Abstract
Background
Iatrogenic pneumothorax is a serious intraoperative complication of immediate breast reconstruction with tissue expanders. However, there is paucity of literature regarding incidence or management of intraoperative pneumothorax in the breast reconstruction patient population.
Methods
We performed a retrospective chart review on prospectively collected data from all patients undergoing immediate breast reconstruction with tissue expanders from 1992 to 2012 to determine institutional incidence. We also searched the Nationwide Inpatient Sample database from 1998–2008 to determine national incidence.
Results
A total of 9653 tissue expanders were placed in 6955 patients at Memorial Sloan Kettering Cancer Center between 1992 and 2012. There were 3 cases of pneumothorax during immediate breast reconstruction with tissue expanders. The incidence of pneumothorax is 0.03% per expander and 0.04% per patient. From the national database, there were 153 cases of pneumothorax during immediate breast reconstruction with tissue expanders in 27, 612 patients. The overall national incidence of pneumothorax is 0.55% per patient.
Conclusion
Our algorithm for management includes a thoracic surgery consultation intraoperatively. A chest tube should be placed at a site distal to the pleurotomy. The site of injury should be repaired primarily or patched as necessary. If the patient remains stable, it is safe to proceed with placement of the tissue expander.
INTRODUCTION
Over 93,000 women underwent breast reconstruction in 2010, an increase of 8% from 2009 (1). 70% of all breast reconstructions are implant-based, often performed in two-stage procedure with tissue expander placement, making it one of the most common plastic surgical reconstructive procedures (2). The overall rate of early complications of tissue expander placement is low, especially for systemic complications with range of 0.2–1.3% in two larger published series (3–4). However, when these systemic complications do occur, they may cause significant morbidity.
One of these potential complications is pneumothorax. However, there is a paucity of literature regarding pneumothorax in the breast reconstruction patient population. Several breast reconstruction case series, whether implant-based or autologous tissue, mention pneumothorax as a complication but the overall numbers of patients are low (5–7). There is no currently reported incidence, whether national or in large case series, of pneumothorax or a widely accepted standard of care for intraoperative management. We present 3 cases of pneumothorax as well as data on national and institutional incidence. We also describe an algorithm for intraoperative management.
METHODS
Approval from the Memorial Sloan Kettering Cancer Center Institutional Review Board was obtained prior to initiation of this study. A review of all tissue expander/implant breast reconstructions by the senior author (P.G.C.) over the 20-year period from 1992 to 2012 was performed. A prospectively maintained breast reconstruction institutional database was analyzed with respect to reconstructive and complication data for 9653 tissue expanders placed in 6955 patients. Pertinent medical records including operative details were reviewed in those identified to have iatrogenic intraoperative pneumothorax.
National incidence of pneumothorax in implant reconstruction was obtained from the Nationwide Inpatient Sample database (NIS) from 1998 to 2008. The NIS is a 20% stratified sample of hospital discharges from non-federal facilities. It is the largest all payer inpatient care database in the United States, with data from 8 million admissions in approximately 1,000 hospitals. It is part of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality (AHRQ), and it is a representative sample of national hospital discharges (8).
Inclusion criteria were patients with breast cancer or increased risk of breast cancer (ICD-9 diagnosis codes 174.0–174.9, 233.0, v16.3, v10.3, v84.01 and v50.41) who underwent total mastectomy (ICD-9 procedure codes 85.4, 85.33-36 and 85.41-48) and implant reconstruction. Implant based reconstructions included immediate tissue expander insertion and immediate permanent implant placement (ICD-9 procedure 85.53-54 and 85.95). Pneumothorax cases were identified as those with ICD-9 diagnosis codes 512.0–1,860.0-1, 860.4-5 and 512.8. National incidence of pneumothorax in implant breast reconstruction was calculated by dividing number of cases/total of implant reconstructions x100.
