic problem directly affecting an estimated 8.3% of the USA population aged 12 years or older [4], with an impact on the user’s family and friends.
Glutamate is the major excitatory neurotransmitter in the mammalian brain and accounts for ~70% of synaptic transmission in the central nervous system [5]. Glutamate and its receptors play important roles in controlling compulsive and uncontrolled drug-use and the high incidence of relapse [6–8]. Opioid addiction has been reported not only to change basal glutamate levels and the presynaptic release of glutamate in various brain regions, but also to alter the expression of glutamate receptors, particularly N-methyl-D-aspartate receptors (NMDARs) in the nucleus accumbens, ventral tegmental area, basolateral amygdala, prefrontal cortex, and hippocampus [9–11]. The hippocampus, which is the center for learning and memory, plays critical roles in the processes by which chronic morphine leads to addictive behaviors, cognitive impairment, and relapse [12–14]. Acute and chronic administration of morphine decreases the extracellular glutamate levels in the CA1 area of the hippocampus [15, 16]. In addition, it has been postulated that one of the neural mechanisms underlying the high rate of relapse is the sudden increase in extracellular glutamate in the hippocampus with exposure to drugs, stress, and conditioned cues. This sudden glutamate increase activates glutamatergic neurons in the hippocampus, which may lead to an increase of dopaminergic neuronal activity in the ventral tegmental area [17]. Some evidence indicates that morphine sensitization alters glutamate receptors in the dorsal hippocampus [18, 19]. So the hippocampus plays a critical role in drug addiction and may also be a crucial region for understanding the relationship between glutamate plasticity and opioid addiction.
Agmatine is endogenously synthesized by the decarboxylation of L-arginine in mammals, and is a putative neurotransmitter and/or neuromodulator [20]. Agmatine is widely distributed in the central nervous system, and has various biological actions [21, 22] including activating imidazoline receptors, blocking NMDARs, and inhibiting nitric oxide synthase activity [21]. In the past decade, a growing body of evidence from our laboratory and others has shown that systemic administration of agmatine enhances opioid analgesia but attenuates opioid-induced tolerance and addiction [23–27]. Moreover, agmatine modulates the adaptation of glutamate transmission in the nucleus accumbens in chronic morphine-treated rats [28]. Thus, there has been growing interest in understanding the relationship between the changes in the glutamate system in other brain regions, such as the hippocampus, and the modulation of opioid addiction by agmatine.
The present study was designed to investigate the effect of agmatine on glutamate transmission in the hippocampus of rats chronically exposed to morphine.
Materials and Methods
Animals
All experiments were carried out in accord with the National Institute of Health Guide for the Care and Use of Laboratory Animals (Publication No. 41-48, revised in 1996) and were approved by the Animal Welfare and Ethics Committee of Beijing Institute of Pharmacology and Toxicology. Efforts were made to minimize the number of animals used and their suffering.
Male Wistar rats (220–250 g) were obtained from the Jingfeng Medical Laboratory Animal Center, China. All rats were housed for at least 1 week before surgery and were maintained in a 12-h light:12-h dark environment (lights on 08:00–20:00) at 24 ± 0.5 °C and 40–50% humidity. The animals were provided free access to food and water.
Drug Administration, In Vivo Microdialysis, and High-Performance Liquid Chromatography (HPLC)/Electrochemical Detection (ECD) Assays
In vivo Microdialysis
The chronic morphine-treated rat model was established and microdialysis was performed as described previously [28]. Briefly, the model was established by subcutaneous (s.c.) injection of morphine (Qinhai Pharmaceutical Factory, Xining, China) twice daily for 5 consecutive days. The doses of morphine were 10 mg/kg on day 1, 20 mg/kg on day 2, 30 mg/kg on day 3, 40 mg/kg on day 4, and 50 mg/kg on day 5. The control group received saline injection according to the same schedule. Agmatine (20 mg/kg, s.c.; Sigma-Aldrich, St. Louis, MO) was injected 30 min before morphine administration. The rats were given naloxone [2 mg/kg, intraperitoneal (i.p.), Sigma-Aldrich] 6 h after the last morphine injection.
A CMA/12 microdialysis guide cannula (CMA Microdialysis, Stockholm, Sweden) was stereotaxically inserted into the right hippocampal CA1 area through the cannula guide (1.7 mm posterior from bregma, 1.1 mm lateral, and 2.5 mm below the surface of the skull (Paxinos and Watson 1998). The efficient dialysis length of the probe was 1 mm. The drugs were administered (according to the above schedule) 16 h after the operation, when the rats had recovered consciousness. After the last morphine treatment, rats were transferred to a free-moving animal system (BAS/100). The microdialysis probes (CMA/12) were inserted into the guide cannula. They were perfused with Ringer’s solution (in mmol/L: 145 NaCl, 2.7 KCl, 2.2 CaCl2, and 1.1 Na2HPO4, pH 7.4) at a constant flow rate of 2 μL/min. Following an equilibration period of 3–4 h, a series of three sequential 30-min samples were taken from each rat to obtain a steady baseline. The drugs were then given and the glutamate levels in the dialysates were determined by HPLC/ECD every 15 min for the first 30 min and every 30 min thereafter until 150 min. The values were expressed as ratios to the pre-injection values. After each microdialysis experiment, the rats were sacrificed by rapid decapitation, and the location of the dialysis probe was verified by histological examination. Data were discarded if the probe was incorrectly positioned.
