Abstract
Although environmental enrichment has been shown to improve functional and histologic outcomes in pre-clinical moderate-to-severe traumatic brain injury (TBI), there are a paucity of pre-clinical data regarding enrichment strategies in the setting of repetitive mild traumatic brain injury (rmTBI). Given the vast numbers of athletes and those in the military who sustain rmTBI, the mounting evidence of the long-term and progressive sequelae of rmTBI, and the lack of targeted therapies to mitigate these sequelae, successful enrichment interventions in rmTBI could have large public health significance. Here, we evaluated enrichment strategies in an established pre-clinical rmTBI model. Seventy-one male C57BL/6 mice were randomized to two different housing conditions, environmental enrichment (EE) or normal condition (NC), then subjected to rmTBI injury (seven injuries in 9 days) or sham injury (anesthesia only). Functional outcomes in all four groups (NC-TBI, EE-TBI, NC-sham, and EE-sham) were assessed by motor, exploratory/anxiety, and mnemonic behavioral tests. At the synaptic level, N-methyl d-aspartate receptor (NMDAR) subunit expression of phosphorylated glutamate receptor 1 (GluR1), phosphorylated Ca2+/calmodulin-dependent protein kinase II (CaMKII), and calpain were evaluated by western blot. Compared to injured NC-TBI mice, EE-TBI mice had improved memory and decreased anxiety and exploratory activity post-injury. Treatment with enrichment also corresponded to normal NMDAR subunit expression, decreased GluR1 phosphorylation, decreased phosphorylated CaMKII, and normal calpain expression post-rmTBI. These data suggest that enrichment strategies may improve functional outcomes and mitigate synaptic changes post-rmTBI. Given that enrichment strategies are feasible in the clinical setting, particularly for athletes and soldiers for whom the risk of repetitive injury is greatest, these data suggest that clinical trials may be warranted.
Keywords: : animal model, concussion, enrichment, mild traumatic brain injury, synaptic plasticity
Introduction
Recently, there has been increased attention to the functional sequelae of repetitive mild traumatic brain injury (rmTBI). Clinically, rmTBI is associated with prolonged and sometimes severe functional impairment, including memory disturbances, behavioral changes, speech irregularities, and gait abnormalities.1–4 Despite this, targeted interventions to treat these sequelae of rmTBI are lacking. Mounting data from clinical and pre-clinical studies suggest that strategies targeting neural plasticity may prevent or treat adverse outcomes after various brain injuries.5 No study of which we are aware has evaluated strategies targeting neural plasticity in the setting of rmTBI.
In pre-clinical models, environmental enrichment (EE) has been shown to promote neural plasticity and cognitive activity in both uninjured and injured animals.5 EE is an experimental strategy where housing is markedly different from that of usual experimental laboratory animals, offering enhanced opportunities for exploratory, physical, and social interactions.5 EE has been shown to affect neuroanatomical and neurochemical changes in healthy mice, including enhanced neural plasticity and improved memory compared to animals housed in normal conditions (NC).6,7 In moderate-to-severe pre-clinical TBI models, EE has been shown to improve functional outcomes, ameliorate post-injury changes in neurotrophins and dopaminergic markers, reduce proinflammatory cytokine response, and improve energy homeostasis post-injury.8–10 Whether these findings are relevant to rmTBI has not been evaluated.
Clinically, rmTBI occurs frequently in high-risk populations, such as collision sport athletes. In these settings, pre- and post-injury treatment with enrichment strategies are clinically feasible and could, in fact, be an important strategy to mitigate the effects of repetitive subconcussive injuries, which may be clinically silent.
Here, we investigated whether EE could modify outcomes in a clinically relevant model of rmTBI that results in persistent deficits in exploratory behavior, balance, and spatial memory. For these studies, we focused primarily on glutamatergic pathways, which have been posited to be central to both acute excitotoxicity and chronic functional deficits post-concussion.11 Previous pre-clinical studies of TBI have shown that biomechanical injury results in hyperacute indiscriminate glutamate release12 and increased activity and expression of glutamate receptors, followed by decreased expression of glutamate receptors at chronic time points post-injury, though these studies modeled more severe forms of TBI.13,14 Although several lines of evidence suggest that EE might mitigate excitotoxicity after concussive injury,5 whether or not EE could protect against chronic glutamate receptor loss post-rmTBI has not been studied. This is important because glutamate receptor loss has been implicated in neurodegenerative diseases, such as Alzheimer's disease (AD),15 and may also be an important mechanism underpinning the progressive functional impairments described post-rmTBI.1–4 Thus, to be a clinically useful intervention, the benefits of EE would have to be sustained at chronic time points post-injury. We therefore evaluated the effects of EE on functional outcomes after rmTBI, as well as glutamate receptor expression and proximal modifiers of glutamate receptor expression (calpains16 and calmodulin-dependent protein kinase II [CaMKII]17), with an emphasis on chronic outcomes post-injury.
Methods
Seventy-one male C57BL/6 mice (5-week-old) were used for these experiments. Mice were housed in 12-h day/night cycles in a pathogen-free environment. Food and water were provided freely. All experiments were approved by the Boston Children's Hospital Institutional Animal Care and Use Committee (Boston, MA) and complied with the National Institutes of Health's “Guide for the Care and Use of Laboratory Animals.”
Housing conditions
Mice were randomized to two different housing conditions: EE (n = 33) or NC (n = 38). EE housing consisted of a Marlau™ cage,18 a two-floor cage that offers features designed to stimulate mice such as running wheels, a climbing ladder, a slide tunnel, and mazes. Mice in EE housing were housed 10–15 animals per Marlau cage starting 3 days before injury and for the duration of the experiments until they were sacrificed. Mice in EE housing were only removed for behavioral testing. Mice in NC housing were housed 5 animals per cage in standard, translucent, plastic mouse cages.
Repetitive mild traumatic brain injury
Mice were randomized to undergo TBI (n = 38) or sham injury (n = 33) resulting in four experimental groups for the remainder of the study: EE-sham (n = 14); EE-TBI (n = 19); NC-TBI (n = 19); or NC-sham (n = 19). The mouse rmTBI model was used as previously described.19 Briefly, mice (5-week-old males) were anesthetized with 4.5% isoflurane for 45 sec in a 70:30 mixture of nitrous oxide and oxygen. Anesthetized mice were then placed on a delicate task wiper (Kimwipe; Kimberly-Clark, Irving, TX) and grasped by the tail. The head was placed directly under a hollow guide tube 71 cm in length. A 54-g metal bolt was then used to deliver the impact to the dorsal aspect of the skull, between the coronal and lambdoid sutures, resulting in rotational acceleration of the head through the Kimwipe. Injured mice underwent seven concussive injuries over 9 days (one injury daily for 5 days, 2 days recovery, then one injury daily for 2 days for a total of seven injuries in 9 days); sham-injured mice underwent anesthesia exposure only. All mice recovered in room air. Loss of consciousness (LOC) was defined as the time from removal of anesthesia to spontaneous righting. For all behavioral testing, experimenters were blinded to injury status, using color-coding stored in a password-protected computer.
At 3 days after the last injury, a randomly chosen subset of mice (NC-sham, NC-TBI, and EE-TBI, n = 4/group) were sacrificed for histology without any behavioral testing; the remaining mice (NC-sham, NC-TBI, and EE-TBI, n = 15/group; EE-sham, n = 14/group) underwent behavior testing starting at 3 weeks after the last injury/anesthesia and were sacrificed 5 weeks after the last injury for histology.
