Abstract
The effects of pomegranate extract (PE) supplementation were evaluated on high-intensity exercise performance, blood flow, vessel diameter, oxygen saturation (SPO2), heart rate (HR), and blood pressure (BP). In a randomized, crossover design, nineteen recreationally resistance trained participants were randomly assigned to PE (1000 mg) or placebo (PL), which were consumed 30 min prior to a repeated sprint ability (RSA) test and repetitions to fatigue (RTF) on bench and leg press. The RSA consisted of ten six-second sprints on a friction-loaded cycle ergometer with 30 s recovery. Brachial artery blood flow and vessel diameter were assessed by ultrasound. Blood flow, vessel diameter, SPO2, HR, and BP were assessed at baseline, 30 min post ingestion, immediately post exercise (IPost), and 30 min post exercise (30minPost). With PE, blood flow significantly increased IPost RSA (mean difference [MD]=18.49 mL·min−1; P<0.05), and IPost and 30minPost RTF (P <0.05) according to confidence intervals (CI). Vessel diameter increased significantly 30minPost RSA according to CI and resulted in a significant interaction IPost and 30minPost RTF (P <0.05). With PE, according to CI, average and peak power output increased significantly in sprint 5 of the RSA (P <0.05). There was no significant difference between PE and PL for bench (P =0.25) or leg press (P =0.15) repetitions. Acute PE supplementation enhanced vessel diameter and blood flow, suggesting possible exercise performance enhancement from increased delivery of substrates and oxygen. The acute timing and capsule form of PE may be advantageous to athletic populations due to ergogenic effects, taste, and convenience.
Keywords: ultrasound, repeated sprints, exercise performance, pre-workout
Introduction
The popularity of pre-workout supplements and ingredients thought to increase nitric oxide production are commonly used before exercise, such as arginine and citrulline, has grown tremendously over the last decade (Jones, 2014). Recently more natural sources, such as pomegranate and red beetroot, have gained attractiveness to athletes by being marketed as an ergogenic aid through increasing nitrate concentration via the endothelial nitric oxide synthase (NOS)-independent pathway or known as the enterosalivary nitrate-nitrite-nitric oxide pathway (Bescós, Sureda, Tur, & Pons, 2012). Through the enterosalivary nitrate-nitrite-NO pathway, nitrate and nitrite are reduced to form nitric oxide (NO), whereas in the NOS-dependent pathway, the amino acid L-arginine is oxidized by NOS enzymes to produce NO (Bescós, Ferrer-Roca, et al., 2012). In the enterosalivary nitrate-nitrite-NO pathway, dietary nitrate is ingested, absorbed in the upper gastrointestinal tract, and circulating nitrate is actively taken up by salivary glands and excreted in the saliva. Nitrate is then reduced to nitrite by bacteria in the mouth, where some is swallowed and converted to NO in the stomach. The other portion of salivary nitrite enters the systemic circulation, which can then be further reduced to NO in blood and tissues. This pathway is especially enhanced under physiological hypoxia and thus may play an important role in NO formation during high-intensity exercise, as well as resistance exercise (Bailey, Vanhatalo, Winyard, & Jones, 2012). Nitric oxide regulates important physiological functions and has been recognized as an important factor in endothelial vascular relaxation during exercise. (Bailey et al., 2012; Hellsten, Nyberg, Jensen, & Mortensen, 2012). Natural components of pomegranate, including polyphenols, may enhance the enterosalivary nitrate-nitrite-NO pathway through catalyzing the non-enzymatic reduction of nitrite in blood and tissues to NO and providing additional benefit for endothelial function (Ignarro, Byrns, Sumi, de Nigris, & Napoli, 2006) Polyphenols specifically in pomegranate, including flavonols, ellagitannins, and anthocyanins, have been demonstrated to be more effective in endothelial-dependent vasodilation than other fruits containing polyphenols (Chong, Macdonald, & Lovegrove, 2010; Mattiello, Trifirò, Jotti, & Pulcinelli, 2009). Polyphenols have been shown to modulate oxidative stress, decrease inflammation, stimulate vasodilation, and promote NO production, which may be beneficial for blood flow and exercise performance (Barona, Aristizabal, Blesso, Volek, & Fernandez, 2012; Scalbert, Johnson, & Saltmarsh, 2005; Tangney & Rasmussen, 2013).
