Abstract
The purpose of this study was to investigate the blood levels of methadone in participants receiving methadone for the treatment of opioid dependence. After stabilization on methadone for four weeks, blood samples from 95 participants were collected between treatment weeks 4 and 12, before and after receiving doses of methadone, and its blood levels were measured. A multiple linear regression model was used to examine the association between methadone blood levels and the outcomes of methadone maintenance treatment (MMT). Outcome differences between participants who had high (≥2) or low (<2) peak-to-trough ratios were also compared using an independent sample t-test. The blood level of methadone was not correlated with the clinical outcome of MMT with the moderate range of doses given. However, the retention of patients who had a free peak-to-trough ratio >2 was significantly poorer than those whose ratio was <2. Thus, monitoring plasma methadone levels is unlikely to be effective for guiding dosing decisions in situations where compliance with MMT is already very high or when the methadone dose is no longer the dominant factor in determining the clinical outcome. However, monitoring plasma methadone levels is still helpful for guiding the dosage for patients with a rapid metabolism.
Keywords: Methadone, Plasma level, Treatment outcome, Metabolism
Introduction
Methadone maintenance treatment (MMT) has been shown to be effective in preventing the harm associated with opiate use. Extensive evidence has accumulated showing that effective strategies are needed to improve its treatment outcome. Plasma methadone levels may guide practitioners’ dosage decisions [1, 2]; however, studies on how to optimize the methadone dose based on its blood levels have shown mixed results that need to be verified in different settings and populations.
MMT patients have shown variable performance in previous studies, due in part to the complexities of methadone metabolism. First, due to its high lipid solubility, 98% of the methadone that reaches the central compartment is rapidly transferred to tissues, only 1%–2% remaining in the blood compartment. Since 60%–90% of the methadone in the blood is bound to plasma proteins, the extent of protein binding is of obvious importance for its activity. The elimination half-life of methadone could also be important for the appearance of withdrawal symptoms [3]. However, the blood level of free methadone and the rate of its metabolism have received little attention in clinical practice. Second, methadone metabolism, including its plasma level and protein binding, differs in different populations and in a single person under different conditions [4–6]. Polymorphisms in the genes coding methadone-metabolizing enzymes and transporter proteins may explain part of the observed variation in the blood levels of methadone among different populations [7].
China has developed an extensive national network of MMT clinics that has cumulatively served >384,500 heroin users. However, the dropout rate during the first 6 months is still high and the average methadone dose administered is lower than that recommended in the guidelines (60–100 mg) [8, 9]. Qualitative research has revealed that uncertainty among clinical staff regarding the optimal dosage strategy causes many to under-dose patients [10]. Since this strategy is derived from research on Western populations, obtaining empirical data on methadone metabolism and blood levels among Chinese patient populations will be helpful to supplement our understanding of possible variations in its metabolism among different ethnic groups.
In order to fill the knowledge gaps discussed above as well as improve the procedures and outcomes of the MMT program in China, with its huge population in need of more effective treatment, this study was designed to obtain direct data on the peak/trough and free/bound blood levels of methadone, along with urine test results from a group of heroin-dependent patients on maintenance treatment after stabilization at four MMT clinics in Shanghai. The aim was to determine the relationship between methadone blood levels and clinical outcomes of MMT in order to improve the personalized dosage strategy and outcomes of the MMT program in China.
Methods
Design
This study was an open label study, in which part of the sample data were obtained from the usual care control group of a randomized controlled trial designed to compare the effects of Cognitive Behavioral Treatment on MMT adherence with the usual care control. Participants were recruited from four MMT clinics in Shanghai, China, and provided with treatment for 12 weeks. Urine samples for toxicology testing were obtained at baseline and weekly thereafter. Blood samples were collected for methadone blood level tests after 4 weeks of treatment. The study was approved by the Review Board of Shanghai Mental Health Centre (SMHC IRB: 2009036) in December 2009.
Participants
Patients were recruited via flyers distributed in MMT clinics attached to Mental Health Centers in the Pudong, Hongkou, Xuhui, and Yangpu districts of Shanghai, China, from December 2009 to June 2010. These Centers are state-owned institutions providing medical management of the prevention, clinical intervention, and rehabilitation of patients with mental health disorders. All participants provided signed informed consent.