Behavior testing
Assessment of motor function
Three weeks after the last injury, motor function was assessed by rotarod testing,19 which consisted of 1 day of training and 2 days of testing (NC-sham, NC-TBI, and EE-TBI, n = 15/group; EE-sham, n = 14/group). Mice were placed on a rotating drum, 4 cm in diameter, suspended within a box. The first day consisted of training and habituation as follows: Each mouse was individually placed on the rotating drum (4 revolutions per minute [rpm]) for 5 min and was immediately placed back on the rotating drum if the mouse fell off the drum during the 5-min training period. Testing was performed on days 2 and 3. Testing consisted of four testing trials per day. Mice were placed on the rotating drum (4 rpm) for 10 sec to acclimate, after which the rod acceleration was increased by 0.1 rpm/sec. Latency to fall was recorded. Mice received a minimum of 5 min of rest between trials.
Assessment of locomotor activity
Locomotor activity and anxiety were assessed 24 days after the last injury with the open field test (NC-sham, NC-TBI, and EE-TBI, n = 10/group; EE-sham, n = 9/group). Mice were placed in an opaque circular arena, 45 cm in diameter with walls 20 cm in height. The arena was placed inside a plastic transparent box with a tracking system and then placed inside a closed container to minimize distractions. The circular arena was broken up in to three concentric circles, an “inner” circle (20 cm in diameter), a “neutral” circle (40 cm diameter), and an “outer” circle (60 cm diameter). Mice were tracked for 10 min using Noldus EthoVision 11.5 (Noldus Information Technology bv, Wageningen, The Netherlands). Time spent in each circle was then recorded: time spent in the inner ring constituted the least anxious behavior, whereas time spent along the perimeter, in the outer ring, constituted anxious behavior.
Assessment of exploratory activity
Exploratory activity was assessed at 27 days after the last injury using the Elevated Plus Maze (EPM; NC-sham, NC-TBI, and EE-TBI, n = 15/group; EE-sham, n = 14/group). The maze is a plus-shaped, elevated platform (85 cm tall) consisting of two closed arms and two open arms (30 × 5 cm) extending opposite one another from a square-shaped decision zone in the center (Lafayette Instruments, Lafayette, IN). Mice were placed in the center of the maze facing one of the closed arms, then allowed to explore the maze for 5 min while being tracked (Noldus Ethovision 11.5; Noldus Information Technology bv). The maze was cleaned with Clidox (1:18:1 Clidox Base/Water/Clidox Activator) between each individual trial. Percent time in each arm was calculated: Less time spent in the open arms constituted less exploratory behavior.
Assessment of spatial learning and memory
Spatial learning and memory was evaluated 30 days after the last injury for 4 consecutive days, using the Morris Water Maze (MWM; NC-sham, NC-TBI, and EE-TBI, n = 15/group; EE-sham, n = 14/group).19, 20 A white circular tub (83 cm diameter, 60 cm deep) was filled with water, approximately 24°C in temperature, to 29 cm deep. Four clearly visible shapes were placed at four points along the walls of the tub, forming four distinct quadrants. A clear, plastic target platform, 10 cm in diameter, was placed in one quadrant, 1 cm below the water's surface. Five hidden trials were performed in which the target platform was not visible. Each hidden trial consisted of four subtrials in which mice were released from each quadrant in a random order. Mice were released facing the wall and given 90 sec to find the platform, mount it, and remain for 7 sec. Mice that failed to locate the platform were gently guided by the experimenter to the platform and allowed to remain there for 10 sec. Mice were subjected to a maximum of two hidden trials a day, with a minimum of 20 min between each subtrial. On day 5, two visible trials were performed in which a red reflector was placed on the platform. Probe trials were conducted on days 4 and 5, during which the platform was removed from the tank. Mice were placed in the quadrant furthest from the platform and given 60 sec to explore the tank. Noldus Ethovision 11.5 software (Noldus Information Technology bv) tracked time spent in the target quadrant where the platform was previously located.
Forced swimming test
The forced swimming test21 was used to evaluate depression-like behavior 38 days post-injury (NC-sham, NC-TBI, and EE-TBI, n = 10/group; EE-sham, n = 9/group). Mice were placed individually in glass cylinders (40 × 18 cm, height × diameter) containing water up to 30 cm at 25°C for 5 min, and the session was recorded with a video camera. An experimenter, blinded to the animal treatments, observed the videotapes and recorded total immobility time. A mouse was considered immobile when floating and making only the necessary movements to keep its head above the water surface. Experiments were performed between 9:00 and 11:00 pm.
Synapse-specific expression by western blot
Mice were sacrificed at 3 days (n = 4/group) and 5 weeks (NC-sham, NC-TBI, and EE-TBI, n = 15/group; EE-sham, n = 14/group) after the last injury for western blot.
Tissue preparation and subcellular fractionation
Mice were deeply anesthetized, perfused with phosphate-buffered saline buffer, and brains were dissected to remove the hippocampus and frontal cerebral cortex (including corpus callosum), which were then immediately placed on dry ice and stored in a −80°C freezer until use. The synaptosome fraction22 was prepared as follows. Tissues were homogenized on ice with a Dounce homogenizer in 10 volumes of Buffer I (320 mM of sucrose, 10 mM of Tris-HCl [pH 7.4], 1 mM of ethylenediaminetetraacetic acid [EDTA], 1 mM ehthylene glycol tetraacetic acid, 1 mM of Na3VO4, and 5 mM of NaF) and protease inhibitor cocktail (Roche Life Science, Indianapolis, IN). Homogenates were centrifuged at 800g at 4°C for 10 min to obtain supernatants (S1) and to remove nuclei and incompletely homogenized material (P1). The S1 supernatants were centrifuged at 11,000g at 4°C for 15 min to obtain a crude synaptosomal fraction (P2) and supernatant (S2). The P2 was suspended in Buffer II (320 mM of sucrose, 10 mM of Tris-HCl [pH 7.4], 1 mM of EDTA, 1 mM of Na3VO4, 5 mM of NaF, 0.5 mM phenylmethylsulfonyl fluoride, 0.1% Triton X100, and cocktail) and then centrifuged at 150,000g at 4°C for 1 hour to obtain (P3) and supernatants (S3). The P3 pellets were suspended in Buffer I containing 0.1% sodium dodecyl sulfate (SDS). Protein concentrations were determined using the Bio-Rad protein assay dye reagent (Bio-Rad Laboratories, Hercules, CA).
Western blots
Equal amounts of protein (20 μg) were separated by 4–15% SDS gradient gel electrophoresis, transferred to polyvinylidenedifluoride membranes, and blocked with 5% nonfat milk. After blocking, proteins were incubated overnight at 4°C with antibodies targeting proteins glutamate receptor 1 (GluR1; 1: 1000; Cell Signaling Technology, Boston, MA), phosphorylated GluR1 (p-GluR1; [s845]; 1:1000; Cell Signaling Technology), p-GluR1 (s831; 1:1000; Abcam, Cambridge, MA), β-actin (1:5000; Abcam), nuclear receptor 1 (NR1; 1:1000; Abcam), N-methyl-d-aspartate (NMDA) receptor subunit 2A (NR2A; 1:1000; Millipore, Billerica, MA), NMDA receptor subunit 2B (NR2B; 1:1000; Abcam), amyloid precursor protein (APP; 1:1000; Millipore), phosphorylated Ca2+/calmodulin-dependent protein kinase II (p-CaMKII; 1:2000; Cell signaling Technology), calpain-1 (1:1000; Abcam, Cambridge, UK), postsynaptic density protein 95 (PSD-95; 1:1000; Cell Signaling Technology), and sodium potassium ATPaes (Na/K-ATPase; 1:1000; Millipore). Proteins were then incubated with horseradish-peroxidase–conjugated secondary antibodies for 1 h at room temperature. Data were scanned and pixel intensities were retrieved directly from blots using Image Quant LAS 4010 (GE Healthcare Biosciences Corp., Piscataway, NJ). Films were exposed at different times to ensure the optimum density, and pictures were assayed using ImageJ software (Sun Microsystems, Inc., Santa Clara, CA). Protein levels were first normalized to internal control levels for each sample and then were measured as fold changes with respect to controls.
