Abstract
The RNA-editing enzyme ADAR1 (adenosine deaminase that acts on RNA) is a bona fide nuclear enzyme that has been cloned from several vertebrate species. Putative nuclear localization signals (NLSs) have been identified in the aminoterminal regions of both human and Xenopus ADAR1. Here we show that neither of these predicted NLSs is biologically active. Instead, we could identify a short basic region located upstream of the RNA-binding domains of Xenopus ADAR1 to be necessary and sufficient for nuclear import. In contrast, the homologous region in human ADAR1 does not display NLS activity. Instead, we could map an NLS in human ADAR1 that overlaps with its third double-stranded RNA-binding domain. Interestingly, the NLS activity displayed by this double-stranded RNA-binding domain does not depend on RNA binding, therefore showing a dual function for this domain. Furthermore, nuclear accumulation of human (hs) ADAR1 is transcription dependent and can be stimulated by LMB, an inhibitor of Crm1-dependent nuclear export, indicating that hsADAR1 can move between the nucleus and cytoplasm. Regulated nuclear import and export of hsADAR1 can provide an excellent mechanism to control nuclear concentration of this editing enzyme thereby preventing hyperediting of structured nuclear RNAs.
INTRODUCTION
Adenosine deaminases that act on RNA (ADARs) are a group of RNA-editing enzymes that convert adenosines to inosines mostly in double-stranded RNA substrates. Because inosines are interpreted as guanosines during translation, an editing event can frequently lead to a codon exchange if the substrate is an mRNA (for review see Bass, 1997).
Substrates for ADARs include both viral and endogenous RNAs. Editing can be highly specific, only affecting a few adenosine residues, or nonspecific, leading to modification of up to 40% of all adenosines present in some RNAs (for review see Bass 1997, and references therein). Nonspecific editing of viral RNAs is believed to be part of a cellular antiviral defense program, an idea that is also supported by the interferon (IFN) inducibility of ADAR expression in mammals (Patterson and Samuel, 1995; George and Samuel, 1999). The extent of nonspecific editing is influenced by neighboring bases, as well as the length of contiguous double-stranded regions present (Polson and Bass, 1994; Lehmann and Bass, 1999).
Among the best studied substrates for site-specific deamination are the RNAs encoding subunits of the glutamate gated ion channel family expressed in the vertebrate brain (for review see O'Connell, 1997). Glutamate receptor B (GluR-B) RNA, for instance, is edited at a total of three sites. Editing at one of these sites leads to modification of a glutamine into an arginine codon (Q/R site) while an arginine codon is modified into a glycine codon (R/G site) at another site. The third site is located within an intron and thus does not give rise to any codon exchange. Editing at both the Q/R and R/G sites alters the electrophysiological properties of the receptor. Gene replacement experiments performed in mice have also shown that Q/R site editing is essential for normal life (Brusa et al., 1995; Higuchi et al., 2000).
The three sites in GluR-B RNA are modified by different members of the ADAR family. The Q/R site is predominantly edited by ADAR2, whereas the R/G site can be edited by both ADAR1 and ADAR2. The intronic site is preferentially edited by ADAR1, indicating that ADARs can discriminate among similar substrates (Melcher et al., 1996; Burns et al., 1997).
To date, three ADAR proteins, termed ADAR1, 2, and 3, are known that have a related and yet different molecular architecture. All three ADAR members have a conserved deaminase domain at their C-terminal end required for enzymatic activity. In their central region, ADAR1 proteins contain three double-stranded RNA-binding domains (dsRBDs), whereas ADAR 2 and 3 proteins only contain two copies of the dsRBD (for review see Bass, 1997; Chen et al., 2000).
In addition, ADAR1 proteins have a relatively long amino terminus that contains two tandemly arranged Z-DNA–binding domains (ZBDs) that are missing in ADAR2 and ADAR3 proteins (Herbert et al., 1997). Although the dsRBDs are known to be important for substrate binding, the function of the ZBDs found in ADAR1 proteins is less clear. It has been proposed that the ZBDs aid in targeting the protein to sites of transcription, thus facilitating the association of the protein with nascent RNAs (Herbert et al., 1998).
It is believed that editing is a cotranscriptional event with supporting evidence coming from both molecular and cytological data. First, some edited sites are defined by base-paired regions formed between intronic and exonic sequences (Herb et al., 1996; Aruscavage and Bass, 2000). Because intron excision occurs predominantly cotranscriptionally, it is assumed that editing may also occur at this stage. This assumption is also supported by the finding that ADAR2-deficient mice not only fail to edit the Q/R site in GluR-B RNA but are also unable to properly splice this RNA (Higuchi et al., 2000). Second, Xenopus laevis ADAR1 can be found associated with nascent transcripts on lampbrush chromosomes (Eckmann and Jantsch, 1999). Finally, immunostaining and biochemical fractionation experiments have indicated that ADAR1 is a predominantly nuclear enzyme (Bass and Weintraub, 1987; O'Connell and Keller, 1994; O'Connell et al., 1995)
To date, putative nuclear localization signals (NLSs) have been identified in the aminoterminal regions of all ADAR variants (Kim et al., 1994; Hough and Bass, 1997). However, deletion experiments performed on Xenopus ADAR1 revealed that the predicted NLSs found in this protein can be removed without affecting nuclear localization (Eckmann and Jantsch, 1999). Furthermore, there is also evidence that some fraction of ADAR1 can be found in the cytoplasm of mammalian cells (Patterson and Samuel, 1995).
We therefore set out to determine the regions in Xenopus and human ADAR1 proteins required for nuclear entry. We can show that a short basic region located upstream of the RNA-binding domains in Xenopus ADAR1 is necessary and sufficient for nuclear entry. In contrast, a region overlapping with the third double-stranded RNA-binding domain in human ADAR1 has NLS activity. Most interestingly, nuclear transport mediated by this dsRBD does not depend on RNA binding, excluding the possibility that this domain exerts NLS activity by binding to an RNA that is imported into the nucleus. Finally, we show that nuclear entry of full-length hsADAR1 is regulated via a presumptive shuttling mechanism, a feature frequently found among RNA-binding proteins (for review see Nakielny and Dreyfuss, 1999).
MATERIALS AND METHODS
ADAR1 cDNAs
Regions to be tested for NLS activity were amplified with the use of suitable primers containing HindIII sites. The amplified fragments were cut with HindIII and cloned in-frame with the pyruvate kinase (PK) cDNA into one of the vectors described below. For Xenopus ADAR1 we used an already cloned cDNA encoding the entire xlADAR1 open reading frame (ORF) as a template. To amplify regions corresponding to regions of the human ADAR1 gene we used a first-strand cDNA produced with random hexamers from HeLa poly A+ RNA with the use of an RNAse H-deficient reverse transcriptase (Life Technologies, Bethesda, MD).
Construction of PK Fusions for Oocyte Injections
A chicken PK cDNA was kindly provided by Dr. Gideon Dreyfuss (Howard Hughes Medical Institute, University of Pennsylvania, Philadelphia, PA; Siomi and Dreyfuss, 1995). The ORF encoding the PK protein was cloned in-frame upstream of seven myc tags followed by the 3′-untranslated region (UTR) of the Xenopus NO38 cDNA (Peculis and Gall, 1992). First, the PK ORF was amplified via polymerase chain reaction. The 5′-primer contained a unique KpnI restriction site for cloning, followed by an ATG for translational initiation and a unique HindIII site that allowed the introduction of amplified fragments to be tested for NLS activity. The 3′-primer contained a unique XhoI restriction site that allowed an in-frame fusion with the seven myc tag-encoding part. The myc tags and the NO38 3′-UTR were amplified via polymerase chain reaction from the previously described C7MA vector (Jantsch and Gall, 1992). The 5′-primer contained an XhoI restriction site to allow a fusion to the PK ORF, and the 3′-primer contained a BamHI restriction site. The PK-encoding fragment was cut with KpnI and XhoI, and the myc-NO38 fragment was cut with XhoI and BamHI. Both fragments were simultaneously ligated into a pBluescript KS vector (Stratagene, La Jolla, CA) cut with KpnI and BamHI. Clones containing both inserts were selected by restriction digest and verified by sequencing. The resulting vector was termed pPK-myc-A+.
Fragments to be tested for NLS activity were cloned into the unique HindIII site of pPK-myc-A+ located downstream of the ATG and upstream of the PK ORF. Again, positive clones were selected by restriction digest and verified via sequencing. The resulting clones were used to synthesize capped RNA that was injected into Xenopus oocytes.
PK Fusions for Transfection Assays
To test ADAR1 fragments for NLS activity in tissue culture cells, one of the following two strategies was used. On the one hand, the entire cassette of pPK-myc-A+ including an already cloned ADAR1 fragment was amplified with the use of two primers. The 5′-primer contained a BsiWI restriction site and the 3′-primer contained a unique XbaI restriction site. The resulting fragment was cut with BsiWI and XbaI and cloned between the KpnI and XbaI sites of a pcDNA3 vector (Invitrogen, La Jolla, CA) in which the original HindIII site was destroyed. On the other hand, some fragments of the ADAR1 cDNA were directly cloned into the unique HindIII site of the PK-myc NO38 cassette in the pCDNA3 vector.
