Abstract
Previous studies have suggested that cortical functional reorganization is associated with motor recovery after stroke and that normal afferent sensory information is very important in that process. In this study, we selected patients who had a stroke in or under the thalamus, with potentially impaired afferent sensory information and analyzed the differences between these patients and healthy controls at three levels: brain regions, the functional connectivity between brain areas, and the whole-brain functional network. Compared with healthy controls, regional homogeneities in the left middle temporal gyrus decreased and functional connectivity between the left middle temporal gyrus and the stroke area increased in the patients. However, there was no significant change in the whole-brain functional network. By focusing on stroke located in or under the thalamus, our study contributes to wider inquiries into understanding and treating stroke.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0064-3) contains supplementary material, which is available to authorized users.
Keywords: ReHo, Stroke, Thalamus, Resting-state
Introduction
Stroke is a common condition that can cause various degrees of neurological defects with a high probability of long-term disability [1–5]. Using functional magnetic resonance imaging (fMRI), previous studies have shown that an activation response to upper limb stimulation may be absent in the somatosensory cortical representation after stroke [6]. Cortical functional reorganization is closely associated with motor recovery following stroke [7]. However, the neural mechanisms of rehabilitation after stroke are unclear because stroke can occur in various regions of the brain. To understand the neural mechanisms of rehabilitation after stroke in a specific location and to provide personalized treatment to patients with strokes in different locations in the future, it is necessary for investigations to isolate stroke locations.
The thalamus has multiple neuronal connections with cortical areas such as those involved in emotion, arousal, and a variety of cognitive functions [8]. Most information is transmitted to the cerebral cortex from the spinal nerves via many subcortical structures. The thalamus and regions below it have more transmission fibers than other regions. Further, most external information from the spinal cord to the brain passes through the thalamus and regions under it. According to the cortico-thalamo-cortico loop theory, after the information from primary sensory afferents reaches the cortex, thalamic regions innervated by cortical drivers in turn relay these signals to other regions via ascending thalamo-cortical projections [9]. The temporal gyrus and the occipital gyrus are important areas in the thalamo-cortical circuit [10]. If stroke occurs in or below the thalamus, many cortical areas involved in the thalamo-cortical circuit, such as the temporal and occipital gyri might be affected and corresponding disorders might occur. For more effective treatment after stroke, it is important to study the changes of brain function after stroke in or under the thalamus.
Resting-state fMRI (rs-fMRI) has been used widely [11–14]. In stroke patients, altered resting-state connectivity and corresponding neurological deficits have been demonstrated in recent studies [2, 11]. And scientists generally study brain function in the resting-state at three levels: (1) brain region, (2) functional connectivity between brain areas, and (3) the whole-brain functional network. In this study, we investigated 8 acute ischemic stroke patients whose stroke was in or under the thalamus and 19 healthy controls using rs-fMRI and analyzed the differences between them using three methods: regional homogeneity (ReHo), functional connectivity, and the graph theoretical approach.
ReHo [15] is a method that maps regional spontaneous activity and is calculated as Kendall’s coefficient of concordance (KCC). The ReHo value reflects, to some extent, the level of brain function. A stroke in or under the thalamus might affect the function of the stroke area and some cortical regions because of the injured sensory afferents and the injured ascending thalamo-cortical projections [9]. Previous studies [16, 17] have shown that stroke patients exhibit changed ReHo. Therefore, we hypothesized that the ReHo of the stroke area and some other cortical areas might be changed.
Functional connectivity is a method that measures the connectivity among different brain regions in different spaces using the connectivity of time series. A previous study [18] has shown that the functional connectivity increases as a compensatory effect after a stroke. This kind of effect might occur in our study. Therefore, we hypothesized that the functional connectivity between the functional regions that were injured would increase because of the compensatory effect.
The whole-brain functional network is generally measured using graph theoretical approaches, and can be displayed graphically by a collection of nodes and edges. Changed topological properties of brain networks have been found in many psychiatric and neurological disorders, including stroke [19–22]. Therefore, we also tried to detect differences in the whole-brain functional network between the patients and healthy controls as an exploratory analysis.