Statistical analysis
All experiments were replicated three times independently. Sample-size calculations were based on our previous studies, accounting for expected performance of uninjured and injured groups and a [clinically meaningful] moderate effect size of intervention. Summarized data are presented as means ± standard error of the mean. MWM and rotarod latencies (with normal distributions) were analyzed by repeated-measures analysis of variance (ANOVA; group × time) or linear regression, controlling for injury (sham or closed head), cage (EE or NC condition), and time (using clustered robust standard errors). EPM and open field were analyzed using a one-way ANOVA followed by a Tukey honest significant difference (HSD) post-hoc test. Open field frequencies were analyzed using a Kruskal-Wallis test. Western blot data were analyzed with one-way ANOVA followed by the intergroup Tukey HSD post-hoc test. Data was graphed using GraphPad Prism (version 5; GraphPad Software Inc., La Jolla, CA) and analyzed with STATA software (StataCorp LP, College Station, TX). Statistical significance was set at *p < 0.05 and **p < 0.01.
Results
There were no convulsions post-injury. There were no injury-related deaths during the study period.
Loss of consciousness
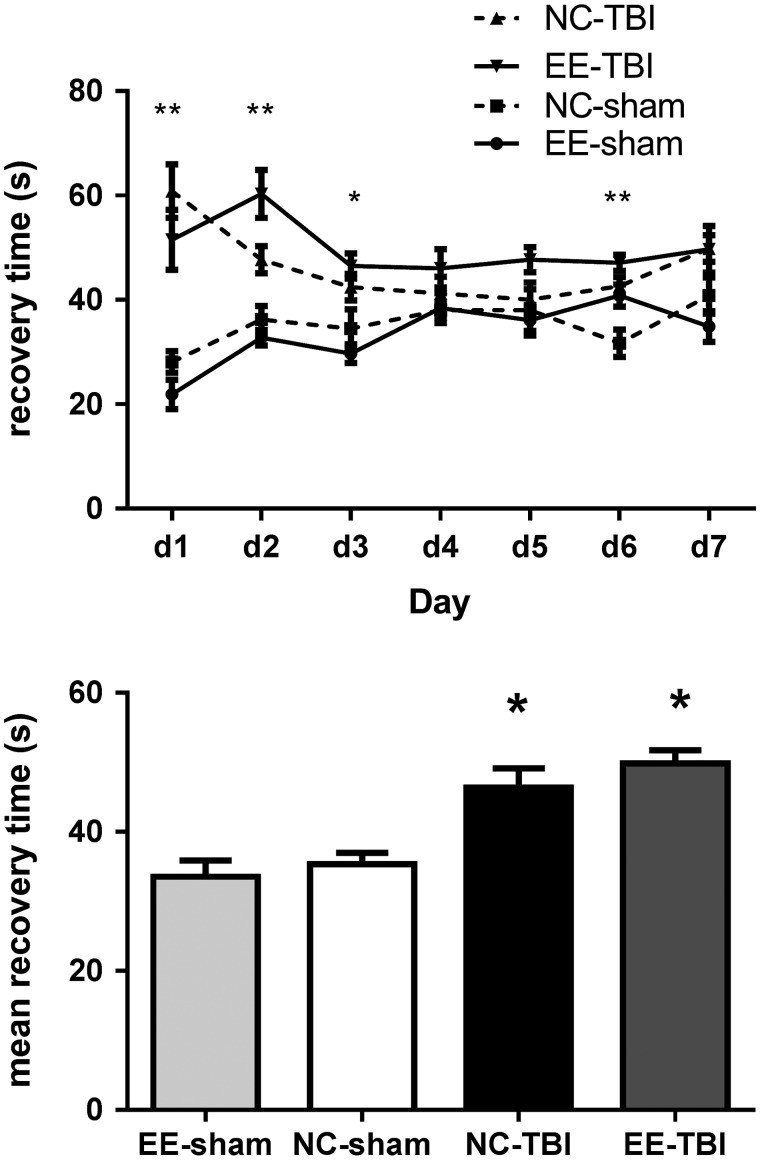
Injured mice had prolonged LOC compared to sham mice (48.1 ± 1.67 vs. 33.9 ± 1.37 sec; p < 0.05), but there was no statistically significant difference between injured EE-TBI and NC-TBI groups (46.4 ± 2.8 vs. 49.8 ± 1.9 sec; p > 0.05). Mice from the EE-sham and NC-sham groups showed no significant difference (32.6 ± 2.2 vs. 35.3 ± 1.6 sec; p > 0.05; Fig. 1).
FIG. 1.
Loss of consciousness (LOC) after rmTBI. Left: Injured mice in both NC-TBI and EE-TBI had increased LOC compared to NC-sham mice on days 1–3 and 6 of injury. EE-sham showed no significant difference when compared to NC-sham except for day 6. Right: NC-TBI and EE-TBI had increased mean LOC over all seven injuries compared to that of NC-sham and EE-sham mice. No difference was found in LOC between the two injured groups housed in different cages (p > 0.1). (**p < 0.001; *p < 0.05). EE, environmental enrichment; NC, normal condition; rmTBI, repetitive mild traumatic brain injury.
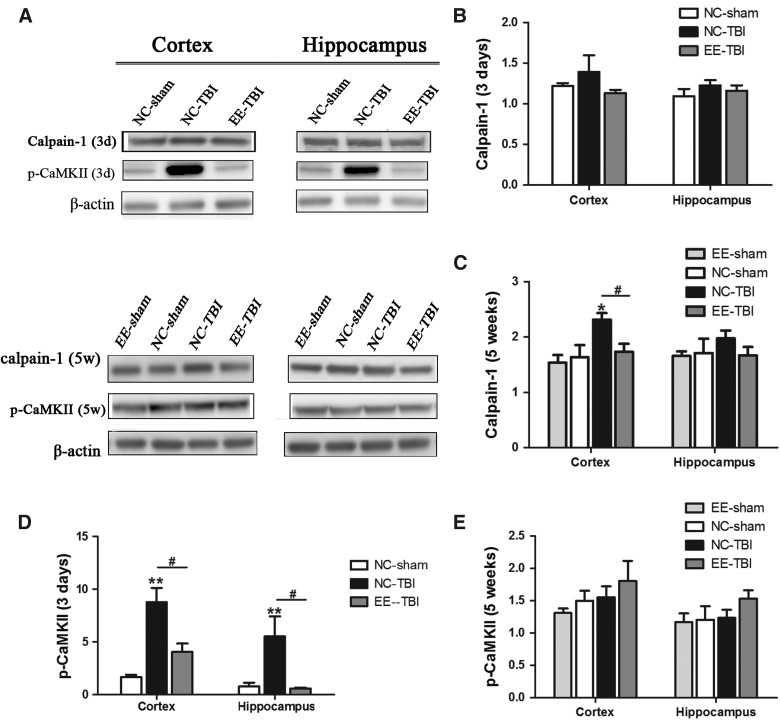
Subacute increase of phosphorylated Ca2+/calmodulin-dependent protein kinase II and delayed increase of calpain-1 post-injury is attenuated by enrichment
CaMKs and calpain-1 are sensitive to intracellular calcium concentration and can be activated when intracellular calcium increases.23,24 Three days after the last injury, there was no difference in calpain-1 cortical expression between groups (Fig. 2A,B), whereas 5 weeks post-injury, calpain-1 expression was increased in cortex of NC-TBI mice compared to NC-sham mice (p < 0.05), but not in EE-TBI compared to NC-sham or EE sham mice (Fig. 2C). There were no significant differences in calpain-1 expression in the hippocampus among groups at either time point.
FIG. 2.
