Abstract
Stem cells have the capacity for self-renewal and differentiation into specialized cells that form and repopulated all tissues and organs, from conception to adult life. Depending on their capacity for differentiation, stem cells are classified as totipotent (ie, zygote), pluripotent (ie, embryonic stem cells), multipotent (ie, neuronal stem cells, hematopoietic stem cells, epithelial stem cells, etc.), and unipotent (ie, spermatogonial stem cells). Adult or tissue-specific stem cells reside in specific niches located in, or nearby, their organ or tissue of origin. There, they have microenvironmental support to remain quiescent, to proliferate as undifferentiated cells (self-renewal), and to differentiate into progenitors or terminally differentiated cells that migrate from the niche to perform specialized functions. The presence of proteins at the cell surface is often used to identify, classify, and isolate stem cells. Among the diverse groups of cell surface proteins used for these purposes, integrin α6, also known as CD49f, may be the only biomarker commonly found in more than 30 different populations of stem cells, including some cancer stem cells. This broad expression among stem cell populations indicates that integrin α6 may play an important and conserved role in stem cell biology, which is reaffirmed by recent demonstrations of its role maintaining self-renewal of pluripotent stem cells and breast and glioblastoma cancer stem cells. Therefore, this review intends to highlight and synthesize new findings on the importance of integrin α6 in stem cell biology.
Keywords: : integrin α6, CD49f, stem cells, niche, self-renewal, differentiation
Introduction
Due to their capacity for self-renewal and differentiation, stem cells play key roles in the development and homeostasis of tissues and organs throughout the life of most living organisms. Soon after fertilization, the zygote divides into individual blastomeres that have identical genetic information and are totipotent in nature. At the morula stage of embryonic development, the first cell lineage differentiation occurs. The blastomeres on the outside of the embryo acquire a trophoblast cell lineage, while the inner blastomeres give rise to the inner cell mass (ICM) of the forming blastocyst. The cells that constitute the ICM give rise to the primitive endoderm and the pluripotent epiblast cells. Isolation and culture in vitro of cells from the ICM result in the establishment of embryonic stem cells (ESCs), which like their counterpart in vivo are pluripotent. The following three primary germ layers: ectoderm, mesoderm, and endoderm, form from the origins of the ICM and together with the germ line originate at the gastrulation stage. From this developmental stage, specific cell lineage differentiation occurs in a coordinated sequence of events that appears to be controlled both temporally and spatially, resulting in the formation of distinct tissues and organs. Diverse and specialized stem cell populations emerge during the development of the fetus, and some are maintained during adult life, repopulating and replenishing cells of each tissue and organ. While the capability of cell lineage differentiation is restricted and unique for each adult stem cell population, all maintain the property of self-renewal. Some stem cells remain active, while others become quiescent, and are “awakened” when needed, to prevent depletion of the stem cell pool and to repopulate their specific tissue cell types.
Several biomarkers have been used to characterize, isolate, expand, and study stem cells. The majority of these biomarkers are proteins located at the cell membrane and have the capability of binding or adhering to other signaling molecules and cells, respectively. Each cell type produces a combination of protein receptors on their surface, creating unique profiles of biomarkers that distinguish them from other cell types. The cell surface markers CD34, CD44, CD90, and CD177 (c-kit) are among the many surface proteins found on stem cells. It is important to note, however, that these proteins are also translated in other somatic cells that are not considered stem cells. For example, CD34 is regarded as a marker of hematopoietic stem cells (HSCs) and hematopoietic progenitor cells [1]. CD34 is also found in other populations of stem cells and progenitor cells like mesenchymal stem cells (MSCs) [2], muscle satellite cells [3], and epithelial progenitors [4], in addition to nonstem cell populations like fibroblasts [5,6].
Integrin α6, also known as CD49f, is among the proteins that have been identified in stem cell populations (Table 1) and somatic cells like keratinocytes [7], platelets [8], epithelial cells, and basal cells of the cornea [9]. This subunit of the α family of integrins was first identified as a stem cell marker in keratinocyte stem cells in 1998, when it was reported that a subpopulation (∼10%) of cells in neonatal human foreskin with the phenotype α6bri10G7dim exhibit highest regenerative capacity compared to other basal cells and were quiescent at the time of isolation [10]. Since then, the expression of integrin α6 has been found in more than 30 stem cell populations, including pluripotent and multipotent stem cells, and cancer stem cells (CSCs) (Table 1). Importantly, the expression of integrin α6 in some of these populations is conserved among several mammalian species. Furthermore, integrin α6 has been identified as the only common gene expressed in stem cell signatures from ESCs, embryonic neuronal stem cells (NSCs), and HSCs [11]. Taken together, these studies point to an important role for this transmembrane receptor in stem cell biology.
Table 1.
