Abstract
The ability of Candida albicans to rapidly and reversibly switch between yeast and filamentous morphologies is crucial to pathogenicity, and it is thought that the filamentous morphology provides some advantage during interaction with the mammalian immune system. Dectin-1 is a receptor that binds β-glucans and is important for macrophage phagocytosis of fungi. The receptor also collaborates with Toll-like receptors for inflammatory activation of phagocytes by fungi. We show that yeast cell wall β-glucan is largely shielded from Dectin-1 by outer wall components. However, the normal mechanisms of yeast budding and cell separation create permanent scars which expose sufficient β-glucan to trigger antimicrobial responses through Dectin-1, including phagocytosis and activation of reactive oxygen production. During filamentous growth, no cell separation or subsequent β-glucan exposure occurs, and the pathogen fails to activate Dectin-1. The data demonstrate a mechanism by which C. albicans shape alone directly contributes to the method by which phagocytes recognize the fungus.
Keywords: C-type lectin, macrophage, phagocytosis, reactive oxygen
Introduction
The ability of Candida albicans to rapidly and reversibly switch between yeast and filamentous morphologies is crucial to pathogenicity, and it is thought that the filamentous morphology provides some advantage during interaction with the mammalian immune system (Lo et al, 1997; Calderone and Fonzi, 2001; Saville et al, 2003). Normal yeast growth is initiated by the formation of a daughter bud on the parent cell. The bud enlarges and ultimately separates from the parent. Environmental cues such as serum induce some yeast to grow in the filamentous form. In this form, cells elongate and daughter cells do not separate from parents (Madhani and Fink, 1998; Berman and Sudbery, 2002). During C. albicans infection, both yeast and filamentous forms can be observed. The requirement for filamentation in infection was demonstrated by Fink and coworkers using C. albicans deficient in two transcription factors required for filamentous growth, Cph1p and Efg1p (Lo et al, 1997). These mutant strains were avirulent compared with wild-type fungi in a murine model of candidiasis. However, Candida lacking the Spt3p transcription factor grow exclusively in the filamentous form, and are also avirulent, suggesting that dimorphism itself is required for pathogenicity (Laprade et al, 2002). A more recent study by Saville et al used C. albicans engineered for tetracycline inducibility of the genetic program for filamentation. They found that the yeast form is required for systemic dispersal, but that the filamentous form is required for pathogenicity (Saville et al, 2003). In addition to the change in cell shape during filamentous growth, expression of a wide range of surface proteins and virulence factors is altered (Gale et al, 1998; Lane et al, 2001), making it difficult to examine the specific role of cell shape in innate immune recognition of C. albicans. In this report, we have explored the effects of dimorphic growth on macrophage recognition of fungi via the phagocytic receptor, Dectin-1.
Dectin-1 is a type II transmembrane receptor containing a single extracellular C-type lectin domain (Ariizumi et al, 2000). Brown and Gordon (2001) demonstrated that the receptor triggers phagocytosis of β-glucan-containing particles when ectopically expressed in normally nonphagocytic cells. β-Glucan is a major structural component of fungal cell walls (Smits et al, 1999). Dectin-1 is expressed widely on phagocytes including macrophages and dendritic cells and contributes to the immunological response to β-glucans (Brown and Gordon, 2003). We have recently demonstrated that, in macrophages and dendritic cells, Dectin-1 and Toll-like receptor 2 collaborate in coordinating inflammatory responses such as cytokine secretion and the production of reactive oxygen species (ROS) in response to β-glucan-containing particles (Gantner et al, 2003).
In this study, we have found that Dectin-1 recognizes C. albicans yeast, but not filaments. Yeast β-glucan is largely inaccessible to Dectin-1 recognition, but deformities produced in the cell wall during budding growth expose patches of β-glucan that the receptor binds. These patches, identified as bud and birth scars, are sufficient to permit activation of Dectin-1, and trigger potent antifungal inflammatory responses in macrophages. C. albicans filaments do not expose β-glucan in bud scars since mother–daughter cell separation does not occur, and thus filamentous Candida fails to activate Dectin-1-mediated defenses.
Results
Dectin-1 binds to C. albicans yeast but not filaments
In order to characterize the ability of Dectin-1 to bind to yeast, we produced a soluble form of the receptor (sDectin) in transiently transfected HEK293 cells and used it to stain zymosan particles. Zymosan particles were bound strongly by the soluble receptor, as assessed by flow cytometry (Figure 1A). Confocal microscopy of zymosan stained with sDectin revealed strong, even staining over the entire surface of the particle (Figure 1A). As predicted by the work of Brown and Gordon (2001), binding of sDectin was completely inhibited by soluble β-glucan (laminarin), but not by mannan or chitin, other components of the yeast cell wall (Figure 1B). Thus, the ligand specificity of sDectin is consistent with published data.
Figure 1.
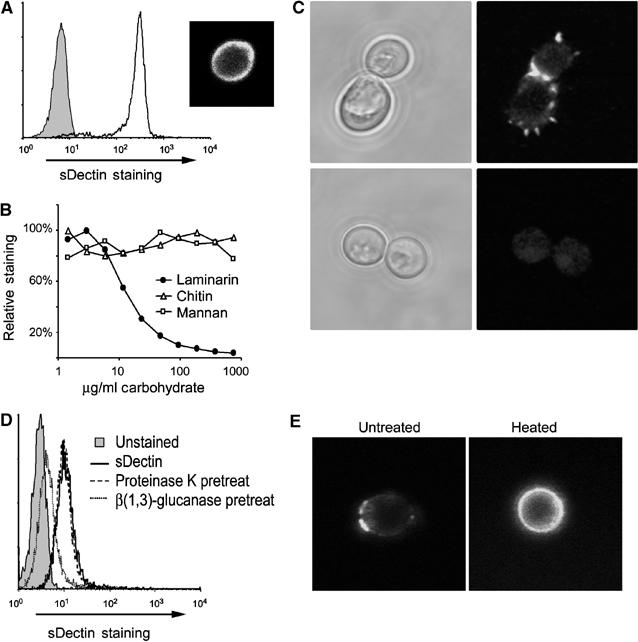
sDectin stains C. albicans yeast. (A) Zymosan particles were labeled with sDectin, and binding was assessed by flow cytometry. sDectin strongly stained zymosan particles (unfilled line and inset confocal image) compared to control-stained particles (filled). (B) Binding of sDectin to zymosan is β-glucan dependant. sDectin binding in the presence of the indicated doses of yeast cell wall components was assessed by flow cytometry and expressed as % mean fluorescence of maximal binding. (C) Binding of sDectin to C. albicans was assessed by confocal microscopy. C. albicans yeast was stained with sDectin, either in the presence (bottom) or absence (top) of laminarin (soluble β-glucan). (D) Binding of sDectin to C. albicans yeast was assessed by flow cytometry. As indicated, yeast were pretreated with proteinase K, or β(1,3)-glucanase. (E) Live (left panel) or heat-killed (right panel) C. albicans yeast were stained with sDectin and imaged by confocal microscopy.