Previous studies on nitrate supplementation have resulted in enhanced exercise tolerance, blood flow, oxygenation, and decreased blood pressure (BP) (Bailey et al., 2009; Bescós et al., 2011; de Nigris et al., 2006; Larsen et al., 2011; Trexler, Smith-ryan, Melvin, Roelofs, & Wingfield, 2014). The majority of previous investigations have examined red beetroot as the NO stimulus (Jones, 2014) as beetroot has been classified as having very high nitrate content of >250 mg/100 g of fresh weight (Santamaria, 2006). Beetroot has been shown to increase time to exhaustion and reduce oxygen cost at a fixed submaximal work rate and improve exercise efficiency (Bailey et al., 2009; Kelly, Vanhatalo, Wilkerson, Wylie, & Jones, 2013). In comparison to beetroot, pomegranate juice has a nitrate concentration of 12.93 mg/L (Hord, Tang, & Bryan, 2009) and pomegranate extract in the current study was an average of 109 ppm/1,000 mg, but few studies have examined exercise effects of pomegranate juice or pomegranate extract (PE). However, additional benefits from pomegranate may result due to the high polyphenol content (Chong et al., 2010; Mattiello et al., 2009). Specifically, pomegranate juice has demonstrated protection of NO against oxidative destruction and enhanced the action of NO due to high polyphenolic content (Ignarro et al., 2006). Polyphenols play an antioxidant role to interact with oxidant radicals, preventing oxidative reactions and promoting NO formation from nitrite (Gago, Lundberg, Barbosa, & Laranjinha, 2007; Rocha, Gago, Barbosa, & Laranjinha, 2009). Rocha et al. (2009) demonstrated increased NO levels approximately 5 to 30 minutes after ingesting polyphenol-containing dietary compounds. Increased NO promotes vasodilation by acting on vascular smooth muscle, which may improve exercise performance due to increased blood flow and oxygenation to working skeletal muscle. Previous research on PE and exercise performance has demonstrated a delay in fatigue during high-intensity running and increased vessel diameter and blood flow (Trexler et al., 2014).
Polyphenols from grapes have also been shown to reduce systolic BP and enhance flow-mediated dilation (FMD), a measure of endothelium-dependent vasodilation (Barona et al., 2012). Polyphenol supplementation had positive cardiovascular effects in men with metabolic syndrome (Barona et al., 2012) and cardiovascular disease (Tangney & Rasmussen, 2013), but not in healthy men (Mierlo, Zock, Knaap, & Draijer, 2010). Where as in elite athletes, acute polyphenol consumption increased FMD and improved endothelial function (Labonté et al., 2013). These data suggest acute ingestion of a natural source of dietary nitrate and polyphenol, such as PE, may be beneficial for improving cardiovascular health and exercise performance (Mathew, Capel-Williams, Berry, & Hall, 2012; Trexler et al., 2014). However, further research examining the effects of PE on blood flow and vessel diameter in a healthy population is warranted.
Dietary nitrate potentially plays an important role, when converted to NO, under periods of low oxygen availability/exercising state (Jones, 2014), and as a result may be beneficial for high-intensity exercise such as repeated sprints or repetitions to fatigue. Additionally, polyphenols may increase NO bioavailability, potentially improving oxidative capacity to improve repeated sprint performance (Girard, Mendez-Villanueva, & Bishop, 2011; Hord et al., 2009). Additional research investigating the effects of PE on high-intensity exercise performance and resistance training would be beneficial for aiding athletes in their training using a natural compound to enhance performance. Therefore, this study aimed to evaluate the effects of acute PE supplementation on high-intensity exercise performance, blood flow, vessel diameter, oxygen saturation (SPO2), heart rate (HR) and BP in healthy men and women.
METHODS
Experimental Approach to the Problem
In a double blind crossover design, participants were randomly assigned, via Random Allocation Software (Isfahan, Iran), to either PE (1000 mg) or placebo (PL) first and then crossed over to the other treatment after a minimum of a 7 day wash out period. Participants abstained from exercise 24 h prior, caffeine, mouthwash, and food for 3 h prior to all exercise testing. Three-day diet logs were completed between visit 1 and 2 and analyzed using The Food Processor software (ESHA Research, Oregon, USA). Five separate visits to the laboratory were completed; the first visit consisted of baseline assessments of height, body mass, and maximal strength tests, and visits 2 through 5 were exercise testing visits. Cardiovascular measurements of blood flow, vessel diameter, BP, HR, and SPO2 were taken on all exercise testing visits at baseline. After baseline cardiovascular measurements, participants ingested either PE or PL at all exercise testing visits and then rested for 30 minutes in a seated position. At 30 min post-ingestion (30minPI), cardiovascular measurements were taken prior to the exercise test warm-up and again immediately post exercise test (IPost). Participants then rested for 30 minutes in a seated position and the cardiovascular measurements were taken 30 min post exercise test (30minPost). The exercise test in visit two consisted of a repeated sprint ability (RSA) test on a cycle ergometer. Visit three consisted of repetitions to fatigue (RTF) calculated at 80% of maximal strength. Visits four and five were identical to visits two and three, with the opposite treatment (Figure 1). Exercise testing visits were performed at the same time of day (+/− 2 h), with 24 to 72 h between same treatments, and 7 to 10 days between different treatment conditions.
Figure 1.