Expression of calpain-1 and p-CaMKII. Expression of calpain-1 and phosphorylated CaMKII were analyzed by western blot. (A) Representative images of calpain-1, p-CaMKII, and β-actin. (3d: 3 days after last injury; 5w: 5 weeks after last injury). (B) Semiquantitative analysis of calpain-1 3 days after injury. (C) Semi quantitative analysis of calpain-1 5 weeks after injury (*p < 0.05, NC-TBI vs. NC sham; #p < 0.05, NC-TBI vs. EE-TBI). (D) Semiquantitative analysis of p-CaMKII 3 days after injury and (E) semiquantitative analysis of p-CaMKII, 5 weeks after injury, using densitometry. β-actin was used as the control. Relative expression of each molecule was compared to β-actin expression (#p < 0.05, NC-TBI vs. EE-TBI; **p < 0.01, NC-TBI vs. NC-sham). (E) There was no significant difference in p-CaMKII expression in the cortex and hippocampus between each group, 5 weeks after last injury. EE, environmental enrichment; NC, normal condition; p-CaMKII, phosphorylated Ca2+/calmodulin-dependent protein kinase II; TBI, repetitive mild traumatic brain injury.
Previous studies have shown that CaMKII is activated through phosphorylation post-TBI.25 Three days after the last injury, a dramatic increase of p-CaMKII expression was observed in both the cortex and hippocampus of the NC-TBI group compared to NC-sham (p < 0.01 in cortex and p < 0.05 in hippocampus; Fig. 2D), but there was no difference in cortical or hippocampal p-CaMKII in the EE-TBI group compared to NC-sham (p > 0.05; Fig. 2D). Five weeks after injury, p-CaMKII expression in all the groups was similar (Fig. 2E).
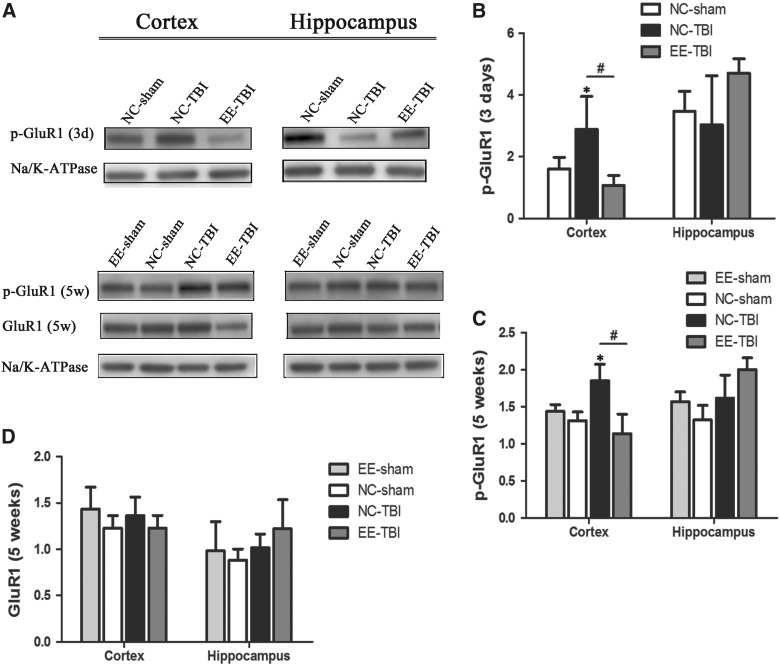
Enrichment is protective against post-injury changes in synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor and glutamate receptor 1 phosphorylation
Three days after the last injury, there were no differences in p-GluR1 expression in either cortex or hippocampus between groups. Five weeks post-rmTBI, p-GluR1 was significantly increased in cortex of NC-TBI mice compared to sham controls and EE-TBI (p < 0.05), whereas there was no difference in cortical p-GluR1 expression between EE-TBI and NC-sham or EE-sham groups (Fig. 3A,B). There was no difference in p-GluR1 expression in hippocampus among groups (Fig. 3C) and no differences between groups in total GluR1 in either cortex or hippocampus (Fig. 3D).
FIG. 3.
Synaptic expression of GluR1 after TBI. GluR1 expression in synaptosome fractions isolated from cortex and hippocampus were examined 3 days (3d) or 5 weeks (5w) after last injury. (A) Representative images of phosphorylated GluR1 (p-GluR1), total GluR1 (GluR1) and sodium potassium ATPase (Na/K-ATPase). (B) Semiquantitative analysis of p-GluR1 3 days after injury. (C) Semiquantitative analysis of p-GluR1 5 weeks after injury and (D) semiquantitative analysis of total GluR1 expression 5 weeks after injury. GluR1 and p-GluR1 expressions were analyzed by densitometry and normalized by Na/K-ATPase expression. (*p < 0.05, NC-sham vs. NC-TBI; #p < 0.05, NC-TBI vs. EE-TBI). EE, environmental enrichment; GluR1, glutamate receptor 1; Na/K-ATPase, sodium potassium ATPase; NC, normal condition; p-GluR1, phosphorylated glutamate receptor 1; TBI, repetitive mild traumatic brain injury.
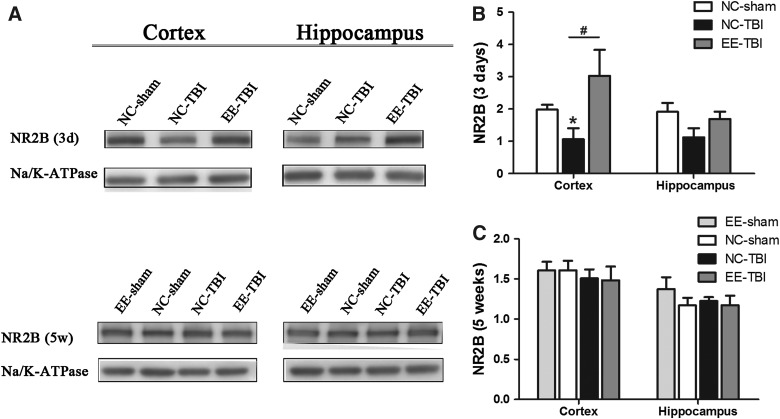
Sub-acute reduction of N-methyl-d-aspartate receptor subunit 2B expression after traumatic brain injury is eliminated by enrichment
NMDA receptors have critical functions in synaptic plasticity, and the NR2B subunit may be particularly vulnerable to the stretch injury characteristic of mTBI.26 Three days after the last rmTBI injury, cortical NR2B was markedly decreased in the NC-TBI group compared to NC-sham controls (p < 0.05; Fig. 4A,B), but there was no difference in cortical NR2B expression in the EE-TBI group compared to NC-sham (p > 0.05). There were no differences in hippocampal NR2B expression between groups 3 days post-injury (Fig. 4B). There were no differences in cortical or hippocampal NR2B expression 5 weeks post-injury (p > 0.05; Fig. 4C). There were no differences between groups in cortical or hippocampal NR1 or NR2A expression at 3 days or 5 weeks after the last injury or sham injury (data not shown). There were no differences between groups in cortical or hippocampal PSD-95 expression at 5 weeks after the last injury or sham injury (p > 0.05).
FIG. 4.
Synaptic NR2B expression after TBI. NR2B expression was detected in synaptosome fractions isolated from the cortex and hippocampus 3 days (3d) or 5 weeks (5w) after last injury using western blot. (A) Representative images of NR2B and Na/K-ATPase expression. (B) Densitometric analysis of NR2B expression 3 days after TBI and (C) densitometric analysis of NR2B expression 5 weeks after TBI. NR2B expression was normalized by Na/K-ATPase expression (*p < 0.05, NC-sham vs. NC-TBI; #p < 0.05, NC-TBI vs. EE-TBI). EE, environmental enrichment; NR2B, N-methyl d-aspartate receptor subtype 2B; Na/K-ATPase, sodium potassium ATPase; NC, normal condition; TBI, repetitive mild traumatic brain injury.
