Abstract
Tobacco consumption is one of the leading causes of preventable death worldwide. However, it is difficult to give up smoking by relying on the help of traditional treatments only. Recent years have witnessed emerging positive evidence that non-invasive brain stimulation (NIBS), such as transcranial magnetic stimulation and transcranial direct-current stimulation, can reduce smoking-related behaviors. Although their potential has been implied by advances in research, several methodological issues restrict the clinical application of NIBS to treating nicotine dependence. In this review, we critically evaluate related studies and give suggestions for future research and applications to meet these challenges.
Keywords: TMS, tDCS, NIBS, Nicotine, Addiction
Introduction
The worldwide leading causes of death, lung cancer and chronic obstructive pulmonary disease, are associated with cigarette smoking [1]. Besides physical health problems, chronic smoking causes functional and structural abnormalities in the brain [2–5]. However, most chronic smokers, including those who are willing to quit, find it difficult to become free of tobacco usage even with the help of intervention methods such as pharmacotherapy [6]. Transcranial magnetic stimulation (TMS) and transcranial direct-current stimulation (tDCS) are methods of non-invasive brain stimulation (NIBS). With the advantage of being able to modulate brain activation non-invasively, these techniques are showing potential as therapy for nicotine addiction [7]. In this article, we systematically review related studies and discuss the potential and challenges of NIBS in treating nicotine addiction.
Potential of NIBS
A Brief Introduction to NIBS
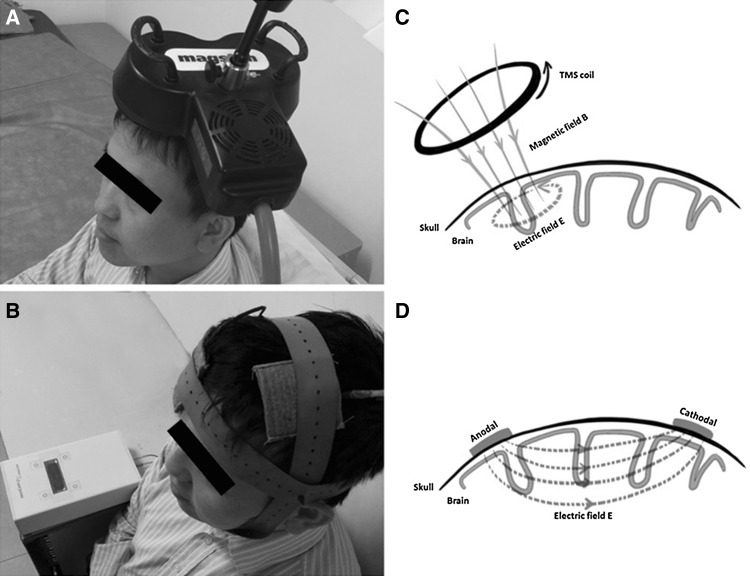
NIBS can selectively excite or inhibit a target brain region by initiating tiny electrical current over the cortex [8]. The two most widely used techniques of NIBS are tDCS and TMS (Fig. 1). tDCS modulates brain activity by weak direct-current stimulation through the intact skull in a safe, efficient, and painless way [9, 10]. It is believed to hyperpolarize (cathodal stimulation) or depolarize (anodal stimulation) neuronal membranes [11], with the assumption of anodal-excitatory and cathodal-inhibitory effects on brain functions [12]. However, this assumption has not held true for all studies [13].
Fig. 1.
Non-invasive brain stimulation. A Transcranial magnetic stimulation (TMS). B Transcranial direct-current stimulation (tDCS). C Mechanism underlying TMS. D Mechanism underlying tDCS
TMS evokes microelectronic current stimulation via a rapidly-alternating magnetic field to temporarily excite or inhibit the activity in a target brain region [14]. Unlike tDCS, it is not only neuromodulatory but also neurostimulatory. The current it induces can depolarize neurons. Repetitive TMS (rTMS) is a widely-used protocol, which stimulates a scalp location at a frequency ranging from 1 to 20 Hz or more [15]. In general, rTMS induces inhibition with stimulation at ~1 Hz, and excitation at ≥5 Hz [16].