Dectin-1 has been reported to recognize C. albicans (Brown and Gordon, 2001), but, unlike zymosan, we found that sDectin does not bind uniformly to the surface of C. albicans (Figure 1C). Binding was concentrated in discrete subdomains on the yeast surface, and was particularly strong in the region between the parent cell and the mature bud (Figure 1C). As with zymosan, binding to C. albicans was completely inhibited by soluble β-glucan. Additionally, while treatment of yeast with proteinase K did not suppress sDectin binding to C. albicans, treatment with highly pure β(1,3)-glucanase abolished binding (Figure 1D). The punctate recognition patter of sDectin on the C. albicans cell surface was not altered by deglycosylating the sDectin, or when using bacterially expressed sDectin (data not shown). Previous reports have suggested that heat-killed C. albicans reveal more β-glucan at the surface than live yeast (Fradin et al, 1996; Suzuki et al, 1998). We observed that, although sDectin binds live yeast in discrete patches, sDectin recognizes the entire cell wall of heat-killed C. albicans (Figure 1E). Taken together, the data demonstrate that Dectin-1 binds specifically to β-glucan, revealed in discrete regions of the cell wall of live C. albicans.
The restricted distribution of Dectin-1-binding sites was not specific to the strain of C. albicans we examined, a clinical isolate (ATCC 90028). Binding of sDectin to another strain of C. albicans (CAI4) and to other Candida species including C. krusei, C. pseudotropicalis, and C. parapsilosis was also restricted to punctuate patches (Supplementary Figure 1). Since we observed this restricted pattern of sDectin staining on a variety of Candida strains, we explored whether the pattern was specific to pathogenic yeast. We observed a similar pattern of punctuate staining on Saccharomyces cerevisiae (Figure 2A). In addition, the magnitude of sDectin binding was quantified by flow cytometry and found to be equivalent between the two species (Figure 2B). In S. cerevisiae (Figure 2C) and in C. albicans (described below; Figure 3F), sDectin binding colocalized with calcofluor white, a stain commonly used to identify bud scars and birth scars. These are permanent deformities in the cell walls of budding yeast that are formed when mother and daughter cells separate. Bud scars and birth scars are known to be rich in chitin, the carbohydrate recognized by calcofluor white. However, while chitin is synthesized and heavily deposited at bud sites, there are no data to suggest that bud sites contain more β-glucan than the surrounding cell wall. Rather, our data suggest that bud scars and birth scars expose β-glucan from the underlying cell wall in a manner that is recognized by Dectin-1.
Figure 2.
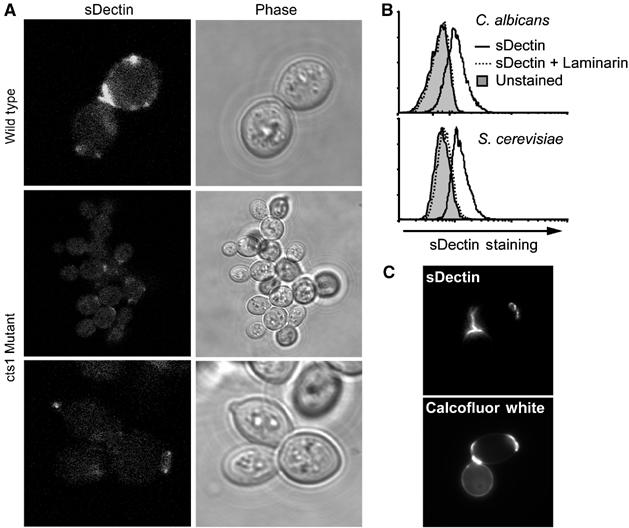
sDectin binds bud scars and birth scars. (A) sDectin binding to S. cerevisiae depends on proper bud separation. Wild-type (top) or endochitinase (cts1)-deficient (middle and lower panels) S. cerevisiae yeast were stained with sDectin and imaged by confocal microscopy. (B) Dectin-1 binds S. cerevisiae and C. albicans equivalently. C. albicans and S. cerevisiae yeast stained with sDectin (solid lines) and analyzed by flow cytometry demonstrate equivalent laminarin-sensitive (dashed line) binding compared to controls (filled). (C) S. cerevisiae bud scars and birth scars were stained with calcofluor white, and staining colocalized with sDectin as assessed by fluorescence microscopy.
Figure 3.
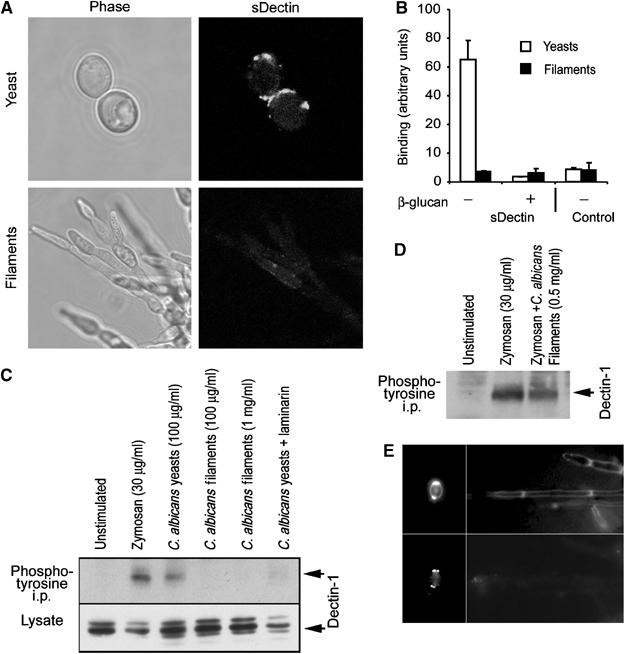
Dectin-1 recognizes C. albicans yeast, but does not bind filaments. (A) Candida yeast (top) or filaments (bottom) stained with sDectin were imaged by confocal microscopy. (B) Binding of sDectin to yeast and filaments was quantified using an enzyme-linked assay. Strong laminarin (β-glucan)-sensitive binding to yeast was detected, but binding to filaments could not be detected over the background (control). (C) Yeast, but not filaments, activate Dectin-1. RAW264.7 cells stably expressing epitope-tagged Dectin-1 were incubated with the indicated stimuli for 15 min. Lysates were prepared, and Dectin-1 expression was confirmed by immunoblotting for the tag. Tyrosine-phosphorylated protein was immunoprecipitated with antiphosphotyrosine antibodies and Dectin-1 was detected by immunoblotting. (D) Candida filaments do not block Dectin-1 activation by zymosan. Cells were stimulated with zymosan in the presence or absence of filaments, as indicated, and Dectin-1 activation was assessed as above. (E) C. albicans filaments formed in vivo do not bind sDectin. C. albicans yeast (left panels) were injected intraperitoneally and filaments were recovered 24 h later (right panels), and stained with calcofluor white (top panels) and sDectin (bottom panels).