Patients who had received a stable dose of methadone for at least 4 weeks in the MMT clinics were invited to participate. They were first-time MMT patients or had not received methadone for several years. Eligibility criteria included being between 18 and 65 years old, having received a Diagnostic and Statistical Manual of Mental Disorders Version 4 (DSM-IV) diagnosis of heroin dependence, and not enrolled in any other study program at the time of recruitment. Patients with serious physical conditions or major mental disorders were excluded from participation.
Measures
The characteristics and drug history data of participants were collected at baseline. All clinic visits were recorded and urine was tested for opiates weekly during the 12-week duration of the study. Assessments included: (1) a Chinese translation of the Addiction Severity Index (ASI) that has good reliability and validity in China [11, 12] was used to assess physical and mental health, employment, family function, drug and alcohol use, and legal information; and (2) the methadone blood level was measured before and after methadone administration to capture the free/bound trough and free/bound peak values.
Procedures
Screening assessments included an interview to collect demographic and drug-use information and lab tests. The clinical files of consenting participants were reviewed to ascertain general health, prescribed medication, urine drug-test results, diagnosis of opioid and other drug dependence, age, pregnancy status, clinic attendance, and stability of residence. Eligible participants were enrolled and provided with MMT for 12 weeks, with urine drug testing weekly. The results of the drug tests were discussed with the patients who were encouraged to use the MMT treatments regularly and to keep their urine results negative. After 4 weeks of treatment, when the dose was stable, two blood samples were taken during one clinic visit. One sample was collected before receiving methadone and this was considered to be the trough level, and a second was collected ~3 h later and this was considered to be the peak level of methadone.
The standardized MMT involves daily visits to the clinic, administration of methadone in the clinic at the dosage determined by the treating physician (based on the client’s condition), regular urine and blood testing, and monitoring in the community by specialized social workers.
The protocol recommends that patients initiating treatment be given a first dose of 15–30 mg at least 4 h after last use of opiates. The physician can re-prescribe methadone within 3–24 h after the first dose if withdrawal is intolerable. After 2 days of this initial stage of treatment, physicians adjust the dose by 5–10 mg to find one suitable for the patient during the next 3–10 days until the patient is maintained at that dose. No minimum or maximum dose is specified, although the protocol suggests 60 mg/day.
The cost of this treatment is 10 Chinese Yuan (1.60 US Dollars) per visit; none of the health insurance packages available in Shanghai cover this cost, so the patients had to pay this fee themselves. Participants could be given a total of 30 US Dollars for participation in the study; 10 US Dollars for screening and 20 US Dollars for providing blood samples for methadone level testing.
Analyses
‘Retention’ was defined as the number of days over the total observation period (12 weeks) of treatment that the participant attended the clinic and received methadone. Compliance (i.e., remaining drug-free) was defined in two ways: the longest continuous period (in weeks) of negative urine tests in the 12 weeks of treatment and the total number of negative urine tests over the 12 weeks. An intention to treat (ITT) analysis was used, so participants who dropped out of the MMT or did not appear for the urine tests were assumed to have positive urine tests.
Statistical analyses were conducted using version 16.0 of SPSS® (SPSS, Inc., Chicago, IL). With adjustment for the dose on the day of blood sampling, the relationship between blood level (free/bound and peak/trough levels) and outcomes (retention and compliance) was analyzed using a multiple linear regression model. Simple linear regression analysis was used to assess the association between daily methadone dose and outcomes. An independent sample t-test was used to compare the differences in outcomes between participants who had high (≥2) or low (<2) peak-to-trough ratios. The significance level for all statistical tests was set at P < 0.05 (two-tailed).