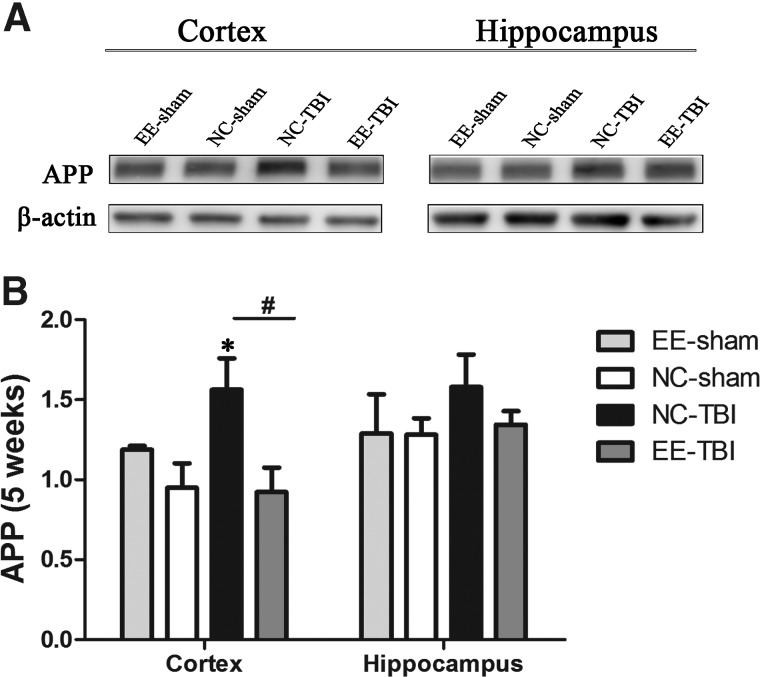
Diminished amyloid precursor protein expression post-injury in enriched environment
APP expression in cortex and hippocampus were examined by western blot 5 weeks after the last injury. APP expression was increased in cortex of NC-TBI mice compared to that in the NC-sham group (p < 0.05; Fig. 5), but there was no difference in cortical APP expression between EE-TBI and NC-sham or EE-sham groups (p > 0.05). No difference in APP expression was detected in the hippocampus among the three groups (p > 0.05).
FIG. 5.
APP expression after injury. APP expression was examined 5 weeks after last injury. Upper: representative images of APP western blots. Lower: relatively quantitative APP expression analyzed by densitometry. Data presented as mean ± standard error of the mean (*p < 0.05 NC-TBI vs. NC-sham; #p < 0.05, NC-TBI vs. EE-TBI). APP, amyloid precursor protein; EE, environmental enrichment; NC, normal condition; TBI, repetitive mild traumatic brain injury.
No effect of enrichment on motor function after repetitive mild traumatic brain injury
Three weeks after the last injury, sham mice and injured mice in both normal cages and enrichment cages had similar latencies to fall on rotarod (79.5 ± 6.9, 79.9 ± 8.8, 77.0 ± 7.1, and 66.2 ± 4.7, respectively, on day 1; p > 0.05; 82.5 ± 7.7, 94.1 ± 78.9, 82.9 ± 6.1, and 78.3 ± 6.3, respectively, on day 2; p > 0.05 for group effect).
Enrichment protects against alterations in locomotor activity and anxiety after repetitive mild traumatic brain injury
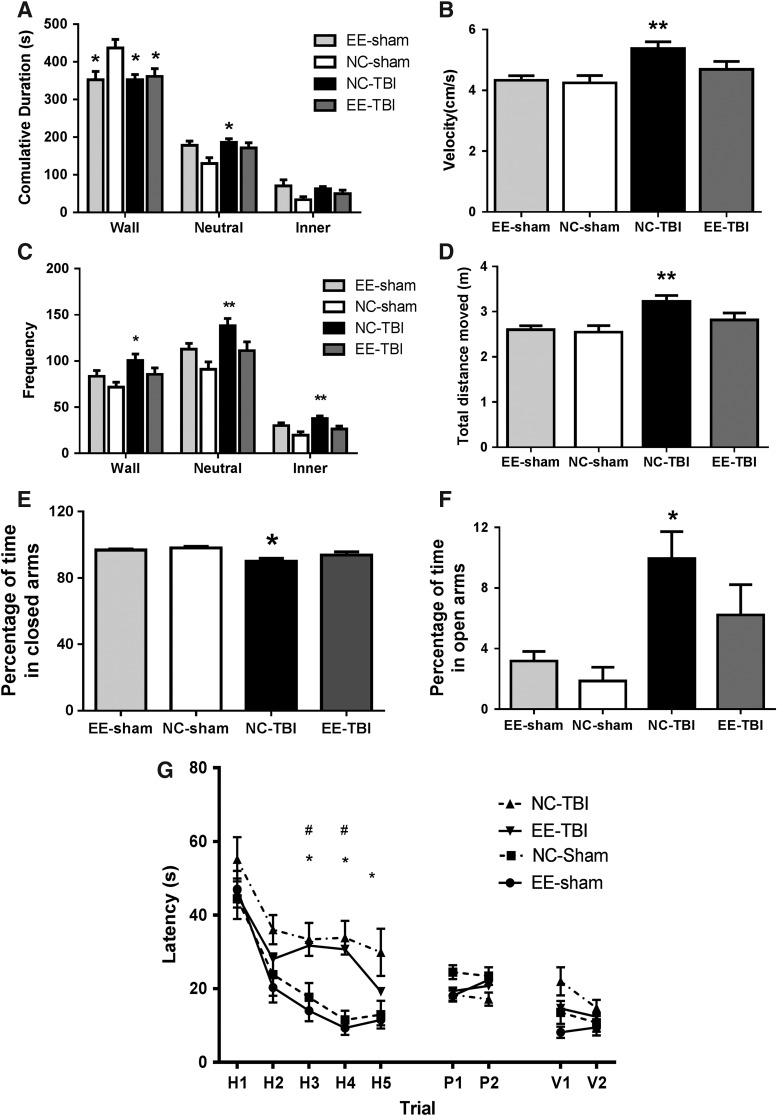
In open field testing, NC-TBI groups and EE-TBI and EE-sham groups spent less time in the wall zones (352.2 ± 13.8, 360.9 ± 20.9, and 352.4 ± 21.8 sec, respectively) compared to NC-sham mice (436.9 ± 22.4 sec; p < 0.05). However, NC-TBI mice spent significantly more time in the neutral zone than NC-sham mice (185.4 ± 10.3 vs. 129.6 ± 15.7 sec; p < 0.05), whereas EE-TBI and EE-sham (171.1 ± 13.7 and 177.6 ± 13.7 sec, respectively) mice were similar to NC-sham mice (p > 0.05). NC-TBI mice showed no difference in time spent in the inner zone when compared to EE-TBI, EE-sham, and NC-sham mice (62.5 ± 6.1 sec vs. 49.6 ± 9.2, 70.1 ± 15.5, and 33.6 ± 7.9 sec, respectively; p > 0.05; Fig. 6A). Mice from the NC-TBI group showed a significant increase in frequency in the wall compared to NC-Sham (100.7 ± 6.9 vs. 71.8 ± 5.2, respectively; p < 0.05), neutral (138.1 ± 7.9 vs. 91 ± 8.0, respectively; p < 0.01), and inner (37.8 ± 2.8 vs. 19.7 ± 3.8, respectively; p < 0.01) zones in open field testing. EE-TBI and EE-sham mice were not significantly different from NC-sham mice (p > 0.05; Fig. 6C).
FIG. 6.