To examine the dynamics of sDectin binding to C. albicans, we imaged yeast cell division in real time in the presence of fluorescently labeled sDectin (Supplementary Figure 2). Prior to separation of mother and daughter cells, sDectin does not bind to the interface between the cells. As the cells separate from each other, sDectin is rapidly bound to the newly exposed surfaces. To further explore the requirement of cell separation in the exposure of Dectin-1 binding sites, we examined S. cerevisiae deficient in endochitinase (cts1), an enzyme that is required for normal separation of budding cells (Kuranda and Robbins, 1991). These mutants grow in grape-like bundles because they fail to efficiently process cell wall chitin at the bud site, and hence fail to properly separate. Compared to wild-type yeast, sDectin binding is largely inhibited (Figure 2A). Taken together, these data demonstrate that most β-glucan in the yeast cell wall is inaccessible to the mammalian β-glucan receptor, Dectin-1, and that β-glucan is exposed only in surface deformities that occur during budding growth and separation.
During filamentous growth, there is no daughter cell separation, and we hypothesized that Dectin-1 recognition might be impaired. C. albicans was grown under conditions that promote filamentous growth, and sDectin failed to bind (Figure 3A) over laminarin-blocked background (data not shown). We observed that our preparations of filamentous fungi contain both true hyphae and pseudohyphae and no difference was detected in sDectin recognition (data not shown). In order to quantify the difference between recognition of yeast and filaments, we employed an enzyme-linked assay. sDectin binding to yeast was readily detected, and was inhibited in the presence of soluble β-glucan. However, binding of sDectin to an equivalent mass of filaments was not detected over the background (Figure 3B). While Dectin-1 readily binds to the yeast form of C. albicans, these data demonstrate that the receptor cannot bind the filamentous form of the pathogen.
Yeast activate Dectin-1 functions, but filaments do not
While we have shown that Dectin-1 physically binds to yeast bud and birth scars, these patches make up only a small fraction of the total yeast cell surface. It is not clear whether enough β-glucan is exposed in these lesions to activate Dectin-1. We therefore explored the biochemical and cell biological consequences of Dectin-1 recognition of C. albicans. As we have previously demonstrated, zymosan-activated Dectin-1 can be immunoprecipitated with antiphosphotyrosine antibodies (Figure 3C) (Gantner et al, 2003). Since Dectin-1 recognizes the entire surface of zymosan and strongly activates the receptor, we explored whether the more limited recognition of small patches of cell wall on C. albicans yeast similarly triggers activation of the receptor. Live C. albicans strongly activated Dectin-1, and this activation was completely inhibited by blocking with laminarin (Figure 3C). Thus, yeast expose enough β-glucan in bud scars to biochemically activate the receptor. However, treatment with an equivalent mass of filaments was not sufficient to activate Dectin-1, nor was a 10-fold higher dose (Figure 3C). Two explanations are possible: first, β-glucan may not be accessible for recognition by Dectin-1; second, filaments could actively inhibit signaling through Dectin-1. We therefore triggered activation of Dectin-1 with zymosan and explored whether filaments could suppress this activation. Filaments had no effect on zymosan-induced activation of the receptor, supporting the conclusion that β-glucan is not functionally accessible on filaments (Figure 3D). It was possible that sequestration of β-glucan in the cell wall was a feature of in vitro growth of filaments, and that in vivo β-glucan might be readily accessible to Dectin-1. We therefore intraperitoneally infected mice with pure C. albicans yeast and harvested filaments from kidney tissue 24 h later. The original yeast stained strongly with sDectin, and this staining colocalized with calcofluor white (Figure 3E). However, the filaments that formed in vivo were detected by calcofluor white, but were not recognized by sDectin (Figure 3E). Thus, filamentous growth in host tissues can result in a cell wall that is not recognized by Dectin-1.
Dectin-1 expression is sufficient to mediate phagocytosis of β-glucan-containing particles by nonphagocytic cells (Brown and Gordon, 2001). We therefore examined whether more limited exposure of β-glucan on the surface of live C. albicans yeast was sufficient to trigger phagocytosis through Dectin-1, in addition to biochemically activating the receptor. C. albicans yeast were stained with the dye FUN-1 which permits discrimination of live and dead yeast, and fed to HEK293 cells stably expressing Dectin-1. Although untransfected HEK293 cells do not bind or internalize C. albicans (data not shown), Dectin-1-expressing cells readily internalized the live yeast as measured by flow cytometry, and this internalization was completely inhibited by soluble β-glucan (Figure 4A). Thus, live yeast activate sufficient Dectin-1 in this reconstitution system to trigger phagocytosis.
Figure 4.