CASE REPORTS
Case 1
The patient was a 59 year old woman with DCIS and a BMI of 24 kg/m2. She elected to undergo bilateral mastectomies with sentinel lymph node biopsies with immediate tissue expander reconstruction. During the dissection of the submuscular pocket, the pleural space was inadvertently entered at the 5th intercostal space, with visualization of the underlying lung through a 1-cm defect. Thoracic surgery was consulted intra-operatively. Given that the injury had been made with a bovie electrocautery, a superficial injury to the lung could not be ruled out. A 20-French chest tube was placed laterally, avoiding the area of the tissue expander. The defect in the intercostal muscle was oversewn to prevent communication between the prosthetic pocket and the pleural space. A tissue expander was placed and the muscle and skin closed in the usual fashion. She woke from anesthesia without any difficulty. A post-operative x-ray showed a small apical pneumothorax. Her chest tube was removed on post-operative day 3 after cessation of airleak and improvement in the pneumothorax. She was discharged on the fourth post-operative day and had no further complications.
Case 2
The patient was a 45 year old woman with left breast cancer with a BMI of 21.8 kg/m2. She elected to undergo immediate breast reconstruction with a tissue expander after a modified radical mastectomy with axillary lymph node dissection. The insertion of the rectus muscle was unusually inferior with very thin intercostal muscles and minimal soft tissue. During this inferior dissection, the parietal pleura sustained a 3-mm injury. It was unclear if the patient sustained an injury to the visceral pleura. A thoracic surgeon evaluated the patient intra-operatively and placed a 16-French chest tube prophylactically through the lateral portion of the mastectomy. A tissue expander was placed and the muscle and skin closed in the usual fashion. She woke from anesthesia without any difficulty. A post-operative x-ray showed a minimal apical pneumothorax. Airleak resolved by post-operative day 1 and her chest tube was discontinued with an x-ray showing no residual pneumothorax. She was discharged on the second post-operative day and had no further complications.
Case 3
The patient was a 45 year old woman with DCIS with a BMI of 20.3 kg/m2. Previous surgical history was significant for a tissue-patch repair of an atrial-septal defect via an infra-mammary anterior thoracotomy at age 30. She elected to undergo bilateral mastectomies with sentinel lymph node biopsies with immediate tissue expander reconstruction. Intra-operatively, the patient was noted to have lung herniation through a 4×12 cm defect in the chest wall intercostal space from the prior thoracotomy starting at the lateral edge of the pectoralis major and extending medially. It was apparent that dissection underneath the pectoralis major would lead to violation of the pleural cavity. Thoracic surgery was called intra-operatively. After discussion with breast and thoracic surgery and the patient’s family, the decision was made to reconstruct the chest wall before proceeding with breast reconstruction.
The dissection proceeded underneath the pectoralis major, leaving behind a very thin layer of pleura, well-vascularized between the gaps in the ribs. The chest wall defect was repaired using acellular dermal matrix (Alloderm) to prevent herniation and separate the pleural space from the prosthetic compartment. Dissection for complete submuscular placement of the tissue expander proceeded in the usual fashion. A red rubber catheter under a water seal was placed underneath the Alloderm re-expand the lung with a Valsalva maneuver. The tissue expander was placed and the muscle, closed. At this point, the red rubber catheter was removed by thoracic surgery. She woke from anesthesia without any difficulty. No pneumothorax was seen on post-operative x-ray. She was discharged on the second post-operative day and had no further complications.
INCIDENCE AND ALGORITHM
There were a total of 9653 tissue expanders placed in 6955 patients at Memorial Sloan Kettering Cancer Center between 1992 and 2012. There were 3 cases of pneumothorax during immediate breast reconstruction with tissue expanders, as described above. The incidence of pneumothorax is 0.03% per expander and 0.04% per patient. From the national database, there were 153 cases of pneumothorax during immediate breast reconstruction with tissue expanders in 27, 612 patients. The overall national incidence of pneumothorax is 0.55% per patient.
