Abstract
Studies conducted in drug addiction suggest a transition in processing of drug-related cues from the ventral to the dorsal component of the striatum. However, this process has not been studied in a behavioral addiction. Assessment of this process in a non-drug addiction can provide insight into the pathophysiology of both substance and behavioral addictions. Thirty-nine male IGD subjects and 23 male matched healthy controls (HCs) participated in functional magnetic resonance imaging (fMRI) during performance of a cue-reactivity task involving alternating presentation of Internet gaming-related stimuli (game cues) and general Internet surfing-related stimuli (control cues). Cue-induced neural activations in the ventral and dorsal striatum were compared between IGD and HC participants. Associations between cue-reactivity within these regions and cue-induced craving and severity and duration of IGD were also explored. IGD participants exhibited higher cue-induced activations within both the ventral and dorsal striatum (DS) when compared to HCs. Within the IGD group, activity within the left ventral striatum (VS) was correlated negatively with cue-induced craving; positive associations were found between activations within the DS (right putamen, pallidum and left caudate) and duration of IGD. Cue-induced activity within the left putamen was negatively associated with right VS volumes among IGD participants. Consistent with studies in substance addictions, our results suggest that a transition from ventral to dorsal striatal processing may occur among individuals with Internet gaming disorder, a condition without the impact of substance intake.
Keywords: cue-reactivity, dorsal striatum, fMRI, Internet gaming disorder, ventral striatum
INTRODUCTION
Internet gaming disorder (IGD), the most prevalent subtype (57.5%) of Internet addiction disorder (IAD) (Chen et al., 2014), is defined as persistent and recurrent use of the Internet to engage in games (American Psychiatric Association, 2013). As with other addictions, IGD is associated with numerous negative consequences across multiple domains, including physical health, academic performance, psychosocial functioning and interpersonal relationships (Kuss and Griffiths, 2012; Petry et al., 2014).
The growing prevalence of IGD and its overall importance within a public mental health context is underscored by its recent inclusion in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as a disorder warranting further study (APA, 2013). Further research is therefore necessary to determine whether IGD warrants inclusion in the category of ‘Addictions and related Disorders’ in future versions of the DSM, as well as to aid in the development of more effective treatment interventions.
Drug addiction has been conceptualized as the endpoint of a series of transitions from initial voluntarily drug use to habitual, compulsive drug use and dependence (Everitt and Robbins, 2013). Within this framework, the transition to dependence is thought to be accompanied by dynamic shifts in neural control over approach behaviors: from initial prefrontal-cortical to striatal processing, and, within the striatum, from ventral to dorsal processing, as mediated by its stratified dopaminergic innervation (Everitt et al., 2008; Everitt and Robbins, 2005). This conceptualization, derived largely from preclinical studies (Koob and Le Moal, 2005; Nestler, 2004), has more recently been experimentally supported by findings from human studies of substance dependence and pathological gambling (Limbrick-Oldfield et al., 2013; Sjoerds et al., 2014; Vollstädt-Klein et al., 2010). For example, negative associations between craving and cue-induced activations in the ventral striatum (VS) (Vollstädt-Klein et al., 2010) and positive associations between craving and cue-induced activations in the dorsal striatum (DS) (Volkow et al., 2006, 2008) have been reported among individuals with substance addictions. However, to our knowledge, no studies have assessed ventral versus dorsal striatal responses to gaming cues in IGD, which may further confirm the validity of this conceptualization in a non-substance addiction.
Existing neuroimaging research indicate alterations in brain reward systems among individuals with IGD/IAD: For example, alterations in dopaminergic neurotransmission within the DS have been reported in IAD (Kim et al., 2011) and studies using cue-reactivity paradigms have reported higher activations within regions of the striatum (caudate, nucleus accumbens; NAcc) among individuals with IGD (Ko et al., 2009b; Sun et al., 2012). These data suggest possible neural functional similarities between IGD and substance addictions; however, further research is needed to characterize the neural correlates of cue-induced craving among individuals with IGD.
Previous fMRI studies conducted in IGD and using cue-reactivity paradigms have employed relatively small sample sizes (≤ 15 individuals per group); e.g., (Ko et al., 2009b; Ko et al., 2013; Sun et al., 2012). Thus, findings require replication in adequately powered samples. In addition, to our knowledge, no studies have systematically assessed neural responses to gaming-related cues within different subdivisions of the striatum; e.g., ventral versus dorsal. This study aimed to replicate previous findings in a larger sample of individuals with IGD (n= 39) and to extend previous findings via investigation of the association between neural responses within different striatal subdivisions in relation to clinical characteristics of IGD (e.g., subjective craving, severity and duration of IGD).
Studies conducted in substance-dependent populations suggest associations between striatal neural structure and clinical characteristics including treatment outcome measures (Das et al., 2012; Janes et al., 2014; Yip et al., 2014). For example, lifetime tobacco use is negatively associated with NAcc volume but positively associated with putamen volume (Das et al., 2012). In addition, dorsal - but not ventral - striatal volume is positively associated with cue-induced craving among smokers (Janes et al., 2014). However, the relationship between striatal neural structure and clinical characteristics among individuals with IGD is comparatively less well understood, although recent data suggest that striatal neural structure may be related to the severity of addiction and cognitive control deficits in this population (Cai et al., 2015). Therefore, a final aim of this study was to explore associations between striatal neural structure and clinical (e.g., duration of IGD) and fMRI (e.g., gaming-cue induced BOLD response in regions of the striatum) measures.