(A–D) Open field testing assayed 24 days after the last injury. (A) Mice from the NC-TBI group spent less time in the wall and neutral zones than NC-sham mice (*p < 0.05). Mice from the EE-TBI group spent less time in the wall zone (*p < 0.05), but showed no difference in time spent in the neutral and inner zones (p > 0.05) when compared to NC-sham and NC-TBI mice. EE-sham mice spent significantly less time in the wall zone than the NC-sham mice (*p < 0.05); however, there was no difference in time spent in the neutral and inner zones (p > 0.05). (B) Mice from the NC-TBI group showed increased velocity (**p < 0.01) compared to NC-sham mice, whereas the velocity of EE-TBI mice was similar to NC-sham mice. No significant difference was observed between NC-sham and EE-sham mice. (C) Mice from the NC-TBI group showed a significant increase in frequency in the wall zone (*p < 0.05) as well as in the neutral and inner zone when compared to NC-sham mice (**p < 0.01). There was no significant difference across all zones between NC-sham and EE-sham. (D) Injured mice in the NC-TBI group also showed a significant increase in distance traveled (**p < 0.01). No significant difference was observed in distance traveled between NC-sham and EE-sham mice. (E and F) Elevated plus maze testing at 27 days after the last injury. The NC-TBI group spent less time in the closed arm zone (E) and more time in open arm zone (F) than the NC-sham mice (*p < 0.05). There was no difference between the mice from the EE-TBI, EE-sham and NC-sham groups. (G) Morris water maze performance of the mice. Thirty days after the last injury, mice underwent MWM testing, with all groups demonstrating time-dependent learning (p < 0.01). NC-sham and EE-sham mice exhibited no difference in performance. Both NC-TBI (n = 15/group) and EE-TBI (n = 15/group) performed worse in the hidden platform testing than NC-sham mice (linear regression injured vs. sham groups, β = 15.6; p < 0.05) and NC-TBI performed worse than EE-TBI (linear regression NC-TBI vs. EE-TBI, β = 6.6; p < 0.05). NC-TBI mice performed worse than NC-sham on hidden trials 3, 4, and 5 whereas EE-TBI mice performed worse than NC-sham on hidden trails 3 and 4 (single trial performance, *p < 0.05, NC-Sham vs. NC-TBI; #p < 0.05, NC-Sham vs. EE-TBI). EE, environmental enrichment; MWM, Morris Water Maze; NC, normal condition; TBI, repetitive mild traumatic brain injury.
Mice from the NC-TBI groups showed a significant increase in velocity (p < 0.01) and total distance moved (p < 0.01) when compared to NC-sham mice (n = 10) assayed 24 days after the last injury. There was no significant difference in velocity and distance traveled between EE-TBI, EE-sham, and NC-sham mice (Fig. 6B,D).
Enrichment protects against changes in exploratory activity after repetitive mild traumatic brain injury
Twenty-seven days after the last injury, the NC-TBI group spent significantly more time in the open arm of the EPM compared to NC-sham (9.9 ± 1.8% vs. 1.9 ± 0.9%; p < 0.01), but there were no differences between NC-sham EE-TBI and EE-sham groups (1.9 ± 0.9% vs. 6.2 ± 2.0% and 3.2 ± 0.6%, respectively; p > 0.05; Fig. 6E,F).
Environmental enrichment protects against long-term cognitive deficits after repetitive mild traumatic brain injury
Thirty days after the last injury, injured mice performed worse than sham mice (linear regression, β = 15.6; p < 0.05; Fig. 6G), and the NC-TBI group performed worse on hidden trials than those in the EE-TBI (linear regression, β = 6.6; p < 0.05). There was no difference in performance among the groups on probe trials or visible platform trials between groups (p > 0.05).
No effect of enrichment on depression after repetitive mild traumatic brain injury
Thirty-eight days after the last injury, depression was evaluated using the forced swim paradigm. Compared to NC-sham, there were no differences in time spent immobile in NC-TBI, EE-sham, or EE-TBI groups (185.5 ± 17.92 vs. 138.1 ± 26.6, 195.6 ± 17.6, and 187.3 ± 16.2; p > 0.05) or in frequency of immobility (1037 ± 141.6 vs. 851.8 ± 149.4, 1012 ± 173.1, and 926.4 ± 113.4; p > 0.05).
Discussion
In this study, we found that treatment with environmental enrichment improved functional outcomes and mitigated synaptic changes post-rmTBI. Previous pre-clinical studies of enrichment have found a beneficial effect of enrichment post-TBI in more severe TBI models associated with intraparenchymal hemorrhage (see Bondi and colleagues for an excellent summary).5 To our knowledge, this is the first study of enrichment in an rmTBI model. We found that treatment with enrichment protects against post-rmTBI changes in p-CaMkII, NR2B, and p-GluR1 associated with improved functional outcomes, including memory paradigms and anxiety-like phenotypes. These data may have clinical implications for athletes engaged in high-risk collision sports and those employed in the military where the risk of rmTBI is significant and where pre- and post-treatment enrichment regimens may, in fact, be feasible. In fact, given the repetitive and sometimes silent nature of TBI in these settings, pre-treatment regimens such as those examined here may be the only viable enrichment strategy. Given that vast numbers of athletes and those in the military sustain repetitive and sometimes clinically silent TBIs, developing enrichment interventions in rmTBI could have large public health significance.
The beneficial effects of enrichment appeared at early time points after repetitive injury. Previous studies suggest that acute increases in extracellular glutamate after more severe TBI are responsible for an excitotoxic cascade post-injury.12 Although the relevance of excitoxicity to rmTBI has not been established, we hypothesized that increases in glutamate post-injury could lead to abrupt increases in intracellular calcium and the resultant activation of several kinases, including Ca2+/calmodulin- dependent protein kinase. Our data suggest that rmTBI does, in fact, lead to an early increase in p-CaMKII post-injury that is attenuated by an enriched environment. However, the mechanism of how the enriched environment suppresses CaMKII phosphorylation remains unclear.
Whereas p-CaMKII was elevated subacutely post-rmTBI, calpain expression was increased at chronic (5 weeks post-rmTBI), but not subacute (3 days post-rmTBI) time points. It is possible that calpain activation at early time points occurred in the absence of changes in protein expression. Further studies are warranted to determine whether rmTBI results in subacute changes in calpain activation and changes in its downstream substrates, such as glycogen synthase kinase 3 beta, p35, and transactive response DNA binding protein 43 kDa, which have all been implicated in the injury cascade of stroke and Alzheimer's disease (AD).27–29 The chronic elevations we found in calpain expression suggest that similar mechanisms may be relevant in our rmTBI model. Interestingly, treatment with enrichment ameliorated this effect, such that EE-TBI mice had calpain levels similar to NC-sham animals.
In addition to evaluating downstream effects of glutamate-mediated excitotoxicity, we sought to evaluate the effect of enrichment on post-rmTBI neurotransmitter receptor expression at the synaptic level. We have previously shown that TBI causes suppression of cortical long term-potentiation (LTP),30 suggesting TBI-induced alterations in synaptic function. We chose to focus on α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation, which has been linked to LTP and synaptic plasticity.31–33 We found that phosphorylation of AMPA GluR1 was increased in the synaptic membrane of the cortex post-rmTBI, and that treatment with enrichment suppressed this post-injury GluR1 phosphorylation. It is notable that we did not find similar changes in the hippocampus, and further experiments are needed to examine whether phosphorylation of AMPA receptors in the hippocampus would develop at later time points in this mouse model.
We also investigated NMDA receptor (NMDAR) expression post-injury, because NMDARs are other key mediators of synaptic dysfunction. Previous studies in more severe TBI models have demonstrated NMDAR subunit loss at subacute time points post-injury.22,34,35 In our rmTBI model, we found profound decreases in cortical NR2B expression post-rmTBI that were reversed with enrichment treatment. We did not find concomitant changes in the NR1 or NR2A subunits post-injury.