Stem Cell Populations Expressing Integrin α6 (CD49f)
| Cardiac stem cells | CD117(+)/Laminin1(+)/CD49f(+) | [77] |
| Cord blood stem cells | CD34(+)/CD90(+)/CD49f(+)/CD117(+) | [78] |
| Corneal epithelial stem cells | CD71(dim)/CD49f(bri) | [79] |
| Dental stem cells | Sca-1(+)/CD49f(bri)/CD44(+) | [80] |
| Embryonic stem cells | SSEA4(+)/SSEA3(+)/TRA-1-60(+)/TRA-1-81(+)/CD49f(+) | [29] |
| Epithelial odontogenic stem cells | CD49f(+) | [81] |
| Epithelial intestine stem cells | PHLDA1(+)/CD49f(+) | [82] |
| Esophageal epithelial stem cells | CD71(dim)CD49f(bri) | [83] |
| Gallbladder stem cells | EpCAM(+)/CD49f(hi) | [84] |
| Hair follicle stem cells | CD34(+)/CD49f(bri) | [85] |
| Hematopoietic stem cells | Thy1(+)/Rho(low)/CD49f(+) | [32] |
| Hepatic stem cells | Adult: CD49f(+)/CD29(+)/c-Kit(−)/Thy1.1(−) Fetal: c-Met(+)/CD49f(+/low)/c-kit(−)/CD45(−)/TER199(−) |
[86,87] |
| Keratinocyte stem cells | CD49f(bri)/10G7(dim); CD71(dim)CD49f(bri) | [10,88–90] |
| Lung epithelial stem cells | EpCAM(hi)/CD49f(+)/CD104(+)/CD24(low) | [91,92] |
| Mammary epithelial stem cells | CD24(+)/CD49f(hi); Sca(hi); CD45(−)/Ter119(−)/CD31(−)/Sca-1(low)/CD24(med)/CD49f(hi) | [33,93,94] |
| Mesenchymal stem cells | PODXL(hi)/CD49f(hi) | [44,95] |
| Merkel cell stem cells | Sca1(+)/CD200(+)/CD49f(+) | [96] |
| Myometrial stem cells | CD49f(+)/CD34(+) | [97] |
| Neural stem cells | CD49f(hi)/CD29(hi)/CD133(+); GFAPv/LeX(+)/CD49f(+))/CD29(+) | [47,48] |
| Oral epithelial stem cells | Ki-67(+)/CD29(+)/CD49f(hi)/keratin 13(low) | [98] |
| Primordial germ cells | SSEA1(+)/SSE3(+)/SSEA4(+)/EMA-1(+)/CD29(+), CD49f(+) | [99] |
| Prostate stem cells | CD45(−)/CD31(−)/Ter119(−)/Sca-1(+)/CD49f(+); Trop2(hi)/CD49f(hi)* | [100–102] |
| Salivary gland stem cells | CD49f(+)/Thy-1(+)/Laminin(+) | [103] |
| Sweat gland myoepithelial stem cells | CD29(hi)/CD49f(hi) | [104] |
| Spermatogonial stem cells | CD29(+)/CD49f(+) | [23] |
| Thymus stem cells | Sca1/(+)PDGFRa(+)/PDGFRb(+)/CD29(+)/CD44(+)/CD49f(+)/CD90(+) | [105] |
| Tracheal epithelial stem cells | CD49f(bri)/Sca1(+)/ALDH(+) | [106] |
| Breast cancer stem cells | CD44(+)/CD49f(+); CD44(+)/CD24(−)/CD49f(+)/NRP2(+) | [57,107] |
| Cervical cancer stem cells | CD34(+)/CD49f(+)/CD133(+); CK-17(+)/p63(+)/All(+)/CD49f(+)/ALDH(hi) | [50,51] |
| Esophageal adenocarcinoma stem cells | CD71(dim)/CD49f(bri) | [83] |
| Glioblastoma cancer stem cells | CD133(+)/CD49f(+) | [49] |
| Hepatocellular carcinoma stem cells | CD133(+)/CD49f(+) | [108] |
| Prostate cancer stem cells | CD49f(+)/CD44(+)/CD133(+); Lin(−)/Sca-1(+)/CD49f(+) | [109,110] |
| Ovarian endometrioma stem cells | Sal-like 4(+)/CD133(+)/Musashi-1(+)/CD49f(+) | [111] |
| Squamous cell carcinoma stem cells | CD34(+)/CD49f(hi)/CD29(hi) | [112] |
The bold text highlights the positive and common expression of CD49f among all the stem cell types listed in the table.
The Binding Partners and Function of Integrin α6
Integrins are a family of type I transmembrane glycoproteins composed of α and β subfamilies. In mammals, the α subfamily has 18 subunits, while 8 subunits comprise the β subfamily. Together, these subunits form at least 24 different heterodimer combinations that have unique affinities for extracellular matrix (ECM) components, including laminins, collagens, and fibronectin, and have specific and nonredundant functions [12]. Integrins play a key role in cell adhesion to ECM ligands and adjacent cells and serve as a link between extracellular contacts and the intracellular cytoskeleton. In addition, integrins work together with receptor tyrosine kinases to communicate bidirectional signals between cells and the ECM [13]. The activation of integrins upon ligand binding triggers signal transduction mechanisms involved in cell differentiation, gene expression, motility, polarity, proliferation, shape, and survival/apoptosis [12,14].
By forming heterodimers with either integrin β1 (CD29) or integrin β4 (CD104), integrin α6 functions as a receptor for the following laminins: LAMA2, LAMA3, LAMB1, LAMB2, LAMB3, LAMC1, and LAMC2. In addition, integrin α6 has two isoforms with distinct cytoplasmic variants, α6A and α6B, which are generated by alternative mRNA splicing [15,16]. Thus, the signaling directed from integrin α6 varies depending on the dominant isoform expressed, by which β chain is partnered to form a heterodimer and by the ligand that is binding [17,18]. As discussed below in detail, the expression of integrin α6 is found in early stages of development and throughout adult life. Embryonic deletion of integrin α6 in mice leads to neonatal death with a phenotype of cerebral malformations and severe skin blistering [19]. The latter resembles skin lesions observed in epidermolysis bullosa, which is observed in mouse deficient in LAMA3 [20], LAMB3 [21], and LAMC2 [22]. All this information indicates the important role of integrin α6 in development and formation of organs.
Expression of integrin α6 in stem cells: from germ cells to somatic stem cells
The expression of integrin α6 has been identified in somatic stem cells and germ cells from early developmental stages through adulthood. The heterodimer of integrin α6β1 is found in spermatogonial stem cells [23] and female primordial germ cells [24]. Spermatogenesis has been reconstituted in infertile male mice after colonization assays in recipient testes using testis cells expressing integrin α6 and integrin β1. Remarkably, the c-kit+ populations isolated from testicular cells are not able to repopulate recipient testes, while the success rate in reconstitution of spermatogenesis doubles using integrin α6+ cells compared to β1+ cells. These findings suggest that integrin α6 selects a pure population of spermatogonial stem cells; while because integrin β1 forms heterodimers with many other α subunits, its selected population may be contaminated with nonstem cells [23]. In female primordial germ cells isolated from mouse embryos at 10.5 embryonic days, which give rise to oocytes, both integrin α6 and β1 are expressed, and their expression is continuous during all stages of oocyte development [24]. It has been demonstrated that the presence of integrin α6β1 in ovulated oocytes is required for fertilization by facilitating the binding and fusion between sperm and egg plasma membranes [25]. In the developing embryo, the expression of integrin α6β1 has been detected at the mRNA level throughout preimplantation development. At the protein level, α6 is detected from two-cell embryos to the late morula/early blastocyst stage. At the early and late blastocyst stage, α6β1 is present in the ICM and all the rest of the cells except in the external surface of the trophectoderm. In vitro culture of outgrowths from blastocyst embryos reveals that only cells from the ICM express integrin α6, while trophoblast outgrowing cells are α6- [26]. In the appropriate in vitro conditions, the ICM outgrowths (integrin α6+ cells) become what are known as ESCs [27,28], which have integrin α6β1 in their undifferentiated and pluripotent state [29,30].
As shown in Table 1, integrin α6 is present in many somatic stem cells, and some studies clearly show its importance in identifying true stem cell populations. Below we describe selected studies that demonstrate the importance of integrin α6 in the most well-studied stem cell populations.