Based on previous findings (reviewed above), we hypothesized: (1) Individuals with IGD would exhibit significantly greater activations in comparison to healthy comparison (HC) participants within both the ventral and dorsal striatum when exposed to gaming-related cues; (2) among IGD participants, cue-induced craving and clinical features of IGD (duration and severity) would be negatively associated with neural responses within the ventral striatum but positively associated with neural responses within the dorsal striatum; (3) there would be correlations between structure and functioning of corresponding striatal regions in IGD participants.
MATERIALS AND METHODS
Participants
Forty-two individuals with Internet gaming disorder (IGD) and 23 healthy comparison (HC) participants were recruited via the Internet and advertisements posted at local universities and selected through online questionnaire and telephone screening. A total of 432 individuals participated in initial screening (see Table S1 in Supporting Information for further details on participant selection).
Participants were recruited according to their weekly Internet gaming time and scores on the Chinese Internet Addiction Scale (CIAS; Chen et al., 2003), which consists of 26 items on a 4-point Likert scale. The reliability and validity of the CIAS among college students has been demonstrated previously (Chen et al., 2003). Inclusion criteria for the IGD group were: (1) a score > 67 on the CIAS (Ko et al., 2009a); (2) > 10 hours per week engaged in Internet gaming, for a minimum of one year; (3) endorsement of Internet gaming as their primary Internet activity; and (4) endorsement of one of three most popular Internet games (CrossFire (CF), World of Warcraft (WOW) and Defense of the Ancients (DOTA; Kuss, 2013) as the only source of Internet-gaming. Inclusion criteria for HC participants were: (1) a score < 60 on the CIAS; (2) never having spent more than 2 hours per week engaged in Internet gaming. Exclusion criteria for all participants were any current or previous use of illegal substances and gambling (including online gambling), any history of psychiatric or neurological illness and current use of psychotropic medications, as assessed by a semi-structured interview.
Given the higher prevalence of IGD in men versus women (Ko et al., 2009a; Meng et al., 2014), only male participants were included. Three subjects in the IGD group were excluded due to excessive head motion during scanning (defined as motion in excess of one voxel); thus the final dataset comprised 39 individuals with IGD and 23 HC individuals. All participants were right-handed.
Tobacco use characteristics were assessed using the Fagerstrom Test for Nicotine Dependence (FTND; Fagerström, 1978), and nicotine-dependent individuals were excluded (i.e., individuals with an FTND score ≥6; Fagerstrom et al., 1990). Alcohol consumption was assessed using the Alcohol Use Disorder Identification Test (AUDIT-C; Bush et al., 1998), and participants with AUDIT-C scores ≥ 5 (Dawson et al., 2005) were instructed to complete the Michigan Alcoholism Screening Test (MAST; Selzer, 1971) for further screening. Individuals with a score ≥ 6 on the MAST were excluded for alcohol dependence. Current depression and anxiety symptoms were assessed using the Beck Depression Inventory (BDI; Beck et al., 1961) and the Beck Anxiety Inventory (BAI; Beck et al., 1988), respectively. The Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University approved the study protocols. All participants provided written informed consent and were paid ¥120 for their participation.
Cue-reactivity task
Stimuli and validation
The cue-reactivity fMRI task included two types of Internet-related stimuli: (1) images specific to Internet gaming (game cues) and (2) images of general Internet-surfing related stimuli (control cues). All images were taken directly from the Internet using screenshots. Gaming-related stimuli (game cues) consisted of screenshot images from three of the most popular Internet games - CF, WOW and DOTA. General Internet surfing stimuli (control cues) consisted of general online screenshot images which were unrelated to gaming; e.g., screenshots of Internet chats, of downloading and of searching and online communicating (see Figure S1).
Validation of task stimuli was conducted among one-hundred and forty four participants (IGD: n = 87; IAD: n = 27; HC: n = 30) using the Self-Assessment Manikin (SAM) picture rating system (Lang et al., 1997) (see Supporting Information for further details on validation of task stimuli).
Task design and presentation
During fMRI scanning Internet gaming cues (30 pictures of CF, WOW or DOTA determined by which game the subject engaged in), control cues (30 non-gaming Internet stimuli) and baseline stimuli (60 mosaic visual stimuli, corresponding degraded stimulus of game and control pictures with no meaning identified) were presented to participants using a block design consisting of 6 Internet-gaming-related blocks (5 stimuli per block), 6 non-gaming-related blocks (5 stimuli per block), and 12 mosaic control blocks (5 stimuli per block). Blocks were divided into two separate runs, each composed of 3 Internet-gaming-related blocks, 3 non-gaming-related blocks and 6 mosaic control blocks. Internet-gaming and non-Internet gaming blocks were always followed by a mosaic control block.
Each block contained 5 pictures with each presented for 3.7 seconds following a 0.3 second fixation cross. Following each Internet-gaming and non-Internet gaming block there was an additional 4 second interval for subjects to complete VAS craving ratings. Each run lasted for 274 seconds and included presentation of an initial fixation cross for 10 seconds, six 4-second VAS and twelve 20-second stimuli blocks (3 game-related blocks, 3 control blocks, and 6 mosaic blocks). The order of the blocks was pseudorandomized (game-baseline, control-baseline, control-baseline, game-baseline; A-B-B-A) to balance the effects of order, whereas the order of the pictures within each block was randomized for each subject (see Figure 1).
Figure 1.