Chronic elevations in APP expression post-rmTBI were also mitigated by enrichment. Increased APP expression post-TBI is likely a marker for axonal damage and may elevate the risk of developing AD and amyloid-beta–related neuronal degeneration.36 In this study, we found an increase in APP expression in the cortex at chronic time points post-TBI, which was attenuated in mice housed in the enriched environment. This, paired with improved functional outcomes, further suggests that enrichment may improve overall axonal repair and behavioral performance. The mechanism for this protective effect, however, remains unknown and should be investigated in future studies.
Enrichment treatment also resulted in improved functional outcomes post-injury. We found that injured mice treated with the EE performed overall similarly to shams on most behavior paradigms, whereas injured mice in the NC environment had impaired performance in the water maze, open field, and EPM tasks. Given that our treatment regimen preceded injury, one mechanism of protection might be related to the concept of “cognitive reserve.” Indeed, sham mice housed in the EE demonstrated better performance in the MWM task than sham mice housed in NCs, suggesting that the EE improved learning and memory in uninjured mice as well. Activity-dependent neuroplasticity has been documented since the pioneering work done by Paul Bach-y-Rita and colleagues,37 and it has been well demonstrated that an EE upregulates expression of growth factors, such as brain-derived neurotrophic factor and nerve growth factor.38,39 These growth factors also contribute to cell survival, axonal repair, and regeneration after brain injury, which may, in part, contribute to the beneficial effects of enrichment we found in this study.
Our data are in line with previous studies, which have shown that enrichment enhances motor and cognitive performance after severe TBI, extending these previous studies to rmTBI.40–46 However, the timing of enrichment (pre- or post-injury) and the relative contribution of increased physical versus cognitive activity within the enrichment paradigm may be important caveats to the current study. The pre-treatment regimen used in the current study supports previous studies that have shown the protective effects of enrichment strategies and physical activity pre-TBI.47,48 Enrichment strategies may have similar efficacy whether they are delivered pre-injury, as in the current study, or post-injury.49 In contrast, there is conflicting evidence regarding the effect of post-injury exercise, with some studies describing a detrimental effect of post-injury voluntary exercise on performance of hippocampal learning tasks,50,51 suggesting that early post-injury exercise may inhibit potential compensatory mechanisms observed post-TBI. And, importantly, even in studies that demonstrate a beneficial effect of post-injury exercise, enrichment strategies after brain injury seem to confer protective effects that exceed those of exercise alone.52
This study suggests that enrichment strategies may be beneficial in treating rmTBI-induced changes in synaptic dysfunction, pathological NMDAR loss, GluR1 phosphorylation, and calpain upregulation, as well as restoring functional outcomes post-injury. To our knowledge, this is the first study to directly address enrichment strategies in the setting of rmTBI. Although enrichment has been attempted in the clinic to treat neurological disorders such as autism,53 the detailed mechanisms of how enriched environments benefit patients is still not fully understood. Further investigation of the mechanisms underlying the protection may provide novel insight into future therapies for rmTBI, identifying new strategies to mitigate the potentially devastating effects of rmTBI.
Acknowledgments
Dr. Meehan receives royalties from ABC-Clio publishing for the sale of his book, Kids, Sports, and Concussion: A guide for coaches and parents, and from Springer International for the book Head and Neck Injuries in Young Athlete and from and royalties from Wolters Kluwer for working as an author for UpToDate. He is under contract with ABC-Clio publishing for a future book entitled, Concussions. His research is funded, in part, by a grant from Harvard Catalyst (the National Football League Players Association mechanism) and by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament.
Dr. Mannix is funded, in part, by the NICHD T32 HD040128, a grant from Harvard Catalyst (the National Football League Players Association mechanism), and by philanthropic support from the National Hockey League Alumni Association through the Corey C. Griffin Pro-Am Tournament.
The research presented in this article is supported by CHB IDDRC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bower J.H., Maraganore D.M., Peterson B.J., McDonnell S.K., Ahlskog J.E., and Rocca W.A. (2003). Head trauma preceding PD: a case-control study. Neurology 60, 1610–1615 [DOI] [PubMed] [Google Scholar]
- 2.Critchley M. (1957). Medical aspects of boxing, particularly from a neurological standpoint. Br. Med. J. 1, 357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grahmann H., and Ule G. (1957). [Diagnosis of chronic cerebral symptoms in boxers (dementia pugilistica & traumatic encephalopathy of boxers)]. [Article in German]. Psychiatr. Neurol. (Basel) 134, 261–283 [PubMed] [Google Scholar]
- 4.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 5.Bondi C.O., Klitsch K.C., Leary J.B., and Kline A.E. (2014). Environmental enrichment as a viable neurorehabilitation strategy for experimental traumatic brain injury. J. Neurotrauma 31, 873–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond M.C., Law F., Rhodes H., Lindner B., Rosenzweig M.R., Krech D., and Bennett E.L. (1966). Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 128, 117–126 [DOI] [PubMed] [Google Scholar]
- 7.Bennett E.L., Diamond M.C., Krech D., and Rosenzweig M.R. (1964). Chemical and anatomical plasticity brain. Science 146, 610–619 [DOI] [PubMed] [Google Scholar]
- 8.Briones T.L., Woods J., and Rogozinska M. (2013). Decreased neuroinflammation and increased brain energy homeostasis following environmental enrichment after mild traumatic brain injury is associated with improvement in cognitive function. Acta Neuropathol. Commun. 1, 57. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wagner A.K., Chen X., Kline A.E., Li Y., Zafonte R.D., and Dixon C.E. (2005). Gender and environmental enrichment impact dopamine transporter expression after experimental traumatic brain injury. Exp. Neurol. 195, 475–483 [DOI] [PubMed] [Google Scholar]
- 10.Ickes B.R., Pham T.M., Sanders L.A., Albeck D.S., Mohammed A.H., and Granholm A.C. (2000). Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp. Neurol. 164, 45–52 [DOI] [PubMed] [Google Scholar]
- 11.Giza C.C., and Hovda D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, Suppl. 4, S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katayama Y., Becker D.P., Tamura T., and Hovda D.A. (1990). Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 73, 889–900 [DOI] [PubMed] [Google Scholar]
- 13.Shohami E., and Biegon A. (2014). Novel approach to the role of NMDA receptors in traumatic brain injury. CNS Neurol. Disord. Drug Targets 13, 567–573 [DOI] [PubMed] [Google Scholar]
- 14.Schumann J., Alexandrovich G.A., Biegon A., and Yaka R. (2008). Inhibition of NR2B phosphorylation restores alterations in NMDA receptor expression and improves functional recovery following traumatic brain injury in mice. J. Neurotrauma 25, 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anggono V., Tsai L.H., and Gotz J. (2016). Glutamate receptors in Alzheimer's Disease: mechanisms and therapies. Neural Plast. 2016, 8256196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doshi S., and Lynch D.R. (2009). Calpain and the glutamatergic synapse. Front. Biosci. (Schol. Ed.) 1, 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rongo C., and Kaplan J.M. (1999). CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402, 195–199 [DOI] [PubMed] [Google Scholar]