Rationale for Using NIBS to Treat Nicotine Addiction
Nicotine, like other drugs of abuse, is associated with abnormal function in the mesolimbic dopamine system and reward-related brain areas, such as the ventral tegmental area, prefrontal cortex, nucleus accumbens, amygdala, and hippocampus [17]. The dorsolateral prefrontal cortex (dlPFC) is widely acknowledged to be an effective stimulation target, as it has been associated with cue-provoked smoking craving [18], which is the primary trigger of relapse [19]. Malfunction of the dlPFC in substance addiction, including nicotine, has been shown to be the mechanism underlying the impaired response inhibition and salience attribution in addicts [20, 21]. Specifically, nicotine may influence the dlPFC in smokers by blocking the α7 nicotinic acetylcholine receptors in glutamate network synapses, which remarkably changes the persistent firing of dlPFC neurons [22]. Thus, modulating the dlPFC by NIBS may result in the release of neurotransmitters such as dopamine and γ-aminobutyric acid in cortical and subcortical regions which may in turn help smokers to get rid of the smoking habit. The potential of NIBS in treating nicotine addiction has recently received some preliminary empirical support.
Evidence Supporting tDCS as a Treatment
The pioneering research on the effect of tDCS on nicotine addicts was performed by Fregni and colleagues [23]. In a randomized, double-blind, sham-controlled crossover study, they demonstrated for the first time that applying weak currents over the dlPFC reduces smoking craving [23]. The cumulative effects of tDCS on smoking craving and consumption have been validated [24]. In another study, Fecteau et al. attempted to modulate the decision-making behavior of smokers via stimulation over the same cortical region. They showed not only a four-day decrease in the number of cigarettes consumed after anodal stimulation but also more rejections of cigarettes but not monetary offers in an Ultimatum Game [25]. Besides the dlPFC, the fronto-parieto-temporal association area has also been proposed as an accessible stimulation site for modulating smoking-related behaviors [26]. The above studies suggest that tDCS has potential in smoking cessation.
Only one study has reported contradictory findings. Xu et al. investigated the tDCS effects on a group of dependent smokers who abstained from smoking overnight on two different occasions [27]. The outcome was that self-reported craving did not show any reduction after 20 min of anodal stimulation of the left dlPFC, though the participants had less negative affect [27]. As this study differed from previous work with regard to the level of abstinence, the findings may indicate the importance of a smoker’s state as a modulator of tDCS effects.
One interesting finding is that tDCS effects on smoking-related behavior may be insensitive to the direction of current flow. Two studies [23, 24] used an electrode protocol with the anode on the left dlPFC and the cathode on the right dlPFC. In contrast, using the reversed placement of electrodes, a study [25] also reported positive tDCS effects on smoking-related behaviors.
Evidence Supporting TMS as a Treatment
Eichhammer and colleagues conducted high-frequency rTMS over the dlPFC in smokers [28]. The results showed that the smoking rate in the active stimulation group was significantly lower than that in the sham group. Although no difference in craving was found, the results illustrated an advantage of TMS in smoking cessation [28]. Meanwhile, Johann et al. demonstrated that rTMS can also reduce cigarette craving [29]; this effect was replicated in a subsequent study using a more rigorous experimental design and a larger group of participants [30]. This study demonstrated that rTMS over the dlPFC reduces both cigarette consumption and craving, though the effects were not robust and seemed to dissipate over time [30]. Furthermore, rTMS may serve as an additional aid in cognitive-behavioral therapy for intermediate nicotine abstinence [31].
Only one study has directly compared high- and low-frequency rTMS. Rose et al. revealed that 10-Hz rTMS over the superior frontal gyrus can elevate craving in the presence of smoking cues, while it lowers the appetite for cigarettes in the presence of neutral cues compared with 1-Hz rTMS [32].
The Challenges of NIBS
Although positive discoveries have been made in this field [8, 33], apparent deficiencies exist [34].
Heterogeneity in the Stimulation Protocol
Both TMS and tDCS have a large set of parameters, and their selection varies among studies. In rTMS, the parameters include stimulation site, intensity, frequency, length of trains of pulses, and the time interval between trains. In tDCS, the parameters include stimulation site, current intensity, duration, electrode shape and size, and the polarity of stimulation (anodal or cathodal). In this young field, although some general principles [35, 36] (mainly safety concerns) have been well-accepted, standard protocols have not yet been developed [37].