Materials and Methods
Participants
Eight patients with acute ischemic stroke (stroke located in or under the thalamus, P group, mean age 54.7 ± 9.6 years) participated in our study. All patients had had a stroke in the previous 7 days, felt limb numbness on one side, and had no psychiatric disorders. We also recruited 19 healthy participants (H group, mean age 57.8 ± 8.9 years) as controls. There were no significant differences in age (P = 0.440) and gender (P = 0.527) between the two groups. Participants were asked to relax, close their eyes, think of nothing systematically, remain motionless, and not fall asleep. After scanning, every participant was asked about his/her waking state during scanning, and none reported having fallen asleep. The demographic profiles and clinical features of all participants are listed in Table S1. Informed consent was given by all participants and the study was approved by the Ethics Committee at the University of Science and Technology of China.
Data Acquisition
All images were acquired on a 3.0 Tesla GE-Signa HDxt MRI scanner (General Electric, Milwaukee, WI) at the Medical Imaging Center at the First Affiliated Hospital of Wannan Medical College. The rs-fMRIs were obtained in axial orientation using the echo-planar imaging sequence TR = 3 s, number of slices = 30, TE = 40 ms, matrix = 96 × 96, FOV = 240 × 240 mm2, FA = 90°, voxel size = 2.5 × 2.5 × 5 mm3, slice thickness = 5 mm with no gap. We had three dummies before the resting scan to reach the signal equilibrium. Then a total of 128 images were acquired on each participant in 6 min and 24 s and all participants were instructed to close their eyes without thinking anything and without falling asleep during the resting-state scanning. After the resting-state scanning, anatomical T1-weighted images were acquired in axial orientation for each participant (TR = 6.28 ms, TE = 2 ms, number of slices = 248, FOV = 240 × 240 mm2, FA = 15°, matrix = 320 × 256, voxel size = 0.75 × 0.9375 × 1.2 mm3, thickness = 1.2 mm). Axial diffusion-weighted images were also acquired to detect clinically silent lesions (TE = minimum, TR = 4800 ms, spacing = 2 mm, slice thickness = 6 mm, NEX = 2). Two professional radiologists (C.L. and A.W. in the author list) diagnosed the infarct locations.
Image Preprocessing
Preprocessing of data was performed using AFNI software (http://afni.nimh.nih.gov/). The first 2 images of each resting scan were removed. The remaining 126 images were realigned, spatially normalized to the Talairach space and resampled to 3 × 3 × 3 mm3. All maps were spatially smoothed using a 10 mm FWHM (full width at half-maximum) filter. Furthermore, 6 head motion parameters were regressed out from all functional time series of every participant. The anatomical images were partitioned into white matter, gray matter, and cerebrospinal fluid. Then average BOLD signals in white matter and ventricles were removed. A band-pass filter (0.01 to 0.08 Hz) was applied to every time series. We made a flip on 4 patients whose stroke locations were on the left side and then analyzed the data using the methods below.
Resting-State fMRI Analysis
ReHo
Individual ReHo maps were generated from each participant by claculating the KCC value of each voxel in the whole brain, consistent with a previous study [23]. We then normalized each map. Finally, we performed a two-tailed, group t-test between the P and H groups. The group-level t-test map was masked by the gray matter of the brain. Multiple comparison correction was performed using the AFNI AlphaSim program(http://afni.nih.gov/afni/docpdf/AlphaSim.pdf) based on Monte Carlo simulation and the corrected P < 0.05 was used (uncorrected P = 0.005, minimal volume = 918 mm3).
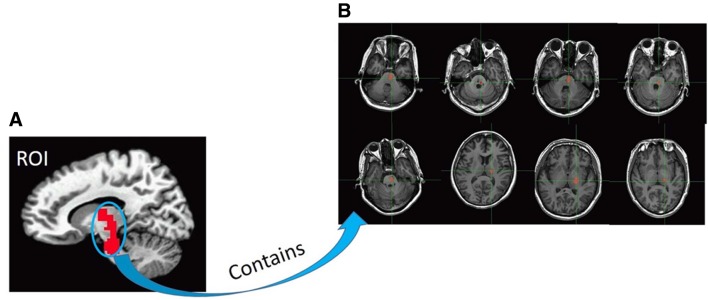
To confirm that the function of the stroke area was impaired, we drew a region-of-interest (ROI) (Fig. 1A) which included each patient’s stroke area. Then we calculated the mean ReHo of the ROI (containing each patient’s stroke area) for each participant and performed a two-tailed, group t-test between the P and H groups.