Results
Demographics and Clinical Data
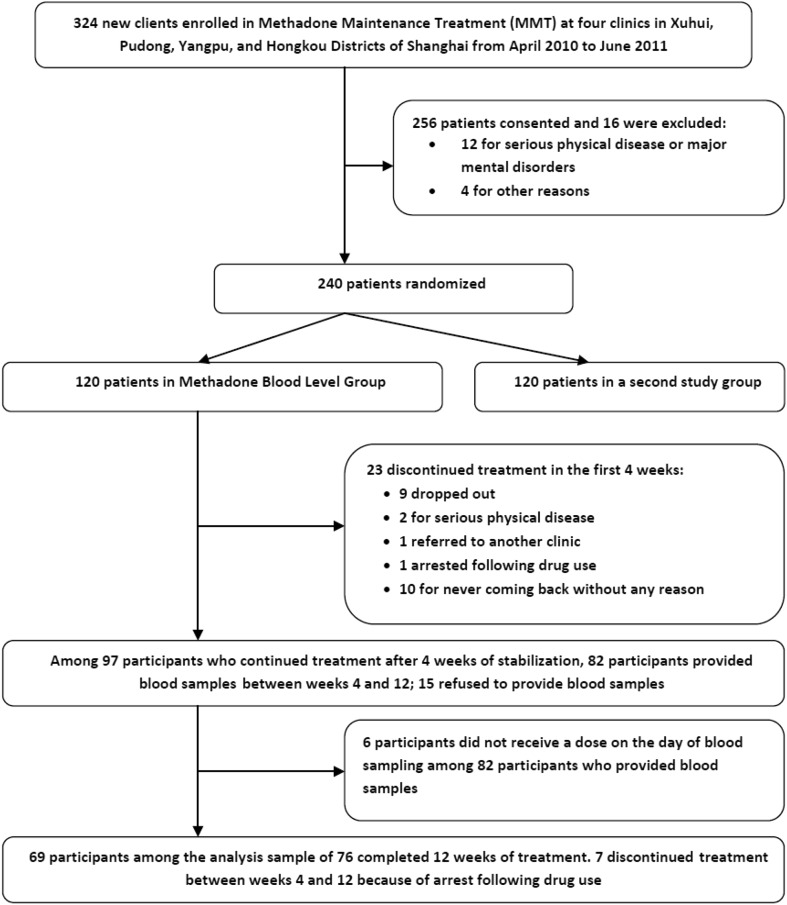
A total of 324 heroin-dependent participants newly entered one of the MMT programs between December 2009 and June 2010 and were invited to participate. Of 256 who were interested, 12 were excluded due to serious physical diseases or major mental disorders, and 4 were excluded for other reasons, leaving 240 enrolled patients. Those who consented were randomized using simple randomization tables generated by the SPSS software package (SEED: 210002) for each of the four clinics; 120 were assigned to the methadone blood level group and 120 to a Cognitive Behavioral Treatment group (used in another study). A total of 82 participants provided blood samples between weeks 4 and 12. Among these, 6 did not receive a dose on the day of blood sampling. At the end of week 12, 69 participants among the analysis sample of 76 had not dropped out. The baseline socio-demographic and drug use characteristics of the analysis sample of 76 is shown in Table 1. Almost 80% were male; >70% were unemployed or had spent time in prison in the 3 years prior to the baseline interview; most (72.4%) had an unstable marital status; they had, on average, a 9-year history of abuse; and more than half had injection behavior. There were no significant differences in socio-demographic and drug use characteristics between the analysis sample of 76 and the rest in the larger group of 120 patients (Fig. 1).
Table 1.
Baseline socio-demographic and drug-use characteristics.
| (n=76) | |
|---|---|
| Socio-demographic characteristics | |
| Gender; male, % (n) | 78.9 (60) |
| Age; years, mean ± SD | 40.1 ± 9.2 |
| Ethnic group: Han, % (n) | 98.7 (75) |
| Years of education | 9.9 ± 2.0 |
| Employment status in 3 years prior to baseline interview, % (n) | |
| Employed (full-time and part-time) | 27.7 (21) |
| Incarcerated | 1.3 (1) |
| Unemployed | 71 (54) |
| Marital status | |
| Married, % (n) | 27.6 (21) |
| Number of criminal offences | 0.3 ± 0.9 |
| Drug use characteristics | |
| Mean lifetime years of heroin use | 8.9 ± 4.1 |
| Average daily dose, g | 0.8 ± 0.7 |
| Injected drug-use, % (n) | 57.9 (44) |
Fig. 1.