Neural Mechanism of NIBS
Although advances have been made, investigations on the particular neuronal mechanisms underlying the NIBS effects on smoking-related behaviors are still lacking. One hypothesis is that the interaction between the dlPFC and the midbrain dopamine system might be a useful target of tDCS and TMS [38]. However, this hypothesis has not yet been tested directly. One of the rare examples is a study by Pripfl and colleagues, who used a combination of TMS and EEG to clarify the mechanisms underlying the TMS-evoked reduction in smoking craving [39]. They conducted high-frequency repetitive TMS over the dlPFC in two groups of smokers, one of which received sham stimuli. The self-reported craving level and delta power in the resting-state EEG, an indicator of the activity in the dopaminergic brain reward system, were recorded. Although TMS effects on both craving and delta power were found, the two measurements did not correlate with each other [39]. A recent fMRI study revealed that the frontal-striatal pathway can be modified by tDCS over the dlPFC in the Balloon Analog Risk Task [40]. However, no behavioral change in that task was found under stimulation; besides, as the experiment was conducted on healthy participants, whether the tDCS effects on the frontal-striatal pathway are associated with reductions in smoking behaviors remains unclear. Although a recent study used a sample of smokers, only the tDCS effects on task-based activations were reported, without evaluating the tDCS effect on functional connectivity [41].
An alternative pathway affected by NIBS is the link between the dlPFC and the hippocampus. The cognitive theory of craving proposes that it reflects the automatic retrieval of related experience, which bias cognitive processing toward smoking-related experience [42]. The neurobiological theory also states that addiction is primarily a malfunction of the hippocampus [43], “the relapse circuits in the brain” [44]. However, whether NIBS can modulate the dlPFC-hippocampus pathway has not been directly tested.
Individual Differences
Recent work has revealed that NIBS effects are vulnerable to individual factors [45, 46]. For instance, one study showed that only people with low performance in working memory benefit from tDCS [47]; also, individual resting-state functional connectivity before stimulation predicts the effect of tDCS on tinnitus [48]. Many factors have to be taken into consideration in tDCS studies, including developmental stage, hormonal levels, plasticity and stability, and even circadian rhythms [45]. These variables might also be confounding factors in TMS studies.
One variable that may be important in NIBS studies of smoking behaviors is the individual’s expectation of smoking during the treatment. Expectancy of smoking is known to be a modulator of cue-reactivity in fMRI studies. For example, McBride et al. discovered that expectancy of and abstinence from cigarettes are possible modulators of the neuronal responses of smokers to a related cue [49]. In people expecting to smoke immediately after the scan, smoking cues activated brain areas implicated in arousal, attention, and cognitive control. These cue-induced activities did not occur in those who were not allowed to smoke for 4 h. Also, trends but not significant differences in cue-related neuronal responses were found between participants with distinct abstinence states. Another noteworthy finding is that applying tDCS to smokers with 12-h abstinence only reduces the negative affect rather than the craving.
Other specific factors that should be noted are the severity of nicotine dependence [50], withdrawal symptoms [34], and gender [51]. These individual factors were not explicitly controlled in the published NIBS studies on smoking behaviors, which questions the generalization of these findings to the clinical context.
Cultural Differences
Cultural difference is another possible variable given that the neuronal substrate underlying smoking behavior may also differ between cultures or ethnicities. Okuyemi evaluated the differences in attention to smoking cues between African-Americans and Caucasians using fMRI [52]. The results showed a strong ethnic effect in several a priori regions of interest. African-Americans responded more in several brain regions than Caucasians both in the contrast between smoking cues and neutral cues and between smokers and non-smokers [52].
One important difference is that between Western and Eastern cultures [53]. No study, to our knowledge, has used task-based fMRI to investigate the differences between western and eastern smokers. However, preliminary evidence for cultural disparities in the morphometry of the brain has been reported [54]. Chinese chronic smokers have a smaller gray matter volume in the cerebellum on both sides of the brain [55], while western smokers only have decreased volume in the right cerebellum [18]. Another study on Chinese chronic smokers found a reduced gray matter volume in the left thalamus [56], while a study on western smokers found a decrement in the right thalamus [57]. These findings suggest a western-eastern difference in the lateralization pattern.