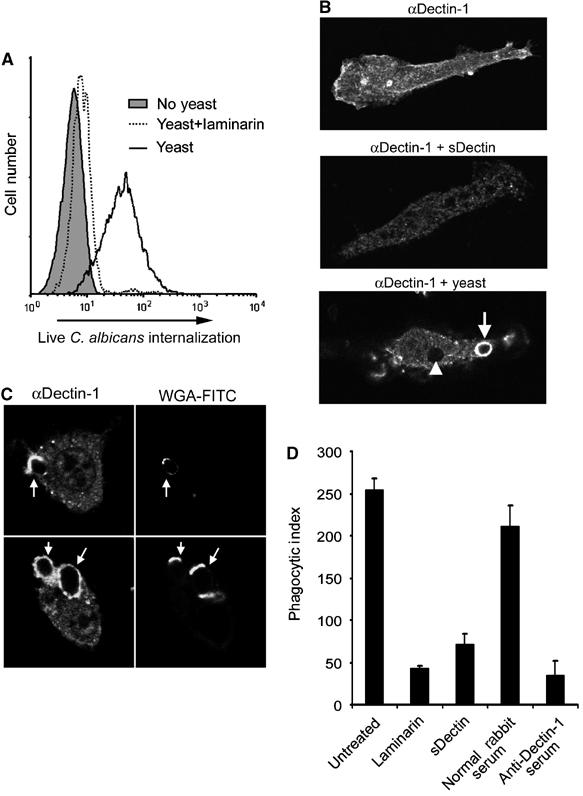
Dectin-1 mediates phagocytosis of C. albicans yeast. (A) Yeast trigger phagocytosis through Dectin-1. HEK293 cells stably expressing Dectin-1 were fed FUN-1-labeled Candida yeast. Internalization of live yeast was detected by flow cytometry. Internalization was completely inhibited by soluble β-glucan (laminarin), as indicated. (B) Dectin-1 is recruited to yeast phagosomes. Bone marrow-derived macrophages were stained with anti-Dectin-1 antibody, showing plasma membrane staining (top), which was blocked by recombinant Dectin-1 (middle). Dectin-1 was enriched on early phagosomes in macrophages fed Candida yeast (bottom, arrow), and staining was diminished on later phagosomes (arrowhead). (C) C. albicans yeast were preincubated with FITC-labeled wheat-germ agglutinin (WGA-FITC) to identify bud and birth scars, and fed to bone marrow macrophages for 10 min. Dectin-1 recruitment to phagosomes was assessed by immunofluorescence microscopy with anti-Dectin-1 antibody. (D) Bone marrow macrophages were pretreated as indicated, and fed Candida yeast for 30 min. Internalization was quantified by microscopy and is expressed as phagocytic index (particles internalized per 100 macrophages).
We have previously shown that epitope-tagged Dectin-1 is recruited to macrophage phagosomes containing zymosan, but not to phagosomes containing IgG-opsonized red blood cells, suggesting that recruitment to phagosomes is a measure of receptor activation (Gantner et al, 2003). We therefore examined whether endogenous Dectin-1 is recruited to phagosomes containing C. albicans yeast. We generated a polyclonal rabbit antibody to mouse Dectin-1, and, by immunofluorescence microscopy, Dectin-1 expression was detected at the plasma membrane of bone marrow macrophages (Figure 4B). The specificity of the staining was confirmed since it was completely blocked by recombinant sDectin (Figure 4B). Dectin-1 was strongly recruited to early actin-coated phagosomes containing zymosan or C. albicans yeast (Figure 4B, arrow; data not shown). Dectin-1 recruitment was transient since the receptor was removed from later phagosomes (Figure 4B, arrowhead). Dectin-1 was typically strongly recruited to the entire surface of the early C. albicans phagosome, even though data suggest that Dectin-1 can bind only to bud and birth scars on the yeast. To examine this further, we labeled C. albicans yeast bud and birth scars with fluorescent wheat-germ agglutinin before feeding them to macrophages (Powell et al, 2003). While Dectin-1 often bound to bud and birth scars, as phagosomes formed Dectin-1 spread around the organism (Figure 4C).
Yeast, but not filaments, activate macrophages through Dectin-1
Since Dectin-1 is recruited to macrophage phagosomes containing C. albicans yeast, we directly examined the contribution of Dectin-1 activation to internalization. We pretreated macrophages with a panel of Dectin-1-blocking agents and measured the phagocytosis of C. albicans yeast. While control macrophages ingested more than 250 yeasts/100 cells, laminarin pretreatment reduced this to less than 50 (Figure 4D), demonstrating a critical role for β-glucan recognition. To confirm that Dectin-1 is responsible for this recognition, we blocked the receptor using two specific strategies. First, while pretreatment of macrophages with preimmune rabbit serum did not inhibit yeast internalization, pretreatment with anti-Dectin-1 antibody strongly inhibited internalization to a degree similar to laminarin (Figure 4D). Second, we blocked Dectin-1-binding sites by precoating yeast with sDectin, a treatment that similarly reduced their internalization by macrophages (Figure 4D). These experiments were also performed using fluorescently labeled yeast and flow cytometry to assess internalization efficiency with identical results (data not shown).
In contrast to phagocytosis, we could measure little or no effect of blocking Dectin-1 on binding of live C. albicans yeast to macrophages at 4°C (data not shown). This observation is consistent with previous reports using mouse and human macrophages (Fradin et al, 1996; Suzuki et al, 1998) and suggests that although Dectin-1 activation is very important for triggering phagocytosis of yeast, additional receptors contribute substantially to initial binding. As yeast mature and produce more progeny, the number of bud scars increases, suggesting the possibility that older yeast might preferentially activate Dectin-1. However, we observed that the birth scar alone was sufficient to trigger Dectin-1-mediated phagocytosis and could not detect significantly more efficient internalization of older yeast (Figure 4C; data not shown).
We have previously demonstrated that Dectin-1 activation is necessary for production of ROS by macrophages stimulated with zymosan (Gantner et al, 2003). Since C. albicans stimulates ROS production by macrophages, and, since this response contributes to immune defense to C. albicans infection (Aratani et al, 2002a, 2002b), we examined the contribution of Dectin-1 to macrophage ROS production in response to yeast and filaments. Yeast induce a maximal ROS response in macrophages at doses as low as 50 μg/ml (Figure 5A), and a detectable response from as little as 5 μg/ml (not shown). However, mature filaments fail to trigger ROS production even at a dose of 200 μg/ml (Figure 5A). Many studies on phagocyte recognition of C. albicans use ‘filaments' formed by short-term germ tube formation in serum. These ‘filaments' are typically mother yeast cells extending short daughter cell filaments. In this form, we observe that sDectin recognizes the yeast mother portion of the ‘filament', but not the new filamentous growth, and that such preparations trigger intermediate amounts of ROS from macrophages (data not shown). As many pathogenic organisms produce virulence factors that inhibit ROS production by macrophages, we examined whether filaments could actively inhibit NADPH oxidase activity induced by Dectin-1. When macrophages were treated with filaments at a dose of 200 μg/ml and zymosan at the same time, we observed no inhibition of the production of ROS triggered by zymosan (Figure 5B).
Figure 5.