Study schema.
Plasma Levels and MMT Outcomes
At the end of 12 weeks of treatment, 69 participants (90.8%) remained in treatment. Over the 12 weeks the participants remained in the MMT for 81.6 ± 8.9 (mean ± SD) days, and the mean longest drug-free interval was 8.0 ± 3.9 weeks. Based on the intention-to-treat analysis which considered missing urine tests to be positive, the mean number of negative urine samples during the 12 weeks was 8.7 ± 3.5. The mean daily methadone dose over the entire 12 weeks was 46.8 ± 24.3 mg, which could be considered to be moderate to low. The mean dose at blood sampling and the mean methadone blood level are listed in Table 2.
Table 2.
Treatment outcomes, methadone doses, and methadone blood levels.
| (n = 76) | |
|---|---|
| Treatment outcomes | |
| Drop-outs during weeks 4–12, % (n) | 9.2 (7) |
| Retention, days | 81.6 ± 8.9 |
| Longest continuous period of negative urine tests, weeks | 8.0 ± 3.9 |
| No. of negative urine samples | 8.7 ± 3.5 |
| Methadone doses and blood levels | |
| Average dose weeks 4–12, mg | 46.8 ± 24.3 |
| Mean dose at blood sampling, mg | 50.9 ± 25.3 |
| Total trough, ng/mL | 222.0 ± 132.3 |
| Free trough, ng/mL | 11.6 ± 7.3 |
| Total peak, ng/mL | 356.2 ± 203.7 |
| Free peak, ng/mL | 18.8 ± 11.4 |
| Free peak to free trough ratio, % | 1.716 ± 0.553 |
| Total peak to total trough ratio, % | 1.766 ± 0.636 |
Correlations and Comparison Between Participants Who Have High (≥2) or Low (<2) Peak-to-Trough Ratios
Five of the 7 who dropped out during the 12 weeks were from the group with a high (≥2) peak-to-trough ratio. The findings from regression prediction of retention and compliance are presented in Table 3. No significant correlation was found between methadone blood level and treatment outcome. Neither were there significant correlations between the average daily methadone dose and retention or compliance. Table 4 shows the results of comparison of treatment outcomes between participants with high (≥2) or low (<2) free peak-to-trough ratios. Participants who had a low free peak-to-trough ratio were more likely to have a long retention (t = 2.343, P = 0.022); however, they were not likely to have a longer ‘longest continuous period of negative urine tests’ (t = 0.279, P = 0.781), and were not likely to have a lower ‘number of negative urine samples’ (t = 0.231, P = 0.808).
Table 3.
Relationship between methadone plasma blood level and treatment outcome during weeks 4–12 after 4 weeks of stabilization on methadone maintenance treatment.
| Variable (n = 76) | Retention, parameter vector, B (95% CI) | Longest continuous period (weeks) of negative urine tests, parameter vector, B (95% CI) | Total number of negative urine tests, parameter vector, B (95% CI) |
|---|---|---|---|
| Free peaka | −0.022 (−0.128, 0.084) | 0.002 (−0.043, 0.048) | −0.003 (−0.045, 0.039) |
| Free trougha | 0.301 (−0.049, 0.661) | 0.147 (−0.007, 0.301) | 0.129 (−0.012, 0.270) |
| Total peaka | −0.017 (−0.112, 0.078) | 0.008 (−0.033, 0.049) | 0.003 (−0.034, 0.041) |
| Total trougha | −0.040 (−0.134, 0.054) | 0.005 (−0.036, 0.046) | 0.001 (−0.037, 0.038) |
| Average dose | 0.001 (−0.084, 0.087) | 0.033 (−0.003, 0.070) | 0.027 (−0.006, 0.060) |
No results were significantly different.
aAdjusted by dose on day of blood sampling.
Table 4.