Fig. 1.
Region of interest (ROI). (A) This ROI contains all stroke areas from each patient. (B) Mask for each patient’s stroke area.
Functional Connectivity
We defined the area in which the ReHo was significantly lower in the P than in the H group as another ROI. We extracted the time series of the two ROIs and calculated the functional connectivity between them for each participant. Then, we performed a one-tailed, group t-test between the P and H groups.
However, a large ROI covering all stroke areas may ignore individual differences and lead to insignificant results. So, to test the reliability of our results, we calculated the individual connectivity between each patient’s individual specific stroke region (Fig. 1B) and the left middle temporal gyrus. We also calculated the corresponding connectivity in healthy participants and took the average of the 19 healthy participants for each connectivity between every stroke area and the left middle temporal gyrus. Then, we performed a one-tailed, group t-test between the P and H groups.
Graph Theoretical Analysis
We performed graph theoretical analysis using Gretna software (http://www.nitrc.org/projects/gretna). After preprocessing, we used an automated anatomical labeling template [24] to extract the time courses of 90 brain regions. We then performed Pearson’s correlation analysis between all possible pairs of the 90 regions to produce a 90 × 90 symmetric correlation matrix for each participant. To increase the normality of the matrix, we performed a Fisher r-to-z transformation.
A previous study [25] suggested that graphs resulting from the above analyses would contain different numbers of edges because of variance in low-level correlations when the same correlation thresholds are used in the correlation matrices of patients and healthy controls. As such, differences across groups in network parameters might not purely reflect the change in topological organization. To control this effect, the network sparsity, which is defined as the number of surviving connections divided by all possible connections, was used as a threshold for producing the graph [22, 25–27].
Following previous studies, we chose the network sparsity as a threshold for producing a binary graph. The range of sparsity was identified as follows: first, to assure that functional connectivity actually existed between nodes, we performed a two-tailed one-sample t-test (P < 0.05, Bonferroni corrected) on the correlation matrices of all participant, and found 1173 significant connections. According to the formula Kcost = E/(N(N–1)/2) (where E is the number of existing edges and N is the number of nodes), an upper bound value of sparsity Kcost = 0.2 was identified. Second, previous studies have shown that functional networks have small-world properties at relatively low cost (i.e., sparsity) [26, 28]. However, the minimum sparsity must assure that each network is fully connected with N nodes. So, the degree K of the graph must be more than ln (N) (here, K > ln (90) ≈ 4.5) [29]. Thus, the lower bound of sparsity was Kcost = N × K/(N(N–1)/2) ≈ 0.1, and K is the average number of edges for each node. Above all, the sparsity was identified as 0.1 ≤ Kcost ≤ 0.2 with an interval of 0.01.
We also calculated shortest path length, clustering coefficients, local efficiency, and global efficiency. We then compared all these between the P and H groups using a two-tailed, group t-test over a wide range of sparsity (P < 0.05, FDR corrected).
Results and Discussion
Our results showed that the mean ReHo of the stroke area (a large ROI covering all stroke areas) in the P group was lower than that in the H group (P = 0.02; Fig. S1). The low ReHo of the stroke area in the P group suggests injured sensory afferents and injured ascending thalamo-cortical projections.