HPLC/ECD Assay
After pre-column derivatization by o-phthalaldehyde (Dikma, Lake Forest, CA, USA), the amount of glutamate in the dialysis samples was quantified by HPLC (Agilent 1100 series, Santa Clara, CA, USA) using the electrochemical detector with the Supelco Hypersil ODS column (150 × 4.6 mm, 5 μm), as previously described [29]. The mobile phase was composed of citric acid-sodium acetate buffer containing Na2-EDTA and 1-octanesulfonate sodium salt with 10% methanol (v/v) in water (pH 3.65). The flow rate was 1 mL/min. The vitreous carbon reference electrode was set at +0.7 V.
Glutamate Release and Uptake Assays
Synaptosome Preparation
Drug treatment was as above. The hippocampus was rapidly dissected on ice 15 min after naloxone injection, and synaptosomes were prepared using the standard method [30]. Protein content was determined by the Bradford method. The synaptosomal fractions were stored on ice and used within 4 h for glutamate release and uptake assays.
Glutamate Release Assay
For basal conditions, synaptosomes (20 μg) were incubated in Krebs–Ringer HEPES buffer (in mmol/L: 140 NaCl, 5 KCl, 5 NaHCO3, 1 MgCl2·6H2O, 1.2 Na2HPO4, 10 glucose, 20 HEPES, pH 7.4, and equilibrated with 95% O2/5% CO2) containing 1.3 mmol/L CaCl2 for 20 min at 37 °C. Incubation was stopped by centrifugation (14,600×g for 10 min at 4 °C). The supernatants were stored at −70 °C until HPLC analysis.
Glutamate Uptake Assay
Synaptosomes (20 μg) were incubated in 400 μL Krebs–Ringer HEPES buffer containing CaCl2, according to a previous study [31]. After incubation (37 °C for 15 min), glutamate uptake was initiated by adding 100 μL of 10 nmol/L (45 Ci/mmol) [3H]-glutamic acid to the reaction system at 37 °C for 4 min. Non-specific uptake was determined by the addition of non-radioactive glutamate (1 mmol/L) into the total volume (500 μL) of the reaction system. The uptake was terminated by filtration on GF/C glass-fiber filters (Whatman, Maidstone, UK) under vacuum. After three washes in 5 ml ice-cold saline and drying, scintillation fluid was added to the filters and the radioactivity was quantified by liquid scintillation counting.
Immunoblotting
Drug treatment was as above, except that the naloxone injection was omitted. The rats were sacrificed 4 h after the last morphine injection and the hippocampus was dissected out. Western blot analysis was performed as described previously [28]. Protein extracts from the hippocampus of morphine-treated rats were separated on 8% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Stockholm, Sweden) by Western-blotting. For detection, the membranes were incubated overnight at 4 °C with the primary antibody anti-NR2B (1:2000, a gift from Professor Jianhong Luo, Zhejiang University, China) or anti-NR1 (1:1000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Anti-β-actin (1:5000, Santa Cruz Biotechnology, Inc.) served as the house-keeping protein. After incubation with the secondary antibodies, bands were visualized using an ECD kit (Applygen Technologies Inc., Beijing, China) according to the manufacturer’s instructions. The blots were scanned and analyzed using ImageJ software (NIH, Bethesda, MD). Values are expressed as the ratio of anti-NR1 or anti-NR2B to anti-β-actin in order to control for variations in protein loading.
Statistical Analysis
All data are presented as the mean ± SEM and were analyzed by one-way or two-way ANOVA for repeated measures followed by the Bonferroni test. All statistical procedures used SAS software (version 6.12, Cary, NC, USA). Significance was reached at values of P < 0.05.