Hematopoietic Stem Cells
Mature blood cell lineages are generated from a network of hierarchically distinct progenitors that arise from self-renewing HSCs. Integrin α6 is expressed in nearly 99% of mouse primitive hematopoietic cells (Lin−/Sca-1+/c-kit+ [LSK]) and in about 90% of committed myeloid progenitors (Lin−/Sca-1−/c-Kit+). Interestingly, the blockage of integrin α6 significantly inhibits the homing of mouse HSCs to the bone marrow (BM) and impairs long-term multilineage reconstitution after competitive repopulation assays. Integrin α6 is also found in human primitive HSCs, suggesting a conserved interspecies functional role during hematopoiesis [31]. More recently, further evidence that integrin α6 (CD49f) plays a key role in human HSC function was demonstrated by showing that single Lin−/CD34+/Cd38−/CD45RA−/Thy+/Rholow/CD49f+ cells engrafted in femur BM give rise to long-term multilineage systemic grafts. In addition, the loss of expression of integrin α6 in human hematopoietic cells distinguishes HSCs from multilineage progenitor populations [32].
Mammary Stem Cells
Integrin α6 has also been identified as a biomarker of mammary stem cells (MaSCs), which are cells that display self-renewal properties and are able to regenerate new mammary tissue in vivo. Transplantation of single cells extracted from adult mouse mammary tissue with the phenotype of CD45−/Ter119−/CD31−/Sca-1low/CD24med/CD49fhigh is able to regenerate mammary glands in the fat pads of virgin host mice [33].
The self-renewal properties of CD45−/Ter119−/CD31−/Sca-1low/CD24med/CD49fhigh cells have also been confirmed after secondary limiting dilution assays prepared from primary outgrowths after transplantation [33]. Interestingly, the expression levels of integrin α6 from high to low are able to differentiate MaSCs from progenitor cells, respectively, in combination with the expression of CD24. CD24med/CD49fhigh cells in Matrigel cultures produce solid colonies with irregular shape, branched ductal appearance, and an irregular-shaped lumen, while CD24high/CD49flow cells generate uniformly spherical acinar structures mainly made off cuboidal epithelium [33].
Mesenchymal Stem Cells
MSCs, which have immunomodulatory and engraftment promoting properties [34], are the subject of several promising clinical trials (ClinicalTrails.gov). First identified from mononuclear cells of the BM [35,36], MSCs are also detected and isolated from other tissues like adipose tissue [37], dental pulp [38], endometrial stroma [39], periodontal ligament [34], placenta [40], and umbilical cord blood (UCB) [41]. The International Society for Cellular Therapy has established that minimum criteria to define a MSC population require: (1) adherence to tissue culture plastic under standard culture conditions, (2) an immunophenotype that includes CD105, CD73, and CD90, while lacking the expression of hematopoietic markers (CD45, CD34, CD14 or CD11b, CD79a, CD19, or HLA-DR), and (3) ability to differentiate into osteoblast, adipocytes, and chondrocytes. Beside these biological markers, other cell surface proteins, including integrin α6 -CD49f- [42], have been used to characterize MSCs [43]. CD49f has been found in MSCs derived from BM [44,45] and UCB [46]. Interestingly, it was reported that the level of CD49f expression in BM-MSC declines with increasing age, while similarly higher levels of expression are found among younger donors of both BM-MSC and UCB-MSCs. This higher expression of CD49f in UCB in relation to adult BM-MSC correlates with a higher lung clearance rate after systemic infusion [46]. The high expression of CD49f and PODXL in BM-MSCs has been also related to significantly better efficiency in generating single cell-derived colonies and in differentiation into mineralizing cells and adipocytes in vitro. Furthermore, after intravenous infusion in mice, the PODLXhi/CD49fhi is less likely to result in lethal pulmonary emboli and these cells survive longer in the lung compared to PODLXlow/CD49flow cells [44].
Neuronal Stem Cells
Neural stem cells (NSC) give rise to all major cell types of the central nervous system and are localized in the ventricular and subventricular zones (SVZ) of the brain, where NSC niches have been identified surrounding vascular endothelial cells [47]. NSCs are identified by the intracellular expression of Sox1, Sox2, Sox3, Nestin, and Musashi and extracellular expression of integrin α6β1; and selection of α6high or β1high cells leads to enrichment of NSCs from neurospheres [48]. The expression of β1 in these cells correlates with the expression of prominin-1 (CD133), one of the most effective markers of NSCs. In the same population of cells, 20% are characterized as integrin α6high cells. Reports show that β1high cells have upregulated expression of Sox2, Sox3, Musashi1, and Bmi1 compared to β1low cells and are able to differentiate into neurons and astrocytes [48]. A similar pattern of α6high/β1high expression has been reported in mouse adult NSCs, and this expression is lost as NSCs differentiate and migrate away from their vascular niche in the SVZ of the brain. Interestingly, in vivo treatment with integrin α6 antibodies in the lateral ventricle of adult mice brains results in migration of NSCs from their niche and proliferation of SVZ lineage cells [47], suggesting that modulation in the expression of integrin α6 induces differentiation of NSC and migration out of the stem cell niche.
Cancer Stem Cells
A distinct population of cells within most tumors have the potential for self-renewal and differentiation into cells that populate new tumors and, therefore, are referred to as tumor-initiating cells or CSCs. In glioblastoma cancer stem cells (GSCs), integrin α6 has been proposed to be an important regulator of self-renewal, proliferation, and tumor formation capacity [49]. An enriched population of GSCs can be obtained from bulk tumors, based on selection of high expression of integrin α6 alone or in combination with CD133 [49]. These cells are able to form tumorspheres in vitro, which confirm their self-renewal capacity. In vivo limiting dilution transplantation assays of integrin α6high cells demonstrates a significant increase in tumor formation with lower numbers of transplanted cells and in a shorter time compared to integrin α6low cells. Knockdown of integrin α6 by shRNA on these cells abrogates the formation of tumorspheres and significantly reduces tumor formation in immune-compromised mice [49], suggesting that this stem cell population is depleted.
Finally, integrin α6 has been used to enrich CSC populations from four different cervical uterine cancer cell lines: HeLa, SiHa, Ca Ski, and C-4 l. The integrin α6+ CSCs obtained from spheroids show self-renewal properties, enhanced tumorigenic capabilities, and increased resistance to ionizing radiation compared to their parental cell lines cultured in monolayer [50,51].