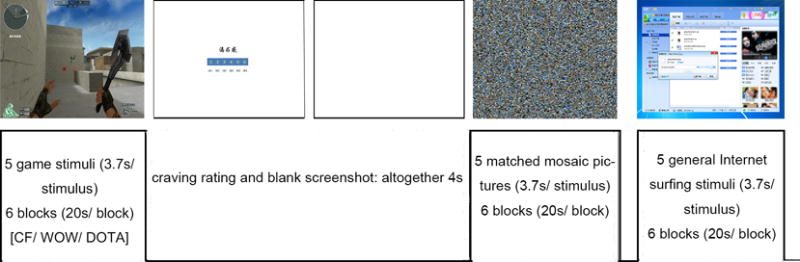
Cue reactivity task
Cue-induced craving was measured by a 6-point visual analogue scale (VAS) (from 1 = ‘not craving at all’ to 6 = ‘severe craving’) asking participants to rate their subjective craving for Internet gaming or general online surfing after the related block was presented.
Image acquisition
Data were acquired using a SIEMENS Trio 3.0 T scanner in the Beijing Normal University Imaging Center for Brain Research. fMRI data were obtained using an EPI sequence with the following parameters: TR = 2000 ms; TE = 25 ms; flip angle = 90°; 64 × 64 matrix size with a resolution of 3 × 3 mm2. Forty-one 3.0 mm axial slices were used to cover the whole cerebrum and most of the cerebellum with no gap. The slices were tilted 30 degrees clockwise from the AC-PC plane to obtain better signals in the orbitofrontal cortex. A high-resolution, T1-weighted sagittal 3-D magnetization prepared rapid gradient-echo sequence was acquired: TR = 2530 ms; TE = 3.39 ms; TI = 1100 ms; FA = 7°; FOV = 256 × 256 mm. 144 slices contiguous sagittal slices were acquired with 1 × 1 mm in-plane resolution and 1.33 mm slab thickness for whole brain coverage.
Data analysis
Behavioral data analysis
Between-group comparisons of demographic, clinical characteristics, and cue-reactivity task performance measures were conducted using independent-sample t-tests or Chi-square tests as appropriate in SPSS 20.0.
Functional image analyses
Imaging data were preprocessed using SPM8 (Welcome Department of Cognitive Neurology, London, UK, http://www.fil.ion.ucl.ac.uk/spm/spm8). Images were manually reoriented to the AC-PC line, realigned to estimate and modify movement parameters, normalized to MNI space, and spatially smoothed with a Gaussian kernel of 5 mm at full-width-half-maximum (FWHM).
Statistical analysis of individual participant imaging data was performed using first-level fixed-effects analyses using a General Linear Model (Friston et al., 1995). Timing of task events for (i.e., game stimuli, control stimuli, craving rating, ISI) were convolved with the canonical hemodynamic response function, with the realignment parameters included as regressors of no interest. To remove low-frequency signal drift, a high-pass filter (128 Hz) was applied. For each subject a contrast image of game-related > control stimuli was constructed to examine regional brain activation related to Internet game cue-reactivity. These contrast images were entered into a second level random-effects analysis using a two-sample t-test design to investigate between-group effects.
Region-of-interest (ROI) analyses
To examine the hypothesized between-group differences in ventral and dorsal striatal responses during cue-reactivity, a priori defined region of interest (ROI) analyses in the striatum were performed. ROIs were defined using bilateral masks for the putamen, caudate nucleus and globus pallidum were derived from the automatic anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) incorporated in the WFU-PickAtlas Tool v2.5.2 (Maldjian et al., 2003). The WFU-Pickatlas does not provide an anatomical mask for the most ventral part of the striatum, comprising the nucleus accumbens. Therefore we selected a bilateral VS mask (Nielsen and Hansen, 2002) defined in the BrainMap database (Fox and Lancaster, 1994), and created a binary mask from the probabilistic mask at P > 0.70 using the Image Calculate-tool in REST. This VS mask was then subtracted from each of the AAL-defined striatal areas to distinguish between ventral and dorsal striatal areas. We report significant brain activations within the ROIs that survived family-wise-error (FWE) correction for multiple comparisons using small volume correction (PSVC-FWE < 0.05).
Whole-brain analyses
Whole-brain statistical maps were voxel-level thresholded at P < 0.001 prior to undergoing cluster-level family-wise-error correction (PFWE < 0.05).
Structural analysis of the striatum
Structural MRI data processing was performed corresponding to an established VBM protocol using the VBM8 toolbox (Structural Brain Mapping Group, University of Jena, Germany; http://dbm.neuro.uni-jena.de/vbm8) for SPM8.
For preprocessing, images were segmented into grey matter, white matter and cerebrospinal fluid tissue probability maps (TPMs). A high-dimensional normalization (DARTEL) was performed using the DARTEL template in MNI space as provided within the VBM8 toolbox. To preserve the total amount of grey matter, spatially normalized grey matter was modulated using the default parameter settings. All images were then smoothed using a 6mm full width half maximum (FWHM) kernel, prior to extraction of individual participant gray matter volumes within the anatomically defined ROIs of VS, caudate and putamen.
Correlation analyses
To test for specific patterns of striatal responses related to clinical characteristics, ROI-based regression analyses with subjective craving ratings and durations of IGD as independent variables were performed within the IGD group. All regression analyses focused on the identified ROIs in striatum, and were conducted using small-volume correction (SVC) with p-values thresholded using FWE correction (PFWE < 0.05).
Correlational analyses with structural volumes were performed in SPSS 20.0 using Pearsons’s r, and considered significant at two-tailed (P < 0.05). For these analyses mean BOLD parameter estimates at the sub regions of the ventral and dorsal striatum were extracted.