Results
Agmatine Attenuated the Elevation of Extracellular Glutamate Levels in the CA1 Region of the Hippocampus in Chronic Morphine-Treated Rats
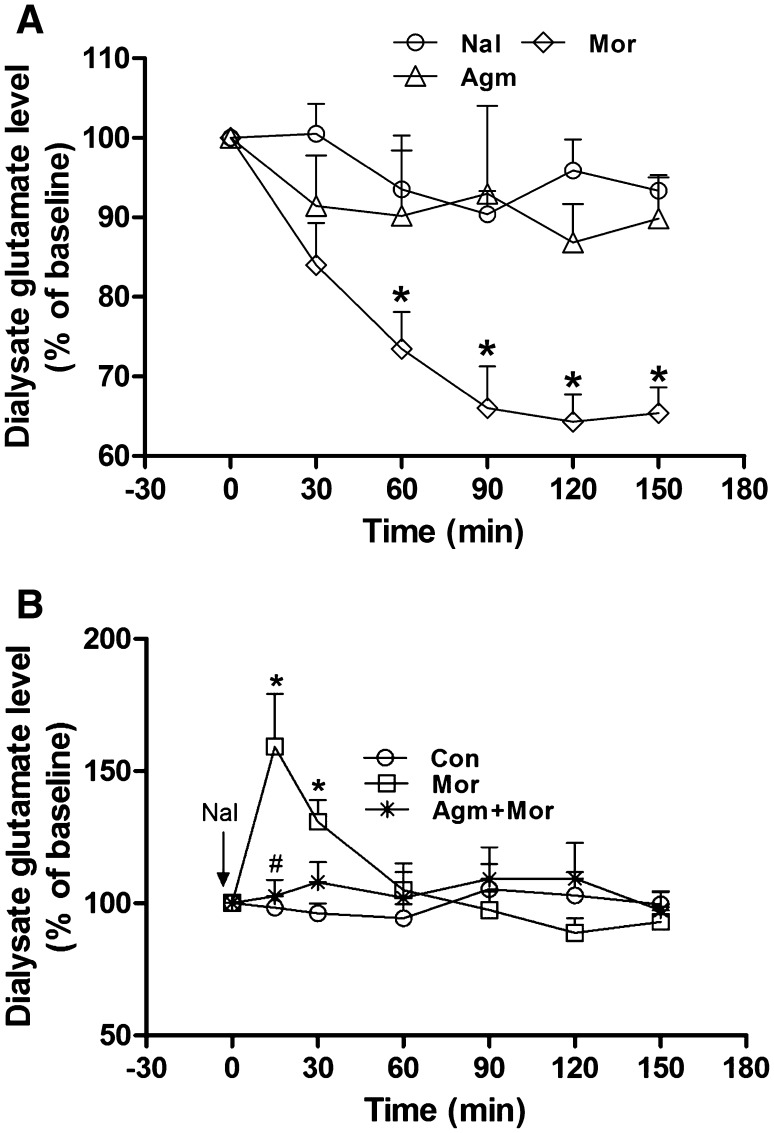
The effects of acute administration of morphine, agmatine, and naloxone on the extracellular glutamate levels in hippocampal CA1 are shown in Fig. 1A. Two-way ANOVA revealed marked differences in treatment, time, and interaction (treatment: F (2, 12) = 59.68, P < 0.0001; time: F (6, 84) = 16.97, P < 0.0001; interaction: F (12, 104) = 5.206, P < 0.0001). Acute administration of morphine (10 mg/kg, s.c.) significantly decreased the level of glutamate in CA1 by 35% at 90 min and lasted until at least 150 min, compared with the baseline level (Fig. 1A). However, acute administration of agmatine (20 mg/kg, s.c.) or naloxone (2 mg/kg, i.p.) did not change the glutamate level in the dialysate, compared with baseline (Fig. 1A). Two-way ANOVA revealed differences in treatment, time, and interaction (treatment: F (2, 12) = 5.364, P < 0.05; time: F (6, 84) = 18.12, P < 0.0001; interaction: F (12, 104) = 22.99, P < 0.0001; Fig. 1B). After chronic morphine treatment, naloxone (2 mg/kg, i.p.) significantly increased the concentration of glutamate, which reached a peak of 159% at 15 min after naloxone injection and returned to baseline after 60 min (Fig. 1B). Agmatine (20 mg/kg, s.c.) co-pretreatment with morphine for 5 days completely abolished the elevation of glutamate induced by naloxone precipitation (Fig. 1B).
Fig. 1.
Agmatine attenuated the naloxone-induced elevation of hippocampal extracellular glutamate in chronic morphine-treated rats. A Effect of acute administration of morphine, agmatine, or naloxone on dialysate glutamate levels in the hippocampal CA1 region (n = 9; *P < 0.05; ***P < 0.001 vs baseline; two-way ANOVA for repeat measures followed by Bonferroni test). Nal naloxone, Mor morphine, Agm agmatine. B Effect of agmatine on the elevation of hippocampal glutamate levels after naloxone injection in chronic morphine-treated rats (n = 5; *P < 0.05 vs Con group, # P < 0.05 vs Mor group; two-way ANOVA for repeat measures followed by Bonferroni test). Con control, Mor morphine, Agm + Mor agmatine concurrent with morphine for 5 days.