The Role of Integrin α6 in the Mechanisms Used to Maintain Self-Renewal in Stem Cells
Despite the common expression of integrin α6 in stem cells (Table 1), little is known about the molecular mechanisms by which this transmembrane receptor is regulated. It is known that Oct4 and Sox2 bind promoters of integrin α6 and support its transcription [45]. It has also been shown that KLF9 represses the transcription of integrin α6 by binding its promoter region [52]. Interestingly, this repressive action of KLF9 on integrin α6 inhibits both glioblastoma cell stemness and tumorigenicity [52].
Our current understanding of the molecular mechanisms by which integrin α6 regulates self-renewal of stem cells comes largely from studies in ESCs and breast CSCs. We recently reported a critical role of integrin α6 in the self-renewal of human ESCs and identified the molecular mechanisms involved in this process [30]. The heterodimer of integrin α6β1 is dominant in undifferentiated human ESCs, regardless of the substrate on which they are cultured (vitronectin-coated plates (CP), fibronectin-CP, laminin 511-CP, Matrigel-CP, or in supporting polymer coatings). The expression of integrin α6 highly correlates with the undifferentiated state of human ESCs and, therefore, with the expression of Oct4 and Sox2, both pluripotent-related transcription factors [30]. Interestingly, human ESCs express the A and B isoforms of integrin α6, and it has been reported that the A isoform is able to prevent the activation of integrin β1 [53]. Indeed, it was observed that β1 signaling in hESCs is inactive, since focal adhesion kinase (FAK) is not phosphorylated in undifferentiated cells. Activation of integrin β1 signaling by activating antibodies induces the phosphorylation of FAK and the differentiation of human ESCs. Furthermore, during differentiation integrin α6 expression decreases, while FAK becomes phosphorylated. FAK is present in the nucleus of undifferentiated hESCs where it co-localizes and interacts with both Oct4 and Sox2, and the overexpression of these transcription factors induces the nuclear localization of FAK. Similarly, during the reprogramming of fibroblasts into induced pluripotent stem cells, integrin α6 is upregulated and FAK is dephosphorylated [30]. Finally, it has been reported that human ESCs synthesize and deposit a laminin 511-rich substrate [54], and the knockdown of laminin in these stem cells induces reduction in integrin α6 expression, phosphorylation of FAK, and degradation of Oct4 and Sox2 [30]. Taken together, these findings suggest a model in which human pluripotent stem cells remodel the microenvironment to sustain integrin α6, prevent integrin β1/FAK activation, and maintain the expression of transcription factors involved in self-renewal and pluripotency (Fig. 1) [30,54].
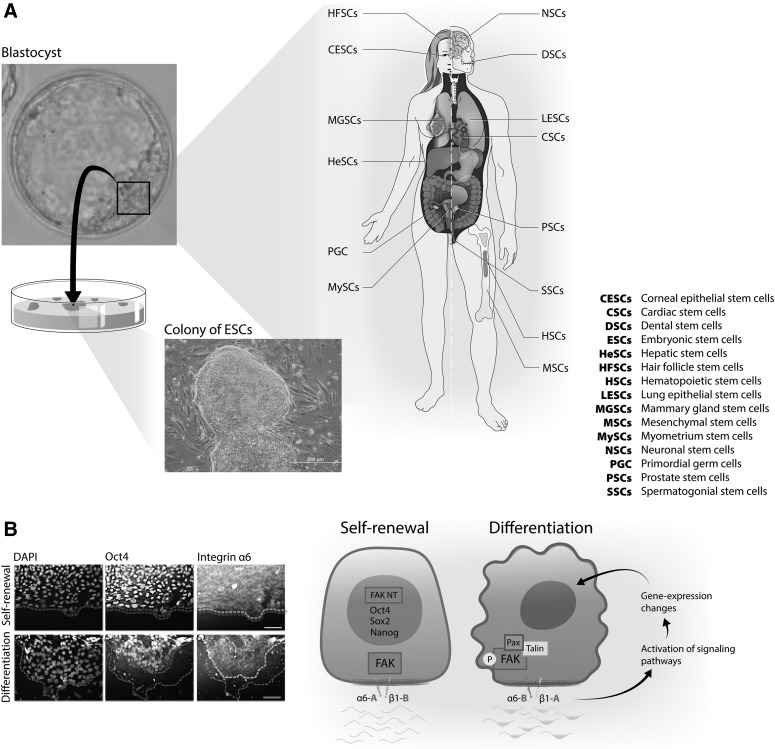
FIG. 1.
Integrin α6 (CD49f) is a conserved biomarker of stem cells involved in their self-renewal. (A) Illustration showing the presence of integrin α6 in diverse stem cell populations. From pluripotent stem cells, the inner cell mass of the blastocyst embryo and ESC to adult stem cells (for a complete list of stem cell types refer to Table 1). (B) Representative micrographs showing coexpression of integrin α6 with the pluripotent transcription factor Oct4 in undifferentiated (top panel) and differentiating (bottom panel) human ESC colonies. The dotted outer line in the micrographs indicates the margins of colonies, while the inner dimmed and bright dotted lines delineate colony areas with positive expression of Oct4 and integrin α6, respectively. Notice tightly coexpression of Oct4 and integrin α6 in both undifferentiated and differentiating colonies. A schematic model illustrating a mechanism by which integrin α6 might regulate self-renewal of stem cells, based on data obtained in human ESCs [30]. The depicted drawing on the left indicates an undifferentiated ESC expressing pluripotent related transcription factors Oct4, Sox2, and Nanog and integrin α6Aβ1B. This protein configuration maintains the FAK inactive and localized in the cytoplasm and the nuclei of undifferentiated cells. Change in translation of integrin α6β1 to α6Bβ1A in the stem cell (drawing on the right) results in recruitment to the plasma membrane and subsequent phosphorylation of FAK, followed by activation of signaling pathways and change in gene expression that include the downregulation of the pluripotent transcription factors and cell differentiation. ESC, embryonic stem cell; FAK, focal adhesion kinase.
The expression of integrin α6 is consistently found in breast CSCs [55–57] and recently it has been shown that the B isoform, rather than the A isoform of α6, defines more precisely the breast CSC population [17]. Phenotypically the integrin α6B-dominant population has a mesenchymal cell morphology compared to the epithelial cell morphology of the α6A-dominant population, and they form heterodimers differently: the α6B heterodimer with integrin β1, while α6A population expresses integrin β4. The expression of ALDH1 and BMI1, well-established stem cell markers, as well as augmented retention of PKH, a marker for slow-cycling cells, is increased in the α6B population. Furthermore, α6B cells have a greater capacity to form mammospheres and to initiate tumors.