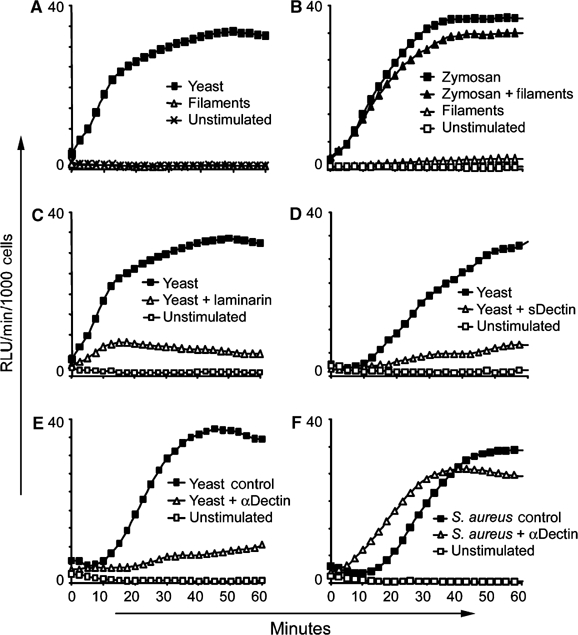
Candida yeast activate production of ROS through Dectin-1, but filaments do not. (A–F) Production of ROS by bone marrow-derived macrophages was measured by luminol-based chemiluminescence. (A) Candida yeast (50 μg/ml) activate production of ROS, while Candida filaments (200 μg/ml) fail to activate. (B) Filaments do not actively inhibit ROS production, since zymosan (30 μg/ml)-induced ROS production was unaffected by coincubation with filaments (200 μg/ml). (C) β-Glucan recognition is critical for ROS production induced by Candida yeast (100 μg/ml) since laminarin (1 mg/ml) blocked the response. (D) Stimulation of ROS production by sDectin-coated yeast (100 μg/ml) was blocked compared to uncoated yeast (100 μg/ml). (E) Preincubation of macrophages with anti-Dectin-1 antibody blocked ROS production induced by yeast (100 μg/ml). Control cells were treated with preimmune rabbit serum. (F) Pretreatment with anti-Dectin-1 serum had no effect on the induction of ROS by S. aureus (250 μg/ml), a stimulus that does not contain β-glucan.
We then confirmed the specificity of Dectin-1 in C. albicans yeast-induced ROS by blocking receptor function. First, preincubation of macrophages with soluble β-glucan ablated the response (Figure 5C). Second, preincubation of yeast with sDectin to obscure Dectin-1-binding sites ablated the ROS response (Figure 5D). Third, we determined that macrophages preincubated with anti-Dectin-1 antiserum failed to produce ROS in response to yeast, while macrophages treated with preimmune serum (or antiserum depleted of Dectin-1 antibodies, not shown) responded normally (Figure 5E). In contrast, the blocking antibody had no effect on ROS production in response to Staphylococcus aureus, which does not contain β-glucan (Figure 5F). Thus, although filaments likely interact with macrophage via other receptors, their failure to activate Dectin-1 is responsible for the lack of ROS production.
Discussion
The ability of C. albicans to reversibly switch between yeast and filamentous morphologies is crucial to pathogenicity. Yeast and filaments interact differently with the immune system, and there has been much work carried out elucidating virulence factors that are induced during filamentous growth. However, it has been difficult to establish whether the morphological differences between yeast and filaments alone lead to differences in the interaction with host immune cells. Here we have directly demonstrated that the basic process of yeast budding growth exposes patches of cell wall that are specifically recognized by the innate immune recognition receptor, Dectin-1. During filamentous growth these patches are not exposed, Dectin-1 cannot recognize the cells, and as a result macrophages respond differently to filaments and yeast.
We have previously demonstrated that Dectin-1 activation enhances inflammatory cytokine and chemokine production in addition to activating antimicrobial killing mechanisms. Dectin-1 activation strongly enhances stimulation of TNFα and IL-12 production in macrophages and dendritic cells in response to Toll-like receptor 2 activation (Gantner et al, 2003). Further, Brown et al (2003) demonstrated that overexpression of Dectin-1 in macrophages was sufficient to enhance TNFα production in response to C. albicans yeast. These data suggest that Dectin-1 recognition may shape the nature of the adaptive immune response to C. albicans by stimulating a specific antifungal immune program. Romani and co-workers observed that dendritic cells pulsed in vitro with yeast and transferred back into mice stimulated Th1 immune responses, while dendritic cells exposed to filaments induced Th2 responses, which are generally considered to be ineffective against Candida (d'Ostiani et al, 2000; Romani et al, 2002). Thus, one important advantage to filamentous growth may be to inappropriately polarize the adaptive immune response. The mechanism for this is not understood; however, it is possible that differential activation of Dectin-1 may play a role. It is likely that avoiding Dectin-1 activation during filamentous growth contributes to C. albicans pathogenicity, although a complete analysis of the contribution of Dectin-1 to host defense against C. albicans infection is beyond the scope of this study. Future work exploring the role of Dectin-1 in host defense against Candida infections will need to take into account the pool of filamentous organisms that are not recognized by the receptor.
In addition to Dectin-1 and Toll-like receptors, a variety of receptors have previously been implicated in recognition of components of the C. albicans cell wall. Examples include the mannose receptor, which recognizes mannans (Martinez-Pomares et al, 1998), and galectin-3, which has been reported to recognize oligomannosides (Fradin et al, 2000). Unlike β-glucan as described here, ligands for these receptors are exposed at the cell surface and are unlikely to be affected by changes in cell morphology. Such receptors undoubtedly contribute to macrophage binding to C. albicans and may additionally trigger signaling that helps to shape the inflammatory response.
We have observed that soluble β-glucan (laminarin in this report) can bind to Dectin-1 without stimulating signaling, while particulate β-glucan (zymosan, or yeast bud/birth scars) activates the receptor. This likely reflects the level of receptor crosslinking required to activate signaling. While receptors such as Toll-like receptors that trigger cytokine and chemokine production can respond to very low concentrations of soluble ligands, phagocytic receptors such as Dectin-1 are activated by much higher concentrations of ligand. This likely reflects the different roles of these types of receptors (Underhill and Gantner, 2004). Phagocytic receptors need to be activated when a microbe is bound and needs to be internalized and killed, while it is useful to activate general inflammatory signaling when microbes are near. In this regard, it is interesting to note that phospholipomannan, an inflammatory glycolipid produced by C. albicans, has been reported to activate signaling through Toll-like receptor 2 at relatively low doses (Jouault et al, 2003).
There are two major models for how phagocytosis may work (Swanson and Baer, 1995). In the ‘zipper' model, membrane extension around the particle is controlled by highly localized signaling through receptors that bind to ligands evenly distributed on the particle. The classic model for this is Fc-receptor-mediated phagocytosis. Studies have demonstrated that, if a particle is not fully IgG-opsonized, Fc-receptors will only bind and extend membrane around the portion of the target that is opsonized (Griffin et al, 1975, 1976). In the ‘trigger' model of phagocytosis, particle binding stimulates broader signaling events that permit membrane extension around particles even in the absence of homogenous ligand distribution on the surface. We have observed that Dectin-1 can mediate phagocytosis of C. albicans yeast even though Dectin-1 binding is limited to discrete patches of the cell wall. The data suggest a ‘trigger' model for phagocytosis initiated by Dectin-1. However, it is also possible that, while Dectin-1 signaling is critical for phagocytosis, additional receptors could participate in coordinating ‘zippering'.