Agmatine Decreased the Glutamate Release and Increased its Uptake in the Hippocampus of Chronic Morphine-Treated Rats
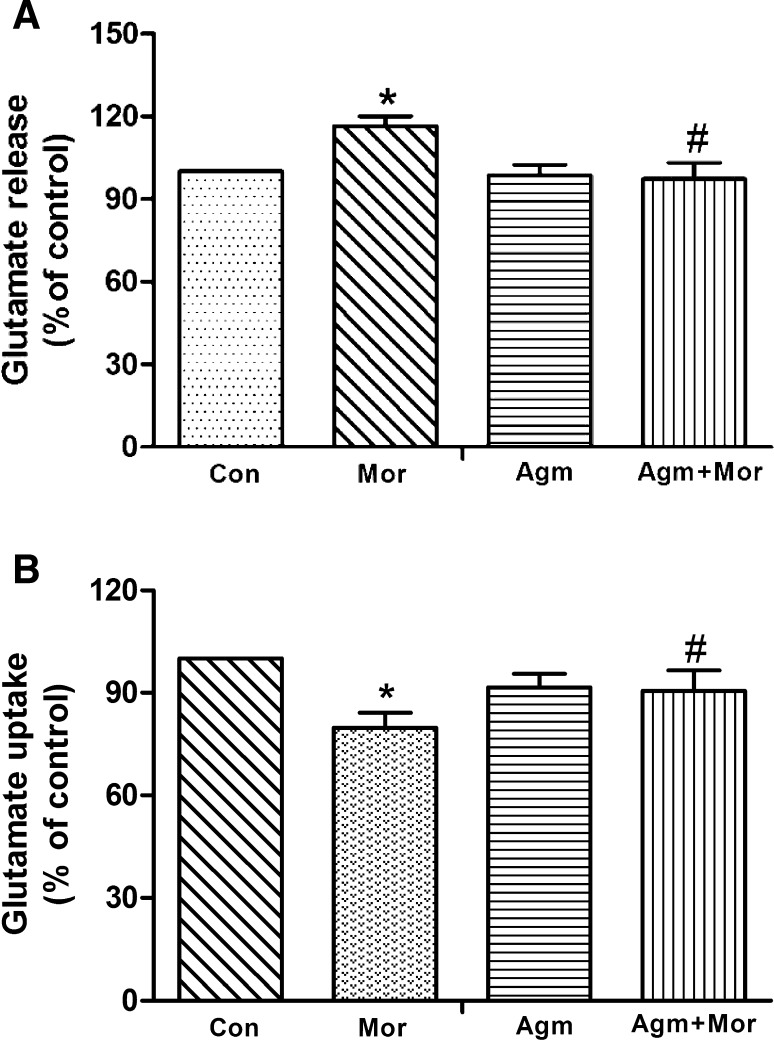
To explore how agmatine attenuated the elevation of extracellular glutamate levels, we measured the release and uptake of glutamate. In hippocampal synaptosomes, the release of glutamate markedly increased by 16% in naloxone-treated rats with chronic exposure to morphine (P < 0.05; Fig. 2A). Agmatine (20 mg/kg, s.c.) co-pretreatment with morphine completely prevented the increase in glutamate release (P < 0.05; Fig. 2A); however, chronic treatment with agmatine alone (20 mg/kg, s.c.) did not alter the glutamate release (Fig. 2A). In [3H]-glutamate uptake assays, we found that glutamate uptake was significantly decreased in hippocampal synaptosomes from chronic morphine-treated rats with naloxone precipitation, and this decrease was prevented by agmatine (20 mg/kg) co-pretreatment with morphine (P < 0.05; Fig. 2B). Chronic treatment with agmatine alone did not affect glutamate reuptake (Fig. 2B). The above results suggested that agmatine attenuates the elevation of hippocampal extracellular glutamate in chronic morphine-treated rats by both decreasing glutamate release and increasing its uptake.
Fig. 2.
Agmatine decreased glutamate release and increased glutamate uptake of hippocampal synaptosomes from chronic morphine-treated rats. A Effect of agmatine on glutamate release in chronic morphine-treated rats. B Effect of agmatine on the uptake of [3H]-glutamate in synaptosomes from chronic morphine-treated rats (n = 9; *P < 0.05 vs Con group, # P < 0.05 vs Mor group, one-way ANOVA followed by Bonferroni test). Con control, Mor morphine, Agm agmatine, Agm + Mor agmatine administered with morphine for 5 days.