Oocyte Injection Assays
For oocyte injections pPK-myc-A+ containing an ADAR1 insert was linearized at a unique site downstream of the NO38 poly A+ tail, and capped RNA was synthesized with the use of T3 RNA polymerase (Stratagene). Fifty oocytes were injected with 50 ng of RNA per oocyte and incubated for 24 h at 16°C in OR-2 (Wallace et al., 1973) to allow translation to occur. Subsequently, oocytes were manually dissected to separate nuclei from cytoplasm. Up to five cytoplasms were collected in NET-2 buffer (Steitz, 1989), sonicated, and centrifuged to remove insoluble material. The supernatant was mixed with an equal volume of 2× SDS sample buffer. Up to 20 germinal vesicles were directly collected into 2× SDS sample buffer. For Western blotting extracts corresponding to 10 germinal vesicles (GVs) or one cytoplasm were separated on a 7% SDS-PAGE gel and blotted onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Blots were detected with the anti-myc monoclonal antibody (mAb) 9E10 (Evan et al., 1985) and a secondary alkaline phosphatase-labeled anti-mouse antibody (Pierce) that was developed with the use of the chromogenic substrate NBT/BCIP.
Tissue Culture and Transfection Assays
HeLa or mouse 3T3 cells grown on coverslips were transfected with the use of a standard calcium phosphate precipitation method (Ausubel, 1987). DNA precipitates were left on cells for 10 h, after which time cells were washed and allowed to express the transfected construct for 24 h. Subsequently, cells were fixed, permeabilized, and stained with the use of mAb 9E10 as previously described (Jantsch and Gall, 1992). For detection of endogenous Xenopus ADAR1 in tissue culture cells, Xenopus XlA6 cells were grown on coverslips and stained with the anti-xlADAR1 antiserum Sat3 (Eckmann and Jantsch, 1999). Microscopic images were taken on a Zeiss fluorescence microscope equipped with filters for fluorescein isothiocyanate (FITC), rhodamine, and 4,6-diamidino-2-phenylindole (DAPI) with the use of a ORCA cooled charge-coupled device camera (Hamamatsu, Middlesex, NJ). Images were imported into Photoshop 5 (Adobe Systems, Mountain View, CA) with the help of a QED plug-in module (QED-Imaging, Pittsburgh, PA).
Drug Treatment of Tissue Culture Cells
To inhibit transcription, actinomycin D (AMD; Sigma, St. Louis, MO) was added to the tissue culture medium at a final concentration of 40 μg/ml 5–10 h before harvesting of the cells. To inhibit nuclear export, leptomycin B (LMB; a kind gift of Minoru Yoshida, University of Tokyo, Japan) was added at a final concentration of 10 ng/ml to the tissue culture cells 5–8 h before fixation. For wash-out experiments cells were cultured for 8 h in the presence of LMB; subsequently, cells were washed extensively with medium and cultured for an additional 12 h before being fixed and processed for staining. To select for cells with low levels of construct expression, they were split onto coverslips in G418-containing medium after transfection. After growth in selective medium for an additional 72 h, cells were fixed and stained. Cells showing only low levels of expression were visually selected under the microscope.
RESULTS
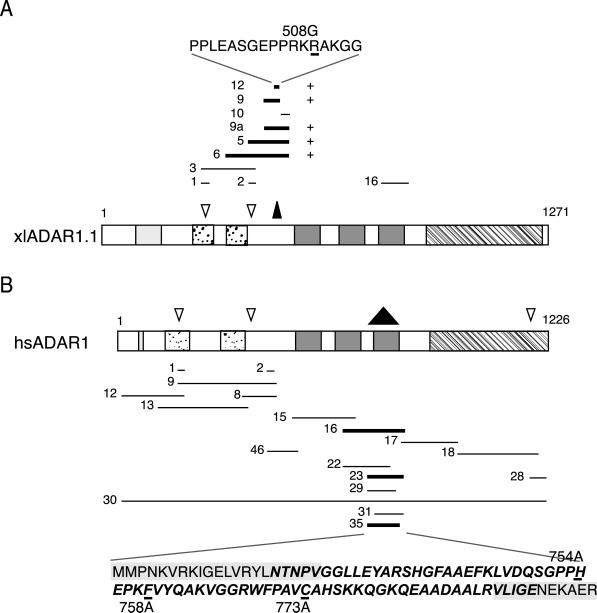
In X. laevis, two cDNAs encoding putative isoforms of the ADAR1 protein have been isolated that are termed xlADAR1.1 and xlADAR1.2 (Hough and Bass, 1997; Eckmann and Jantsch, 1999). The major difference between the two translation products can be found in their amino-terminal ends where xlADAR1.1 has a 145-amino acid-long insertion that is composed of several copies of an 11-amino acid-long repeat that is missing from the xlADAR1.2 cDNA. Furthermore, no 5′-AUG methionine start codon has been defined in the ADAR1.2 cDNA, making numbering of the putative protein difficult. Therefore, for the remainder of this paper amino acid data and their numbering will always refer to the xlADAR1.1 cDNA (GenBank accession no. U88065).
A Nonconserved Basic Region Serves as an NLS in xlADAR1
xlADAR1.1 contains two short stretches of basic amino acids within its amino-terminal third, each of which was proposed to serve as a bipartite NLS (Hough and Bass, 1997). The two putative NLSs located between aa 293–307 and 427–435 in the xlADAR1.1 protein, respectively, can also be found conserved in the xlADAR1.2 cDNA (accession no. U88066) and with minor variations in human and rat ADAR1, where the corresponding regions have also been identified as putative NLSs (Kim et al., 1994; O'Connell et al., 1995).
In previous experiments we produced deletion variants of xlADAR1.1 in which the first of the two putative NLSs found in this sequence was deleted. Oocyte injection experiments showed that the truncated versions of the protein were still able to accumulate in the GV, indicating that an active NLS sequence was still retained in the truncated protein (Eckmann and Jantsch, 1999). We therefore wanted to determine which region in xlADAR1.1 would serve as the active NLS. To do this various regions of the xlADAR1.1 cDNA sequence were cloned in-frame and upstream of a cDNA encoding the chicken PK protein that had six myc-tags at its C-terminal end. The 3′-UTR of the Xenopus NO38 cDNA was added at the 3′-end of this construct to ensure stability of RNAs when injected into Xenopus oocytes (Jantsch and Gall, 1992). The resulting constructs were linearized, and capped in vitro transcripts were synthesized and injected into Xenopus oocytes. After an appropriate incubation period, oocytes were hand enucleated and cytoplasmic and nuclear extracts were tested by Western blotting for nuclear accumulation of the protein with the use of the anti-myc mAb 9E10.
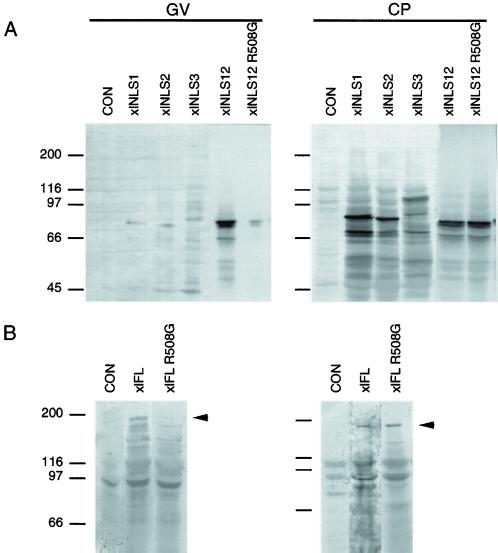
A series of overlapping fragments all located in the amino-terminal region of the xlADAR1.1 protein were tested (Figure 1A). Much to our surprise none of the two putative NLSs showed any NLS activity. Instead, a third region located between aa 496–592 upstream of the first dsRBD was able to translocate the myc-tagged PK reporter protein to oocyte nuclei (Figure 2). We termed this region xlNLS12. To verify the results obtained from Xenopus oocyte injection experiments, some constructs including xlNLS12 were also expressed as PK-myc fusion proteins in HeLa tissue culture cells. The results obtained from these experiments were in complete agreement with the oocyte injection experiments. Again, xlNLS12 did exhibit NLS activity in all transfected cells,whereas the other putative NLS regions were inactive (Figure 3; Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). xlNLS12 is a 17 amino acid-long fragment that contains a cluster of four basic amino acids at its C-terminal end and thus somewhat resembles the group of classical mono- or bipartite NLSs (Dingwall and Laskey, 1991). To determine whether the basic residues found in xlNLS12 were indeed required for nuclear import, we exchanged a single arginine (R) residue in this construct for a glycine (G). The resulting construct xlNLS12-R508G failed to show NLS activity either in Xenopus oocytes (Figure 2) or in HeLa cells (Figure 3).