Despite the evidence outlined above, the factor of culture has not received further attention with regard to the effects of NIBS on smoking behavior. We performed a simple search on the Web of Science with the following strategy: Topic: “transcranial direct current stimulation” or “TMS”; Address: “China”, “Germany”, or “USA”; Research domains: “science technology”; Research areas: “neuroscience neurology” or “psychology”. The results revealed that, so far, only 33 tDCS studies and 334 TMS studies have been done in mainland China, while in the USA there have been >600 tDCS studies and thousands of TMS studies. The statistics may not be accurate, as we did not manually check each publication. However, the general pattern reflects a situation that is worthy of note by researchers in China. Among these studies, only few have aimed at investigating the therapeutic effect of these techniques on nicotine addiction. Only one study on the effects of NIBS on smoking-related behaviors has been published by researchers in China [26]. That study used a unique protocol that directly compared the findings in Asian smokers with those in western smokers. As general functional differences between Westerners and East Asians have been reported [53], the problem of simple generalization of western findings to an Asian context is evident.
Perspectives
Combining Behavioral and Neuroimaging Measures
Most of the previous studies on the NIBS effects rely on the measurement of self-reported craving given that this is believed to be the primary motivation for relapse [58]. However, the existence of an association between subjective craving and relapse has been contested [59]. It is inappropriate to rely too much on craving reports. Alternative approaches, such as EEG and fMRI, can be considered.
Combining behavioral and neuroimaging measurements can also clarify the neuronal mechanisms underlying the NIBS effects. Future studies may directly investigate the roles of two important pathways in the NIBS effects: the dlPFC-striatal pathway and the dlPFC-hippocampal pathway.
Optimizing NIBS Protocols Using Brain Connectomics
The functions of the human brain are characterized by both local specialization and global integration. The field of connectomics is contributing new knowledge and tools to reveal the functional organization of the human brain [60, 61]. The state-of-the-art progress of connectomics may serve as a tool for optimizing the protocols of TMS and tDCS [62, 63]. On the one hand, the choice and validation of stimulation targets may be guided by connectomics. On the other hand, brain network analysis could be used in future studies on the mechanisms of the TMS and tDCS effects on nicotine addiction to reveal the underlying neuronal mechanism from the network point of view, which may improve the understanding of NIBS and promote its clinical application.
Individualized NIBS Protocols
Individualized NIBS protocols are the right direction, given that NIBS effects are modulated by many individual factors. To achieve this goal, a “localizer” fMRI scan might be an ideal solution to guide the selection of the target brain region for each participant. Such an approach has empirical support. For instance, Sack et al. have verified that individual fMRI-guided TMS neuro-navigation yields a greater effect size than the MRI-guided neuro-navigation coordinates of group results and the 10–20 EEG location [64]. Clark et al. demonstrated that tDCS guided by fMRI significantly improves the ability to learn to identify concealed objects [65], which is a good example of using an individualized protocol to help people to attain expertise. We suggest that the validation of individualized NIBS treatment protocols may be a valuable research direction. For studies using the group-based protocol, we suggest that individual factors, such as expectancy and abstinence level, must be well-controlled or set as covariates.
Multi-Center and Cross-Cultural Studies
Culture-led neuronal differences may be variables for NIBS effects on smokers, as cultural differences seem to play a role in brain activity among smokers. No conclusions can be drawn since, so far, this has neither been tested directly nor can the results from western countries be compared with those from the east due to the small numbers and incomparable experimental designs. Therefore, to make this question clear, we recommend a direct check of the cross-cultural NIBS effects on smokers as well as more studies from East Asian countries with designs comparable to those from the west.