The expression of A and B isoforms of integrin α6 is regulated by alternative splicing mechanisms [58], and the ESRP1-splicing factor has been identified as a key regulator of α6A while it also functions to repress α6B [17]. Interestingly, VEGF signaling also promotes the initiation of triple negative breast cancer tumors through suppression of ESRP1 and, consequently, by the induction of α6B expression [17,57]. Supporting the concept that integrin α6B is a determinant in breast CSCs, laminin511 is found to be expressed in the niche that supports self-renewal of breast CSCs by engaging with integrin α6B [59]. The interaction between laminin511 and integrin α6B activates TAZ [59], a transducer of the Hippo pathway that has been associated in the self-renewal and tumor-initiation capacities of breast CSCs [60].
Integrin α6 Links Stem Cells to Laminins Present in Their Niche
Stem cells reside in specialized microenvironments termed the stem cell niche. The niche provides cues in the form of cell-ECM, cell-cell contact, and secreted factors that regulate the fate of the residing stem cell population. Specific characteristics among niches vary [61–63]. However, a common finding among stem cell niches is the presence of the laminins. These ECM proteins, which are the ligands for integrin α6, have been found in the niches of several somatic stem cells such as corneal [64], colonic [65], epithelial [66], hair follicle [67], hematopoietic [68,69], hepatic [70], spermatogonia [23], neuronal [47,71], as well as in the blastocyst embryo [72], where the ICM is formed, and in the niche of glioblastoma [49] and breast CSCs [59]. The deposition of laminins in stem cell niches may originate from nonstem cells that are part of the niche. For example, vascular endothelial cells [73], which are key elements in several stem cell niches [47,74–76]. However, recent evidence suggests that stem cells secrete laminins as well and may therefore contribute to the formation and connection to the stem cell niche, and with their self-renewal mechanisms. In vitro human ESCs deposit laminin511 [30,54], and knockdown of laminin in these stem cells demonstrates connection to protein levels of integrin α6 and Oct4 [30]. Breast CSCs produce laminin511, which functions as a ligand for α6Bβ1 expressed in these cells, promoting self-renewal and tumor initiation as well [59]. The deposition of laminins by stem cells in the niche may be regulated by integrin signaling through integrin-linked kinase (ILK), as demonstrated recently in the niche of CD34+/CD49fhigh hair follicle stem cells (HFSCs) [67]. Deletion of ILK leads to changes in the laminin isoforms present in the stem cell niche, which results in activation of HFSCs and in their exhaustion [67]. This suggests a critical role between laminins in the niche and integrins in stem cells regulating their activities, and the fact that integrin α6 is present in more than 30 stem cell populations (Table 1) gives merit to further investigation of this specific integrin subunit in the maintenance and function of stem cells.
Conclusion
Stem cells play key roles in homeostasis by replenishing tissues with differentiated cells that are lost because of natural causes or during injury. Stem cells share the properties of self-renewal and differentiation, as well as the need for a specialized niche in which to reside. In this literature review, we shed light onto another common characteristic, the expression and functional role of integrin α6 (CD49f). We synthesized data from several lines of evidence in a broad range of stem cells to support the case that integrin α6 can be considered as an authentic and reliable stem cell marker because of its common expression and function in homing and connecting stem cells to their niches and for its role in regulating self-renewal mechanisms in stem cells.
Acknowledgments
The National Institutes of Health grant R01DE016530-08 supported this work. The authors thank AlphaMed Press for permission to use a modified version of a figure published in Stem Cells 2016;34:1753-64 (DOI:10.1002/stem.2349) by the authors.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Civin CI, Strauss LC, Brovall C, Fackler MJ, Schwartz JF. and Shaper JH. (1984). Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol 133:157–165 [PubMed] [Google Scholar]
- 2.Simmons PJ. and Torok-Storb B. (1991). CD34 expression by stromal precursors in normal human adult bone marrow. Blood 78:2848–2853 [PubMed] [Google Scholar]
- 3.Dupas T, Rouaud T, Rouger K, Lieubeau B, Cario-Toumaniantz C, Fontaine-Perus J, Gardahaut MF. and Auda-Boucher G. (2011). Fetal muscle contains different CD34+ cell subsets that distinctly differentiate into adipogenic, angiogenic and myogenic lineages. Stem Cell Res 7:230–243 [DOI] [PubMed] [Google Scholar]
- 4.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M. and Fuchs E. (2004). Defining the epithelial stem cell niche in skin. Science 303:359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J, Greaves MF. and Molgaard HV. (1991). The gene encoding the stem cell antigen, CD34, is conserved in mouse and expressed in haemopoietic progenitor cell lines, brain, and embryonic fibroblasts. Int Immunol 3:175–184 [DOI] [PubMed] [Google Scholar]
- 6.Sidney LE, Branch MJ, Dunphy SE, Dua HS. and Hopkinson A. (2014). Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 32:1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kligys KR, Wu Y, Hopkinson SB, Kaur S, Platanias LC. and Jones JC. (2012). Alpha6beta4 integrin, a master regulator of expression of integrins in human keratinocytes. J Biol Chem 287:17975–17984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neuber K, Hilger RA. and Konig W. (1992). Differential increase in 12-HETE release and CD29/CD49f expression of platelets from normal donors and from patients with atopic dermatitis by Staphylococcus aureus. Int Arch Allergy Immunol 98:339–342 [DOI] [PubMed] [Google Scholar]
- 9.Schlotzer-Schrehardt U. and Kruse FE. (2005). Identification and characterization of limbal stem cells. Exp Eye Res 81:247–264 [DOI] [PubMed] [Google Scholar]
- 10.Li A, Simmons PJ. and Kaur P. (1998). Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 95:3902–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortunel NO, Otu HH, Ng HH, Chen J, Mu X, Chevassut T, Li X, Joseph M, Bailey C, et al. (2003). Comment on “‘Stemness’: transcriptional profiling of embryonic and adult stem cells” and “a stem cell molecular signature”. Science 302:393; author reply 393 [DOI] [PubMed] [Google Scholar]
- 12.Hynes RO. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110:673–687 [DOI] [PubMed] [Google Scholar]
- 13.Gilcrease MZ. (2007). Integrin signaling in epithelial cells. Cancer Lett 247:1–25 [DOI] [PubMed] [Google Scholar]