Figure 1.
Schematic representation of Xenopus and human ADAR1 proteins and fragments tested for NLS activity. Only most important fragments are shown. Fragments that displayed NLS activity are highlighted by bold lines. Important elements found in those proteins are indicated as follows: ZBDs (hatched boxes), dsRBDs (dark gray boxes), deaminase domain (striped box). Previously identified putative NLSs are indicated by open arrowheads. The actual active NLSs are marked by inverted black triangles. (A) Schematic drawing of Xenopus ADAR1.1 and fragments tested from this protein. The sequence of the minimal xlNLS12 fragment is displayed on top. The single-point mutation R508G that abolishes NLS activity both in xlNLS12 and in the full-length protein is indicated. (B) Schematic drawing of human ADAR1. The sequence of the minimal active hsNLS35 fragment is shown at the bottom. The dsRBD consensus sequence is highlighted in bold face italics. Deletions into this region that abolish NLS activity are boxed in gray. Two mutations, H754A and F758A, that abolish RNA binding but do not affect NLS activity as well as a C773A mutation that changes a Cys found conserved in some mammalian ADAR1 proteins are indicated.
Figure 2.
xlNLS12 is both necessary and sufficient for nuclear import in Xenopus oocytes. Oocytes were injected with RNAs encoding myc-tagged xlNLS-PK reporter constructs, myc-tagged full-length (xlFL) or mutated xlADAR1 (xlFL R508G). Extracts of hand-enucleated oocytes corresponding to one cytoplasm (CP) and five GVs were loaded on a 7% SDS-PAGE gel and blotted, and myc-tagged proteins were detected with mAb 9E10. (A) Constructs covering the two putative amino-terminal NLSs (xlNLS1 and xlNLS2) or the entire amino-terminal region of xlADAR1 (xlNLS3) show no nuclear accumulation. In contrast, xlNLS12 shows clear nuclear accumulation, whereas the point mutation xlNLS12 R508G does not. Uninjected oocytes show no signal (CON). (B) Full-length xlADAR (xlFL) accumulates in the nucleus, whereas the point mutation R508G in this protein (xlFL R508G) abolishes nuclear accumulation. To fit the intensity of bands the lane containing the xlFL extract was exposed longer.
Figure 3.
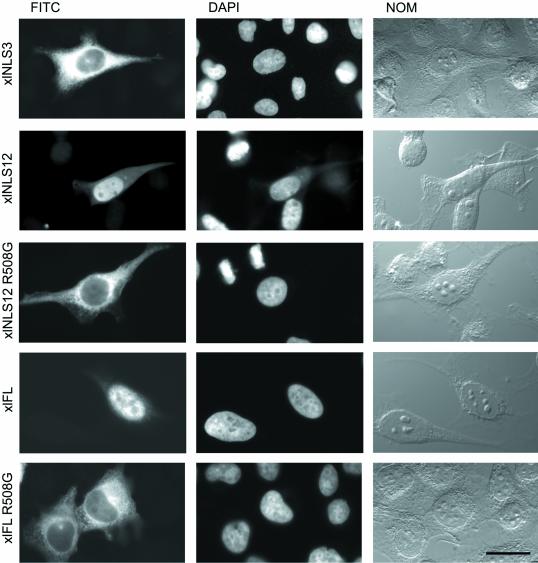
xlNLS12 is necessary and sufficient for nuclear import in HeLa cells. HeLa cells grown on coverslips were transiently transfected with the PK-myc constructs indicated. After fixation cells were permeabilized and stained for the presence of myc-tagged protein with the use of mAb 9E10 and a secondary FITC-labeled antibody (FITC). xlNLS12 displays NLS activity, whereas the R508G point mutation in this construct does not. myc-tagged full-length xlADAR1.1 protein accumulates in the nucleus (xlFL). The single-point mutation R508G in xlADAR1.1 abolishes nuclear accumulation (xlFL R508G). Nuclei were stained with DAPI and images of whole cells were taken by differential interference contrast (NOM). Scale bar, 20 μm.
xlNLS12 Is Necessary and Sufficient for Nuclear Import
The above experiments showed that xlNLS12 was sufficient for nuclear import of a PK reporter protein. To test whether xlNLS12 was the only NLS present in xlADAR1.1 and thus also necessary for nuclear import of the wild type protein, we introduced the R508G mutation into full-length xlADAR1.1. This point mutation dramatically impaired nuclear import both in Xenopus oocytes and in HeLa cells, indicating that xlNLS12 is both necessary and sufficient for nuclear import of xlADAR1.1 (Figures 2 and 3).
The NLS in Human ADAR1 Overlaps a dsRBD
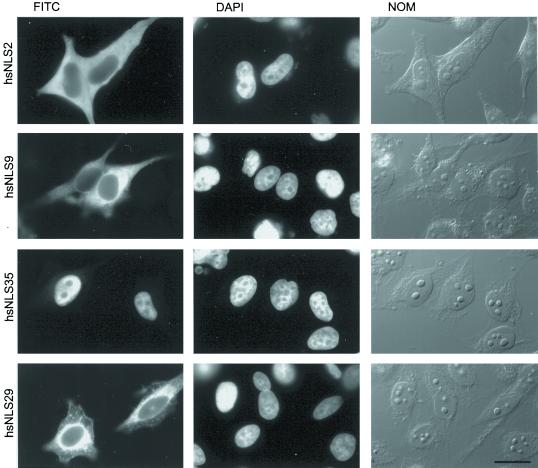
Sequence alignments of xlADAR1.1 with human and rat ADAR1 proteins showed that the region corresponding to xlNLS12 is not conserved in mammalian ADARs (Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). Instead, like for xlADAR1.1, two putative NLSs can be found highly conserved among all ADAR1 members that are located N-terminally to the xlNLS12 region (Kim et al., 1994; see Figure 1B). We therefore tested whether any of the putative NLSs or the region corresponding to xlNLS12 in human ADAR1 could show NLS activity on the PK-myc reporter construct. Surprisingly, neither the putative NLSs nor the region corresponding to xlNLS12 in hsADAR1 displayed NLS activity, nor did the entire N-terminal region of hsADAR1 (Figures 1B and 4).
Figure 4.
The third dsRBD of hsADAR1 has NLS activity. Various fragments of the hsADAR1 protein were fused to a PK-myc reporter construct, transfected into HeLa cells, and tested for NLS activity. HsNLS2, the region corresponding to the NLS found in xlADAR1 displays no NLS activity. Similarly, a large amino-terminal fragment of hsADAR1, harboring two additional putative NLSs, is also inactive (hsNLS9). In contrast, the third dsRBD of hsADAR1 displays NLS activity (hsNLS35). Deletion into this dsRBD from either the C-terminal end (hsNLS29) or the N-terminal end (Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results) completely abolishes NLS activity. FITC, Localization of myc-tagged protein as seen after antibody staining in the FITC channel. DAPI, Nuclei counterstained with DAPI. Whole cells were imaged by differential interference contrast (NOM). Scale bar, 20 μm.
We therefore started a systematic search for an active NLS in hsADAR1. Overlapping fragments each encoding ∼200 amino acids of the protein were cloned upstream of the PK-myc reporter construct and tested by tissue culture transfection for NLS activity. This screen covered essentially the entire hsADAR1 protein. One fragment (hsNLS16) extending from the middle of the second dsRBD to the end of the third dsRBD was able to confer NLS activity. Further deletion analysis indicated that the NLS overlapped entirely the third dsRBD of hsADAR1. We termed this minimal fragment hsNLS35 (Figures 1B and 4).
The dsRBD is a highly conserved 70 amino acid-long motif. Structure analysis of several dsRBDs has revealed a conserved structure of two α-helices located at the N- and C-terminal end of the domain and three antiparallel β-sheets in its center (Bycroft et al., 1995; Kharrat et al., 1995; Ryter and Schultz, 1998). Two regions required for RNA contact are located in the loop regions between β1 and β2, as well as between β3 and α2. A third region that makes contact with RNA is located at the beginning of helix 2 (Ryter and Schultz, 1998). The second C-terminal α-helix is rich in basic amino acid residues. Because typical NLSs are also basic in nature we asked whether this second α-helix might be sufficient to act as an NLS. Two deletions that removed either the entire second α-helix or only part of it were made. Both deletions, hsNLS22 and hsNLS29, almost completely destroyed the NLS activity found in dsRBD3 (Figures 1B and 4; Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). However, the smaller of the two deletions (hsNLS29) removed only the C-terminal end of helix 2 that does not contain any basic residues, indicating that the basic charge of helix 2 alone is not sufficient to act as an NLS. Furthermore, N-terminal deletions of the dsRBD that retained the entire C-terminal α-helix also failed to exhibit NLS activity (construct hsNLS31 in Figure 1B; Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). It thus appeared that only the entire dsRBD could exhibit NLS activity but not a subregion of this domain.