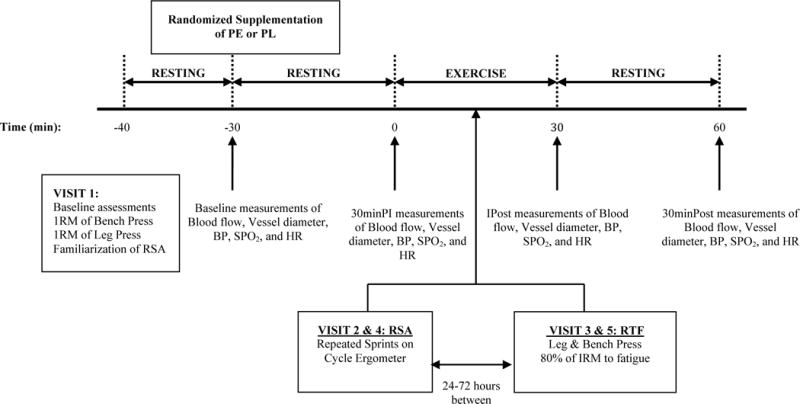
Overview of the study experimental design. Pomegranate Extract (PE); Placebo (PL); 1 repetition maximum (1RM); Blood pressure (BP); Oxygen saturation (SPO2); Heart rate (HR); Repeated sprint ability (RSA); Repetitions to fatigue (RTF); 30 minutes post ingestion (30minPI); immediately post exercise (IPost); 30 minutes post exercise (30minPost).
Subjects
Twenty-one individuals (10 male, 11 female) were recruited to participate in the study; two males dropped out prior to randomization due to reasons unrelated to the study. Nineteen recreationally resistance-trained individuals (mean ± SD; age: 22.1 ± 1.9 y; height: 170.4 ± 12.4 cm; body mass: 68.7 ± 15.9 kg) between the ages of 18–35 years participated. Participants were recreationally active, with at least three hours per week of resistance training for at least three months, determined by an exercise status questionnaire. Exclusion criteria consisted of any injury or health risk that contraindicated exercise; use of dietary supplements within 12 weeks prior to study enrollment; use of any medication with vasodilatory effects (for example – inhaler); and any known allergy or sensitivity to the test ingredient or placebo. All methodology was approved by the University’s Institutional Review Board, and all participants provided written informed consent prior to participation. All experimental methodology conformed to the requirements stipulated in the Declaration of Helsinki.
Procedures
Supplementation
Using a computer generated allocation sequence, in a double-blind fashion, participants were randomized to either 1000mg of PE (True Pomegranate Extract, Stiebs Nature Elevated, Madera, CA, USA) or PL (95% maltodextrin, 5% purple carrot and hibiscus for color) for visits 2 and 3 and then crossed over to the opposite treatment for visits 4 and 5. Capsules were blinded in identical opaque capsules, and were ingested in the lab with 6 oz. of water. Per the 1,000 mg dose, nitrate content was an average of 109 parts per million and polyphenols were an average of 3500 μmol/L. Certificate of analyses for nitrate and polyphenol concentration was provided by the manufacturer of the capsules directly to the research group, contracted by the distributing company (Stiebs Nature Elevated). Participants were asked to maintain their current diet throughout the duration of the study and to consume the same foods prior to each testing session.
Cardiovascular Measurements
Heart rate was measured using a Polar monitor (Polar FT1, Polar USA, Port Washington, NY, USA). Oxygen saturation was measured using a fingertip pulse oximeter (Contec Medical Systems Co, Qinhuangdao, China). Blood pressure was measured with an automatic BP cuff (Omron Healthcare, Lake Forest, IL, USA). Blood flow and vessel diameter were measured using a GE logiq-e B-mode ultrasound (GE Healthcare, Wisconsin, USA) with vascular, pulse wave, and color flow settings. Blood flow was measured using a protocol previously published by Uehata et al. (Uehata et al., 1997) using the brachial artery of the right arm (Trexler et al., 2014). For the resting measurements, a BP cuff was placed on the upper arm for one minute inflated to 180 mmHg. The cuff was taken off and the ultrasound probe (GE: 12L-RS) was placed longitudinally on the upper arm, 5–15 cm above the antecubital fossa, over the brachial artery to measure blood flow for at minimum four heartbeats. Diameter of the artery was quantified using the ultrasound software ‘measure’ function by drawing a straight line between the walls of the artery; diameter and flow values were determined from the ultrasound’s internal software. Blood flow and vessel diameter measurements IPost and 30minPost did not use the cuff prior to measurement. Reliability measures for blood flow produced an intraclass correlation coefficient (ICC) of 0.86 and standard error of the measurement (SEM) of 5.92 mL·min−1; reliability for vessel diameter resulted in an ICC of 0.82 and SEM of 0.027 cm.
Maximal Strength
One-repetition maximal (1RM) strength was assessed for bench press and leg press (York Barbell Co., York, PA, USA), as previously described by Adams et al. (Adams et al., 1999). Participants completed a five-minute self-selected warm-up and were familiarized with the equipment. Each participant then performed 8–10 repetitions at 50% of their predicted 1RM. After a one-minute rest period, participants performed 4–6 repetitions at 80% of their predicted 1RM. Following a one-minute rest period, the weight was increased to the estimated 1RM load, and participants attempted to lift the weight one time. After each successful attempt, the weight was increased according to participant’s perceived effort until a failed attempt occurred. Two to three min of rest was given between each 1RM attempt, and the 1RM was found within five attempts to avoid fatigue. The heaviest weight successfully lifted was recorded as the 1RM. After testing maximal strength, participants were familiarized with the RSA protocol.