- 14.Watt FM. (2002). Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J 21:3919–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogervorst F, Kuikman I, van Kessel AG. and Sonnenberg A. (1991). Molecular cloning of the human alpha 6 integrin subunit. Alternative splicing of alpha 6 mRNA and chromosomal localization of the alpha 6 and beta 4 genes. Eur J Biochem 199:425–433 [DOI] [PubMed] [Google Scholar]
- 16.Tamura RN, Cooper HM, Collo G. and Quaranta V. (1991). Cell type-specific integrin variants with alternative alpha chain cytoplasmic domains. Proc Natl Acad Sci U S A 88:10183–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goel HL, Gritsko T, Pursell B, Chang C, Shultz LD, Greiner DL, Norum JH, Toftgard R, Shaw LM. and Mercurio AM. (2014). Regulated splicing of the alpha6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep 7:747–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dogic D, Rousselle P. and Aumailley M. (1998). Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J Cell Sci 111 (Pt 6):793–802 [DOI] [PubMed] [Google Scholar]
- 19.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A. and Le Meur M. (1996). Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13:370–373 [DOI] [PubMed] [Google Scholar]
- 20.McGrath JA, Kivirikko S, Ciatti S, Moss C, Dunnill GS, Eady RA, Rodeck CH, Christiano AM. and Uitto J. (1995). A homozygous nonsense mutation in the alpha 3 chain gene of laminin 5 (LAMA3) in Herlitz junctional epidermolysis bullosa: prenatal exclusion in a fetus at risk. Genomics 29:282–284 [DOI] [PubMed] [Google Scholar]
- 21.Kuster JE, Guarnieri MH, Ault JG, Flaherty L. and Swiatek PJ. (1997). IAP insertion in the murine LamB3 gene results in junctional epidermolysis bullosa. Mamm Genome 8:673–681 [DOI] [PubMed] [Google Scholar]
- 22.Pulkkinen L, Christiano AM, Airenne T, Haakana H, Tryggvason K. and Uitto J. (1994). Mutations in the gamma 2 chain gene (LAMC2) of kalinin/laminin 5 in the junctional forms of epidermolysis bullosa. Nat Genet 6:293–297 [DOI] [PubMed] [Google Scholar]
- 23.Shinohara T, Avarbock MR. and Brinster RL. (1999). beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 96:5504–5509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuccotti M, Giorgi Rossi P, Fiorillo E, Garagna S, Forabosco A. and Redi CA. (1998). Timing of gene expression and oolemma localization of mouse alpha6 and beta1 integrin subunits during oogenesis. Dev Biol 200:27–34 [DOI] [PubMed] [Google Scholar]
- 25.Almeida EA, Huovila AP, Sutherland AE, Stephens LE, Calarco PG, Shaw LM, Mercurio AM, Sonnenberg A, Primakoff P, Myles DG. and White JM. (1995). Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81:1095–1104 [DOI] [PubMed] [Google Scholar]
- 26.Sutherland AE, Calarco PG. and Damsky CH. (1993). Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 119:1175–1186 [DOI] [PubMed] [Google Scholar]
- 27.Evans MJ. and Kaufman MH. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156 [DOI] [PubMed] [Google Scholar]
- 28.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS. and Jones JM. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- 29.Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD. and Carpenter MK. (2001). Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol 19:971–974 [DOI] [PubMed] [Google Scholar]
- 30.Villa-Diaz LG, Kim JK, Laperle A, Palecek SP. and Krebsbach PH. (2016). Inhibition of focal adhesion kinase signaling by integrin alpha6beta1 supports human pluripotent stem cell self-renewal. Stem Cells 34:1753–1764 [DOI] [PubMed] [Google Scholar]
- 31.Qian H, Tryggvason K, Jacobsen SE. and Ekblom M. (2006). Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood 107:3503–3510 [DOI] [PubMed] [Google Scholar]
- 32.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I. and Dick JE. (2011). Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333:218–221 [DOI] [PubMed] [Google Scholar]
- 33.Stingl J, Eirew P, Ricketson I, Shackleton M, Vaillant F, Choi D, Li HI. and Eaves CJ. (2006). Purification and unique properties of mammary epithelial stem cells. Nature 439:993–997 [DOI] [PubMed] [Google Scholar]
- 34.Ozawa K, Sato K, Oh I, Ozaki K, Uchibori R, Obara Y, Kikuchi Y, Ito T, Okada T, et al. (2008). Cell and gene therapy using mesenchymal stem cells (MSCs). J Autoimmun 30:121–127 [DOI] [PubMed] [Google Scholar]
- 35.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S. and Marshak DR. (1999). Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- 36.Krebsbach PH, Kuznetsov SA, Bianco P. and Robey PG. (1999). Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med 10:165–181 [DOI] [PubMed] [Google Scholar]
- 37.Katz AJ, Tholpady A, Tholpady SS, Shang H. and Ogle RC. (2005). Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells 23:412–423 [DOI] [PubMed] [Google Scholar]
- 38.Shi S. and Gronthos S. (2003). Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696–704 [DOI] [PubMed] [Google Scholar]
- 39.Schwab KE. and Gargett CE. (2007). Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 22:2903–2911 [DOI] [PubMed] [Google Scholar]
- 40.Igura K, Zhang X, Takahashi K, Mitsuru A, Yamaguchi S. and Takashi TA. (2004). Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 6:543–553 [DOI] [PubMed] [Google Scholar]
- 41.Romanov YA, Svintsitskaya VA. and Smirnov VN. (2003). Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21:105–110 [DOI] [PubMed] [Google Scholar]
- 42.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR. and Mosca JD. (2003). Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci 10:228–241 [DOI] [PubMed] [Google Scholar]
- 43.Lv FJ, Tuan RS, Cheung KM. and Leung VY. (2014). Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419 [DOI] [PubMed] [Google Scholar]
- 44.Lee RH, Seo MJ, Pulin AA, Gregory CA, Ylostalo J. and Prockop DJ. (2009). The CD34-like protein PODXL and alpha6-integrin (CD49f) identify early progenitor MSCs with increased clonogenicity and migration to infarcted heart in mice. Blood 113:816–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu KR, Yang SR, Jung JW, Kim H, Ko K, Han DW, Park SB, Choi SW, Kang SK, Scholer H. and Kang KS. (2012). CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells 30:876–887 [DOI] [PubMed] [Google Scholar]
- 46.Nystedt J, Anderson H, Tikkanen J, Pietila M, Hirvonen T, Takalo R, Heiskanen A, Satomaa T, Natunen S, et al. (2013). Cell surface structures influence lung clearance rate of systemically infused mesenchymal stromal cells. Stem Cells 31:317–326 [DOI] [PubMed] [Google Scholar]
- 47.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B. and Temple S. (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3:289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hall PE, Lathia JD, Miller NG, Caldwell MA. and Ffrench-Constant C. (2006). Integrins are markers of human neural stem cells. Stem Cells 24:2078–2084 [DOI] [PubMed] [Google Scholar]