RNA Binding Is Not Required for NLS Activity
Deletion analysis of hsADAR1 has shown that the third dsRBD in this protein is most important for RNA binding and enzyme activity (Lai et al., 1995; Liu et al., 1998). We therefore reasoned that the NLS activity exerted by the third dsRBD in hsADAR1 might depend on active RNA binding. Nuclear import could be mediated by binding to an RNA that itself is imported into the nucleus. Small nuclear (sn) RNAs, for instance, are synthesized in the nucleus, exported to the cytoplasm where they become modified, associate with proteins, and are subsequently reimported into the nucleus where they are required for nuclear splicing (Mattaj, 1988). However, such a piggy-back mechanism would require a constant stream of RNA from the cytoplasm to the nucleus. To test this model we inhibited transcription by AMD treatment of cells. Inhibition of RNA synthesis should, in principle, also inhibit the import of newly synthesized RNAs into the nucleus. However, AMD treatment had no effect on the nuclear accumulation of hsNLS35, the minimal active NLS covering the third dsRBD (Figure 5). Although this result indicated that nuclear import does not depend on import of a newly synthesized RNA, it did not exclude the possibility that active RNA binding was required for nuclear accumulation of the hsNLS35 construct. For instance, nuclear transport might still be mediated by binding to an RNA that shuttles between the nucleus and cytoplasm irrespective of ongoing transcription. Therefore, to test the requirement of active RNA binding for nuclear import of the third dsRBD in more detail, we created mutations within the dsRBD that would abolish or greatly reduce RNA binding. Two mutations were introduced. One mutation, H754A, exchanged a single histidine (H) residue for an alanine (A), whereas the other mutation H754A plus F758A exchanged a phenylalanine (F) for an alanine (A), in addition. Both the histidine and phenylalanine residues are highly conserved in dsRBDs. Structural data indicate that the histidine residue makes RNA contact, whereas the phenylalanine is required for proper folding of the dsRBD (Ryter and Schultz, 1998). Mutational analysis of several dsRBDs has also shown that either residue is essential for RNA binding (Bycroft et al., 1995; Krovat and Jantsch, 1996; Ramos et al., 2000).
Figure 5.
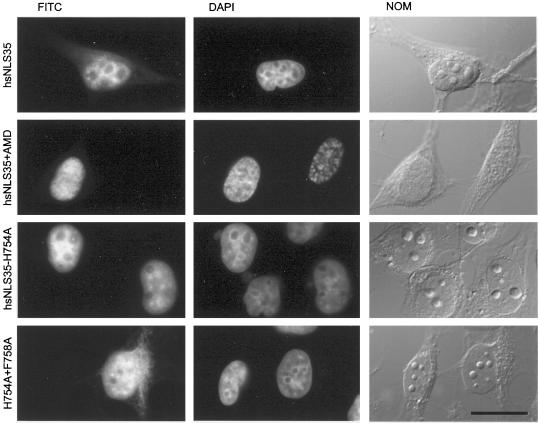
RNA binding is not required for NLS activity of hsNLS35, the third dsRBD of hsADAR1. To determine whether hsNLS35 acts via binding to an RNA that is imported into the nucleus, transcription was inhibited with the use of AMD. The third dsRBD has NLS activity (hsNLS35) even in the absence of transcription (hsNLS35+AMD). A single (hsNLS35-H754A) or double-point mutation (H754A+F758A) in this domain that abolish RNA binding has no effect on NLS activity. myc-tagged proteins are shown in the FITC channel (FITC), and nuclei were stained with DAPI (DAPI). Whole cells were imaged by differential interference contrast (NOM). Scale bar, 20 μm.
When transfected into tissue culture cells, the H754A had no effect on nuclear accumulation of a dsRBD3-PK-myc reporter construct, whereas the H754A plus F758A showed slightly reduced but still relatively strong nuclear accumulation, indicating that active RNA binding is not required for the NLS activity exerted by this dsRBD (Figure 5).
So far, NLS activity had not been reported for any other dsRBD. We therefore tested whether the third dsRBD of Xenopus ADAR1 would also display NLS activity. However, the resulting fragment (xlNLS16) did not display any NLS activity when cloned into the PK-myc reporter construct, indicating that not all dsRBDs have intrinsic NLS activity (Figure 1A; Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). The third dsRBD of human ADAR1 is highly homologous to the one in Xenopus ADAR1 and differs only in 12 of 70 amino acids. Of those, Cys 773 in hsADAR can also be found in rat and mouse ADAR1 but not in any other dsRBD. Thus, to determine whether this amino acid is required for NLS activity of dsRBD3 in hsADAR1 we mutated C773 into an alanine in hsNLS35. Interestingly, the resulting C773A mutation showed normal nuclear accumulation, indicating that this amino acid is not required for NLS activity (Figure 1B; Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results).
Nuclear Entry of Full-length hsADAR1 Is Regulated
Our data showed that the third dsRBD of hsADAR1 is sufficient to meditate nuclear localization of a PK-myc reporter construct. To determine whether this region was also necessary for nuclear import, we deleted this region from the full-length hsADAR1 protein. As a control, full-length hsADAR fused to the PK-myc construct (FL-PK-myc) was transfected as well. Most interestingly, FL-PK-myc was predominantly cytoplasmic. Even after removal of the PK part of the fusion construct, the protein stayed almost exclusively cytoplasmic (Figure 6A). We therefore counterstained the cells with an antiserum directed against hsADAR to determine the localization of the endogenous ADAR1 protein. Staining with this α-II antiserum (a kind gift from Professor Walter Keller, Biozentrum Basel, Switzerland) showed that the endogenous protein was predominantly but not exclusively nuclear (Figure 6B). It was obvious, however, that the endogenous ADAR1 concentration was much lower than that of the ectopically expressed protein. This suggested that the nuclear accumulation of ectopically expressed hsADAR1 protein was somehow regulated.
Figure 6.
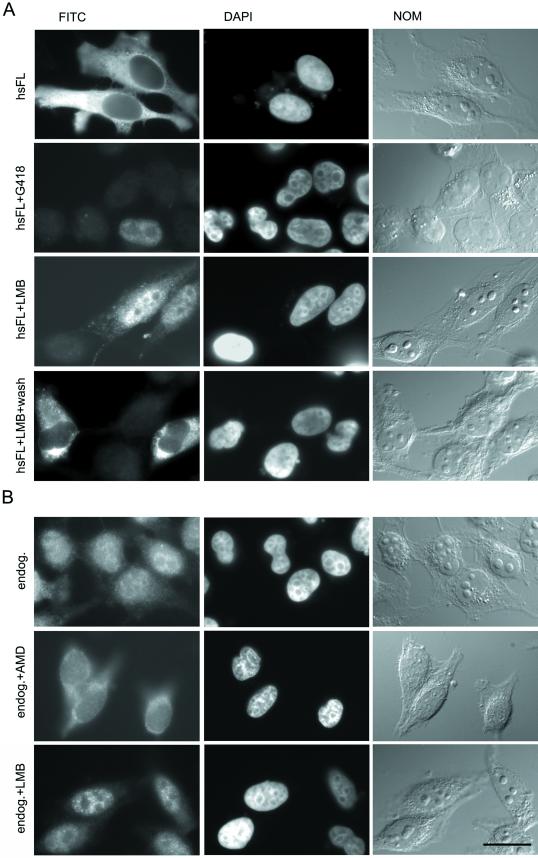
Nuclear accumulation of hsADAR1 is regulated. (A) Nuclear accumulation of transiently transfected hsADAR1 is concentration dependent and can be stimulated by LMB. Strong transient expression of myc-tagged hsADAR1 leads to cytoplasmic accumulation of this protein (hsFL). In cells selected for low levels of expression after G418 treatment, the protein can efficiently move to the nucleus (hsFL+G418). LMB treatment leads to nuclear accumulation of full-length hsADAR1 (hsFL+LMB), suggesting that cytoplasmic accumulation of overexpressed hsADAR1 is caused by increased nuclear export rather than reduced nuclear import. As can be seen cytoplasmic staining can still be observed in most of the cells. In only 20% of cells is complete nuclear accumulation of the transfected protein observed. Removal of LMB leads to redistribution of ectopically expressed hsADAR into the cytoplasm, indicating that the observed block of nuclear export by LMB treatment is reversible (hsFL+LMB+wash). (B) Nuclear accumulation of endogenous hsADAR1 is transcription dependent but can be stimulated by LMB. Staining with an antibody directed against endogenous hsADAR1 indicates that the protein is predominantly but not exclusively nuclear in normally growing cells (endog.). Inhibition of transcription by AMD treatment relocates the protein from the nucleus to the cytoplasm, indicating that ongoing transcription is required for nuclear accumulation or retention of the endogenous protein (endog.+AMD). LMB treatment leads to nuclear accumulation of endogenous hsADAR1 protein (endog.+LMB). The cytoplasmic staining seen in untreated cells is reduced, whereas nuclear staining is increased after LMB treatment. FITC, Localization of myc-tagged transfected (A) or endogenous (B) protein seen after antibody staining in the FITC channel. DAPI, Staining of nuclei with DAPI. NOM, Images of whole cells taken by Nomarski optics. Bar, 20 μm.