Repeated Sprint Ability
On a friction-loaded cycle ergometer (Monark 894E, Stockholm, Sweden), participants warmed up for ten minutes at a self-selected pace. The RSA consisted of ten six-second maximal sprints with 30s of passive recovery between each sprint with a load applied of 65 g/kg of body mass (Gaitanos, Williams, Boobis, & Brooks, 1993). Participants remained seated on the cycle ergometer during the entire test. Average and peak power output were recorded in watts for each sprint using the default system (Monark ATS Software; Monark, Stockholm, Sweden). Previous test re-test reliability from our lab for peak power had an ICC of 0.96 and SEM 19.7 Watts.
Repetitions to Fatigue
The results from the 1RM assessments were used to determine the weight load for each participant for the RTF sessions. Following a self-selected warm-up that was consistent for each visit, one set at 80% of 1RM was completed to fatigue on bench press and leg press, respectively, with three minutes of rest between each exercise (Duncan, Smith, Cook, & James, 2012). Bench press reliability resulted in an ICC of 0.89 and SEM 1.28 repetitions, while leg press resulted in an ICC of 0.88 and SEM 2.3 repetitions.
Statistical Analyses
Data were assessed for normality using the Shapiro-Wilks test. Variables were further analyzed using repeated measures ANOVAs for bench and leg press RTF. Separate factorial repeated measures ANOVAs [treatment (PE vs. PL) × time (sprint 1 vs. sprint 2 … vs. sprint 10)] were used to assess peak and average power output for RSA tests. Separate factorial repeated measures ANOVAs [treatment (PE vs. PL) × time (baseline vs. 30minPI vs. IPost vs. 30minPost)] were used to assess blood flow, vessel diameter, BP, HR, and SPO2 for RSA and RTF tests. Bonferroni post hoc comparisons were used when necessary. For RSA vessel diameter, a linear-mixed model ANCOVA was employed to covary for significant differences at baseline. Change scores from PE to PL were calculated for each participant and 95% confidence intervals (CI) were placed around the mean change score. If the 95% CI included zero, the mean change score was not different from zero, which can be interpreted as no significant change. If the 95% CI interval did not include zero, the mean change score was considered statistically significant (P ≤0.05). SPSS Version 20 (IBM; Chicago, IL, USA) was used to perform the ANOVA models and ANCOVA with an alpha level of P <0.05 for statistical significance. The 95% CI were created and calculated in Microsoft Excel (Version 2011, Microsoft Corporation; The Microsoft Network, LLC, Richmond, WA, USA). Sample size was calculated a priori. Sample size and power calculations were completed a priori using nQuery Advisor. Assuming an alpha level of 0.05 and using data from Trexler et al., when the sample size in each sequence group was 8 (total sample size of 16) an 80% power would be sufficient to detect a 0.030 difference in vessel diameter assuming a standard deviation of 0.040
Results
Repeated sprint ability (RSA)
There was no significant two-way interaction (P =0.17) or main effect for treatment (P =0.37) for average power. There was a significant main effect for time (P < 0.0001; Table 1). When 95% CI were used, average power was significantly higher in sprint 5 for the PE treatment, compared to PL. When analyzing peak power, there was no two-way interaction (p=0.37) or main effect for treatment (P =0.19). There was a significant main effect for time (P <0.001; Table 1). When 95% CI were employed, sprints 5 and 7 were significantly higher with PE.
Table 1.
Average and peak power output for pomegranate extract (PE) and placebo (PL) during repeated sprint ability (RSA) test (Mean ± SD).
| Average Power Output (Watts) | Peak Power Output (Watts) | |||
|---|---|---|---|---|
|
| ||||
| PE | PL | PE | PL | |
| Sprint 1 | 488.2 ± 196.6* | 484.1 ± 215.0* | 661.0 ± 228.3* | 689.4 ± 275.9* |
| Sprint 2 | 447.6 ± 188.7* | 461.8 ± 212.2* | 688.8 ± 382.5* | 607.8 ± 286.4* |
| Sprint 3 | 401.8 ± 194.9* | 400.9 ± 210.7* | 607.1 ± 321.8* | 571.9 ± 267.6* |
| Sprint 4 | 375.1 ± 195.2* | 362.4 ± 206.8* | 518.4 ± 218.5* | 510.1 ± 265.9* |
| Sprint 5 | 366.5 ± 193.8* | 341.8 ± 183.8* | 509.3 ± 245.1* | 477.5 ± 226.4* |
| Sprint 6 | 349.5 ± 194.6 | 347.1 ± 184.1 | 490.0 ± 233.4 | 492.2 ± 232.0 |
| Sprint 7 | 342.0 ± 181.9 | 324.1 ±173.7 | 488.3 ± 225.4 | 452.9 ± 215.3 |
| Sprint 8 | 323.4 ± 181.7 | 330.0 ± 167.4 | 460.5 ± 230.8 | 465.1 ± 221.0 |
| Sprint 9 | 341.5 ± 173.9 | 329.9 ± 173.6 | 477.4 ± 215.7 | 470.3 ± 225.9 |
| Sprint 10 | 333.4 ± 181.7 | 318.7 ± 188.3 | 476.8 ± 230.4 | 472.6 ± 234.4 |
denotes significant main effect for time (P <0.05). There were no significant main effects for treatment.