The targets of innate immune recognition receptors must be conserved molecular structures that are produced only by specific microbes and not by self (pathogen-associated molecular patterns (PAMPs)) (Janeway, 1989; Janeway and Medzhitov, 2002). Structural polysaccharide components of fungal cell walls, such as β-glucan, chitin, and mannan, are highly conserved between fungi, and, since the cell wall is critical to fungal growth and survival, its constituents are ideal PAMPs (Calderone and Braun, 1991). Our data emphasize that the mechanisms by which good PAMPs are visible to the immune system must be conserved. Toll-like receptor ligands (such as LPS, lipoproteins, flagellin) are largely recognized as soluble molecules, and as such cannot be shielded from recognition (Underhill and Ozinsky, 2002; Takeda et al, 2003). PAMPs recognized by phagocytic receptors must be exposed on the particle surface. Our findings indicate that although β-glucan in the fungal cell wall is mostly obscured by outer wall components, the structural demands of budding growth disrupt portions of the wall and reveal the underlying β-glucan layer. Thus, the process of budding growth, as much as the molecular structure of β-glucan, is the target for Dectin-1 recognition. During filamentous growth, the cell wall structure is not altered to reveal the underlying β-glucan, and Dectin-1 is not activated. The data demonstrate a mechanism by which C. albicans shape alone directly contributes to the method by which phagocytes recognize the fungus. It is possible that exploring methods for restoring activation of Dectin-1 during infection may improve the clinical control of C. albicans.
Materials and methods
Reagents
Zymosan (Sigma) and S. aureus (clinical isolate) were prepared as described previously (Underhill et al, 1999). Laminarin (soluble β-glucan from Laminaria digitata, Sigma) and mannan (from S. cerevisiae, Sigma) were prepared as 10 mg/ml stocks in RPMI, sterile filtered and stored frozen until use. Chitin (from crab shell, Sigma) was similarly prepared, but without filtering.
Yeast cultures
G85 (wild type) and G30032 (cts1Δ) are isogenic strains of S. cerevisiae S1278b and were the kind gifts of Dr Tim Galitski (Institute for Systems Biology). The cts1 deletion was made using a PCR-based strategy that replaces the gene open reading frame (ORF) by the KanMX4 ‘barcode' deletion allele (Winzeler et al, 1999). C. albicans (‘wild type' in this manuscript, ATCC 90028), C. albicans (CAI4 laboratory strain, genotype ura3∷1 imm434/ura3∷1 imm434, a kind gift of Dr G Fink), C. parapsitosis (ATCC22019), C. pseudotropicalis (ATCC 40764), C. krusei (ATCC 6259), and S. cerevisiae were maintained as stocks on YPD plates. For preparation of fresh yeast, C. albicans was seeded into Sabouraud dextrose broth (SDB) and incubated with shaking at 37°C overnight. For preparation of filaments, C. albicans was seeded into RPMI 1640 (Mediatech) and incubated with shaking at 37°C overnight. Cells were typically >95% live at the time of use, as determined by FUN-1 (Molecular probes) staining (Pina-Vaz et al, 2001). S. cerevisiae yeast were grown overnight in YPD at 30°C. Yeast and filamentous cultures were washed at least three times in sterile PBS before use. For experiments requiring stimulation of macrophages with fungi, an aliquot of each culture was pelleted, dried under vacuum, and weighed to determine the dry weight. We typically recovered 6 × 104 yeast/μg dry weight. Aliquots of the live cultures representing the indicated dry weights of yeast or filaments were used for stimulations. For preparation of heat-killed yeast, organisms were incubated for 20 min and 90°C. For preparation of C. albicans yeast with fluorescently labeled bud and birth scars, yeast were incubated for 30 min in PBS with 0.5 mg/ml FITC-labeled wheat-germ agglutinin (lectin from Triticum vulgaris; Sigma) (Powell et al, 2003). Labeled yeast were washed once with PBS, lightly fixed with 2.5% formalin in PBS for 10 min to prevent removal of the lectin, and washed with PBS before use.
Cell culture
HEK 293 (ATCC# CRL-1573) and HEK 293T (ATCC# CRL-11268) were maintained in DMEM (Invitrogen), and the mouse macrophage cell line RAW 264.7 (ATCC# TIB-71) was maintained in RPMI with 25 mM HEPES (Invitrogen). Both media were made complete with 10% heat-inactivated fetal bovine serum (Hyclone), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (Gibco). Generation of RAW 264.7 cells expressing V5-tagged murine Dectin-1 has been described previously (Gantner et al, 2003). HEK293 cells expressing GFP-tagged Dectin-1 were generated by cotransfecting cells with pTZ/GFP-Dectin-1 (an expression vector for full-length murine Dectin-1 fused to GFP at the amino-terminus under control of a tetracycline-regulatable promoter) and pTTIN, an expression vector directing production of a bicistronic mRNA coding for the tetracycline-regulated transcriptional activator (Tak, derived from pTetTak, Invitrogen) and neomycin resistance under control of a tetracycline-regulated promoter. Stably transfected cell lines were selected with neomycin (in the absence of tetracycline) and cloned. Bone marrow-derived macrophages were prepared from bone marrow collected from C57Bl/6 mice (Jackson labs), cultured for 5 days in complete RPMI supplemented with 50 ng/ml recombinant human M-CSF (Chiron Corp.). For activation assays, cells were washed and plated overnight in media with M-CSF and recombinant murine IFN-γ (50 U/ml) (PeproTech).
Soluble Dectin-1 and antibody production
The cDNA coding region for the C-terminal extracellular domain of murine Dectin-1 (amino acids 69–244) was cloned into an expression vector (pHA/Tet) that provides an amino-terminal Ig kappa chain signal peptide and a hemagglutin (HA) epitope tag, all under control of a tetracycline-regulated promoter. This vector was transiently transfected together with pTetTak (Invitrogen), a vector encoding the tetracycline-regulated transcriptional activator for expression by the Tet-inducible promoter, into HEK293T cells using Polyfect (Qiagen) according to the manufacturer's instructions. Culture supernatants were harvested 2 days post-transfection, concentrated four-fold by centrifugation through a 5 kDa cutoff filter, and stored at −20°C. For some experiments, the glycosylated protein was treated with PNGase F (New England Biolabs) according to the manufacturer's recommendations. Deglycosylation caused the receptor to run as a single sharp band by denaturing SDS–PAGE (data not shown).