Because we had already shown that constructs expressing only the minimal NLS sequence (hsNLS35) efficiently accumulated in the nucleus, we could exclude the possibility that full-length hsADAR could saturate the import machinery, thereby leading to cytoplasmic retention of the protein. We thus considered whether the full-length protein might be retained in the cytoplasm via an anchoring mechanism, similar to the one that regulates nuclear entry of NF-κB (Verma et al., 1995). Because expression of hsADAR1 is regulated by IFN at the transcriptional level, it appeared conceivable that nuclear entry or cytoplasmic retention might also be triggered by an IFN-dependent regulatory mechanism (Patterson and Samuel, 1995). However, treatment of cells with IFN-α, IFN-γ, or ocadaic acid or cotransfection of the IFN-stimulated kinase PKR had no effect on the predominantly cytoplasmic localization of transfected full-length hsADAR1 (Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results).
To test whether cytoplasmic accumulation of ectopically expressed protein was a consequence of the strong overexpression from the cytomegalovirus promoter, we selected for neomycin-resistant cells that had the transgene integrated into the genome. Stable integrants typically show only moderate levels of expression. Cells were grown for several cell cycles in G418-containing medium, reseeded on coverslips, and stained for myc-tagged hsADAR. Cells showing moderate to low expression of the transgene were selected under the microscope. Interestingly, cells expressing only low levels of myc-tagged hsADAR showed predominantly nuclear accumulation of the protein, indicating that the protein can move to the nucleus but that nuclear accumulation can rapidly be saturated upon overexpression (Figure 6A).
hsADAR Has the Characteristics of a Shuttling Protein
One hint of how nuclear entry of hsADAR might be regulated came from experiments designed to determine whether the minimal NLS required ongoing transcription for nuclear import. When cells treated with AMD were stained with antiserum αII directed against endogenous ADAR1 protein, we noticed that the nuclear concentration of hsADAR1 decreased in all cells, leading to cytoplasmic accumulation of the protein (Figure 6B). These results indicated that nuclear accumulation of full-length hsADAR1 but not of hsNLS35, the minimal NLS, is transcription dependent.
Transcription-dependent nuclear accumulation has been previously described for several proteins that mostly belong to the group of heterogeneous nuclear ribonucleoprotein (hnRNP) proteins (Pinol-Roma and Dreyfuss, 1992; Michael et al., 1997). This phenomenon has been attributed to a shuttling mechanism whereby a protein is both imported and exported from the nucleus in a regulated manner. Like import, nuclear export is regulated by specific export signals present in the shuttling protein. The nuclear export signals (NESs) can either overlap the import signal or can be found separate from an NLS (Nakielny and Dreyfuss, 1999). NESs are recognized by export receptors that mediate their transport through nuclear pores to the cytoplasm.
An export receptor responsible for the export of the leucine-rich NES is Crm1 (Fornerod et al., 1997). The drug LMB can inhibit Crm1 activity and thus inhibit export of leucine-rich NESs from the nucleus (Kudo et al., 1999). LMB treatment has a strong effect on the nuclear export of snRNAs but has also a mild effect on nuclear export of mRNA (Fornerod et al., 1997; Watanabe et al., 1999). Therefore, to test whether nuclear accumulation of hsADAR1 was indeed caused by increased nuclear export, we treated cells transfected with hsADAR1 with LMB. This treatment led to accumulation of the transfected protein in the nucleus, supporting the idea that hsADAR1 is exported from the nucleus (Figure 6). Nuclear accumulation of hsADAR1 upon LMB treatment was reversible because removal of the drug restored the cytoplasmic localization of the protein. However, LMB treatment did not lead to complete nuclear accumulation of FL-PK-myc because a fraction of the protein was still localized in the cytoplasm. Cytoplasmic protein levels varied between different cells, which most likely reflects the level of ectopically expressed protein in those cells. Thus, in ∼80% of transfected cells cytoplasmic staining was clearly visible over a stronger nuclear signal (Figure 6A), whereas complete nuclear accumulation of transfected hsADAR1 was observed in only 20% of cells. LMB treatment led not only to nuclear accumulation of transfected hsADAR1 but also to nuclear accumulation of the endogenous protein. Nuclear staining with αII antiserum was more focused and prominent in LMB-treated cells than in untreated control cells, indicating that minor fractions of endogenous hsADAR1 reside in the cytoplasm as well (Figure 6). The fact that LMB treatment did not lead to complete nuclear accumulation of transfected hsADAR1 suggests that nuclear export of this protein might either not depend exclusively on Crm1 or, alternatively, might depend only indirectly on this export receptor.
The Third dsRBD in hsADAR1 Is Important for Nuclear Import
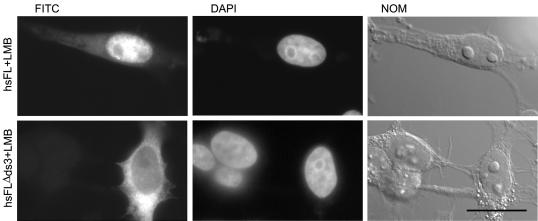
To determine whether the third dsRBD in hsADAR1 was the only active NLS found in this protein, we deleted the corresponding region from myc-tagged, full-length hsADAR1. The resulting construct, hsFLΔds3, was transfected into HeLa cells in parallel to wild-type full-length (hsFL) hsADAR1. To allow the detection of nuclear accumulation of the proteins, cells were treated with LMB before fixation and staining with mAb 9E10. As expected, the hsFLΔds3 construct showed greatly reduced nuclear accumulation when compared with FL hsADAR1 in LMB-treated cells, indicating that the third dsRBD in hsADAR1 is important for nuclear import (Figure 7). Interestingly, a fraction of hsFLΔds3 could still be detected in the nucleus, suggesting that other cryptic NLSs might exist in hsADAR1. We therefore tested whether the amino-terminal part of hsADAR1 (construct hsNLS9), harboring the other putative NLSs in this protein, would display NLS activity after LMB treatment. Surprisingly, even after LMB treatment this region did not display NLS activity, indicating that other regions within hsADAR1 may be responsible for the observed residual NLS activity retained in the hsFLΔds3 construct.
Figure 7.
The third dsRBD is important for nuclear import of hsADAR1. To determine the importance of the third dsRBD for nuclear import of hsADAR1, this domain was removed from the full-length protein. To avoid nuclear export of the overexpressed proteins cells were treated with LMB before fixation and staining. myc-tagged, full-length hsADAR accumulates in the nucleus (hsFL+LMB), whereas deletion of the third dsRBD in this protein leads to cytoplasmic accumulation (hsFLΔds3). FITC, Localization of myc-tagged constructs seen after antibody staining. DAPI, Localization of nuclei as seen in the DAPI channel. NOM, Whole cell images were taken by Nomarski optics. Scale bar, 20 μm.
No Evidence for Nuclear Export of Xenopus ADAR1
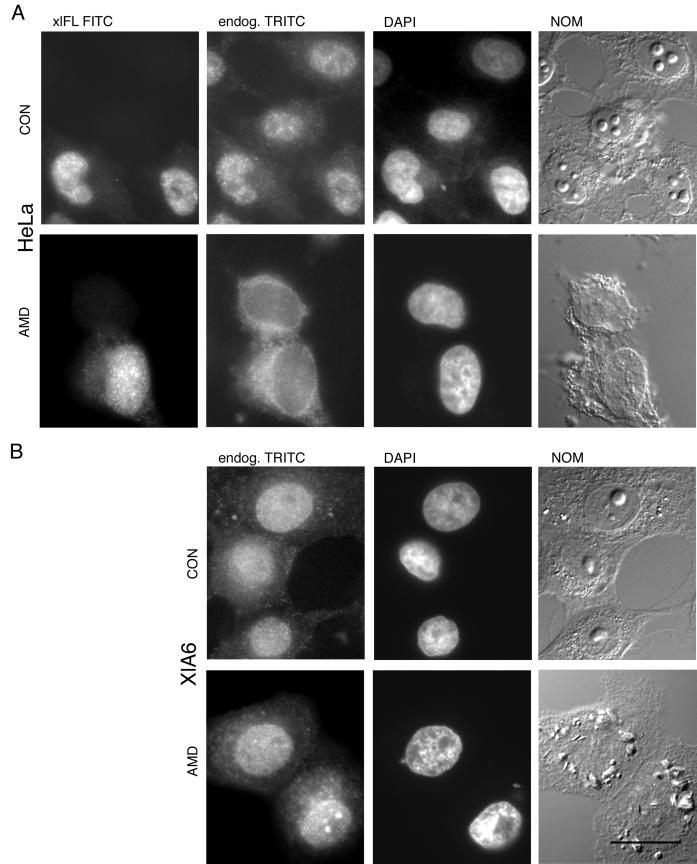
Because human ADAR1 seems to be exported from the nucleus, we wanted to determine whether Xenopus ADAR1 would behave similarly. We had already shown that Xenopus ADAR1, in contrast to its human homologue, was exclusively nuclear even when overexpressed in HeLa cells (compare Figure 3). We therefore tested whether nuclear accumulation of a transiently transfected, full-length xlADAR1-PK fusion construct (xlFL) was transcription dependent in HeLa cells. By costaining for both the transfected xlFL construct and the endogenous human ADAR1 protein with the use of specific antibodies for either protein, we could show that only the endogenous hsADAR1 protein showed transcription-dependent nuclear accumulation, whereas nuclear accumulation of xlADAR1 was insensitive to treatment with AMD (Figure 8A).