For vessel diameter, there was no significant two-way interaction (P =0.78) and no significant main effect for treatment (P =0.530). There was a significant main effect for time (P =0.041) with 30minPost higher than baseline. When 95% CI were employed, diameter was significantly greater at 30minPost with the PE treatment.
For blood flow, there was no significant two-way interaction (P =0.13) and no main effect for treatment (P =0.06). There was a main effect for time (P =0.001), demonstrating greater baseline flow, compared to 30minPI; 30minPost greater than 30minPI; and IPost greater than baseline, 30minPI, and 30minPost PE. The 95% CI demonstrated a significant difference for blood flow IPost when PE was consumed (Mean difference [MD] = 18.49 mL·min−1, P =0.05).
There were no significant two-way interactions, main effects for treatment, or main effects for time for diastolic BP and SPO2. There was no significant two-way interaction or main effect for treatment for HR (P =0.81) and systolic BP (P =0.32). There was a significant main effect for time for HR (P <0.0001) and systolic BP (P =0.001; Table 2).
Table 2.
Average heart rate and blood pressure for pomegranate extract (PE) and placebo (PL) during repeated sprint ability (RSA) test and repetitions to fatigue (RTF) test (Mean ± SD).
| Heart Rate (bpm) | Blood Pressure (mmHg) | |||
|---|---|---|---|---|
|
| ||||
| PE | PL | PE | PL | |
| RSA | ||||
| Baseline | 71.8 ± 10.4 | 73.1 ± 12.4 | 116/73 ± 11.5 | 116/76 ± 12.6 |
| 30minPI | 69.3 ± 12.6* | 68.4 ± 9.0* | 117/75 ± 11.4 | 119/74 ± 7.8 |
| IPost | 152.2 ± 14.1* | 151.5 ± 17.8* | 138/73 ± 16.8* | 134/74 ± 20.5* |
| 30minPost | 90.0 ± 11.6* | 91.6 ± 14.7* | 110/72 ± 9.1* | 108/71 ± 7.6* |
| RTF | ||||
| Baseline | 70.9 ± 13.4 | 68.8 ± 11.0 | 118/74 ± 10.7 | 117/73 ± 11.7 |
| 30minPI | 66.8 ± 13.2 | 64.9 ± 8.9 | 117/70 ± 12.2 | 116/74 ± 11.1 |
| IPostBench | 134.5 ± 21.9* | 138.6 ± 18.4* | 125/67 ± 12.4* | 125/67 ± 12.3* |
| IPostLeg | 149.5 ± 17.0* | 150.7 ± 16.3* | 131/71 ± 13.6* | 135/71 ± 16.7* |
| 30minPost | 73.7 ± 11.4 | 74.2 ± 9.3 | 109/71 ± 17.6 | 114/68 ± 10.8 |
denotes significant main effect for time (P <0.05). There was no treatment effect for heart rate or blood pressure.
Repetitions to fatigue (RTF)
There was no significant difference for treatment effects for repetitions completed for bench press (P =0.25) or leg press (P =0.15) when comparing PE to PL. However, bench press (MD= 0.63 reps) and leg press (MD= 1.9 reps) repetitions were higher with the PE treatment compared to PL.
For vessel diameter, there was a significant interaction (P =0.008), main effect for time (P =0.0001), and main effect for treatment (P =0.03; Table 3). Vessel diameter was significantly greater with the PE treatment for IPostBench (MD= 0.029 cm; P =0.025), IPostLeg (MD= 0.042 cm; P =0.001), and 30minPost (MD= 0.027 cm; P =0.029). The 95% CI also demonstrated a significant difference between treatments for IpostBench, IPostLeg, and 30minPost.
Table 3.
Vessel diameter and blood flow for pomegranate extract (PE) and placebo (PL) during repetitions to fatigue (Mean ± SD).
| Vessel Diameter (cm) | Blood Flow (mL·min−1) | |||
|---|---|---|---|---|
|
| ||||
| PE | PL | PE | PL | |
| Baseline | 0.36 ± 0.06 | 0.36 ± 0.05 | 36.0 ± 23.9 | 38.0 ± 17.9 |
| 30minPI | 0.37 ± 0.05 | 0.36 ± 0.06 | 27.5 ± 11.6 | 26.3 ± 10.9 |
| IPostBench | 0.40 ± 0.06†* | 0.37 ± 0.05 | 179.9 ± 60.3* | 163.2 ± 60.2* |
| IPostLeg | 0.41 ± 0.06†* | 0.37 ± 0.06 | 94.1 ± 43.2* | 76.2 ± 35.7* |
| 30minPost | 0.39 ± 0.06† | 0.36 ± 0.06 | 41.4 ± 20.7 | 31.3 ± 14.7 |
denotes significant main effect for treatment (P <0.05).
denotes significant main effect for time (p<0.05).
There was no significant interaction (P = 0.25) or main effect for treatment (P =0.117) for blood flow. There was a significant main time effect for blood flow (P <0.001; Table 3). When evaluating 95% CI, there was a significant difference in IPostLeg and 30minPost blood flow with PE.