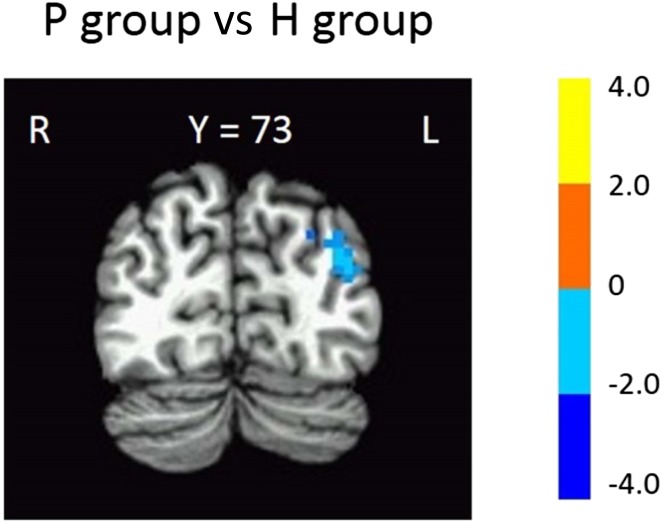
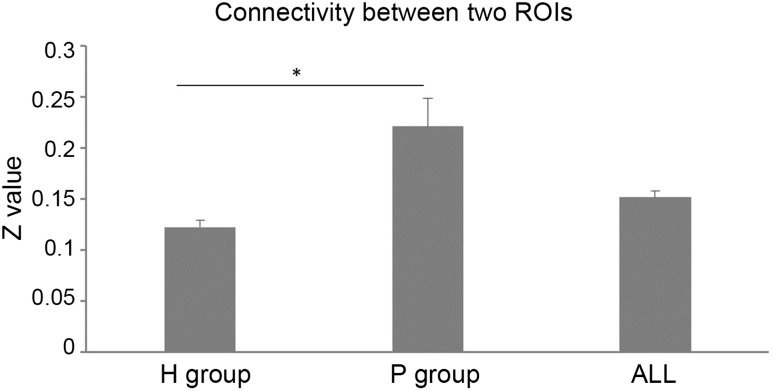
Compared with the H group, the P group had significantly lower ReHo in the left middle temporal gyrus/left superior occipital gyrus (Fig. 2, Table 1) whereas the functional connectivity between the left middle temporal gyrus/left superior occipital gyrus and the stroke area (a large ROI covering all stroke areas) was higher with marginal significance in the P group than in the H group (Fig. 3, P = 0.08). Further, confirmatory individual connectivity analysis (using individual specific ROIs) showed a similar trend (P = 0.09) with results obtained using a large ROI covering all stroke areas.
Fig. 2.
Change of regional homogeneity. Significantly lower ReHo in the left middle temporal gyrus in the P group (stroke patients) than in the H group (healthy controls) (FWE corrected P < 0.05). T-score bars are shown on the right.
Table 1.
Significant differences in ReHo between patient and healthy groups.
| Region | Talairach coordinates | BAa | Number of voxels | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| P group < H group | ||||||
| Left middle temporal gyrus | +37.5 | +73.5 | +23.5 | 19 | 47 | 1269 |
aBrodmann’s area.
Fig. 3.
Change of functional connectivity. Marginally significant increase in functional connectivity between the left middle temporal gyrus and the stroke area in the P group (stroke patients) compared with the H group (healthy controls) (P = 0.08). *P < 0.1. ALL indicates all participants (P group + H group).
The left middle temporal gyrus/left superior occipital gyrus are near BA19 and are involved in the integration of different kinds of sensory input such as somatosensory, visual, and auditory [30]. Another important function of the left middle temporal gyrus is that it is involved in short-term verbal memory [31, 32]. The decreased ReHo in the left middle temporal gyrus/left superior occipital gyrus in the P group might suggest that integration of sensory information and short-term verbal memory are impaired. The increased connectivity between the left middle temporal gyrus/left superior occipital gyrus and the stroke area including the thalamus might reflect compensation. These findings are consistent with those obtained in a previous study [32].
Previous studies have shown significant connectivity between the thalamus and numerous functional regions including the temporal gyrus [33–35]. The temporal gyrus is an important area in the thalamo-cortical circuit [10] and a damaged thalamus could affect the temporal area [8]. Therefore, in our study, the low ReHo of the temporal area might be associated with the impaired thalamo-temporal circuit because of the impaired information input from the thalamus. Furthermore, our results of functional connectivity between these two areas also support this point. Further investigation of this conclusion is needed because our current results lacked behavioral data.