Figure 8.
xlADAR1 is constitutively nuclear in HeLa and Xenopus cells. (A) To determine whether nuclear accumulation of xlADAR1 is transcription dependent, HeLa cells were transfected with a myc-tagged, full-length xlADAR1 protein (xlFL). The ectopically expressed protein is constitutively nuclear in both the absence (CON) and presence of the transcriptional inhibitor AMD (AMD) as can be seen in the FITC channel. In contrast, staining with an antiserum specific for the human ADAR1 protein indicates that this protein is translocated to the cytoplasm upon AMD treatment (endog. TRITC). Note that the localization of endogenous hsADAR1 is not affected by overexpression of the Xenopus ADAR1 protein, suggesting different import mechanisms for those two proteins. (B) Also in Xenopus cells xlADAR1 is constitutively nuclear. Endogenous xlADAR1 protein was visualized in the tetramethylrhodamine (TRITC) channel with a rabbit polyclonal antiserum specific for Xenopus ADAR1 (endog. TRITC) in the absence (CON) or presence of AMD (AMD). DAPI, Nuclei were visualized by DAPI staining. NOM, Whole cells were imaged by Nomarski optics. Scale bar, 20 μm.
At the same time, this experiment showed that nuclear accumulation of the endogenous human ADAR1 protein was not perturbed by the overexpressed Xenopus protein, indicating that the import machinery used by the human protein was not saturated by Xenopus ADAR1. This in turn suggests that the two proteins might use different import machineries (Figure 8A).
Because humans and frogs are evolutionary rather distant within the vertebrate lineage, we considered the possibility that Xenopus ADAR1 might fail to be exported in HeLa cells because of the lack of some specific regulatory factors but might do so in Xenopus cells. We therefore tested the localization of endogenous xlADAR1 in Xenopus tissue culture cells and its response to treatment with the transcriptional inhibitor AMD. Interestingly, the nuclear localization of xlADAR1 remained unchanged even in the absence of ongoing transcription, suggesting that xlADAR1, unlike its human homologue, is not exported from the nucleus (Figure 8B).
DISCUSSION
Xenopus and human ADAR1 proteins contain putative NLSs in their amino-terminal regions. We have tested the corresponding fragments for NLS activity with the use of a PK reporter construct and could show that none of the predicted regions displayed NLS activity. Instead, a short basic fragment located upstream of the three double-stranded RNA-binding domains was found to be necessary and sufficient for nuclear targeting of Xenopus ADAR1, whereas in the human protein the third dsRBD is most important for nuclear targeting. Thus, our study clearly underscores the importance of experimentally testing NLS function. Furthermore, we could show that nuclear accumulation of human but not Xenopus ADAR1 is transcription dependent and that the human protein displays the characteristics of a shuttling protein.
Two Evolutionary Divergent NLSs in Xenopus and Human ADAR1 Proteins
The fact that different regions in Xenopus and human ADAR1 are required for nuclear targeting is surprising given that both proteins have an overall identity of 51.2% and a similarity of 66.5% at the protein level and are similar in their overall architecture. It was thus generally assumed that the putative NLSs found conserved in both proteins would serve as the actual NLS elements. In this context, it should also be noted that we tested the third dsRBD of xlADAR1 in LMB-treated cells and could show that dsRBD3 of the Xenopus protein has no NLS activity. Similarly, the amino-terminal region of human ADAR1, corresponding to the NLS of xlADAR1, is inactive, even in LMB-treated cells (Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). Thus, either human or Xenopus ADAR acquired a new NLS while losing another one. This in turn raises the question at which time in evolution the new NLS was acquired and whether intermediate forms of ADAR carrying two NLSs can be found along the evolutionary line from Xenopus to humans in lower mammals, reptiles, or birds. Recently, ADAR1-encoding cDNAs were isolated from fugu and zebrafish (Slavov et al., 2000b). Alignment of the corresponding fish proteins with Xenopus and mammalian ADAR1 proteins indicates that the basic NLS of xlADAR1 is found only in the Xenopus protein and is absent in all other ADAR1 members. The third dsRBD, in contrast, is most conserved among mammalian ADAR1 proteins but also rather similar in fish ADAR1 proteins. In Xenopus this region seems most divergent. It is thus possible that the third dsRBD has NLS activity in most vertebrate species and that only in Xenopus or amphibians this region has lost NLS activity while a new basic NLS has evolved.
The existence of different NLSs in homologous proteins raises the question which evolutionary advantage one NLS would have over the other. Two models can be envisaged to address this question. On the one hand, the evolution of new ADAR variants might have increased the need for a new NLS sequence. ADAR2 proteins, for instance, have not yet been found in amphibia. Those proteins lack the amino-terminal part found in ADAR1 that also harbors the NLS found in xlADAR1. The evolution of an ADAR multiprotein family with some members lacking an amino-terminal part might thus have made the evolution of a new NLS necessary. An NLS once evolved in ADAR2 might then have been introduced into ADAR1 via recombination. It should be noted, however, that ADAR2 proteins were recently also discovered in fish, suggesting that homologous proteins might also be present in amphibia (Slavov et al., 2000a). It will thus be of interest to screen for amphibian ADAR2 homologues and to compare the NLSs in ADAR1 and ADAR2 proteins.
On the other hand, we could show that hsADAR1 has some characteristics of a shuttling protein, whereas xlADAR1 seems exclusively nuclear. Whether hsADAR is a true shuttling protein is currently not clear. It is possible, however, that the unconventional NLS represented by dsRBD3 of hsADAR allows better fine tuning and modulation of nuclear import and export than a constitutively active, conventional basic NLS would do.
Mechanism of Nuclear Import
Our experiments showed that a short basic region located in the amino-terminal part of xlADAR1 is both necessary and sufficient for nuclear import. Albeit basic in nature, this region does not fit the NLS consensus sequence known from other proteins perfectly. In fact, two other basic regions found in this protein would fit the NLS consensus sequence much better but they do not exhibit NLS activity. However, a recent mutational analysis of mono- and bipartite NLSs showed that some amino acids have only minor effects on importin-α binding. Taking this into consideration, the NLS of Xenopus ADAR1 resembles a mutated NLS of the myc protein that would still bind to importin-α with a KD in the nanomolar range (Hodel et al., 2001). Nonetheless, further experiments will be required to determine whether the NLS in xlADAR1 utilizes the standard import machinery consisting of importin-α and β known from other basic NLSs or whether other import factors are required for nuclear transport of this protein (Jans et al., 2000).
The situation is even more complex for the NLS in human ADAR1, which entirely overlaps the third dsRBD in this protein. Minor deletions into the dsRBD consensus region from either end rapidly abolished NLS activity. Whereas the C-terminal part of the dsRBD is basic in nature and might thus be considered a potential basic NLS, the amino-terminal part of the dsRBD is not. The fact that deletions from the amino-terminal end abolish NLS activity therefore indicates that the basic charge of the dsRBD is not sufficient for nuclear import. Additionally, we could show that single-point mutations that abolish RNA binding did not affect NLS activity of the dsRBD, excluding the possibility that nuclear entry is mediated by binding of the protein to an RNA that itself is imported into the nucleus. Presently, it is therefore not clear which mechanism and which nuclear import factors are used by this dsRBD for nuclear entry. However, the fact that overexpression of Xenopus ADAR1 in HeLa cells does not affect the nuclear localization of the endogenous human protein can be seen as an indication that those two ADAR1 proteins use different import machineries.
Overlapping NLS and RNA-binding regions have already been noted in other RNA-binding proteins (LaCasse and Lefebvre, 1995). Nonetheless, to our knowledge this is the first time that NLS activity could be demonstrated for a dsRBD. Our finding, that only dsRBD3 of hsADAR1 but not of the homologous Xenopus protein displays NLS activity raises the question which residues within dsRBD3 of hsADAR1 make it act as an NLS. So far we have mutated a single cysteine residue that can be found in the center of the third dsRBD of mammalian ADARs but not in Xenopus ADAR1 or in other dsRBDs. However, this mutation did not abolish NLS activity of dsRBD3 of hsADAR1 (Eckmann, Neunteufl, Pfaffstetter, and Jantsch, unpublished results). Careful mutational analysis will therefore be required to determine why dsRBD3 of human but not Xenopus ADAR1 can act as an NLS. This analysis should also provide evidence whether cryptic NLSs might be present in other dsRBDs. Deletion of dsRBD3, for instance, does not completely eliminate nuclear entry of the human ADAR1 protein. It is thus possible, that weak cryptic NLSs might reside in the other dsRBDs of this protein.