RESULTS
Demographic, and behavioral characteristics
Demographic characteristics of IGD and HC participants are shown in Table 1. IGD and HC groups did not differ significantly in age or years of education. Consistent with the inclusion criteria, IGD participants had significantly higher CIAS scores, and longer years of Internet use/Internet gaming than healthy controls. Six of the 23 HC participants reported occasionally engaging in Internet gaming. However, also consistent with study inclusion criteria, the IGD group spent significantly more time on Internet games weekly compared to the subgroup of controls reporting occasional Internet gaming. Consist with our hypotheses, the IGD group had higher subjective craving ratings following exposure to Internet gaming stimuli, but did not differ from HC participants in craving ratings following exposure to non-game Internet surfing cues.
Table 1.
Demographic characteristics between IGD subjects and healthy controls.
| Variable | IGD subjects (n = 39) |
Healthy controls (n = 23) |
t/χ2 value |
|---|---|---|---|
|
| |||
| mean ± S.D. | mean ± S.D. | ||
| Demographics | |||
| Age | 22.64 ± 2.12 | 23.09 ± 2.13 | −0.798 |
| Years of education | 15.64 ± 1.42 | 15.91 ± 1.47 | −0.717 |
| Internet-related and other characteristics | |||
| CIAS | 75.59 ± 8.82 | 45.65 ± 9.80 | 12.389*** |
| Years of Internet use | 8.95 ± 2.52 | 7.17 ± 3.58 | 2.286* |
| Years of Internet gaming | 6.62 ± 2.82 | 5.80 ± 3.19 | 2.093* |
| Time spent on Internet gaming (hours per week) |
18.92 ± 9.85 | 0.92 ± 0.20a | 4.436*** |
| Duration of current gaming state (months) |
15.24 ± 14.38 | – | – |
| Alcohol use (at least once per month) |
33 | 17 | 1.06 |
| AUDIT-C | 3.48 ± 2.12b | 2.41 ± 1.33c | 2.19* |
| Cigarette use (at least once per month) |
5 | 0 | 3.21 |
| FTND | 2.00 ± 1.87 | – | – |
| Depression severity (BDI score) |
7.49 ± 5.84 | 2.91 ± 3.12 | 3.99*** |
| Anxiety severity (BAI score) |
5.05 ± 5.17 | 3.18 ± 3.23 | 1.74 |
| Behavioral characteristics | |||
| Craving for Internet gaming | 4.85 ± 1.19 | 1.52 ± 0.67 | 14.10*** |
| Craving for Internet surfing | 3.40 ± 1.19 | 2.94 ± 1.10 | 1.51 |
P < 0.05;
P < 0.01;
P < 0.001.
S.D. = standard deviation; IGD = Internet gaming disorder; CIAS = Chen Internet addiction scale; AUDIT-C = Alcohol use disorder identification test; FTND = Fagerstrom test for nicotine dependence; BAI = Back Anxiety Inventory; BDI = Beck Depression Inventory.
n = 6;
n = 33;
n = 17.
According to AUDIT-C and MAST, 33 of the 39 IGD participants and 17 of the 23 HC participants were occasional alcohol drinkers (non-dependent drinkers), and the former reported higher scores of AUDIT-C relative to the HC group. None of the participants met criteria for alcohol dependence, as defined by a score ≥ 6 on the MAST. Five IGD participants and no controls reported current cigarette smoking. Participants with IGD scored significantly higher than controls on the BDI.
fMRI results
Region-of-interest analysis
Within-group comparisons
Within the IGD group, exposure to Internet-gaming stimuli (versus general Internet stimuli) was associated with increased neural responses within dorsal striatal ROIs (right pallidum (t = 4.41, PFWE = 0.003, [21 3 3]) and right putamen (t = 4.22, PFWE = 0.016, [21 6 3])) but not within ventral striatal ROIs. Among HC participants, exposure to Internet-gaming stimuli (versus general Internet stimuli) was not associated with any differences in neural responses within either the VS or DS.
Between-group comparisons
Small-volume-corrected (SVC) group comparisons of gaming-related > control stimuli showed higher cue-induced brain activations in the IGD group compared to the HC group (Table 2). Specifically, when compared with the HC group, IGD participants exhibited increased activation within the right VS, bilateral caudate, bilateral putamen and bilateral pallidum ROIs (PSVC-FWE < 0.05).
Table 2.
Findings from ROI analyses comparing BOLD responses during exposure to gaming cues (contrast ‘Internet-gaming > general Internet-surfing stimuli’) between IGD group and HC group; positive t-values indicate IGD > HC (PSVC-FWE < 0.05)
| ROI | Hemisphere | PFWE | Peak MNI (mm) | Peak t value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| VS | |||||||
| VS | R | 0.004 | 18 | 9 | −3 | 3.94 | |
| VS | L | 0.062 | −15 | 6 | −3 | 2.84 | |
| DS | |||||||
| Caudate | R | 0.002 | 12 | 12 | 9 | 4.69 | |
| Pallidum | R | 0.001 | 15 | 6 | 0 | 4.53 | |
| Putamen | R | 0.002 | 21 | 12 | 3 | 4.78 | |
| Caudate | L | 0.022 | −15 | −3 | 18 | 3.94 | |
| Pallidum | L | 0.001 | −18 | 6 | 3 | 4.64 | |
| Putamen | L | 0.001 | −18 | 3 | 6 | 4.81 | |
IGD = Internet gaming disorder; HC = healthy control; R= right; L= left; VS = ventral striatum; DS = dorsal striatum
Whole-brain analysis
Whole-brain analyses confirmed the ROI findings of increased BOLD responses within bilateral putamen, caudate, and pallidum among IGD participants (in comparison to healthy controls). Whole-brain analyses further indicated increased activity among IGD individuals within regions previously implicated in cue-reactivity, including the precuneus, IFG, DLPFC, cerebellum, cingulate gyrus and occipital gyrus. Whole-brain analyses further indicated decreased activations within the right precentral gyrus, insula and operculum among IGD participants when compared to HC participants (PFWE < 0.05) (see Table 3; Figure 2).