Before data analysis, we flipped all stroke locations that were on the left to the right. However, we found decreased ReHo in the left middle temporal gyrus. In our study, most strokes occurred in the inferior brain stem near the pyramidal decussation and this might explain the decreased ReHo of the middle temporal gyrus on the other side. More studies are required to further support this conclusion.
Besides, our results showed no significant difference in topological properties between the P and H groups (all P > 0.5; Fig. S2), suggesting that strokes in or under the thalamus did not significantly influence the topological properties of the whole-brain functional network in the resting state.
In this study, we targeted strokes located in or below the thalamus when we recruited stroke patients. We did not find significant changes in many brain regions as well as in the whole-brain functional network. These results are inconsistent with those obtained in a previous study in which stroke locations were not isolated [17, 21]. This suggests that specific stroke locations might cause specific local changes but not changes in the whole-brain functional network. However, a potential limitation of our study is that the number of stroke patients was small.
There were several limitations in this study. First, the relatively large age range of participants (43 to 73 years) might have affected the results. However, this large range might also help to generalize our results to a wide range of age groups. Second, the sample size of the P group was relatively small. The sample size of 8 was very small for a resting-state study, so type II errors might have occurred. Future replicative studies should use large samples to confirm or deny our findings. Third, there was no score for symptoms. Fourth, the P value of functional connectivity was 0.08. We believed that this was caused by the small sample of patients. Finally, as this study involved acute cases, it is necessary to do serial follow-ups to investigate sequential changes of ReHo over time; a task we shall undertake in the next phase of this study.
Conclusions
We investigated the differences between patients with specific strokes (in or under the thalamus) and healthy controls. Our results suggest that a stroke in this location might impair local brain function but might not significantly influence the topological properties of the whole-brain functional network. As far as we know, our study is the first to investigate differences between stroke patients whose stroke is located in or under the thalamus and healthy controls using resting-state fMRI. Our results may provide a new direction for treatment, thus, if strokes in this location can cause functional impairment of cortical areas such as the left middle temporal gyrus, then the corresponding cortical areas may present as potential therapeutic targets. Different tools and techniques (e.g., transcranial magnetic stimulation or transcranial direct current stimulation) can be used to stimulate these targets to make them work normally. This study is a positive contribution to wider studies searching for comprehensive understanding and treatment of stroke.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31230032, 31171083, and 31471071), Fundamental Research Funds for the Central Universities of China (WK2070000033), the Natural Science Foundation of Anhui Province, China (1208085MH179), and Hefei Science Center, CAS “User with Potential” (2015HSC-UP017). Some of the numerical calculations in this study were performed with the supercomputing system at the Supercomputing Centre of USTC.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0064-3) contains supplementary material, which is available to authorized users.
Contributor Information
Jian Zhai, Email: yjszhaij@126.com.
Xiaochu Zhang, Email: zxcustc@ustc.edu.cn.
References
- 1.Varsou O, Macleod MJ, Schwarzbauer C. Functional connectivity magnetic resonance imaging in stroke: an evidence-based clinical review. Int J Stroke. 2014;9:191–198. doi: 10.1111/ijs.12033. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Han T, Qin W, Zhang J, Liu H, Li Y, et al. Altered functional connectivity of cognitive-related cerebellar subregions in well-recovered stroke patients. Neural Plast 2013, 2013. doi:10.1155/2013/452439. [DOI] [PMC free article] [PubMed]
- 3.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, et al. Heart disease and stroke statistics-2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 4.Hosp JA, Luft AR. Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plast 2011, 2011. doi:10.1155/2011/871296. [DOI] [PMC free article] [PubMed]
- 5.Faralli A, Bigoni M, Mauro A, Rossi F, Carulli D. Noninvasive strategies to promote functional recovery after stroke. Neural Plast 2013, 2013. doi:10.1155/2013/854597. [DOI] [PMC free article] [PubMed]