In human ADAR1 dsRBD3 has been identified as most important for RNA binding and editing (Lai et al., 1995; Liu and Samuel, 1996; Liu et al., 1997), whereas in Xenopus ADAR1 the second dsRBD seems most important for RNA binding (Brooks et al., 1998). The fact that dsRBD3 of hsADAR also harbors its NLS underscores the importance of this dsRBD for hsADAR1 function. It also shows that the multiple dsRBDs found in some RNA-binding proteins might have additional functions other than RNA binding, a fact recently also demonstrated for the multiple dsRBDs of the Drosophila Staufen protein (Micklem et al., 2000).
Deletion of dsRBD3 from full-length hsADAR1 strongly reduces, but does not completely abolish, nuclear accumulation of the protein in LMB-treated cells. This finding can be interpreted in two ways. On the one hand, it is possible that additional cryptic NLSs are present in hsADAR1. However, given that we could not detect NLS activity in any of the other tested fragments that spanned the entire hsADAR1 protein, this possibility appears rather unlikely. On the other hand, nuclear accumulation of the FLΔds3 construct might be a consequence of LMB treatment. After mitosis when the nuclear envelope is reformed cytoplasmic proteins are typically expelled from the nucleus most likely via regulated nuclear export. Inhibition of nuclear export could therefore lead to nuclear occurrence of proteins that lack an NLS simply by failure to export them from the nucleus. Nuclear occurrence of the FLΔds3 construct might therefore be a consequence of the LMB treatment leading to failure to expel the protein from the nucleus after mitosis.
Is hsADAR1 a Shuttling Protein?
Our data demonstrates that nuclear entry of human but not Xenopus ADAR1 protein is regulated. Overexpression of hsADAR1 leads to cytoplasmic accumulation of the protein, whereas the endogenous protein is predominantly nuclear. It was previously realized that a 150-kDa full-length ADAR1 protein can be found in the nucleus and cytoplasm, whereas a smaller, possibly proteolytic fragment of the protein can be found exclusively in the nucleus (Patterson and Samuel, 1995).
The observation that the minimal NLS of hsADAR leads to efficient nuclear accumulation, together with the fact that LMB treatment enhances nuclear accumulation of full-length hsADAR1, strongly suggests the existence of a Crm1-dependent NES in this protein. If the previously described p110 version of hsADAR1 indeed represents a proteolytic fragment of the full-length protein, this in turn would suggest that the p110 protein has lost its NES because of proteolytic cleavage. Given that p110 is enzymatically active and that the catalytic deaminase domain is located in the C-terminal end of all ADARs, it is most likely that the presumptive NES can be found in the amino-terminal part of the protein. In fact, preliminary data obtained in our laboratory demonstrate the existence of an NES located in the amino terminus of hsADAR1. Sequence analysis of this region reveals a leucine-rich element that could potentially serve as a Crm1-dependent export signal. Experiments are under way to clarify whether this element represents the active NES and whether Crm1 is the true export receptor for this protein. Currently, it also has to be considered that LMB treatment leads to an alteration of the nucleo-cytoplasmic distribution of other factors required for proper nuclear export of hsADAR1, thereby also preventing the export of this protein.
The coexistence of nuclear import and export signals is a hallmark of shuttling proteins (Nakielny and Dreyfuss, 1999). Typically, shuttling proteins continuously move between the nucleus and cytoplasm as first demonstrated for hnRNP proteins (Pinol-Roma and Dreyfuss, 1992). However, NESs have also been found in proteins that are predominantly nuclear and normally do not shuttle. In the hnRNP C protein, for instance, nuclear export is prevented by a nuclear retention signal that overrides the function of the NES present in this protein (Nakielny and Dreyfuss, 1996). Cytoplasmic accumulation of hsADAR1 occurs both in the absence of transcription and upon overexpression of the protein. Furthermore, LMB treatment leads to nuclear accumulation of overexpressed and endogenous hsADAR1. Taken together, these data are compatible with, but do not formally prove, continuous shuttling of the protein between the nucleus and cytoplasm. Our data would also be consistent with a model in which nuclear export of hsADAR1 is prevented by a transcription-dependent, saturable nuclear retention mechanism. Binding to nuclear substrate RNA, for instance, might tether the protein in the nucleus. In the absence of transcription no substrate RNA would be synthesized, allowing export of the protein into the cytoplasm. Similarly, overexpression of hsADAR1 might saturate all available binding sites on a nuclear RNA, again allowing nuclear export of excess, unbound hsADAR1 protein. If this model is correct, one would predict that one of the three dsRBDs in this protein might act as a nuclear retention signal, preventing nuclear export by binding to nuclear RNA. Further experiments will therefore be required to determine whether hsADAR1 is a true shuttling protein and, if so, whether the protein accompanies an RNA from the nucleus to the cytoplasm.
It is also interesting to note that only human but not Xenopus ADAR1 is translocated to the cytoplasm in the absence of transcription. This suggests that these two proteins not only contain different import signals but might also differ with respect to their ability to become exported from the nucleus. It is therefore tempting to speculate that the unconventional NLS represented by the third dsRBD in hsADAR1 is a prerequisite for controlled nuclear import and export that might only have evolved in mammals but not in lower vertebrates.
ADAR1-mediated RNA editing is believed to be a nuclear process. In vitro, the enzyme can act on almost any double-stranded substrate of sufficient length, showing little site specificity. Despite the rather promiscuous behavior and the relative abundance of this enzyme, only a few candidate substrates for ADAR1 are known to date, suggesting that ADAR1-mediated editing is a rare process in vivo. This in turn raises the question how inadvertent editing of structured nuclear RNA is prevented. On the one hand, this might be achieved by proteins that compete for binding sites on the RNA but also by specific conformations an RNA might have to fold into to allow access to the base to be edited, examples for both cases have been demonstrated (Saccomanno and Bass, 1994; Ohman et al., 2000). On the other hand, the extent of nuclear editing might be regulated by controling nuclear ADAR1 levels. If sufficient substrate RNA is available, the enzyme could stay in the nucleus, retained via binding to RNA. In the absence of substrate RNA, however, nuclear retention of ADAR1 would be prevented, leading to its export from the nucleus, thereby impeding binding of the enzyme to non substrate RNAs. Regulated nuclear import and export could therefore provide an elegant means to avoid hyperediting of nuclear RNAs.
ACKNOWLEDGMENTS
The authors would like to thank. Dr. Minoru Yoshida (University of Tokyo, Japan) for providing the leptomycin B and Professor Walter Keller (Biozentrum Basel, Switzerland) for the kind gift of antiserum α-II that was used to detect endogenous human ADAR1.We also thank the members of the department for helpful discussions and critical reading of the manuscript. This work was supported by Austrian Science Foundation grant P11444 to M.F.J.