Conclusions
We have discussed the potential and challenges of using NIBS in treating nicotine addiction. Although its potential has been suggested by recent studies, several methodological issues restrict the clinical application of NIBS. We give several suggestions to meet these challenges. First, the neural mechanisms underlying NIBS may be directly tested with specific hypotheses. Second, knowledge from brain connectomics may be used to guide NIBS protocols. Third, validation of individualized NIBS protocols may be a direction for future research. Finally, cross-cultural studies on NIBS effects in Asian and Western smokers are needed.
Acknowledgements
This review was supported by grants from the National Natural Science Foundation of China (31471071 and 31500917), China Postdoctoral Science Foundation (2016M592051), Fundamental Research Funds for the Central Universities of China (WK2070000033), and Hefei Science Center, CAS “User with Potential”, China (2015HSC-UP017).
References
- 1.Wang ZL. Association between chronic obstructive pulmonary disease and lung cancer: the missing link. Chin Med J. 2013;126:154–165. [PubMed] [Google Scholar]
- 2.Lin F, Wu G, Zhu L, Lei H. Altered brain functional networks in heavy smokers. Addict Biol 2015, 20: 809–819. [DOI] [PubMed]
- 3.Ray R, Schnoll RA, Lerman C. Nicotine dependence: biology, behavior, and treatment. Annu Rev Med. 2009;60:247–260. doi: 10.1146/annurev.med.60.041707.160511. [DOI] [PubMed] [Google Scholar]
- 4.Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA. Factors underlying prefrontal and insula structural alterations in smokers. Neuroimage. 2011;54:42–48. doi: 10.1016/j.neuroimage.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Salmeron BJ, Ross TJ, Gu H, Geng X, Yang Y, et al. Anatomical differences and network characteristics underlying smoking cue reactivity. Neuroimage. 2011;54:131–141. doi: 10.1016/j.neuroimage.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rigotti NA. Treatment of tobacco use and dependence. N Engl J Med. 2002;346:506–512. doi: 10.1056/NEJMcp012279. [DOI] [PubMed] [Google Scholar]
- 7.Hone-Blanchet A, Ciraulo DA, Pascual-Leone A, Fecteau S. Noninvasive brain stimulation to suppress craving in substance use disorders: review of human evidence and methodological considerations for future work. Neurosci Biobehav Rev 2015, 59: 184–200. [DOI] [PMC free article] [PubMed]
- 8.Jansen JM, Daams JG, Koeter MW, Veltman DJ, van den Brink W, Goudriaan AE. Effects of non-invasive neurostimulation on craving: a meta-analysis. Neurosci Biobehav Rev. 2013;37:2472–2480. doi: 10.1016/j.neubiorev.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Nitsche M, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang LZ, Zhang W, Shi B, Yang Z, Wei Z, Gu F, et al. Electrical stimulation over bilateral occipito-temporal regions reduces N170 in the right hemisphere and the composite face effect. PLoS One. 2014;9:e115772. doi: 10.1371/journal.pone.0115772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. The Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 12.Jacobson L, Koslowsky M, Lavidor M. tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res. 2012;216:1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- 13.George MS, Aston-Jones G. Noninvasive techniques for probing neurocircuitry and treating illness: vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) Neuropsychopharmacology. 2010;35:301–316. doi: 10.1038/npp.2009.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balconi M. Dorsolateral prefrontal cortex, working memory and episodic memory processes: insight through transcranial magnetic stimulation techniques. Neurosci Bull. 2013;29:381–389. doi: 10.1007/s12264-013-1309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 16.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 17.Hou H, Wang C, Jia S, Hu S, Tian M. Brain dopaminergic system changes in drug addiction: a review of positron emission tomography findings. Neurosci Bull. 2014;30:765–776. doi: 10.1007/s12264-014-1469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/S0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- 19.Beaver JD, Long CJ, Cole DM, Durcan MJ, Bannon LC, Mishra RG, et al. The effects of nicotine replacement on cognitive brain activity during smoking withdrawal studied with simultaneous fMRI/EEG. Neuropsychopharmacology. 2011;36:1792–1800. doi: 10.1038/npp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Paspalas CD, Jin LE, Picciotto MR, Arnsten AF, Wang M. Nicotinic α7 receptors enhance NMDA cognitive circuits in dorsolateral prefrontal cortex. Proc Natl Acad Sci USA. 2013;110:12078–12083. doi: 10.1073/pnas.1307849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry. 2008;69:32–40. doi: 10.4088/JCP.v69n0105. [DOI] [PubMed] [Google Scholar]