Agmatine Prevented the Down-Regulation of NMDA NR2B Subunit Expression in the Hippocampus of Chronic Morphine-Treated Rats
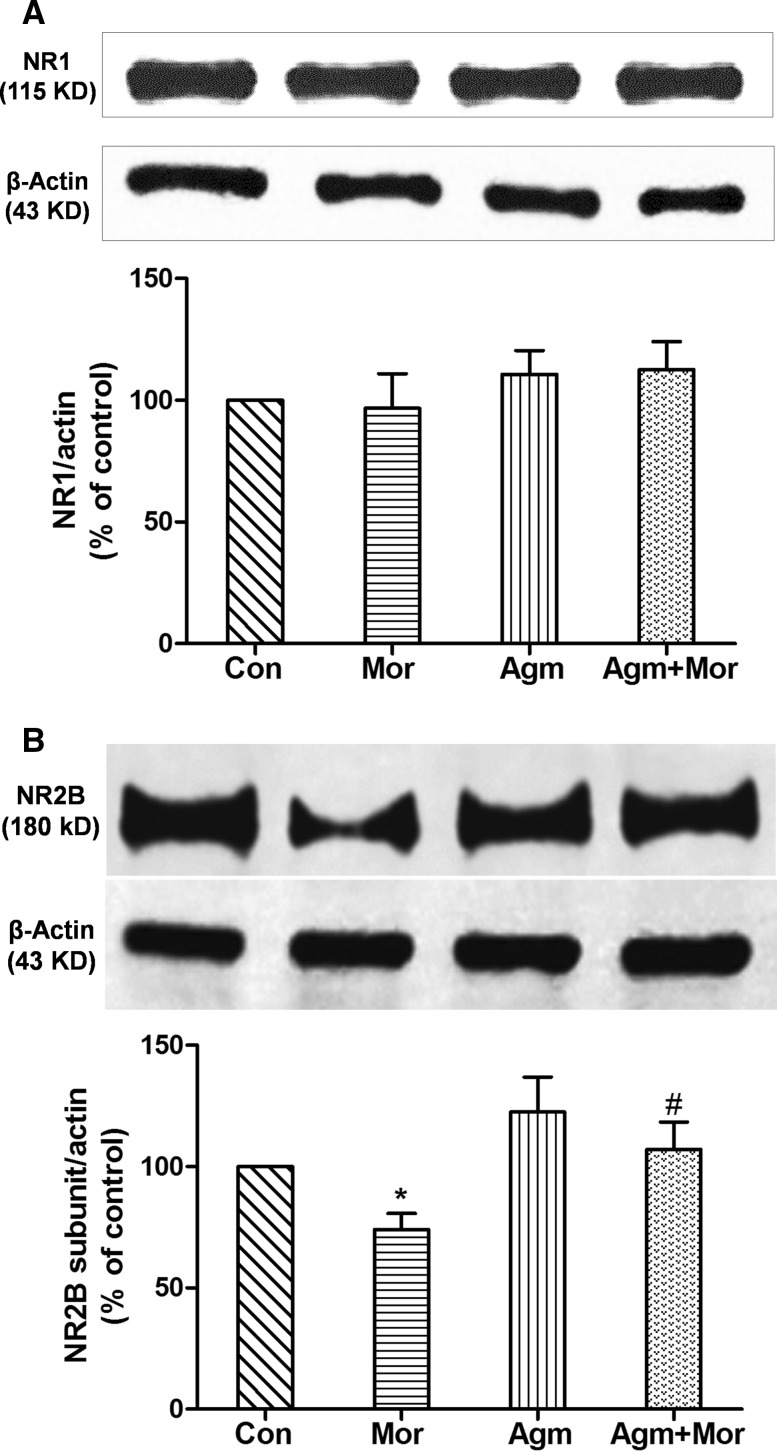
In chronic morphine-treated rats, the level of hippocampal NR1 subunit did not change, while the level of the NR2B subunit was decreased by 26% (P < 0.05; Fig. 3A, B). These results suggested that the subunit structure of the NMDARs in the hippocampus changes during chronic morphine treatment, while their density remains unchanged. Chronic administration of agmatine (20 mg/kg, s.c.) with morphine completely prevented the morphine-induced reduction of the NR2B subunit level (P < 0.05; Fig. 3B). Agmatine alone had no effect on the expression of both NR1 and NR2B subunits (Fig. 3A, B).
Fig. 3.
Agmatine prevented down-regulation of the NR2B subunit, but not the NR1 subunit, of NMDARs in the hippocampus of chronic morphine-treated rats (n = 10; *P < 0.05 vs Con group, # P < 0.05 vs Mor group, one-way ANOVA followed by Bonferroni test). Con control, Mor morphine, Agm agmatine, Agm + Mor agmatine administered with morphine for 5 days.
Discussion
Accumulating evidence suggests that alterations in the glutamate system trigger a cascade of adaptations underlying the physical and psychological dependence on morphine, or behavioral sensitization in animal models [5, 32]. According to our previous studies, agmatine (20 mg/kg) attenuates opioid-induced tolerance, physical dependence, and addiction, as well as reversing the adaptations of glutamate neurotransmission in the nucleus accumbens induced by chronic exposure to morphine [23, 25, 28, 33]. In the present study, we found that agmatine prevented the adaptations of the glutamate system, including extracellular glutamate concentration and NMDAR expression, in the hippocampus during chronic morphine treatment, which might be one of mechanisms by which agmatine modulates opioid addiction.