REFERENCES
- Aruscavage PJ, Bass BL. A phylogenetic analysis reveals an unusual sequence conservation within introns involved in RNA editing. RNA. 2000;6:257–269. doi: 10.1017/s1355838200991921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1987. [Google Scholar]
- Bass BL. RNA editing and hypermutation by adenosine deamination. Trends Biochem Sci. 1997;22:157–162. doi: 10.1016/s0968-0004(97)01035-9. [DOI] [PubMed] [Google Scholar]
- Bass BL, Weintraub H. A developmentally regulated activity that unwinds RNA duplexes. Cell. 1987;48:607–613. doi: 10.1016/0092-8674(87)90239-x. [DOI] [PubMed] [Google Scholar]
- Brooks R, Eckmann CR, Jantsch MF. The double-stranded RNA-binding domains of Xenopus laevis ADAR1 exhibit different RNA-binding behaviors. FEBS Lett. 1998;434:121–126. doi: 10.1016/s0014-5793(98)00963-6. [DOI] [PubMed] [Google Scholar]
- Brusa R, Zimmermann F, Koh DS, Feldmeyer D, Gass P, Seeburg PH, Sprengel R. Early-onset epilepsy and postnatal lethality associated with an editing-deficient GluR-B allele in mice. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, Emeson RB. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Bycroft M, Grunert S, Murzin AG, Proctor M, St. Johnston D. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Cho DS, Wang Q, Lai F, Carter KC, Nishikura K. A third member of the RNA-specific adenosine deaminase gene family, ADAR3, contains both single- and double-stranded RNA binding domains. RNA. 2000;6:755–767. doi: 10.1017/s1355838200000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C, Laskey RA. Nuclear targeting sequences: a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Eckmann CR, Jantsch MF. The RNA-editing enzyme ADAR1 is localized to the nascent ribonucleoprotein matrix on Xenopus lampbrush chromosomes but specifically associates with an atypical loop. J Cell Biol. 1999;144:603–615. doi: 10.1083/jcb.144.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- George CX, Samuel CE. Characterization of the 5′-flanking region of the human RNA-specific adenosine deaminase ADAR1 gene and identification of an interferon-inducible ADAR1 promoter. Gene. 1999;229:203–213. doi: 10.1016/s0378-1119(99)00017-7. [DOI] [PubMed] [Google Scholar]
- Herb A, Higuchi M, Sprengel R, Seeburg PH. Q/R site editing in kainate receptor GluR5 and GluR6 pre-mRNAs requires distant intronic sequences. Proc Natl Acad Sci USA. 1996;93:1875–1880. doi: 10.1073/pnas.93.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Alfken J, Kim YG, Mian IS, Nishikura K, Rich A. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A, Schade M, Lowenhaupt K, Alfken J, Schwartz T, Shlyakhtenko LS, Lyubchenko YL, Rich A. The Zalpha domain from human ADAR1 binds to the Z-DNA conformer of many different sequences. Nucleic Acids Res. 1998;26:3486–3493. doi: 10.1093/nar/26.15.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Maas S, Single FN, Hartner J, Rozov A, Burnashev N, Feldmeyer D, Sprengel R, Seeburg PH. Point mutation in an AMPA receptor gene rescues lethality in mice deficient in the RNA-editing enzyme ADAR2. Nature. 2000;406:78–81. doi: 10.1038/35017558. [DOI] [PubMed] [Google Scholar]
- Hodel MR, Corbett AH, Hodel AE. Dissection of a nuclear localization signal. J Biol Chem. 2001;276:1317–1325. doi: 10.1074/jbc.M008522200. [DOI] [PubMed] [Google Scholar]
- Hough RF, Bass BL. Analysis of Xenopus dsRNA adenosine deaminase cDNAs reveals similarities to DNA methyltransferases. RNA. 1997;3:356–370. [PMC free article] [PubMed] [Google Scholar]
- Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Jantsch MF, Gall JG. Assembly and localization of the U1-specific snRNP C protein in the amphibian oocyte. J Cell Biol. 1992;119:1037–1046. doi: 10.1083/jcb.119.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharrat A, Macias MJ, Gibson TJ, Nilges M, Pastore A. Structure of the dsRNA binding domain of E. coli RNase III. EMBO J. 1995;14:3572–3584. doi: 10.1002/j.1460-2075.1995.tb07363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim U, Wang Y, Sanford T, Zeng Y, Nishikura K. Molecular cloning of cDNA for double-stranded RNA adenosine deaminase, a candidate enzyme for nuclear RNA editing. Proc Natl Acad Sci USA. 1994;91:11457–11461. doi: 10.1073/pnas.91.24.11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krovat BC, Jantsch MF. Comparative mutational analysis of the double-stranded RNA binding domains of Xenopus laevis RNA-binding protein A. J Biol Chem. 1996;271:28112–28119. doi: 10.1074/jbc.271.45.28112. [DOI] [PubMed] [Google Scholar]
- Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCasse EC, Lefebvre YA. Nuclear localization signals overlap DNA- or RNA-binding domains in nucleic acid-binding proteins. Nucleic Acids Res. 1995;23:1647–1656. doi: 10.1093/nar/23.10.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Drakas R, Nishikura K. Mutagenic analysis of double-stranded RNA adenosine deaminase, a candidate enzyme for RNA editing of glutamate-gated ion channel transcripts. J Biol Chem. 1995;270:17098–17105. doi: 10.1074/jbc.270.29.17098. [DOI] [PubMed] [Google Scholar]
- Lehmann KA, Bass BL. The importance of internal loops within RNA substrates of ADAR1. J Mol Biol. 1999;291:1–13. doi: 10.1006/jmbi.1999.2914. [DOI] [PubMed] [Google Scholar]
- Liu Y, George CX, Patterson JB, Samuel CE. Functionally distinct double-stranded RNA-binding domains associated with alternative splice site variants of the interferon-inducible double-stranded RNA-specific adenosine deaminase. J Biol Chem. 1997;272:4419–4428. doi: 10.1074/jbc.272.7.4419. [DOI] [PubMed] [Google Scholar]
- Liu Y, Herbert A, Rich A, Samuel CE. Double-stranded RNA-specific adenosine deaminase: nucleic acid binding properties. Methods. 1998;15:199–205. doi: 10.1006/meth.1998.0624. [DOI] [PubMed] [Google Scholar]
- Liu Y, Samuel CE. Mechanism of interferon action: functionally distinct RNA-binding and catalytic domains in the interferon-inducible, double-stranded RNA-specific adenosine deaminase. J Virol. 1996;70:1961–1968. doi: 10.1128/jvi.70.3.1961-1968.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattaj IW. UsnRNP assembly and transport. In: Birnstiel ML, editor. Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Berlin: Springer Verlag; 1988. pp. 100–114. [Google Scholar]
- Melcher T, Maas S, Herb A, Sprengel R, Seeburg PH, Higuchi M. A mammalian RNA editing enzyme. Nature. 1996;379:460–464. doi: 10.1038/379460a0. [DOI] [PubMed] [Google Scholar]
- Michael WM, Eder PS, Dreyfuss G. The K nuclear shuttling domain: a novel signal for nuclear import and nuclear export in the hnRNP K protein. EMBO J. 1997;16:3587–3598. doi: 10.1093/emboj/16.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem DR, Adams J, Grunert S, St. Johnston D. Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 2000;19:1366–1377. doi: 10.1093/emboj/19.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. The hnRNP C proteins contain a nuclear retention sequence that can override nuclear export signals. J Cell Biol. 1996;134:1365–1373. doi: 10.1083/jcb.134.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S, Dreyfuss G. Transport of proteins and RNAs in and out of the nucleus. Cell. 1999;99:677–690. doi: 10.1016/s0092-8674(00)81666-9. [DOI] [PubMed] [Google Scholar]
- O'Connell MA. RNA editing: rewriting receptors. Curr Biol. 1997;7:R437–439. doi: 10.1016/s0960-9822(06)00212-0. [DOI] [PubMed] [Google Scholar]
- O'Connell MA, Keller W. Purification and properties of double-stranded RNA-specific adenosine deaminase from calf thymus. Proc Natl Acad Sci USA. 1994;91:10596–10600. doi: 10.1073/pnas.91.22.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W. Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Mol Cell Biol. 1995;15:1389–1397. doi: 10.1128/mcb.15.3.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman M, Kallman AM, Bass BL. In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. RNA. 2000;6:687–697. doi: 10.1017/s1355838200000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JB, Samuel CE. Expression and regulation by interferon of a double-stranded-RNA-specific adenosine deaminase from human cells: evidence for two forms of the deaminase. Mol Cell Biol. 1995;15:5376–5388. doi: 10.1128/mcb.15.10.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peculis BA, Gall JG. Localization of the nucleolar protein NO38 in amphibian oocytes. J Cell Biol. 1992;116:1–14. doi: 10.1083/jcb.116.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinol-Roma S, Dreyfuss G. Shuttling of pre-mRNA binding proteins between nucleus and cytoplasm. Nature. 1992;355:730–732. doi: 10.1038/355730a0. [DOI] [PubMed] [Google Scholar]
- Polson AG, Bass BL. Preferential selection of adenosines for modification by double-stranded RNA adenosine deaminase. EMBO J. 1994;13:5701–5711. doi: 10.1002/j.1460-2075.1994.tb06908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St. Johnston D, Varani G. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J. 2000;19:997–1009. doi: 10.1093/emboj/19.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccomanno L, Bass BL. The cytoplasm of Xenopus oocytes contains a factor that protects double-stranded RNA from adenosine-to-inosine modification. Mol Cell Biol. 1994;14:5425–5432. doi: 10.1128/mcb.14.8.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. A nuclear localization domain in the hnRNP A1 protein. J Cell Biol. 1995;129:551–560. doi: 10.1083/jcb.129.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavov D, Clark M, Gardiner K. Comparative analysis of the RED1 and RED2 A-to-I RNA editing genes from mammals, pufferfish and zebrafish. Gene. 2000a;250:41–51. doi: 10.1016/s0378-1119(00)00174-8. [DOI] [PubMed] [Google Scholar]
- Slavov D, Crnogorac-Jurcevic T, Clark M, Gardiner K. Comparative analysis of the DRADA A-to-I RNA editing gene from mammals, pufferfish and zebrafish. Gene. 2000b;250:53–60. doi: 10.1016/s0378-1119(00)00175-x. [DOI] [PubMed] [Google Scholar]
- Steitz JA. Immunoprecipitation of ribonucleoproteins using autoantibodies. Methods Enzymol. 1989;180:468–481. doi: 10.1016/0076-6879(89)80118-1. [DOI] [PubMed] [Google Scholar]
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Wallace RA, Jared DW, Dumont JN, Sega MW. Protein incorporation by isolated amphibian oocytes: 3. Optimum incubation conditions. J Exp Zool. 1973;184:321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukuda M, Yoshida M, Yanagida M, Nishida E. Involvement of CRM1, a nuclear export receptor, in mRNA export in mammalian cells and fission yeast. Genes Cells. 1999;4:291–297. doi: 10.1046/j.1365-2443.1999.00259.x. [DOI] [PubMed] [Google Scholar]