Table 3.
Whole-brain analyses comparing BOLD responses (Internet-gaming > general Internet-surfing stimuli) between IGD and HC participants; (voxel-level P < 0.001, PFWE < 0.05)
| Hemisphere | Brain regions | BA | Cluster size | Cluster PFWE | Peak MNI (mm) | peak t value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| IGD > HC | ||||||||
| R+L | Precuneus/middle occipital gyrus/fusiform gyrus/parahippocampal gyrus/posterior cingulate/brainstem/postcentral gyrus/inferior parietal gyrus/middle temporal gyrus/cerebellum/declive/superior temporal gyrus/midbrain/angular gyrus/associative visual cortex/inferior temporal gyrus/culmen/inferior temporal gyrus/lingual gyrus/hippocampus | 19, 20, 30, 37, 39 | 3821 | < 0.001 | 12 | −54 | 15 | 7.29 |
| R | IFG/DLPFC/middle frontal gyrus/superior frontal gyrus/precentral gyrus | 8, 9, 46 | 737 | < 0.001 | 51 | 12 | 24 | 6.83 |
| L | Precentral gyrus/IFG/DLPFC/middle frontal gyrus/superior frontal gyrus | 6, 8, 9, 46 | 1007 | < 0.001 | −51 | 6 | 42 | 6.78 |
| L+R | Cerebellum/uvula/declive/vermis | 684 | < 0.001 | −9 | −51 | −45 | 5.87 | |
| L+R | Cingulate gyrus/cingulum/posterior cingulate | 31 | 65 | 0.004 | −3 | −30 | 42 | 5.41 |
| L+R | Cingulum/anterior cingulate | 24 | 79 | 0.001 | −3 | 21 | 15 | 5.21 |
| L | Putamen/pallidum/caudate | 93 | 0.001 | −18 | 3 | 6 | 4.81 | |
| R | Putamen/caudate/pallidum | 147 | < 0.001 | 21 | 12 | 3 | 4.69 | |
| HC > IGD | ||||||||
| R | Operculum/insula | 13 | 50 | 0.016 | 45 | −18 | 15 | 5.08 |
| R | Precentral gyrus/postcentral gyrus/primary motor cortex/primary somatosensory cortex | 3, 4 | 161 | < 0.001 | 36 | −24 | 54 | 4.77 |
| R | Precentral/operculum/superior temporal gyrus/postcentral/premotor cortex | 6, 43 | 54 | 0.011 | 60 | −6 | 12 | 4.67 |
IGD = Internet gaming disorder; HC = healthy control; BA = Brodmann’s area; R= right; L= left; IFG = inferior frontal gyrus; DLPFC = dorsolateral prefrontal cortex.
Figure 2.
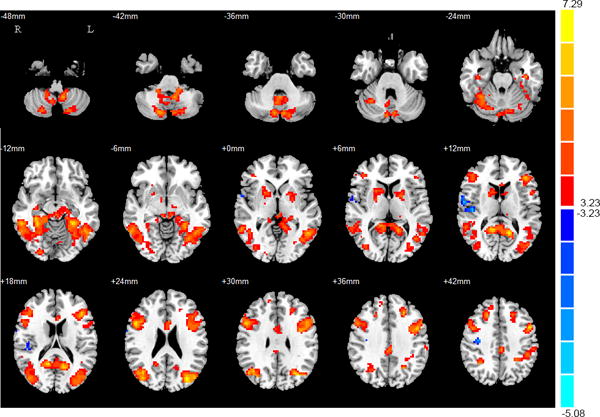
Whole-brain analyses for cue-reactivity in IGD group compared with HC group in the contrast of ‘Internet gaming > general Internet-surfing stimuli’, voxel-level P < 0.001, cluster-corrected PFWE < 0.05.
Regression analyses within the IGD group
Within the IGD group, ROI-based regression analyses indicated a significant negative association between gaming-cue induced subjective craving ratings and gaming-cue induced BOLD responses within the left VS (t = −3.78, PFWE = 0.009, [peak: x, y, z] = [−12 12 −15]; Figure 3). While a similar pattern was observed for the right VS ROI, this association did not reach statistical significance (t = −2.99, PFWE = 0.055, [15 12 −15]). For illustrative purposes, mean BOLD parameter estimates at the identified cluster peaks for the left VS were extracted using a 3mm sphere and are plotted against craving ratings in Figure 3.
Figure 3.