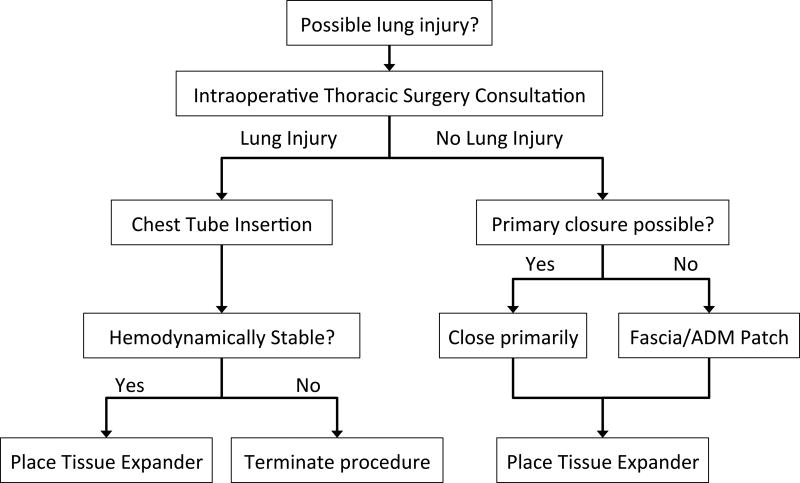
Based on our clinical experience, we propose an algorithm for management of suspected lung injury during immediate breast reconstruction with tissue expanders. First, the possibility of lung injury is evaluated in conjunction with a thoracic surgeon. If it has been determined definitely that no lung injury occurred, the defect must then be evaluated and closed primarily if possible or with either fascia or acellular dermal matrix. After this has been repaired, it is safe to proceed with placement of the tissue expander. If there remains a significant possibility that the lung sustained an injury, the patient should have a chest tube placed intraoperatively at a site distant to the pleurotomy and away from the tissue expander. If the patient has remained hemodynamically stable, placement of the tissue expander may proceed. See Figure 1. All patients with a lung injury suspected intraoperatively should undergo a postoperative chest xray. Management of postoperative pneumothorax should follow standard of care treatment for that institution in conjunction with a thoracic surgery consultation.
Figure 1.
Algorithm for Intraoperative Management of Suspected Lung Injury during Tissue Expander Placement
DISCUSSION
Pneumothorax remains a serious though rare complication of tissue expander placement for breast reconstruction. It is important to be alert to this possibility intraoperatively. Delay of diagnosis may be fatal as the patient can quickly become hemodynamically unstable (9). Iatrogenic pneumothoraces can result from transthoracic needle lung biopsies, central line placement, thoracentesis, transbronchial lung biopsies, pleural biopsies and positive-pressure ventilation (10). The majority of cases in the plastic surgery literature relate to breast augmentation. In a survey of members of the California Society of Plastic Surgeons, 1 in 3 surgeons had experienced pneumothorax related to breast augmentation (11). Over one third of all pneumothoraces in breast augmentation have been reported as secondary to injection of local anesthesia (11, 12). Other suspected causes include barotrauma related to air in subpectoral pocket pressed inside pleural cavity as a result of the implant (13).
Pleural injury that occurs during tissue expander placement represents a fundamentally different problem. In the cases we report, the pneumothorax does not result from a simple needle puncture but rather a direct injury to the lung by electrocautery with an unknown extent of involvement. In addition, the mastectomy may have removed some muscle and fascia overlying the area of injury. This is especially true given that our patients were women with low BMI and intercostals are thin anteriorly, a risk factor for pneumothorax in breast augmentation (11). The combination of an undetermined extent of injury with a paucity of soft tissue coverage suggests that the pneumothorax be treated conservatively.
Why not simply evacuate the potential pneumothorax with a red rubber catheter? Unfortunately, after pleural injury, simple evacuation does not guarantee resolution of the pneumothorax. 20% of patients who had pneumothoraces sustained intraoperatively during open nephrectomy had residual pneumothorax required postoperative chest tube placement (14). Waiting to place a chest tube postoperatively for residual pneumothorax may not only be frightening and potentially traumatic for the patient but also places the patient, who has already sustained an intraoperative complication, at risk for a tension pneumothorax. Placing the chest tube immediately in the OR at the time of injury is preferably because the patient is already sedated and will have the pneumothorax treated definitively prior to emergence from anesthesia. This management agrees with the recommendations of the anesthesiology and pulmonary literature as well (15).
We have also shown that it is safe to proceed with tissue expander placement if the patient is stable, the lung expanded and a chest tube placed. This forms the basis for yet another argument in favor of chest tube placement. Wickman reported 1 patient out of 30 with a pneumothorax but it is difficult to extrapolate a true incidence from such a small sample size (5). Our data also represents the first published national incidence as well as incidence from a large case series from a single institution.