- 49.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB. and Rich JN. (2010). Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell 6:421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lopez J, Poitevin A, Mendoza-Martinez V, Perez-Plasencia C. and Garcia-Carranca A. (2012). Cancer-initiating cells derived from established cervical cell lines exhibit stem-cell markers and increased radioresistance. BMC Cancer 12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ortiz-Sanchez E, Santiago-Lopez L, Cruz-Dominguez VB, Toledo-Guzman ME, Hernandez-Cueto D, Muniz-Hernandez S, Garrido E, Cantu-De Leon D. and Garcia-Carranca A. (2016). Characterization of cervical cancer stem cell-like cells: phenotyping, stemness, and Human Papilloma Virus co-receptor expression. Oncotarget 7:31943–31954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying M, Tilghman J, Wei Y, Guerrero-Cazares H, Quinones-Hinojosa A, Ji H. and Laterra J. (2014). Kruppel-like factor-9 (KLF9) inhibits glioblastoma stemness through global transcription repression and integrin alpha6 inhibition. J Biol Chem 289:32742–32756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE. and Horwitz AF. (1999). Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol 144:1295–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Laperle A, Hsiao C, Lampe M, Mortier J, Saha K, Palecek SP. and Masters KS. (2015). Alpha-5 laminin synthesized by human pluripotent stem cells promotes self-renewal. Stem Cell Reports 11:195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vieira AF, Ricardo S, Ablett MP, Dionisio MR, Mendes N, Albergaria A, Farnie G, Gerhard R, Cameselle-Teijeiro JF, et al. (2012). P-cadherin is coexpressed with CD44 and CD49f and mediates stem cell properties in basal-like breast cancer. Stem Cells 30:854–864 [DOI] [PubMed] [Google Scholar]
- 56.Meyer MJ, Fleming JM, Lin AF, Hussnain SA, Ginsburg E. and Vonderhaar BK. (2010). CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res 70:4624–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goel HL, Pursell B, Chang C, Shaw LM, Mao J, Simin K, Kumar P, Vander Kooi CW, Shultz LD, et al. (2013). GLI1 regulates a novel neuropilin-2/alpha6beta1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol Med 5:488–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y. and Carstens RP. (2010). An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J 29:3286–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang C, Goel HL, Gao H, Pursell B, Shultz LD, Greiner DL, Ingerpuu S, Patarroyo M, Cao S, et al. (2015). A laminin 511 matrix is regulated by TAZ and functions as the ligand for the alpha6Bbeta1 integrin to sustain breast cancer stem cells. Genes Dev 29:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, et al. (2011). The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147:759–772 [DOI] [PubMed] [Google Scholar]
- 61.Spradling A, Drummond-Barbosa D. and Kai T. (2001). Stem cells find their niche. Nature 414:98–104 [DOI] [PubMed] [Google Scholar]
- 62.Fuchs E, Tumbar T. and Guasch G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116:769–778 [DOI] [PubMed] [Google Scholar]
- 63.Watt FM. and Hogan BL. (2000). Out of Eden: stem cells and their niches. Science 287:1427–1430 [DOI] [PubMed] [Google Scholar]
- 64.Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC. and Li DQ. (2004). Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells 22:355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujimoto K, Beauchamp RD. and Whitehead RH. (2002). Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology 123:1941–1948 [DOI] [PubMed] [Google Scholar]
- 66.Jones PH. and Watt FM. (1993). Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73:713–724 [DOI] [PubMed] [Google Scholar]
- 67.Morgner J, Ghatak S, Jakobi T, Dieterich C, Aumailley M. and Wickstrom SA. (2015). Integrin-linked kinase regulates the niche of quiescent epidermal stem cells. Nat Commun 6:8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siler U, Rousselle P, Muller CA. and Klein G. (2002). Laminin gamma2 chain as a stromal cell marker of the human bone marrow microenvironment. Br J Haematol 119:212–220 [DOI] [PubMed] [Google Scholar]
- 69.Gu Y, Sorokin L, Durbeej M, Hjalt T, Jonsson JI. and Ekblom M. (1999). Characterization of bone marrow laminins and identification of alpha5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-Mix cells. Blood 93:2533–2542 [PubMed] [Google Scholar]
- 70.Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H. and Taniguchi H. (2000). Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 32:1230–1239 [DOI] [PubMed] [Google Scholar]
- 71.Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP. and Ffrench-Constant C. (2007). Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol 505:630–643 [DOI] [PubMed] [Google Scholar]
- 72.Leivo I, Vaheri A, Timpl R. and Wartiovaara J. (1980). Appearance and distribution of collagens and laminin in the early mouse embryo. Dev Biol 76:100–114 [DOI] [PubMed] [Google Scholar]
- 73.Vartanian KB, Kirkpatrick SJ, McCarty OJ, Vu TQ, Hanson SR. and Hinds MT. (2009). Distinct extracellular matrix microenvironments of progenitor and carotid endothelial cells. J Biomed Mater Res A 91:528–539 [DOI] [PubMed] [Google Scholar]
- 74.Palmer TD, Willhoite AR. and Gage FH. (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494 [DOI] [PubMed] [Google Scholar]
- 75.Louissaint A, Jr., Rao S, Leventhal C. and Goldman SA. (2002). Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960 [DOI] [PubMed] [Google Scholar]
- 76.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C. and Morrison SJ. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121 [DOI] [PubMed] [Google Scholar]
- 77.Castaldo C, Di Meglio F, Nurzynska D, Romano G, Maiello C, Bancone C, Muller P, Bohm M, Cotrufo M. and Montagnani S. (2008). CD117-positive cells in adult human heart are localized in the subepicardium, and their activation is associated with laminin-1 and alpha6 integrin expression. Stem Cells 26:1723–1731 [DOI] [PubMed] [Google Scholar]
- 78.Chaurasia P, Gajzer DC, Schaniel C, D'Souza S. and Hoffman R. (2014). Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest 124:2378–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hayashi R, Yamato M, Saito T, Oshima T, Okano T, Tano Y. and Nishida K. (2008). Enrichment of corneal epithelial stem/progenitor cells using cell surface markers, integrin alpha6 and CD71. Biochem Biophys Res Commun 367:256–263 [DOI] [PubMed] [Google Scholar]
- 80.Chang JY, Wang C, Jin C, Yang C, Huang Y, Liu J, McKeehan WL, D'Souza RN. and Wang F. (2013). Self-renewal and multilineage differentiation of mouse dental epithelial stem cells. Stem Cell Res 11:990–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiong J, Mrozik K, Gronthos S. and Bartold PM. (2012). Epithelial cell rests of Malassez contain unique stem cell populations capable of undergoing epithelial-mesenchymal transition. Stem Cells Dev 21:2012–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakthianandeswaren A, Christie M, D'Andreti C, Tsui C, Jorissen RN, Li S, Fleming NI, Gibbs P, Lipton L, et al. (2011). PHLDA1 expression marks the putative epithelial stem cells and contributes to intestinal tumorigenesis. Cancer Res 71:3709–3719 [DOI] [PubMed] [Google Scholar]