The extracellular domain of mouse Dectin-1 was also produced by expression in Escherichia coli. The coding region for the extracellular domain was cloned into pTrcHis2-TOPO (Invitrogen), and expressed according to the manufacturer's instructions. Inclusion bodies containing the protein were purified, the protein was solubilized under denaturing conditions, and it was refolded by dialysis (Steinle et al, 2001). The bacterially expressed protein was used to produce polyclonal rabbit antisera at R&R Rabbitry (Stanwood, WA). The anti-Dectin antisera were screened for specific staining of Dectin-1-expressing HEK293 cells, and by immunoblot of the protein produced in the HEK293 cell supernatants above (data not shown).
Flow cytometry and microscopy with soluble Dectin-1
Mock-transfected or sDectin-transfected HEK293 cell culture supernatants were incubated on ice for 1 h with an FITC-coupled antibody to the HA epitope tag (HA.11-FITC, Covance) to generate a fluorescently labeled soluble dectin that could be stored at −20°C until use. Unlabeled soluble Dectin-1 could also be used to stain particles when followed by a second incubation with the epitope tag antibody (data not shown). Particles in 1 μl of 10 mg/ml zymosan, 5 μl of fresh yeast culture, or 30 μl of filament culture were collected by centrifugation, and incubated in 30 μl FITC-labeled soluble Dectin-1 for 1 h on ice. In some experiments, the FITC-labeled soluble Dectin-1 was preincubated with various carbohydrates at the indicated concentrations for 20 min before use. In some experiments yeast were additionally stained with calcofluor white (Fluostain I, Sigma). In some experiments yeast were pretreated with proteinase K (Sigma, 2.5 U/ml) or β-1,3-D-glucanase (Fluka, 10 U/ml) in PBS for 1 h at 37°C. As indicated, in some experiments, particles were stained with bacterially expressed biotinylated Dectin-1 (10 μg/ml) and staining was detected with streptavidin-FITC (Sigma). The particles were washed by centrifugation once with 1 ml of 10% calf serum in PBS, resuspended in 0.5 ml PBS with 2.5% formalin, and analyzed by flow cytometry on a FACScaliber (BD Biosciences) using CellQuest™ software. Alternatively, washed particles were mounted on glass slides and examined using a SP2 laser-scanning confocal microscope (Leica). All compared images were collected and processed identically. Bone marrow macrophages were stained with rabbit antisera to Dectin-1 using previously described methods (Underhill et al, 1999). All staining experiments were performed at least three times with similar results.
Filaments formed in vivo
For recovery of filaments formed in vivo, wild-type C. albicans yeast was incubated in sterile water for 4 h at 37°C. Yeast were washed three times with sterile PBS. C57Bl/6 mice were injected intraperitoneally with 108 yeast. After 24 h of incubation, mice were killed, and Candida filaments were recovered from the surface of the kidney by gentle scraping. The original yeast culture and the recovered filaments were stained with sDectin and calcofluor white and imaged by fluorescence microscopy, as described above.
Enzyme-linked sDectin-binding assay
In all, 50 μg Candida yeast or filaments were incubated in HEK293 supernatants containing HA sDectin and HA.11 antibody (Covance), for 30 min on ice, either in the presence or absence of laminarin at 1 mg/ml. Controls were incubated in mock-transfected HEK293 supernatants and HA.11. Candida was pelleted and washed in complete DMEM, followed by incubation in goat anti-mouse HRP (Jackson Immunoresearch) in complete DMEM for 60 min on ice. Candida was pelleted and washed in complete DMEM, and resuspended in PBS. Bound yeast and filaments were pipetted into a 96-well plate, and incubated with 100 μl TMB peroxidase substrate (KPL) for approximately 20 min, and the reaction was stopped with sulfuric acid (Sigma). Colorimetric quantitation was performed on a PowerWaveX microplate spectrophotometer at 450 nm (Bio-Tek Instruments). The binding experiment was completed three times with similar results.
Functional assays
Data for all functional assays were collected in at least three separate experiments, and one representative experiment is shown. For Dectin-1-blocking experiments, mouse bone marrow macrophages were preincubated with laminarin (1 mg/ml) or rabbit sera (10%) at 37°C for 30 min, followed by a fresh media change. For laminarin-blocking experiments, fresh laminarin was included with the stimuli. In some experiments, sDectin-coated yeast were prepared by preincubating washed yeast with 400 μg/ml bacterially expressed sDectin in PBS for 30 min on ice. The production of ROS was assayed by luminol-enhanced chemiluminescence as previously described (Gantner et al, 2003). Phosphotyrosine immunoprecipitation of Dectin-1 was performed as previously described (Gantner et al, 2003).
Phagocytosis of yeast was performed by incubating bone marrow-derived macrophage with the indicated reagents for 30 min at 37°C, and then changing media to fresh RPMI containing 50 μg wild-type C. albicans yeast, or yeast that had been preincubated with sDectin, followed by brief centrifugation to settle yeast onto macrophages, and incubating at 37°C for 30 min. Cells were then washed vigorously with cold PBS, and lifted with PBS containing 5 mM EDTA and 1 mM sodium azide. Cells were stained with calcofluor white to detect noninternalized yeast, mounted, and examined microscopically. Phagocytic index was assessed by counting number of internalized yeast particles per 100 phagocytes.
For measurements of C. albicans phagocytosis by Dectin-1-expressing HEK293 cells, 100 μl overnight culture of C. albicans yeast grown in SDB was pelleted and resuspended in 0.5 ml 2% glucose, 10 mM HEPES (pH 7.0) containing 10 μM of the vital dye, FUN-1 (Molecular Probes). The cells were incubated for 30 min at room temperature and washed several times in glucose buffer. The cells were >90% viable. A total of 200 000 cells were placed into each well of a 24-well plate containing Dectin-1-expressing 293 cells, and settled to the bottom by brief centrifugation. Phagocytosis was allowed to proceed for 20 min at 37°C before cells were harvested, uninternalized extracellular yeast were stripped off the surface by proteinase K treatment (50 μg/ml, 20 min), and internalization of live yeast (FUN-1, red channel) was measured by flow cytometry.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 2 Legend
Acknowledgments
The work was supported by the National Institutes of Health grant #RO1 GM62995 to DMU. We are grateful for reagents and helpful discussions provided by Dr Tim Galitski, Dr John Aitchison, and members of their laboratories.