- 6.Dijkhuizen RM, van der Marel K, Otte WM, Hoff EI, van der Zijden JP, van der Toorn A, et al. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res. 2012;3:36–43. doi: 10.1007/s12975-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, et al. A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 8.Shim YS, Kim JS, Shon YM, Chung YA, Ahn KJ, Yang DW. A serial study of regional cerebral blood flow deficits in patients with left anterior thalamic infarction: anatomical and neuropsychological correlates. J Neurol Sci. 2008;266:84–91. doi: 10.1016/j.jns.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Basso MA, Uhlrich D, Bickford ME. Cortical function: a view from the thalamus. Neuron. 2005;45:485–488. doi: 10.1016/j.neuron.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 11.Park CH, Chang WH, Ohn SH, Kim ST, Bang OY, Pascual-Leone A, et al. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42:1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Yuan Y, Bai F, Shu H, You J, Li L, et al. Altered functional connectivity networks of hippocampal subregions in remitted late-onset depression: a longitudinal resting-state study. Neurosci Bull. 2015;31:13–21. doi: 10.1007/s12264-014-1489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Du H, Chen N, Guo J, Gong Q, Zhang J, et al. Regional homogeneity abnormalities in patients with tensiontype headache: a resting-state fMRI study. Neurosci Bull. 2014;30:949–955. doi: 10.1007/s12264-013-1468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lei X, Yang T, Wu T. Functional neuroimaging of extraversion-introversion. Neurosci Bull. 2015;31:663–675. doi: 10.1007/s12264-015-1565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YH, Yuan R, Huang YC, Yeh MY, Lin CP, Biswal BB. Disruption of brain connectivity in acute stroke patients with early impairment in consciousness. Front Psychol. 2014;4:956. doi: 10.3389/fpsyg.2013.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin D, Luo Y, Song F, Xu D, Peterson BS, Sun L, et al. Functional reorganization associated with outcome in hand function after stroke revealed by regional homogeneity. Neuroradiology. 2013;55:761–770. doi: 10.1007/s00234-013-1146-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Liu H, Wang L, Yang J, Yan R, Zhang J, et al. Relationship between functional connectivity and motor function assessment in stroke patients with hemiplegia: a resting-state functional MRI study. Neuroradiology. 2016;58:503–511. doi: 10.1007/s00234-016-1646-5. [DOI] [PubMed] [Google Scholar]
- 19.Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Altered functional connectivity and small-world in mesial temporal lobe epilepsy. PLoS One. 2010;5:e8525. doi: 10.1371/journal.pone.0008525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- 21.Yin D, Song F, Xu D, Sun L, Men W, Zang L, et al. Altered topological properties of the cortical motor-related network in patients with subcortical stroke revealed by graph theoretical analysis. Hum Brain Mapp. 2014;35:3343–3359. doi: 10.1002/hbm.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Zhu C, He Y, Zang Y, Cao Q, Zhang H, et al. Altered small-world brain functional networks in children with attention-deficit/hyperactivity disorder. Hum Brain Mapp. 2009;30:638–649. doi: 10.1002/hbm.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu C, Li C, Wu H, Wu Y, Hu S, Zhu Y, et al. Gender differences in cerebral regional homogeneity of adult healthy volunteers: A resting-state fMRI study. Biomed Res Int 2015, 2015. doi:10.1155/2015/183074. [DOI] [PMC free article] [PubMed]
- 24.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 25.He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci. 2008;28:4756–4766. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- 28.Bassett DS, Bullmore ED. Small-world brain networks. Neuroscientist. 2006;12:512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 29.Achard S, Salvador R, Whitcher B, Suckling J, Bullmore ED. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bing D, Chen L. Clinical Neuroanatomy. 1. Beijing: People’s Medical Publishing House; 2007. pp. 349–362. [Google Scholar]
- 31.Raettig T, Kotz SA. Auditory processing of different types of pseudo-words: an event-related fMRI study. Neuroimage. 2008;39:1420–1428. doi: 10.1016/j.neuroimage.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Zhou B, Liu Y, Zhang Z, An N, Yao H, Wang P, et al. Impaired functional connectivity of the thalamus in Alzheimer’s disease and mild cognitive impairment: a resting-state fMRI study. Curr Alzheimer Res. 2013;10:754–766. doi: 10.2174/15672050113109990146. [DOI] [PubMed] [Google Scholar]
- 33.Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex. 2010;20:1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.