- 24.Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fregni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neurosci Lett. 2009;463:82–86. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 25.Fecteau S, Agosta S, Hone-Blanchet A, Fregni F, Boggio P, Ciraulo D, et al. Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend. 2014;140:78–84. doi: 10.1016/j.drugalcdep.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng Z, Liu C, Yu C, Ma Y. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates smoking behavior. J Psychiatr Res. 2014;54:19–25. doi: 10.1016/j.jpsychires.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Fregni F, Brody AL, Rahman AS. Transcranial direct current stimulation reduces negative affect but not cigarette craving in overnight abstinent smokers. Front Psychiatry 2013, 4. [DOI] [PMC free article] [PubMed]
- 28.Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry. 2003;64:951–953. doi: 10.4088/JCP.v64n0815. [DOI] [PubMed] [Google Scholar]
- 29.Johann M, Wiegand R, Kharraz A, Bobbe G, Sommer G, Hajak G, et al. Repetitive transcranial magnetic stimulation in nicotine dependence. Psychiatrische Praxis. 2003;30:129–131. doi: 10.1055/s-2003-39733. [DOI] [PubMed] [Google Scholar]
- 30.Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104:653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- 31.Dieler AC, Dresler T, Joachim K, Deckert J, Herrmann MJ, Fallgatter AJ. Can intermittent theta burst stimulation as add-on to psychotherapy improve nicotine abstinence? Results from a pilot study. Eur Addict Res. 2014;20:248–253. doi: 10.1159/000357941. [DOI] [PubMed] [Google Scholar]
- 32.Rose JE, McClernon FJ, Froeliger B, Behm FM, Preud’homme X, Krystal AD. Repetitive transcranial magnetic stimulation of the superior frontal gyrus modulates craving for cigarettes. Biol Psychiatry. 2011;70:794–799. doi: 10.1016/j.biopsych.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Feil J, Zangen A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev. 2010;34:559–574. doi: 10.1016/j.neubiorev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 34.McClernon FJ, Kozink RV, Rose JE. Individual differences in nicotine dependence, withdrawal symptoms, and sex predict transient fMRI-BOLD responses to smoking cues. Neuropsychopharmacology. 2008;33:2148–2157. doi: 10.1038/sj.npp.1301618. [DOI] [PubMed] [Google Scholar]
- 35.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Screening questionnaire before TMS: An update. Clin Neurophysiol. 2011;122:1686–1686. doi: 10.1016/j.clinph.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 37.Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic efficacy of neurostimulation for depression: techniques, current modalities, and future challenges. Neurosci Bull. 2016;32:115–126. doi: 10.1007/s12264-015-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wing VC, Barr MS, Wass CE, Lipsman N, Lozano AM, Daskalakis ZJ, et al. Brain stimulation methods to treat tobacco addiction. Brain Stimul. 2013;6:221–230. doi: 10.1016/j.brs.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Pripfl J, Tomova L, Riecansky I, Lamm C. Transcranial magnetic stimulation of the left dorsolateral prefrontal cortex decreases cue-induced nicotine craving and EEG delta power. Brain Stimul. 2014;7:226–233. doi: 10.1016/j.brs.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: A tDCS-fMRI study. Hum Brain Mapp. 2014;35:3673–3686. doi: 10.1002/hbm.22429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reichenbach N, Karch S, Klemme J, Keeser D, Ludwig H, Zeren A, et al. EPA-1578-Modifications of human brain activity due to transcranial direct current stimulation (tdcs) in patients with nicotine dependence. Eur Psychiatry. 2014;29:1. doi: 10.1016/S0924-9338(14)78734-6. [DOI] [Google Scholar]
- 42.Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: the elaborated intrusion theory of desire. Psychol Rev. 2005;112:446. doi: 10.1037/0033-295X.112.2.446. [DOI] [PubMed] [Google Scholar]