There was no significant interaction or main effect for treatment for HR, systolic BP, or diastolic BP. There was a significant main time effect for HR (P < 0.0001), systolic BP (P =0.001), and diastolic BP (P =0.009; Table 2). SPO2 resulted in no significant interaction, main effect for time or treatment.
There was no dietary intake difference across time (mean ± SD; calories: 2384.4 ± 616.3 kcal; carbohydrate: 263.6 ± 88.2 g; fat: 89.8 ± 21.4 g; protein: 133.0 ± 48.4 g).
Discussion
The current study demonstrated beneficial effects of 1,000 mg of PE on blood flow and vessel diameter immediately following the RTF test and 30 minutes post RSA and RTF. There may be a potential improvement in repeated sprints as a result of increased vessel diameter and blood flow, as there was an improvement in peak power in sprint 5 and 7 and average power output in sprint 5, suggesting an ergogenic effect from PE supplementation. As a result of only a few sprints of the 10 increasing, research should further evaluate the ergogenic effects of PE in repeated exercise tests.
Previous research has shown nitrate supplementation may increase blood flow and vessel diameter (Ferguson et al., 2013; Trexler et al., 2014). However, compared to the nitrate concentration of PE, the rich content of polyphenols in PE may be more of a cause for the beneficial effects due to polyphenols capability of increasing bioavailability of NO (Ignarro et al., 2006). In a similar study done previously in our lab, Trexler et al. (Trexler et al., 2014) demonstrated an increase in vessel diameter 30minPost (Δ=0.021 ± 0.04 cm) and an increase in blood flow 30minPI (Δ=11.0 ± 20.8 mL·min−1) of PE compared to a placebo. The current study results were similar for vessel diameter 30minPost (Δ=0.027 ± 0.04 cm) and blood flow 30minPI (Δ=4.03 ± 18.9 mL·min−1), supporting previous research with enhanced blood flow IPost leg press in the RTF and immediately post RSA. Additionally, in the current study, vasodilation was enhanced 30minPost in the RSA, IPost bench and leg press, and 30minPost in the RTF as a result of PE supplementation.
As a result of high concentration of polyphenols and nitrates, pomegranate may further increase the delivery of blood, oxygen, and energy substrates to skeletal muscle. Specific to pomegranate, the primary active polyphenols have been reported to be: flavonols, ellagitannins, and anthocyanins (Chong et al., 2010; Mattiello et al., 2009). Previous polyphenol research resulted in increased FMD and reduced BP (Barona et al., 2012). In elite athletes, FMD increased after consuming a polyphenol-rich drink from cranberries and grape seeds, with FMD peaking at 60 minutes and returning to baseline 3 hours after ingestion potentially due to increased NO concentrations (Labonté et al., 2013). In the same study, there were no significant differences between placebo and the polyphenol-rich drink in time to completion, average wattage, or perceived exertion after a time-trial. Yet, HR was significantly lower 2 and 5 minutes post exercise. In contrast, the current study demonstrated no difference between PE and PL for HR, BP, or SPO2 in recreationally trained individuals. Pomegranate juice has resulted in lower BP and improved endothelial function in clinical populations when consumed chronically (Asgary et al., 2014). The current study was completed acutely in a young, healthy population and may explain the lack of change in HR or BP. As a result of previous research and lack of findings in this study, cardiovascular benefits of pomegranate and polyphenols may require chronic consumption and/or clinical populations with cardiovascular impairments. Future research may evaluate chronic consumption and acute consumption of PE in individuals with compromised cardiovascular health such as hypertension or endothelial dysfunction.
The culmination of previous research on nitrate supplementation on exercise performance has primarily examined red beetroot, while few studies have examined pomegranate juice or PE. Beetroot has been shown to increase NO through the same enterosalivary nitrate-nitrite-NO pathway (Bailey et al., 2010; Bescós, Sureda, et al., 2012). While there is thought that beetroot may potentially improve performance through the same mechanism (Jones, 2014), additional ergogenic benefit from PE may result from the high polyphenol content that differs in type and concentrations from red beetroot (Chong et al., 2010; Mattiello et al., 2009). Trombold et al. (Trombold, Reinfeld, Casler, & Coyle, 2011) demonstrated that chronic consumption of pomegranate juice attenuated muscular weakness, reduced soreness, and improved recovery, potentially as result of the high antioxidant content. PE increased running time to exhaustion and delayed fatigue at exercise intensities of 90% and 100% of peak velocity (Trexler et al., 2014). Results from Bailey et al. (Bailey et al., 2009), when using beetroot, also demonstrated an increased time to exhaustion and reduced oxygen cost at a fixed submaximal work rate. The current study evaluated high-intensity tests, demonstrating an improvement in average and peak power output halfway through the RSA as a result of one dose of PE supplementation. When using beetroot juice for seven days, Kelly et al. (Kelly et al., 2013) demonstrated improved exercise tolerance at 60%, 70%, and 80% of peak power, however, maximal peak power (100%) was not improved, which may be due to the polyphenol content difference in beetroot and pomegranate or supplementation timing. Collectively, acute (≤6 days) beetroot juice intake has failed to elicit improvements in maximal oxygen uptake (Bailey et al., 2009, 2010; Kelly et al., 2013; Wylie et al., 2013). In contrast, chronic supplementation of beetroot (three weeks) and acute supplementation of PE (one dose) may be advantageous for submaximal aerobic exercise. The current study is the first to demonstrate a potential benefit of PE on power output in a repeated sprint bout, however because only few sprints increased, more data is needed to interpret the full effects of PE.