References
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Dinauer MC, Maeda N, Koyama H (2002a) Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis 185: 1833–1837 [DOI] [PubMed] [Google Scholar]
- Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki K, Dinauer MC, Maeda N, Koyama H (2002b) Relative contributions of myeloperoxidase and NADPH-oxidase to the early host defense against pulmonary infections with Candida albicans and Aspergillus fumigatus. Med Mycol 40: 557–563 [DOI] [PubMed] [Google Scholar]
- Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R III, Kumamoto T, Edelbaum D, Morita A, Bergstresser PR, Takashima A (2000) Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem 275: 20157–20167 [DOI] [PubMed] [Google Scholar]
- Berman J, Sudbery PE (2002) Candida albicans: a molecular revolution built on lessons from budding yeast. Nat Rev Genet 3: 918–930 [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S (2001) Immune recognition. A new receptor for beta-glucans. Nature 413: 36–37 [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S (2003) Fungal beta-glucans and mammalian immunity. Immunity 19: 311–315 [DOI] [PubMed] [Google Scholar]
- Brown GD, Herre J, Williams DL, Willment JA, Marshall AS, Gordon S (2003) Dectin-1 mediates the biological effects of beta-glucans. J Exp Med 197: 1119–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone RA, Braun PC (1991) Adherence and receptor relationships of Candida albicans. Microbiol Rev 55: 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderone RA, Fonzi WA (2001) Virulence factors of Candida albicans. Trends Microbiol 9: 327–335 [DOI] [PubMed] [Google Scholar]
- d'Ostiani CF, Del Sero G, Bacci A, Montagnoli C, Spreca A, Mencacci A, Ricciardi-Castagnoli P, Romani L (2000) Dendritic cells discriminate between yeasts and hyphae of the fungus Candida albicans. Implications for initiation of T helper cell immunity in vitro and in vivo. J Exp Med 191: 1661–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fradin C, Jouault T, Mallet A, Mallet JM, Camus D, Sinay P, Poulain D (1996) Beta-1,2-linked oligomannosides inhibit Candida albicans binding to murine macrophage. J Leukoc Biol 60: 81–87 [DOI] [PubMed] [Google Scholar]
- Fradin C, Poulain D, Jouault T (2000) beta-1,2-linked oligomannosides from Candida albicans bind to a 32-kilodalton macrophage membrane protein homologous to the mammalian lectin galectin-3. Infect Immun 68: 4391–4398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale CA, Bendel CM, McClellan M, Hauser M, Becker JM, Berman J, Hostetter MK (1998) Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279: 1355–1358 [DOI] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM (2003) Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med 197: 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin FM Jr, Griffin JA, Leider JE, Silverstein SC (1975) Studies on the mechanism of phagocytosis. I. Requirements for circumferential attachment of particle-bound ligands to specific receptors on the macrophage plasma membrane. J Exp Med 142: 1263–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin FM Jr, Griffin JA, Silverstein SC (1976) Studies on the mechanism of phagocytosis. II. The interaction of macrophages with anti-immunoglobulin IgG-coated bone marrow-derived lymphocytes. J Exp Med 144: 788–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway CA Jr (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54 (Part 1): 1–13 [DOI] [PubMed] [Google Scholar]
- Janeway CA Jr, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20: 197–216 [DOI] [PubMed] [Google Scholar]
- Jouault T, Ibata-Ombetta S, Takeuchi O, Trinel PA, Sacchetti P, Lefebvre P, Akira S, Poulain D (2003) Candida albicans phospholipomannan is sensed through toll-like receptors. J Infect Dis 188: 165–172 [DOI] [PubMed] [Google Scholar]
- Kuranda MJ, Robbins PW (1991) Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J Biol Chem 266: 19758–19767 [PubMed] [Google Scholar]
- Lane S, Birse C, Zhou S, Matson R, Liu H (2001) DNA array studies demonstrate convergent regulation of virulence factors by Cph1, Cph2, and Efg1 in Candida albicans. J Biol Chem 276: 48988–48996 [DOI] [PubMed] [Google Scholar]
- Laprade L, Boyartchuk VL, Dietrich WF, Winston F (2002) Spt3 plays opposite roles in filamentous growth in Saccharomyces cerevisiae and Candida albicans and is required for C. albicans virulence. Genetics 161: 509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HJ, Kohler JR, DiDomenico B, Loebenberg D, Cacciapuoti A, Fink GR (1997) Nonfilamentous C. albicans mutants are avirulent. Cell 90: 939–949 [DOI] [PubMed] [Google Scholar]
- Madhani HD, Fink GR (1998) The control of filamentous differentiation and virulence in fungi. Trends Cell Biol 8: 348–353 [DOI] [PubMed] [Google Scholar]
- Martinez-Pomares L, Mahoney JA, Kaposzta R, Linehan SA, Stahl PD, Gordon S (1998) A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum. J Biol Chem 273: 23376–23380 [DOI] [PubMed] [Google Scholar]
- Pina-Vaz C, Sansonetty F, Rodrigues AG, Costa-Oliveira S, Tavares C, Martinez-de-Oliveira J (2001) Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. Clin Microbiol Infect 7: 609–618 [DOI] [PubMed] [Google Scholar]
- Powell CD, Quain DE, Smart KA (2003) Chitin scar breaks in aged Saccharomyces cerevisiae. Microbiology 149: 3129–3137 [DOI] [PubMed] [Google Scholar]
- Romani L, Bistoni F, Puccetti P (2002) Fungi, dendritic cells and receptors: a host perspective of fungal virulence. Trends Microbiol 10: 508–514 [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits GJ, Kapteyn JC, van den Ende H, Klis FM (1999) Cell wall dynamics in yeast. Curr Opin Microbiol 2: 348–352 [DOI] [PubMed] [Google Scholar]
- Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, Spies T (2001) Interactions of human NKG2D with its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics 53: 279–287 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ohno N, Ohshima Y, Yadomae T (1998) Soluble mannan and beta-glucan inhibit the uptake of Malassezia furfur by human monocytic cell line, THP-1. FEMS Immunol Med Microbiol 21: 223–230 [DOI] [PubMed] [Google Scholar]
- Swanson JA, Baer SC (1995) Phagocytosis by zippers and triggers. Trends Cell Biol 5: 89–93 [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S (2003) Toll-like receptors. Annu Rev Immunol 21: 335–376 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Gantner B (2004) Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect 6: 1368–1373 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A (2002) Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol 14: 103–110 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401: 811–815 [DOI] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M'Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms R, Beronneau S, Voet M, Bolckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, Davis RW (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 2 Legend