- 43.Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 44.Vorel SR, Liu X, Hayes RJ, Spector JA, Gardner EL. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 45.Krause B, Kadosh RC. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front Syst Neurosci 2014, 8. [DOI] [PMC free article] [PubMed]
- 46.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci. 2008;12:447–454. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Tseng P, Hsu T-Y, Chang C-F, Tzeng OJ, Hung DL, Muggleton NG, et al. Unleashing potential: transcranial direct current stimulation over the right posterior parietal cortex improves change detection in low-performing individuals. J Neurosci. 2012;32:10554–10561. doi: 10.1523/JNEUROSCI.0362-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vanneste S, Focquaert F, Van de Heyning P, De Ridder D. Different resting state brain activity and functional connectivity in patients who respond and not respond to bifrontal tDCS for tinnitus suppression. Exp Brain Res. 2011;210:217–227. doi: 10.1007/s00221-011-2617-z. [DOI] [PubMed] [Google Scholar]
- 49.McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 50.Smolka MN, Bühler M, Klein S, Zimmermann U, Mann K, Heinz A, et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology. 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- 51.Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav. 2014;39:789–792. doi: 10.1016/j.addbeh.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okuyemi KS, Powell JN, Savage CR, Hall SB, Nollen N, Holsen LM, et al. Enhanced cue-elicited brain activation in African American compared with Caucasian smokers: an fMRI study. Addict Biol. 2006;11:97–106. doi: 10.1111/j.1369-1600.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- 53.Han S, Ma Y. Cultural differences in human brain activity: A quantitative meta-analysis. NeuroImage. 2014;99:293–300. doi: 10.1016/j.neuroimage.2014.05.062. [DOI] [PubMed] [Google Scholar]
- 54.Pan P, Shi H, Zhong J, Xiao P, Shen Y, Wu L, et al. Chronic smoking and brain gray matter changes: evidence from meta-analysis of voxel-based morphometry studies. Neurol Sci. 2013;34:813–817. doi: 10.1007/s10072-012-1256-x. [DOI] [PubMed] [Google Scholar]
- 55.Yu R, Zhao L, Lu L. Regional grey and white matter changes in heavy male smokers. PLoS One. 2011;6:e27440. doi: 10.1371/journal.pone.0027440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17:977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- 57.Almeida OP, Garrido GJ, Lautenschlager NT, Hulse GK, Jamrozik K, Flicker L. Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. Am J Geriatr Psychiatry. 2008;16:92–98. doi: 10.1097/JGP.0b013e318157cad2. [DOI] [PubMed] [Google Scholar]
- 58.Skinner MD, Aubin HJ. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34:606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Wray JM, Gass JC, Tiffany ST. A Systematic Review of the Relationships Between Craving and Smoking Cessation. Nicotine Tob Res. 2013;15:1167–1182. doi: 10.1093/ntr/nts268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sporns O. Contributions and challenges for network models in cognitive neuroscience. Nat Neurosci. 2014;17:652–660. doi: 10.1038/nn.3690. [DOI] [PubMed] [Google Scholar]
- 61.Sporns O. Towards network substrates of brain disorders. Brain. 2014;137:2117–2118. doi: 10.1093/brain/awu148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sale MV, Mattingley JB, Zalesky A, Cocchi L. Imaging human brain networks to improve the clinical efficacy of non-invasive brain stimulation. Neurosci Biobehav Rev. 2015;57:187–198. doi: 10.1016/j.neubiorev.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Fox MD, Buckner RL, Liu H, Chakravarty MM, Lozano AM, Pascual-Leone A. Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc Natl Acad Sci USA. 2014;111:E4367–E4375. doi: 10.1073/pnas.1405003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sack AT. Cohen Kadosh R, Schuhmann T, Moerel M, Walsh V, Goebel R. Optimizing functional accuracy of TMS in cognitive studies: a comparison of methods. J Cogn Neurosci. 2009;21:207–221. doi: 10.1162/jocn.2009.21126. [DOI] [PubMed] [Google Scholar]
- 65.Clark VP, Coffman BA, Mayer AR, Weisend MP, Lane TDR, Calhoun VD, et al. TDCS guided using fMRI significantly accelerates learning to identify concealed objects. Neuroimage. 2012;59:117–128. doi: 10.1016/j.neuroimage.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]