Comparison of treatment outcomes between participants with high (≥2) or low (<2) free peak-to-trough ratios.
| Variable (n = 76) | Free peak-to-trough ratio <2 (n = 56) | Free peak-to-trough ratio ≥2 (n = 20) | t value | P value |
|---|---|---|---|---|
| Retention, mean ± SD, days | 82.9 ± 6.1 | 77.7 ± 13.6 | 2.342 | 0.022 |
| Longest continuous period of negative urine tests, weeks | 8.0 ± 3.9 | 7.8 ± 4.2 | 0.279 | 0.781 |
| No. of negative urine samples | 8.7 ± 3.5 | 8.5 ± 3.8 | 0.231 | 0.818 |
Discussion
To our knowledge, this is the first study in which direct data were obtained on the blood levels of methadone in a Chinese population. We examined the relationship between methadone blood level and clinical outcome among heroin-dependent patients on maintenance treatment after stabilization at MMT clinics in Shanghai, China. Our findings suggest that methadone blood levels are not significant predictors to MMT outcome when participants are given relatively low doses of methadone. Our results also suggest that participants with a high free peak-to-trough ratio perform worse on retention than those who have a low ratio.
We believe the main reasons our study failed to replicate western studies that have demonstrated a correlation between methadone blood level and MMT clinical outcome [2, 13] are the social and political factors which led to the unexpected high compliance with a low range of daily doses of methadone (only 7 of 76 participants dropped out, and the mean daily dose was ~50 mg). Factors contributing to the high compliance in this study included the fact that the effectiveness of anti-drug social workers in Shanghai has been gradually improving [14] and the diligence of the Shanghai public security system in combating drug abuse and drug trafficking during the 2010 Shanghai World Exposition (this study was conducted during the Exposition). Thus, plasma monitoring is not of much value when the MMT outcome is strongly affected by environmental factors.
We also found that the retention of participants who had a free peak-to-trough ratio >2 was significantly poorer than those whose ratio was <2. Since arrest on the charge of relapse was the main reason why they dropped out of the voluntary outpatient MMT program (7 patients were arrested on this charge), and the overall proportion of relapses was relatively small (<15%), it is unlikely that the dropouts led to biased results. Prior evidence has shown that the rate of decline of the methadone plasma level from peak to trough is correlated with the severity of withdrawal [13, 15]. Thus, poor retention among patients who had a high free peak-to-trough ratio could be explained by the rapid decline in plasma concentrations to levels below the effective concentration, which also could translate into dropout due to withdrawal toward the end of the dosing interval. Evidence has shown that the trough blood level of methadone can be used to decide whether the dose is adequate and effective since it is correlated with withdrawal symptoms [16, 17]. However, use of the trough blood level as a guide to dosing for patients with a rapid decline in plasma concentration is not likely to be helpful. For those cases who have rapid metabolism, to split the daily dose according to the peak-to-trough ratio, rather than increasing the dose according to trough blood concentration, would be a more suitable strategy [13].
Limitations and Implications
The study findings should be considered within the context of the limitations. First, due to lack of withdrawal data, we were not able to check whether the daily dose was adequate from a clinical perspective. Second, all participants were recruited from the MMT programs in Shanghai, so it might not be possible to generalize our results to heroin-dependent patients who have enrolled in MMT in other areas of China.
The most important message from the study is that monitoring plasma methadone levels is unlikely to be effective for guiding dosing decisions in situations where compliance with MMT is already very high or when the methadone dose is no longer the dominant factor in determining the clinical outcome. Optimizing the methadone dose should not be based on blood levels alone, but plasma monitoring should still be involved in this complex decision-making strategy due to its advantage in identifying and guiding the dosage for patients with a rapid metabolism.
Acknowledgements
This work was supported by the Research Project of Shanghai Municipal Health and Family Planning Commission, China (2013SY011 and 2014ZYJB0002), the National Natural Science Foundation of China (81271468), Doctoral Supervisor Funding from the Ministry of Education of China (20120073110089), and Research Funding from Shanghai Key Laboratory of Severe Mental illness, China (13dz2260500). We thank the patients and staff of the four Shanghai MMT clinics who participated in this study.