Glutamate is the major excitatory neurotransmitter in the brain and adaptations of glutamatergic neurotransmission are crucial for drug addiction. Many studies have demonstrated that the hippocampus is an important component of the reward circuit, and that adaptations of hippocampal glutamatergic neurotransmission contribute to reward-related learning and memory, withdrawal, drug seeking, and reinstatement. Therefore, interventions that regulate either synaptic and non-synaptic extracellular glutamate, which is controlled by glutamate release and reuptake, or the function and expression of postsynaptic glutamate receptors, can influence drug addiction [34]. Agmatine is considered to be a novel neurotransmitter and/or neuromodulator, modulating the release of some neurotransmitters and hormones. However, reports about the effects of agmatine on glutamate levels are limited. Previous studies have reported that agmatine blocks the increase of extracellular glutamate during pentylenetetrazol-induced seizures [35]. In the current study, we found that acute administration of agmatine alone (20 mg/kg) did not affect extracellular glutamate levels in the hippocampus. However, chronic agmatine co-pretreatment with morphine prevented the increase of extracellular glutamate levels induced by naloxone precipitation in chronic morphine-treated rats, which is consistent with our previous findings in the nucleus accumbens. Therefore, the attenuation of the increase in extracellular glutamate may be one of the neurochemical mechanisms underlying the agmatine-mediated inhibitory effect on opioid addiction.
In general, extracellular glutamate levels are controlled by its release and reuptake in both neurons and glial cells [36]. Unlike in the nucleus accumbens, agmatine prevented both the increase in glutamate release and the decrease in glutamate uptake in the hippocampus when co-administered with morphine, which might contribute to the inhibitory effect of agmatine on the elevation of hippocampal extracellular glutamate caused by chronic morphine treatment and naloxone precipitation. Previous studies have indicated that naloxone-precipitated withdrawal and cue- or drug-induced relapse lead to the enhancement of neuronal glutamate release in chronic opioid-treated animals, whereas the decrease in basal extracellular glutamate levels during chronic opioid treatment is due to reduced cystine-glutamate exchange in astrocytes [16, 36, 37]. In the present study, chronic morphine treatment and naloxone precipitation increased glutamate release from hippocampal synaptosomes, and agmatine co-pretreatment with morphine abolished this phenomenon, suggesting that the ability of agmatine to attenuate the naloxone-induced elevation of extracellular glutamate is due to its reduction of neuronal glutamate release. Neurotransmitter release from presynaptic membranes is Ca2+-dependent. Previous studies have shown that agmatine attenuates voltage-gated Ca2+ channel currents in isolated neurohypophysial terminals and in cultured rat hippocampal neurons [38, 39]; this might contribute to the inhibition of glutamate release by agmatine.
The elimination of extracellular glutamate is governed by glutamate transporters, the most influential of which is glutamate transporter 1 (GLT1). Repeated treatment with morphine induces a significant increase in the protein levels of membrane-bound GLT1 [40]. The increasing expression of GLT1 directly promotes glutamate reuptake and decreases extracellular glutamate concentrations [41]. Although glial GLT1 was thought to be primarily responsible for extracellular glutamate reuptake in most brain regions in drug addiction, Xu et al. reported that neuronal GLT1 contributes to hippocampal glutamate reuptake during morphine withdrawal [40]. In the present study, the [3H]-glutamate uptake assay revealed that glutamate uptake into hippocampal synaptosomes markedly decreased in chronic morphine-treated rats and agmatine co-treatment with morphine completely reversed the decrease, suggesting that the neuronal mechanism of glutamate reuptake at least in part contributes to the regulation of extracellular glutamate levels by agmatine. However, we found that naloxone treatment for 15 min decreased glutamate reuptake by hippocampal synaptosomes from chronic morphine-treated rats, which was inconsistent with the findings of Xu et al. This contradiction might be due to the difference in the time of naloxone-induced withdrawal, which in Xu’s report was 1 h after naloxone administration [40].
The adaptations of glutamate transmission involve both glutamate levels and glutamate receptors. NMDARs are primarily located in postsynaptic membranes that mediate fast excitatory neurotransmission. NMDARs are assembled from NR1, NR2 (NR2A, NR2B, NR2C, NR2D), and NR3 (NR3A, NR3B) subunits. The NR1 subunit is the essential component, and its number represents the expression level of NMDARs. The various isoforms of the NR2 subunit modify the electrophysiological characteristics of the receptor. The NR2B subunit tends to transform into the NR2A subunit, resulting in the functional diversity of NMDARs [42]. First, the deactivation time of NR1/NR2A assemblies is shorter, and the EC50 for glutamate or glycine is higher than in NR1/NR2B assemblies. Second, the speed of excitatory postsynaptic current decay is different. Finally, NR2A assembles as a functionally distinct triheteromeric NMDAR (i.e. NR1/NR2A/NR3) more easily than the other NR2 subunit isoforms, and this channel has reduced relative Ca2+ permeability [43]. Accumulating evidence suggests that NMDARs, particularly those containing the NR2B subunit, play a crucial role in opioid addiction [44]. Chronic exposure to morphine markedly decreases the expression of the NR2B subunit in the nucleus accumbens [45], frontal cortex, and striatum [45]. In rat hippocampus and hypothalamus, chronic morphine treatment fails to affect the allosteric binding but significantly decreases the expression of NR2B [46–48]. In the present study, we showed that in the hippocampus of chronic morphine-treated rats, expression of the NR2B subunit, but not the NR1 subunit, was significantly down-regulated, and agmatine reversed this change. This suggested that agmatine not only prevents alteration of the extracellular glutamate level, but also reverses the adaptation of NMDAR expression. However, the electrophysiological characteristics of NMDARs in the hippocampus of chronic morphine-treated rats and the effect of agmatine on them remain unclear.