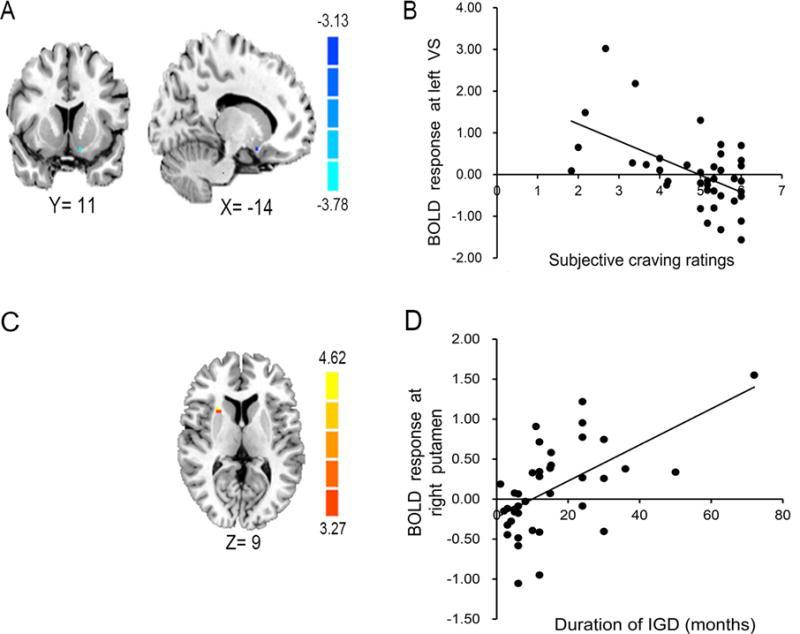
Findings from ROI-based regression analyses within the IGD group. (A) Negative association between gaming-cue induced craving ratings and cue-reactivity within the left ventral striatum (peak SVC activation within the left VS shown). (B) Mean BOLD parameter estimates at the identified cluster peak for the left VS were extracted using a 3 mm sphere and are plotted against cue-induced craving ratings for illustrative purposes. (C) Positive association between durations of IGD and cue-reactivity within the right putamen (peak SVC activation within the right putamen shown). (D) Mean BOLD parameter estimates at the identified cluster peak for the right putamen were extracted using a 3 mm sphere and are plotted against durations of IGD for illustrative purposes.
Regression analyses further indicated significant positive associations between durations of IGD and cue-induced activity within the right putamen (t = 4.62, PFWE = 0.006, [27 15 9]; Figure 3), right pallidum (t = 5.05, PFWE < 0.001, [15 −3 0]; t = 4.70, PFWE = 0.001, [24 −9 0]) and left caudate (t = 3.77, PFWE = 0.05, [−12 12 18]). For illustrative purposes, mean BOLD parameter estimates at the identified cluster peaks for the right putamen were extracted using a 3mm sphere and are plotted in Figure 3.
Among IGD participants, ROI-based regression analyses indicated no significant associations between severity of IGD and cue-induced BOLD responses within sub-regions of the striatum.
Relationship to striatal volumes
Contrasts between groups
There were no significant between-group differences in grey matter volumes within our a priori ROIs.
Relationship between volumes and brain activity
Within the IGD group, there was a significant negative association between right VS volumes and cue-induced activity within the left putamen (Pearson’s r= −0.347, P = 0.031; Figure 4). The correlation between right VS volumes and activity in the left putamen in HC group was not significant (Pearson’s r= 0.211, P = 0.33). Comparison of r-values using Fisher’s r-to-Z transformation indicated a significant between-group difference in associations between right VS volumes and cue-induced activity within the left putamen (Z = 2.07, P < .05).
Figure 4.
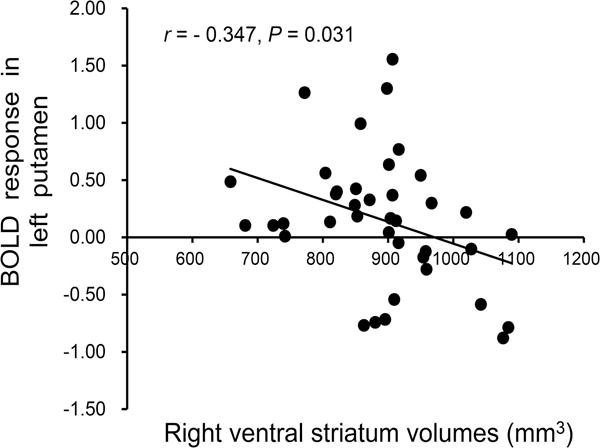
Negative association between right VS volumes and game-cue induced brain activation in the left putamen among IGD participants.
DISCUSSION
Consistent with our primary hypothesis, individuals with IGD exhibited increased activation within ventral and dorsal striatal ROIs during exposure to gaming-related cues, when compared to healthy controls. These data are consistent with previous findings from studies on cue-reactivity conducted in substance-using populations, e.g., (Engelmann et al., 2012; Schacht et al., 2013), as well as those from studies on pathological gambling (Limbrick-Oldfield et al., 2013), suggesting similarities across addiction subtypes.
Further consistent with findings from substance-using populations (Grüsser et al., 2004; Vollstädt-Klein et al., 2010), and non-substance addictions (Kim et al., 2011), between-group differences were most pronounced for the dorsal (versus ventral) ROIs, suggesting a transition from ventral to dorsal striatal processing of addiction-related cues among individuals with IGD. This interpretation is further supported by our finding of activation within dorsal – but not ventral – striatal ROIs in response to Internet-gaming-related cues among IGD participants (within-group comparison).
Consistent with previous studies (Schacht et al., 2013; Vollstädt-Klein et al., 2010), a negative association between cue-reactivity in the left VS and gaming-cue-induced craving was observed, suggesting decreased involvement of the ventral striatum in cue processing in individuals with IGD (as would be consistent with a shift to dorsal striatal processing) (Everitt and Robbins, 2013). However, contrary to this hypothesis, associations between BOLD responses within DS and craving were not found, which may indicate neurobiological differences between individuals with IGD compared to those with substance dependence. Further research directly comparing relationships between craving and neural responses with ventral and dorsal components of the striatum between individuals with IGD and those with substance addictions is needed.
Positive associations between duration of IGD and cue-induced activities within the dorsal striatum are consistent with those from a study conducted in alcohol dependence (Sjoerds et al., 2014), and with neurobiological models positing a transition at the neural level from ventral to dorsal striatal processing over addictive behaviors, as well as a progression from medial to more lateral parts of the dorsal striatum (Everitt et al., 2008; Everitt and Robbins, 2013). To our knowledge, this is the first report of an association between dorsal striatal activity and duration of addiction among individuals with a non-substance addiction. Together with previous findings, our data therefore suggest validity for this transition in different addiction subtypes, including those not involving exposure to an exogenous substance with impact on dopaminergic functioning.