References
- 1.Loimer N, Schmid R. The use of plasma levels to optimize methadone maintenance treatment. Drug Alcohol Depend. 1992;30(3):241–246. doi: 10.1016/0376-8716(92)90058-K. [DOI] [PubMed] [Google Scholar]
- 2.Mohamad N, Salehuddin RM, Ghazali B, Bakar NHA, Musa N, Ibrahim MA, et al. Plasma methadone level monitoring in methadone maintenance therapy: A personalised methadone therapy. In: Gowder S (Ed.), New insights into toxicity and drug testing. InTech; 2013, 236–237.
- 3.Nilsson MI, Grönbladh L, Widerlöv E, Änggård E. Pharmacokinetics of methadone in methadone maintenance treatment: characterization of therapeutic failures. Eur J Clin Pharmacol. 1983;25(4):497–501. doi: 10.1007/BF00542117. [DOI] [PubMed] [Google Scholar]
- 4.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone. Clin Pharmacokinet. 2002;41(14):1153–1193. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 5.Abramson FP. Methadone plasma protein binding: Alterations in cancer and displacement from α1-acid glycoprotein. Clin Pharmacol Ther. 1982;32(5):652–658. doi: 10.1038/clpt.1982.217. [DOI] [PubMed] [Google Scholar]
- 6.Calvo R, Aguirre C, Troconiz I, López J, Garrido M. Alpha1-acid glycoprotein and serum protein binding of methadone in heroin addicts during withdrawal. Int J Clin Pharmacol Ther. 2000;38(1):35–40. doi: 10.5414/CPP38035. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Kantelip J, Schieveen PG, Davani S. Interindividual variability of methadone response: impact of genetic polymorphism. Mol Diagn Ther. 2008;12(2):109. doi: 10.1007/BF03256276. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan SG, Wu Z, Rou K, Pang L, Luo W, Wang C, et al. Who uses methadone services in China? Monitoring the world’s largest methadone programme. Addiction. 2015;110(S1):29–39. doi: 10.1111/add.12781. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan SG, Wu Z, Detels R. National methadone maintenance treatment working group. Time to first treatment interruption in the Chinese methadone maintenance treatment programme. Drug Alcohol Depend. 2013;133(2):427–432. doi: 10.1016/j.drugalcdep.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 10.Lin C, Detels R. A qualitative study exploring the reason for low dosage of methadone prescribed in the MMT clinics in China. Drug Alcohol Depend. 2011;117(1):45–49. doi: 10.1016/j.drugalcdep.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao M, Li X, Hao W, Wang Z, Zhang M, Xu D. A preliminary study of the reliability and validity of the Addiction Severity Index. J Chin Med Res. 2004;4(8):679–680. [Google Scholar]
- 12.Luo W, Wu Z, Wei X, Jia W, Zhang Q, Li L, et al. Chinese assimilation of the fifth edition of Addiction severity index scale and the evaluations on its applications in addiction status investigation. Chin J Drug Depend. 2007;16(5):373–376. [Google Scholar]
- 13.Dyer KR, Foster DJ, White JM, Somogyi AA, Menelaou A, Bochner F. Steady-state pharmacokinetics and pharmacodynamics in methadone maintenance patients: Comparison of those who do and do not experience withdrawal and concentration-effect relationships. Clin Pharmacol Ther. 1999;65(6):685–694. doi: 10.1016/S0009-9236(99)90090-5. [DOI] [PubMed] [Google Scholar]
- 14.Sun A. Review and prospective for self-organized peer education. Anti-drug Social Work. 2010;4(17):5–8. [Google Scholar]
- 15.Crettol S, Déglon JJ, Besson J, Croquette-Krokkar M, Gothuey I, Hämmig R, et al. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78(6):593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Chitasombat P, Willenbring ML, Maddux T, Severeide N. Reliability of methadone plasma levels in methadone maintenance. Am J Addict. 1995;4(4):351–355. doi: 10.1111/j.1521-0391.1995.tb00274.x. [DOI] [Google Scholar]
- 17.Drozdick J, Berghella V, Hill M, Kaltenbach K. Methadone trough levels in pregnancy. Am J Obstet Gynecol. 2002;187(5):1184–1188. doi: 10.1067/mob.2002.127132. [DOI] [PubMed] [Google Scholar]