- 18.Fares R.P., Belmeguenai A., Sanchez P.E., Kouchi H.Y., Bodennec J., Morales A., Georges B., Bonnet C., Bouvard S., Sloviter R.S., and Bezin L. (2013). Standardized environmental enrichment supports enhanced brain plasticity in healthy rats and prevents cognitive impairment in epileptic rats. PLoS One 8, e53888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mannix R., Berglass J., Berkner J., Moleus P., Qiu J., Andrews N., Gunner G., Berglass L., Jantzie L.L., Robinson S., and Meehan W.P., 3rd (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 121, 1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 21.Porsolt R.D., Bertin A., and Jalfre M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336 [PubMed] [Google Scholar]
- 22.Mannix R., Berkner J., Mei Z., Alcon S., Hashim J., Robinson S., Jantzie L., Meehan W.P., 3rd, and Qiu J. (2017). Adolescent mice demonstrate a distinct pattern of injury after repetitive mild traumatic brain injury. J. Neurotrauma. 34, 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vosler P.S., Brennan C.S., and Chen J. (2008). Calpain-mediated signaling mechanisms in neuronal injury and neurodegeneration. Mol. Neurobiol. 38, 78–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont G., Houart G., and De Koninck P. (2003). Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium 34, 485–497 [DOI] [PubMed] [Google Scholar]
- 25.Atkins C.M., Chen S., Alonso O.F., Dietrich W.D., and Hu B.R. (2006). Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J. Cereb. Blood Flow Metab. 26, 1507–1518 [DOI] [PubMed] [Google Scholar]
- 26.Singh P., Doshi S., Spaethling J.M., Hockenberry A.J., Patel T.P., Geddes-Klein D.M., Lynch D.R., and Meaney D.F. (2012). N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. J. Biol. Chem. 287, 4348–4359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang X.L., Tan M.S., Tan L. and Yu J.T. (2016). The role of TDP-43 in Alzheimer's disease. Mol. Neurobiol. 53, 3349–3359 [DOI] [PubMed] [Google Scholar]
- 28.Mines M.A., Beurel E., and Jope R.S. (2011). Regulation of cell survival mechanisms in Alzheimer's disease by glycogen synthase kinase-3. Int. J. Alzheimers Dis. 2011, 861072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang A., Liew H., Kim Y.M., Choi H., Kim S., Lee S.H., Ohshima T., Mikoshiba K., and Suh Y.H. (2013). p35 deficiency accelerates HMGB-1-mediated neuronal death in the early stages of an Alzheimer's disease mouse model. Curr. Alzheimer Res. 10, 829–843 [DOI] [PubMed] [Google Scholar]
- 30.Kondo A., Shahpasand K., Mannix R., Qiu J., Moncaster J., Chen C.H., Yao Y., Lin Y.M., Driver J.A., Sun Y., Wei S., Luo M.L., Albayram O., Huang P., Rotenberg A., Ryo A., Goldstein L.E., Pascual-Leone A., McKee A.C., Meehan W., Zhou X.Z., and Lu K.P. (2015). Antibody against early driver of neurodegeneration cis P-tau blocks brain injury and tauopathy. Nature 523, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H.K., Takamiya K., Han J.S., Man H., Kim C.H., Rumbaugh G., Yu S., Ding L., He C., Petralia R.S., Wenthold R.J., Gallagher M., and Huganir R.L. (2003). Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 [DOI] [PubMed] [Google Scholar]
- 32.Lee H.K., Barbarosie M., Kameyama K., Bear M.F., and Huganir R.L. (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959 [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y., Shi S.H., Esteban J.A., Piccini A., Poncer J.C., and Malinow R. (2000). Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287, 2262–2267 [DOI] [PubMed] [Google Scholar]
- 34.Giza C.C., Maria N.S., and Hovda D.A. (2006). N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J. Neurotrauma 23, 950–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osteen C.L., Giza C.C., and Hovda D.A. (2004). Injury-induced alterations in N-methyl-D-aspartate receptor subunit composition contribute to prolonged 45calcium accumulation following lateral fluid percussion. Neuroscience 128, 305–322 [DOI] [PubMed] [Google Scholar]
- 36.Johnson V.E., Stewart W., and Smith D.H. (2010). Traumatic brain injury and amyloid-beta pathology: a link to Alzheimer's disease? Nat. Rev. Neurosci. 11, 361–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bach-y-Rita P., Collins C.C., Saunders F.A., White B., and Scadden L. (1969). Vision substitution by tactile image projection. Nature 221, 963–964 [DOI] [PubMed] [Google Scholar]
- 38.Zhang X.Q., Mu J.W., Wang H.B., Jolkkonen J., Liu T.T., Xiao T., Zhao M., Zhang C.D., and Zhao C.S. (2016). Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol. Med. Rep. 14, 2231–2237 [DOI] [PubMed] [Google Scholar]
- 39.Torasdotter M., Metsis M., Henriksson B.G., Winblad B., and Mohammed A.H. (1998). Environmental enrichment results in higher levels of nerve growth factor mRNA in the rat visual cortex and hippocampus. Behav. Brain Res. 93, 83–90 [DOI] [PubMed] [Google Scholar]
- 40.Sozda C.N., Hoffman A.N., Olsen A.S., Cheng J.P., Zafonte R.D., and Kline A.E. (2010). Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma 27, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin S.S., Bales J.W., Yan H.Q., Kline A.E., Wagner A.K., Lyons-Weiler J., and Dixon C.E. (2013). The effect of environmental enrichment on substantia nigra gene expression after traumatic brain injury in rats. J. Neurotrauma 30, 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., Matter A.M., Cheng J.P., and Kline A.E. (2013). Environmental enrichment promotes robust functional and histological benefits in female rats after controlled cortical impact injury. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matter A.M., Folweiler K.A., Curatolo L.M., and Kline A.E. (2011). Temporal effects of environmental enrichment-mediated functional improvement after experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 25, 558–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kline A.E., Wagner A.K., Westergom B.P., Malena R.R., Zafonte R.D., Olsen A.S., Sozda C.N., Luthra P., Panda M., Cheng J.P., and Aslam H.A. (2007). Acute treatment with the 5-HT(1A) receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav. Brain Res. 177, 186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kline A.E., Olsen A.S., Sozda C.N., Hoffman A.N., and Cheng J.P. (2012). Evaluation of a combined treatment paradigm consisting of environmental enrichment and the 5-HT1A receptor agonist buspirone after experimental traumatic brain injury. J. Neurotrauma 29, 1960–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kline A.E., McAloon R.L., Henderson K.A., Bansal U.K., Ganti B.M., Ahmed R.H., Gibbs R.B., and Sozda C.N. (2010). Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu Y.L., Zhang L.W., Ma N., Ye L.L., Wang de X., and Gao X. (2014). Cognitive improvement of mice induced by exercise prior to traumatic brain injury is associated with cytochrome c oxidase. Neurosci. Lett. 570, 86–91 [DOI] [PubMed] [Google Scholar]
- 48.Zhao Z., Sabirzhanov B., Wu J., Faden A.I., and Stoica B.A. (2015). Voluntary exercise preconditioning activates multiple antiapoptotic mechanisms and improves neurological recovery after experimental traumatic brain injury. J. Neurotrauma 32, 1347–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Held J.M., Gordon J., and Gentile A.M. (1985). Environmental influences on locomotor recovery following cortical lesions in rats. Behav. Neurosci. 99, 678–690 [DOI] [PubMed] [Google Scholar]
- 50.Crane A.T., Fink K.D., and Smith J.S. (2012). The effects of acute voluntary wheel running on recovery of function following medial frontal cortical contusions in rats. Restor. Neurol. Neurosci. 30, 325–333 [DOI] [PubMed] [Google Scholar]
- 51.Griesbach G.S., Hovda D.A., Molteni R., Wu A., and Gomez-Pinilla F. (2004). Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience 125, 129–139 [DOI] [PubMed] [Google Scholar]
- 52.Gentile A.M., Beheshti Z. and Held J.M. (1987). Enrichment versus exercise effects on motor impairments following cortical removals in rats. Behav. Neural Biol. 47, 321–332 [DOI] [PubMed] [Google Scholar]
- 53.Woo C.C., Donnelly J.H., Steinberg-Epstein R., and Leon M. (2015). Environmental enrichment as a therapy for autism: a clinical trial replication and extension. Behav. Neurosci. 129, 412–422 [DOI] [PMC free article] [PubMed] [Google Scholar]