In conclusion, we have demonstrated that, in the hippocampus of chronic morphine-treated rats, agmatine prevents or attenuates chronic morphine-induced adaptations of the hippocampal glutamate transmission system, including attenuating the elevation of extracellular glutamate levels through the modulation of glutamate release and reuptake and preventing the down-regulation of the NR2B subunit expression of NMDARs. This may be one of neurobiological mechanisms by which agmatine attenuates opioid addiction.
Acknowledgments
This work was supported by grants from the National Basic Research Development Program of China (2015CB553504), the National Natural Science Foundation of China (30930040 and 81102426), and was a Project of the National Science and Technology Support Program of China (2012BAI01B07).
Footnotes
Xiao-Fei Wang and Tai-Yun Zhao have contributed equally to this work.
Contributor Information
Ning Wu, Email: wuning7671@126.com.
Jin Li, Email: jinli9802@163.com.
References
- 1.Filipowicz WBS, Sonenber N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:13. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aldworth J AK, Bishop E. Results from the 2006 National Survey on Drug Use and Health: national findings. 2007.
- 5.Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–664. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain Res Rev. 2008;58:192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Koob GF. Neuroadaptive mechanisms of addiction: studies on the extended amygdala. Eur Neuropsychopharmacol. 2003;13:442–452. doi: 10.1016/j.euroneuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Enrico P, Mura MA, Esposito G, Serra P, Migheli R, De Natale G, et al. Effect of naloxone on morphine-induced changes in striatal dopamine metabolism and glutamate, ascorbic acid and uric acid release in freely moving rats. Brain Res. 1998;797:94–102. doi: 10.1016/S0006-8993(98)00371-0. [DOI] [PubMed] [Google Scholar]
- 10.Siggins GR, Martin G, Roberto M, Nie Z, Madamba S, De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- 11.Glass MJ, Kruzich PJ, Kreek MJ, Pickel VM. Decreased plasma membrane targeting of NMDA-NR1 receptor subunit in dendrites of medial nucleus tractus solitarius neurons in rats self-administering morphine. Synapse. 2004;53:191–201. doi: 10.1002/syn.20049. [DOI] [PubMed] [Google Scholar]
- 12.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JT, Christie MJ, Manzoni O. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 14.Berke JD, Eichenbaum HB. Drug addiction and the hippocampus. Science. 2001;294:1235. doi: 10.1126/science.294.5545.1235a. [DOI] [PubMed] [Google Scholar]
- 15.Farahmandfar M, Karimian SM, Zarrindast MR, Kadivar M, Afrouzi H, Naghdi N. Morphine sensitization increases the extracellular level of glutamate in CA1 of rat hippocampus via mu-opioid receptor. Neurosci Lett. 2011;494:130–134. doi: 10.1016/j.neulet.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 16.Guo M, Xu NJ, Li YT, Yang JY, Wu CF, Pei G. Morphine modulates glutamate release in the hippocampal CA1 area in mice. Neurosci Lett. 2005;381:12–15. doi: 10.1016/j.neulet.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 17.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 18.Sepehrizadeh Z, Sahebgharani M, Ahmadi S, Shapourabadi MB, Bozchlou SH, Zarrindast MR. Morphine-induced behavioral sensitization increased the mRNA expression of NMDA receptor subunits in the rat amygdala. Pharmacology. 2008;81:333–343. doi: 10.1159/000122959. [DOI] [PubMed] [Google Scholar]
- 19.Sepehrizadeh Z, Bahrololoumi Shapourabadi M, Ahmadi S, Hashemi Bozchlou S, Zarrindast MR, Sahebgharani M. Decreased AMPA GluR2, but not GluR3, mRNA expression in rat amygdala and dorsal hippocampus following morphine-induced behavioural sensitization. Clin Exp Pharmacol Physiol 2008, 35: 1321–1330. [DOI] [PubMed]
- 20.Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000;21:187–193. doi: 10.1016/S0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 21.Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, et al. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013;18:880–893. doi: 10.1016/j.drudis.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Uzbay TI. The pharmacological importance of agmatine in the brain. Neurosci Biobehav Rev. 2012;36:502–519. doi: 10.1016/j.neubiorev.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Wei XL, Su RB, Lu XQ, Liu Y, Yu SZ, Yuan BL, et al. Inhibition by agmatine on morphine-induced conditioned place preference in rats. Eur J Pharmacol. 2005;515:99–106. doi: 10.1016/j.ejphar.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Li X, Pei G, Qin BY. Analgesic effect of agmatine and its enhancement on morphine analgesia in mice and rats. Zhongguo Yao Li Xue Bao. 1999;20:81–85. [PubMed] [Google Scholar]