Contrary to findings from alcohol dependence (Sjoerds et al., 2014), no significant associations were found between severity of IGD and cue-induced activations within ROIs of the striatum. It is possible that this finding may relate to methodological differences between IGD and alcohol dependence severity measures, or else might reflect a neurobiological difference between IGD and alcohol dependence. Further research directly comparing these two clinical populations is needed to determine these and other hypotheses.
Despite the between-group differences in neural functional striatal responses, no significant between-group differences in striatal volumes were observed. This finding is contrary to those from some previous studies conducted among individuals with IGD (Cai et al., 2015; Kühn et al., 2011) and may relate to between-study differences in inclusionary criteria for IGD. Though volumetric differences in the striatum have previously been confirmed among different substance addiction classes (Berman et al., 2008; Janes et al., 2014), the direction of differences is not consistent across studies, nor is the relationship with severity of addiction (Cai et al., 2015; Howell et al., 2013). Given the habitual nature of cue-induced craving (Janes et al., 2014), our finding of a negative correlation between right ventral striatal volume and left putamen activity during reward cue-reactivity suggests a possible structural neural basis for the transition of ventral to dorsal striatal processing. However, further research using longitudinal designs is needed to establish causality.
Whole-brain analyses confirmed our ROI findings and further indicated that, in comparison to HC participants, individuals with IGD had decreased gaming cue-induced brain activations within regions including the right insula and operculum. Individual differences in insular neural function and structure are associated with differences in avoidance learning and fear conditioning following exposure to aversive stimuli (Hartley et al., 2011; Samanez-Larkin et al., 2008). As such, it is possible that the decreased insula activity observed among IGD participants in this study may relate to alterations in aversion learning. While highly preliminary, this hypothesis is supported by neuropsychological data indicating decreased sensitivity to losses and decreased utilization of feedback during decision-making among individuals with IGD (Yao et al., 2014; Yao et al., 2015). More generally, decreased insular activity among individuals with IGD is consistent with reports from other behavioral and substance addictions (Balodis et al., 2013; Paulus and Stewart, 2014), suggesting insular involvement across different addiction subtypes.
Strengths and Limitations
This study has several strengths, including a relatively robust sample size in comparison to previous fMRI cue-reactivity studies conducted among individuals with IGD; e.g., (Ko et al., 2009b; Ko et al., 2013; Sun et al., 2012). To our knowledge, this is also the first study focusing on the function of both the ventral and dorsal striatum in response to Internet gaming-related cues among individuals with IGD. Associations between striatal function and structure and clinical features of IGD (e.g., craving ratings, duration of IGD) were also assessed, providing integrated assessment of ventral versus dorsal striatal cue-processing in a non-substance addiction.
This study also has several limitations, including the cross-sectional design which prevents us from making causal conclusions related to ventral versus dorsal striatal activations. While our findings are consistent with those from studies conducted in substance-using populations (Sjoerds et al., 2014; Vollstädt-Klein et al., 2010), longitudinal research is needed to further characterize the relationship between ventral and dorsal striatal activations across the course of IGD and other addictions.
Given the prevalence of Internet use in daily life, and the popularity of Internet games among young adults as a leisure activity, we recruited less severe IGD participants for this study, which represents a large proportion of the population of Chinese universities. However, it is possible that this methodology may have resulted in underestimation of effects. A final limitation of this study is our inclusion of only male participants, thus further research is needed to determine the relationship between ventral versus dorsal striatal activity among women with IGD.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31170990 and No. 81100992), project of Humanities and Social Sciences from Ministry of Education in China (No. 15YJA190010), and T32 DA007238-23 and CASAColumbia (SWY).
Footnotes
The address where the work was carried out: 3.0 T Siemens Trio scanner at State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University
The authors declare no competing financial interests.
Authors Contribution
XYF and JTZ were responsible for the study concept and design. ZJS, LL, LJW, BL, SSM and YWY contributed to the acquisition of MRI data. LL, JTZ, and SWY assisted with data analysis and interpretation of findings. LL drafted the manuscript. JTZ, SWY and XYF provided critical revision of the manuscript for intellectual content. All authors critically reviewed and approved the final version of the manuscript submitted for publication.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, White MA, Stevens MC, Pearlson GD, Sinha R, Grilo CM, Potenza MN. Monetary reward processing in obese individuals with and without binge eating disorder. Biol Psychiatry. 2013;73:877–886. doi: 10.1016/j.biopsych.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of consulting and clinical psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Annals of the New York Academy of Sciences. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Archives of internal medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Cai C, Yuan K, Yin J, Feng D, Bi Y, Li Y, Yu D, Jin C, Qin W, Tian J. Striatum morphometry is associated with cognitive control deficits and symptom severity in internet gaming disorder. Brain imaging and behavior. 2015 doi: 10.1007/s11682-015-9358-8. [DOI] [PubMed] [Google Scholar]
- Chen CY, Huang MF, Yen JY, Chen CS, Liu GC, Yen CF, Ko CH. Brain correlates of response inhibition in Internet gaming disorder. Psychiatry and clinical neurosciences. 2014;69:201–209. doi: 10.1111/pcn.12224. [DOI] [PubMed] [Google Scholar]