SUMMARY
Pneumothorax is an extremely rare complication of implant-based breast reconstruction. Thin patients are at increased risk. If there is suspicion of a pleural injury, we recommend thoracic surgery consultation and chest tube placement intraoperatively. After these interventions, reconstruction may be safely completed in a hemodynamically stable patient.
Footnotes
DISCLOSURES
None of the authors has any financial disclosures or commercial associations.
References
- 1.American Society of Plastic Surgeons. [Accessed August 15, 2011];Report of 2010 Plastic Surgery Statistics. Available at: http://www.plasticsurgery.org/Documents/news-resources/statistics/2010-statisticss/Overall-Trends/2010-reconstructive-trends-statistics-trends.pdf.
- 2.Cordeiro PG. Breast Reconstruction after Surgery for Breast Cancer. N Engl J Med. 2008;359:1590–1601. doi: 10.1056/NEJMct0802899. [DOI] [PubMed] [Google Scholar]
- 3.Cordeiro PG, McCarthy C. A Single Surgeon's 12-Year Experience with Tissue Expander/Implant Breast Reconstruction: Part I. A Prospective Analysis of Early Complications. Plast Recon Surg. 2006;118:825–831. doi: 10.1097/01.prs.0000232362.82402.e8. [DOI] [PubMed] [Google Scholar]
- 4.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: Two-year results of the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2002;109:2265–2274. doi: 10.1097/00006534-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Wickman M, Sandelin K, Arver B. Technical aspects and outcome after prophylactic mastectomy and immediate breast reconstruction in 30 consecutive high-risk patients. Plast Reconstr Surg. 2003;111:1069–1077. doi: 10.1097/01.PRS.0000046250.95557.C6. [DOI] [PubMed] [Google Scholar]
- 6.Dupin CL, Allen RJ, Glass CA, et al. The internal mammary artery and vein as a recipient site for free-flap breast reconstruction: a report of 110 consecutive cases. Plast Reconstr Surg. 1996;98:685–689. doi: 10.1097/00006534-199609001-00013. [DOI] [PubMed] [Google Scholar]
- 7.Patel AJ, Malata CM. Intercostal drain insertion for pneumothorax following free flap breast reconstruction--a near miss! J Plast Reconstr Aesthet Surg. 2010;63:1929–31. doi: 10.1016/j.bjps.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 8.HCUP Nationwide Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality R, MD; 2008. [Accessed July 2011]. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 9.Williamson JA, Webb RK, Van der Walt JH, et al. The Australian Incident Monitoring Study. Pneumothorax: an analysis of 2000 incident reports. Anaesth Intensive Care. 1993;21:642–5. doi: 10.1177/0310057X9302100525. [DOI] [PubMed] [Google Scholar]
- 10.Mulholland MW. The Greenfield's Surgery: Scientific Principles and Practice. Fourth. Williams & Wilkins; 2006. [Google Scholar]
- 11.Osborn JM, Stevenson TR. Pneumothorax as a complication of breast augmentation. Plast Reconstr Surg. 2005;116:1122–1126. doi: 10.1097/01.prs.0000179182.58036.a7. [DOI] [PubMed] [Google Scholar]
- 12.Kaye AD, Eaton WM, Jahr JS, et al. Local anesthesia infiltration as a cause of intraoperative tension pneumothorax in a young healthy woman undergoing breast augmentation with general anesthesia. J Clin Anesth. 1995;7:422–424. doi: 10.1016/0952-8180(95)00059-q. [DOI] [PubMed] [Google Scholar]
- 13.Fayman MS, Beeton A, Potgieter E. Barotrauma: an unrecognized mechanism for pneumothorax in breast augmentation. Plast Reconstr Surg. 2005;116:1825–1826. doi: 10.1097/01.prs.0000188852.01898.70. [DOI] [PubMed] [Google Scholar]
- 14.Atmaca AF, Canda AE, Serefoglu EC, et al. The incidence and management of pleural injuries occurring during open nephrectomy. Advances in Urology. 2009 doi: 10.1155/2009/948906. Epub 2009 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baumann MH, Noppen M. Pneumothorax. Respirology. 2004;9:157–164. doi: 10.1111/j.1440-1843.2004.00577.x. [DOI] [PubMed] [Google Scholar]