- 83.Zhao R, Quaroni L. and Casson AG. (2012). Identification and characterization of stemlike cells in human esophageal adenocarcinoma and normal epithelial cell lines. J Thorac Cardiovasc Surg 144:1192–1199 [DOI] [PubMed] [Google Scholar]
- 84.Manohar R, Komori J, Guzik L, Stolz DB, Chandran UR, LaFramboise WA. and Lagasse E. (2011). Identification and expansion of a unique stem cell population from adult mouse gallbladder. Hepatology 54:1830–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM. and Tennant RW. (2003). Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120:501–511 [DOI] [PubMed] [Google Scholar]
- 86.Fujikawa T, Hirose T, Fujii H, Oe S, Yasuchika K, Azuma H. and Yamaoka Y. (2003). Purification of adult hepatic progenitor cells using green fluorescent protein (GFP)-transgenic mice and fluorescence-activated cell sorting. J Hepatol 39:162–170 [DOI] [PubMed] [Google Scholar]
- 87.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H. and Taniguchi H. (2002). Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol 156:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Webb A, Li A. and Kaur P. (2004). Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 72:387–395 [DOI] [PubMed] [Google Scholar]
- 89.Terunuma A, Kapoor V, Yee C, Telford WG, Udey MC. and Vogel JC. (2007). Stem cell activity of human side population and alpha6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells 25:664–669 [DOI] [PubMed] [Google Scholar]
- 90.Tani H, Morris RJ. and Kaur P. (2000). Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 97:10960–10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chernaya O, Shinin V, Liu Y. and Minshall RD. (2014). Behavioral heterogeneity of adult mouse lung epithelial progenitor cells. Stem Cells Dev 23:2744–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McQualter JL, Yuen K, Williams B. and Bertoncello I. (2010). Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci U S A 107:1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matulka LA, Triplett AA. and Wagner KU. (2007). Parity-induced mammary epithelial cells are multipotent and express cell surface markers associated with stem cells. Dev Biol 303:29–44 [DOI] [PubMed] [Google Scholar]
- 94.Deugnier MA, Faraldo MM, Teuliere J, Thiery JP, Medina D. and Glukhova MA. (2006). Isolation of mouse mammary epithelial progenitor cells with basal characteristics from the Comma-Dbeta cell line. Dev Biol 293:414–425 [DOI] [PubMed] [Google Scholar]
- 95.Yang Z, Dong P, Fu X, Li Q, Ma S, Wu D, Kang N, Liu X, Yan L. and Xiao R. (2015). CD49f acts as an inflammation sensor to regulate differentiation, adhesion, and migration of human mesenchymal stem cells. Stem Cells 33:2798–2810 [DOI] [PubMed] [Google Scholar]
- 96.Woo SH, Stumpfova M, Jensen UB, Lumpkin EA. and Owens DM. (2010). Identification of epidermal progenitors for the Merkel cell lineage. Development 137:3965–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ono M, Kajitani T, Uchida H, Arase T, Oda H, Uchida S, Ota K, Nagashima T, Masuda H, et al. (2015). CD34 and CD49f double-positive and lineage marker-negative cells isolated from human myometrium exhibit stem cell-like properties involved in pregnancy-induced uterine remodeling. Biol Reprod 93:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Igarashi T, Shimmura S, Yoshida S, Tonogi M, Shinozaki N. and Yamane GY. (2008). Isolation of oral epithelial progenitors using collagen IV. Oral Dis 14:413–418 [DOI] [PubMed] [Google Scholar]
- 99.Jung JG, Kim DK, Park TS, Lee SD, Lim JM. and Han JY. (2005). Development of novel markers for the characterization of chicken primordial germ cells. Stem Cells 23:689–698 [DOI] [PubMed] [Google Scholar]
- 100.Lawson DA, Xin L, Lukacs RU, Cheng D. and Witte ON. (2007). Isolation and functional characterization of murine prostate stem cells. Proc Natl Acad Sci U S A 104:181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barclay WW, Axanova LS, Chen W, Romero L, Maund SL, Soker S, Lees CJ. and Cramer SD. (2008). Characterization of adult prostatic progenitor/stem cells exhibiting self-renewal and multilineage differentiation. Stem Cells 26:600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP. and Witte ON. (2008). Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. Proc Natl Acad Sci U S A 105:20882–20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M. and Endo F. (2007). Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells 9:191–205 [DOI] [PubMed] [Google Scholar]
- 104.Kurata R, Futaki S, Nakano I, Tanemura A, Murota H, Katayama I. and Sekiguchi K. (2014). Isolation and characterization of sweat gland myoepithelial cells from human skin. Cell Struct Funct 39:101–112 [DOI] [PubMed] [Google Scholar]
- 105.Osada M, Singh VJ, Wu K, Sant'Angelo DB. and Pezzano M. (2013). Label retention identifies a multipotent mesenchymal stem cell-like population in the postnatal thymus. PLoS One 8:e83024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghosh M, Helm KM, Smith RW, Giordanengo MS, Li B, Shen H. and Reynolds SD. (2011). A single cell functions as a tissue-specific stem cell and the in vitro niche-forming cell. Am J Respir Cell Mol Biol 45:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.To K, Fotovati A, Reipas KM, Law JH, Hu K, Wang J, Astanehe A, Davies AH, Lee L, et al. (2010). Y-box binding protein-1 induces the expression of CD44 and CD49f leading to enhanced self-renewal, mammosphere growth, and drug resistance. Cancer Res 70:2840–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rountree CB, Senadheera S, Mato JM, Crooks GM. and Lu SC. (2008). Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology 47:1288–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yamamoto H, Masters JR, Dasgupta P, Chandra A, Popert R, Freeman A. and Ahmed A. (2012). CD49f is an efficient marker of monolayer- and spheroid colony-forming cells of the benign and malignant human prostate. PLoS One 7:e46979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mulholland DJ, Xin L, Morim A, Lawson D, Witte O. and Wu H. (2009). Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res 69:8555–8562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chan RW, Ng EH. and Yeung WS. (2011). Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. Am J Pathol 178:2832–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schober M. and Fuchs E. (2011). Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci U S A 108:10544–10549 [DOI] [PMC free article] [PubMed] [Google Scholar]