- 25.Li J, Li X, Pei G, Qin BY. Effects of agmatine on tolerance to and substance dependence on morphine in mice. Zhongguo Yao Li Xue Bao. 1999;20:232–238. [PubMed] [Google Scholar]
- 26.Aricioglu-Kartal F, Uzbay IT. Inhibitory effect of agmatine on naloxone-precipitated abstinence syndrome in morphine dependent rats. Life Sci. 1997;61:1775–1781. doi: 10.1016/S0024-3205(97)00801-1. [DOI] [PubMed] [Google Scholar]
- 27.Kolesnikov Y, Jain S, Pasternak GW. Modulation of opioid analgesia by agmatine. Eur J Pharmacol. 1996;296:17–22. doi: 10.1016/0014-2999(95)00669-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang XF, Wu N, Su RB, Lu XQ, Liu Y, Li J. Agmatine modulates neuroadaptations of glutamate transmission in the nucleus accumbens of repeated morphine-treated rats. Eur J Pharmacol. 2011;650:200–205. doi: 10.1016/j.ejphar.2010.09.071. [DOI] [PubMed] [Google Scholar]
- 29.Wen ZH, Chang YC, Cherng CH, Wang JJ, Tao PL, Wong CS. Increasing of intrathecal CSF excitatory amino acids concentration following morphine challenge in morphine-tolerant rats. Brain Res. 2004;995:253–259. doi: 10.1016/j.brainres.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz J, Fitzgerald LW, Charlton M, Lane S, Trevisan L, Guitart X, et al. Biochemical actions of chronic ethanol exposure in the mesolimbic dopamine system. Synapse. 1995;21:289–298. doi: 10.1002/syn.890210403. [DOI] [PubMed] [Google Scholar]
- 31.Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei XL, Su RB, Wu N, Lu XQ, Zheng JQ, Li J. Agmatine inhibits morphine-induced locomotion sensitization and morphine-induced changes in striatal dopamine and metabolites in rats. Eur Neuropsychopharmacol. 2007;17:790–799. doi: 10.1016/j.euroneuro.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng Y, LeBlanc MH, Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci Lett. 2005;390:129–133. doi: 10.1016/j.neulet.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 37.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 1998;56:237–267. doi: 10.1016/S0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 38.Wang G, Gorbatyuk OS, Dayanithi G, Ouyang W, Wang J, Milner TA, et al. Evidence for endogenous agmatine in hypothalamo-neurohypophysial tract and its modulation on vasopressin release and Ca2 + channels. Brain Res. 2002;932:25–36. doi: 10.1016/S0006-8993(02)02260-6. [DOI] [PubMed] [Google Scholar]
- 39.Weng XC, Gai XD, Zheng JQ, Li J. Agmatine blocked voltage-gated calcium channel in cultured rat hippocampal neurons. Acta Pharmacol Sin. 2003;24:746–750. [PubMed] [Google Scholar]
- 40.Xu NJ, Bao L, Fan HP, Bao GB, Pu L, Lu YJ, et al. Morphine withdrawal increases glutamate uptake and surface expression of glutamate transporter GLT1 at hippocampal synapses. J Neurosci. 2003;23:4775–4784. doi: 10.1523/JNEUROSCI.23-11-04775.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, et al. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin G, Guadano-Ferraz A, Morte B, Ahmed S, Koob GF, De Lecea L, et al. Chronic morphine treatment alters N-methyl-D-aspartate receptors in freshly isolated neurons from nucleus accumbens. J Pharmacol Exp Ther. 2004;311:265–273. doi: 10.1124/jpet.104.067504. [DOI] [PubMed] [Google Scholar]
- 43.Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/S0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- 44.Narita M, Aoki T, Suzuki T. Molecular evidence for the involvement of NR2B subunit containing N-methyl-D-aspartate receptors in the development of morphine-induced place preference. Neuroscience. 2000;101:601–606. doi: 10.1016/S0306-4522(00)00405-X. [DOI] [PubMed] [Google Scholar]
- 45.Kao JH, Huang EY, Tao PL. NR2B subunit of NMDA receptor at nucleus accumbens is involved in morphine rewarding effect by siRNA study. Drug Alcohol Depend. 2011;118:366–374. doi: 10.1016/j.drugalcdep.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Johansson T, Elfverson M, Zhou Q, Nyberg F. Allosteric modulation of the NMDA receptor by neurosteroids in rat brain and the impact of long term morphine administration. Biochem Biophys Res Commun. 2010;401:504–508. doi: 10.1016/j.bbrc.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 47.Turchan J, Maj M, Przewlocka B. The effect of drugs of abuse on NMDAR1 receptor expression in the rat limbic system. Drug Alcohol Depend. 2003;72:193–196. doi: 10.1016/S0376-8716(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 48.Oh S, Kim JI, Chung MW, Ho IK. Modulation of NMDA receptor subunit mRNA in butorphanol-tolerant and -withdrawing rats. Neurochem Res. 2000;25:1603–1611. doi: 10.1023/A:1026618603795. [DOI] [PubMed] [Google Scholar]