- Chen S, Weng L, Su Y, Wu H, Yang P. Development of a Chinese Internet addiction scale and its psychometric study. Chinese Journal of Psychology. 2003;45:279–294. [Google Scholar]
- Das D, Cherbuin N, Anstey KJ, Sachdev PS, Easteal S. Lifetime cigarette smoking is associated with striatal volume measures. Addiction biology. 2012;17:817–825. doi: 10.1111/j.1369-1600.2010.00301.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT‐C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clinical and Experimental Research. 2005;29:844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60:252–262. doi: 10.1016/j.neuroimage.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neuroscience & Biobehavioral Reviews. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Fagerström K-O. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL. Neuroscience on the net. Science. 1994;266:994–996. doi: 10.1126/science.7973682. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline J, Grasby P, Williams S, Frackowiak RS, Turner R. Analysis of fMRI time-series revisited. Neuroimage. 1995;2:45–53. doi: 10.1006/nimg.1995.1007. [DOI] [PubMed] [Google Scholar]
- Grüsser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell NA, Worbe Y, Lange I, Tait R, Irvine M, Banca P, Harrison NA, Bullmore ET, Hutchison WD, Voon V. Increased ventral striatal volume in college-aged binge drinkers. PloS one. 2013;8:e74164. doi: 10.1371/journal.pone.0074164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Park MTM, Farmer S, Chakravarty MM. Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology. 2014;40:406–411. doi: 10.1038/npp.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Romanowski A, Schilling C, Lorenz R, Mörsen C, Seiferth N, Banaschewski T, Barbot A, Barker G, Büchel C. The neural basis of video gaming. Translational Psychiatry. 2011;1:e53. doi: 10.1038/tp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Baik S-H, Park CS, Kim SJ, Choi SW, Kim SE. Reduced striatal dopamine D2 receptors in people with Internet addiction. Neuroreport. 2011;22:407–411. doi: 10.1097/WNR.0b013e328346e16e. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Yen J-Y, Chen S-H, Yang M-J, Lin H-C, Yen C-F. Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Comprehensive psychiatry. 2009a;50:378–384. doi: 10.1016/j.comppsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu G-C, Hsiao S, Yen J-Y, Yang M-J, Lin W-C, Yen C-F, Chen C-S. Brain activities associated with gaming urge of online gaming addiction. Journal of psychiatric research. 2009b;43:739–747. doi: 10.1016/j.jpsychires.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Ko CH, Liu GC, Yen JY, Chen CY, Yen CF, Chen CS. Brain correlates of craving for online gaming under cue exposure in subjects with Internet gaming addiction and in remitted subjects. Addiction biology. 2013;18:559–569. doi: 10.1111/j.1369-1600.2011.00405.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of addiction. Academic Press, Elsevier; Oxford, UK: 2005. [Google Scholar]
- Kuss DJ. Internet gaming addiction: current perspectives. Psychology research and behavior management. 2013;6:125–137. doi: 10.2147/PRBM.S39476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuss DJ, Griffiths MD. Internet and gaming addiction: a systematic literature review of neuroimaging studies. Brain Sciences. 2012;2:347–374. doi: 10.3390/brainsci2030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, Fla: The Center for Research in Psychophysiology, University of Florida; 1997. [Google Scholar]
- Limbrick-Oldfield EH, van Holst RJ, Clark L. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? NeuroImage: Clinical. 2013;2:385–393. doi: 10.1016/j.nicl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meng Y, Deng W, Wang H, Guo W, Li T. The prefrontal dysfunction in individuals with Internet gaming disorder: a meta-analysis of functional magnetic resonance imaging studies. Addiction biology. 2014;20:799–808. doi: 10.1111/adb.12154. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacology. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Nielsen FA, Hansen LK. Automatic anatomical labeling of Talairach coordinates and generation of volumes of interest via the BrainMap database. NeuroImage 2002; 16. Presented at the 8th International Conference on Functional Mapping of the Human Brain; 2–6 June 2002; Sendai, Japan. 2002. Available on CD-Rom. [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Rehbein F, Gentile DA, Lemmens JS, Rumpf HJ, Mößle T, Bischof G, Tao R, Fung DS, Borges G. An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction. 2014;109:1399–1406. doi: 10.1111/add.12457. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual Differences in Insular Sensitivity During Loss Anticipation Predict Avoidance Learning. Psychological Science. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addiction biology. 2013;18:121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;12:1653–1658. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Sjoerds Z, van den Brink W, Beekman AT, Penninx BW, Veltman DJ. Cue reactivity is associated with duration and severity of alcohol dependence: an fMRI study. PloS one. 2014;9:e84560. doi: 10.1371/journal.pone.0084560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Ying H, Seetohul RM, Xuemei W, Ya Z, Qian L, Guoqing X, Ye S. Brain fMRI study of crave induced by cue pictures in online game addicts (male adolescents) Behavioural brain research. 2012;233:563–576. doi: 10.1016/j.bbr.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39:1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- Yao YW, Chen PR, Chen C, Wang LJ, Zhang JT, Xue G, Deng LY, Liu QX, Yip SW, Fang XY. Failure to utilize feedback causes decision-making deficits among excessive Internet gamers. Psychiatry Res. 2014;219:583–588. doi: 10.1016/j.psychres.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Yao YW, Chen PR, Li S, Wang LJ, Zhang JT, Yip SW, Chen G, Deng LY, Liu QX, Fang XY. Decision-Making for Risky Gains and Losses among College Students with Internet Gaming Disorder. PLoS One. 2015;10:e0116471. doi: 10.1371/journal.pone.0116471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, DeVito EE, Kober H, Worhunsky PD, Carroll KM, Potenza MN. Pretreatment measures of brain structure and reward-processing brain function in cannabis dependence: an exploratory study of relationships with abstinence during behavioral treatment. Drug and alcohol dependence. 2014;140:33–41. doi: 10.1016/j.drugalcdep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