Despite the positive findings in the present study, limitations do exist. The results of this study are limited to acute consumption, thus future research may focus on varying PE dosing and length of consumption for ergogenic effects. Research with varying modes and duration of exercise may better determine the ergogenic utility of PE. Additionally, the current study administered the extract in a capsule form which may delay the salivary component of the enterosalivary nitrate-nitrite-NO pathway; therefore a sublingual administration may provide a more immediate effect. Oxygen saturation was measured at the fingertip for ease of access with no significant differences observed; whereas measuring SPO2 at the working skeletal muscle may be more advantageous. Moreover, the participants in this study were young, recreationally active, resistance-trained individuals with no adverse health conditions. Additional research on untrained individuals, elite athletes, or individuals with health conditions or diseases would help to determine the generalizability effects of PE.
In conclusion, acute supplementation of PE resulted in enhanced vessel diameter, blood flow, and peak and average power output. These improvements are likely due to the nitrate and polyphenol content in PE, which have likely resulted in increased NO to enhance endothelial function and enhance exercise performance. While the majority of previous research on beetroot and pomegranate juice had participants ingest supplements chronically, the present study demonstrated PE to be effective when ingested 30 minutes before exercise. The acute timing and capsule form of PE may be advantageous to the athletic population due to ergogenic effects, taste, compliance, convenience, and PE being a natural compound. Power output and repetitions were increased after PE ingestion suggesting a potential for increasing training volume. Additionally, combining PE with other ergogenic aids may be advantageous as a pre-workout supplement to further augment performance.
Acknowledgments
The project described was by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 1KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The pomegranate extract was donated by Stiebs Nature Elevated, Madera, CA. The treatment and placebo products were encapsulated and blinded by Dymatize Nutrition, Dallas, TX.
Footnotes
There were no conflicts of interest for any of the researchers or the company with the pomegranate extract donation.
References
- Adams KJ, Barnard KL, Swank AM, Mann E, Kushnick MR, Denny DM. Combined High-Intensity Strength and Aerobic Training in Diverse Phase II Cardiac Rehabilitation Patients. Journal of Cardiopulmonary Rehabilitation. 1999;19(4):209–215. doi: 10.1097/00008483-199907000-00001. [DOI] [PubMed] [Google Scholar]
- Asgary S, Sahebkar A, Afshani MR, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytotherapy Research: PTR. 2014;28(2):193–9. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, Dimenna FJ, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. 2010:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Bailey SJ, Vanhatalo A, Winyard PG, Jones AM. The nitrate-nitrite-nitric oxide pathway: Its role in human exercise physiology. European Journal of Sport Science. 2012;12(4):309–320. [Google Scholar]
- Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Dietary JAM. Dietary nitrate supplementation reduces the O 2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of Applied Physiology. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- Barona J, Aristizabal JC, Blesso CN, Volek JS, Fernandez ML. Grape Polyphenols Reduce Blood Pressure and Increase Flow-Mediated Vasodilation in Men with Metabolic Syndrome 1. Journal of Nutrition. 2012;142:1626–1632. doi: 10.3945/jn.112.162743.antioxidant. [DOI] [PubMed] [Google Scholar]
- Bescós R, Ferrer-Roca V, Galilea Pa, Roig A, Drobnic F, Sureda A, Pons A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Medicine and Science in Sports and Exercise. 2012;44(12):2400–9. doi: 10.1249/MSS.0b013e3182687e5c. [DOI] [PubMed] [Google Scholar]
- Bescós R, Rodríguez Fa, Iglesias X, Ferrer MD, Iborra E, Pons A. Acute administration of inorganic nitrate reduces VO(2peak) in endurance athletes. Medicine and Science in Sports and Exercise. 2011;43(10):1979–86. doi: 10.1249/MSS.0b013e318217d439. [DOI] [PubMed] [Google Scholar]
- Bescós R, Sureda A, Tur Ja, Pons A. The effect of nitric-oxide-related supplements on human performance. Sports Medicine (Auckland, N.Z.) 2012;42(2):99–117. doi: 10.2165/11596860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Chong MF-F, Macdonald R, Lovegrove Ja. Fruit polyphenols and CVD risk: a review of human intervention studies. The British Journal of Nutrition. 2010;104(Suppl):S28–39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- de Nigris F, Williams-Ignarro S, Botti C, Sica V, Ignarro LJ, Napoli C. Pomegranate juice reduces oxidized low-density lipoprotein downregulation of endothelial nitric oxide synthase in human coronary endothelial cells. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society. 2006;15(3):259–63. doi: 10.1016/j.niox.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Smith M, Cook K, James RS. The acute effect of a caffeine-containing energy drink on mood state, readiness to invest effort, and resistance exercise to failure. Journal of Strength and Conditioning Research / National Strength & Conditioning Association. 2012;26(10):2858–2865. doi: 10.1519/JSC.0b013e318241e124. [DOI] [PubMed] [Google Scholar]
- Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Poole DC. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. The Journal of Physiology. 2013;591(Pt 2):547–57. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gago B, Lundberg JO, Barbosa RM, Laranjinha J. Red wine-dependent reduction of nitrite to nitric oxide in the stomach. Free Radical Biology and Medicine. 2007;43(9):1233–1242. doi: 10.1016/j.freeradbiomed.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Gaitanos GC, Williams C, Boobis LH, Brooks S. Human muscle metabolism during intermittent maximal exercise. Journal of Applied Physiology. 1993 doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- Girard O, Mendez-Villanueva A, Bishop D. Repeated-sprint ability - part I: factors contributing to fatigue. Sports Medicine (Auckland, N.Z.) 2011;41(8):673–94. doi: 10.2165/11590550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Nyberg M, Jensen LG, Mortensen SP. Vasodilator interactions in skeletal muscle blood flow regulation. The Journal of Physiology. 2012;590(Pt 24):6297–305. doi: 10.1113/jphysiol.2012.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. 2009;(6):1–10. doi: 10.3945/ajcn.2008.27131.INTRODUCTION. [DOI] [PubMed] [Google Scholar]
- Ignarro LJ, Byrns RE, Sumi D, de Nigris F, Napoli C. Pomegranate juice protects nitric oxide against oxidative destruction and enhances the biological actions of nitric oxide. Nitric Oxide: Biology and Chemistry / Official Journal of the Nitric Oxide Society. 2006;15(2):93–102. doi: 10.1016/j.niox.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Jones AM. Dietary nitrate supplementation and exercise performance. Sports Medicine (Auckland, N.Z.) 2014;44(Suppl 1):S35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J, Vanhatalo A, Wilkerson DP, Wylie LJ, Jones AM. Effects of nitrate on the power-duration relationship for severe-intensity exercise. Medicine and Science in Sports and Exercise. 2013;45(9):1798–806. doi: 10.1249/MSS.0b013e31828e885c. [DOI] [PubMed] [Google Scholar]
- Labonté K, Couillard C, Motard-Bélanger A, Paradis ME, Couture P, Lamarche B. Acute Effects of Polyphenols from Cranberries and Grape Seeds on Endothelial Function and Performance in Elite Athletes. Sports. 2013;1(3):55–68. doi: 10.3390/sports1030055. [DOI] [Google Scholar]
- Larsen FJ, Schiffer Ta, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13(2):149–59. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Mathew AS, Capel-Williams GM, Berry SEE, Hall WL. Acute effects of pomegranate extract on postprandial lipaemia, vascular function and blood pressure. Plant Foods for Human Nutrition (Dordrecht, Netherlands) 2012;67(4):351–7. doi: 10.1007/s11130-012-0318-9. [DOI] [PubMed] [Google Scholar]
- Mattiello T, Trifirò E, Jotti GS, Pulcinelli FM. Effects of pomegranate juice and extract polyphenols on platelet function. Journal of Medicinal Food. 2009;12(2):334–9. doi: 10.1089/jmf.2007.0640. [DOI] [PubMed] [Google Scholar]
- Van Mierlo LAJ, Zock PL, Van Der Knaap HCM, Draijer R. Grape Polyphenols Do Not Affect Vascular Function in Healthy Men. The Journal of Nutrition. 2010;140:1769–1773. doi: 10.3945/jn.110.125518.and. [DOI] [PubMed] [Google Scholar]
- Rocha BS, Gago B, Barbosa RM, Laranjinha J. Dietary polyphenols generate nitric oxide from nitrite in the stomach and induce smooth muscle relaxation. Toxicology. 2009;265(1–2):41–48. doi: 10.1016/j.tox.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Santamaria P. Nitrate in vegetables: toxicity, content, intake and EC regulation. Journal of the Science of Food and Agriculture. 2006;86(1):10–17. doi: 10.1002/jsfa.2351. [DOI] [Google Scholar]
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. The American Journal of Clinical Nutrition. 2005;81(1 Suppl):215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Current Atherosclerosis Reports. 2013;15(5):324. doi: 10.1007/s11883-013-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler ET, Smith-ryan AE, Melvin MN, Roelofs EJ, Wingfield HL. Effects of pomegranate extract on blood flow and running. Applied Physiology Nutrition Metabolism. 2014;39:1038–1042. doi: 10.1139/apnm-2014-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trombold JR, Reinfeld AS, Casler JR, Coyle EF. The Effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. The Journal of Strength and Conditioning Research. 2011;25(7):1782–1788. doi: 10.1519/JSC.0b013e318220d992. [DOI] [PubMed] [Google Scholar]
- Uehata a, Lieberman EH, Gerhard MD, Anderson TJ, Ganz P, Polak JF, Yeung aC. Noninvasive assessment of endothelium-dependent flow-mediated dilation of the brachial artery. Vascular Medicine (London, England) 1997 Jan; doi: 10.1177/1358863X9700200203. [DOI] [PubMed] [Google Scholar]
- Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. Journal of Applied Physiology. 2013;115:325–36. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]


