Abstract
Scope and Significance: There are ∼185,000 amputations each year and nearly 2 million amputees currently living in the United States. Approximately 25% of these amputees will experience chronic pain issues secondary to localized neuroma pain and/or phantom limb pain.
Problem: The significant discomfort caused by neuroma and phantom limb pain interferes with prosthesis wear, subjecting amputees to the additional physical and psychological morbidity associated with chronic immobility. Although numerous neuroma treatments are described, none of these methods are consistently effective in eliminating symptoms.
Translational Relevance: Targeted muscle reinnervation (TMR) is a surgical technique involving the transfer of residual peripheral nerves to redundant target muscle motor nerves, restoring physiological continuity and encouraging organized nerve regeneration to decrease and potentially prevent the chaotic and misdirected nerve growth, which can contribute to pain experienced within the residual limb.
Clinical Relevance: TMR represents one of the more promising treatments for neuroma pain. Prior research into “secondary” TMR performed in a delayed manner after amputation has shown great improvement in treating amputee pain issues because of peripheral nerve dysfunction. “Primary” TMR performed at the time of amputation suggests that it may prevent neuroma formation while avoiding the risks associated with a delayed procedure. In addition, TMR permits the target muscles to act as bioamplifiers to direct bioprosthetic control and function.
Summary: TMR has the potential to treat pain from neuromas while enabling amputee patients to return to their activities of daily living and improve prosthetic use and tolerance. Recent research in the areas of secondary (i.e., delayed) and primary TMR aims to optimize efficacy and efficiency and demonstrates great potential for establishing a new standard of care for amputees.
Keywords: : residual limb, neuroma, phantom limb pain, phantom limb sensation, targeted reinnervation, targeted muscle reinnervation

Ian L. Valerio, MD, MBA, MS, FACS
Scope and Significance
In the United States, there are ∼185,000 amputations each year and nearly 2 million amputees are currently living with their residual limb(s).1 Nearly half of these amputations are secondary to traumatic injuries or oncological treatment needs, with the remaining being secondary to vascular disease, diabetic complications, infection, sepsis, and other vasculopathies. Amputation is associated with various limitations on activities of daily living, and adjusting to and coping with these changes and/or potential mobility challenges can contribute to further physical and psychological morbidity.1
Adequate control of residual limb pain is an important contributor to an amputee's motivation and ability to tolerate and utilize a prosthetic (Fig. 1). Approximately 25% of major limb amputees will develop chronic localized pain because of symptomatic neuromas within their residual limb.2–6 These neuromas consist of disorganized nerve fibers intertwined with scar tissue, and they commonly form at the end of a severed or damaged nerve. Thus, neuromas arise as a result of uncoordinated regenerated nerve fibers that can lead to symptomatic and focal pain that can be aggravated with prosthetic wear or use. Because of these pathological changes to an amputated peripheral nerve, the associated neuromas can result in focal pain that is difficult to treat by traditional medical or surgical measures. The resulting painful neuroma can frequently make the wear and use of a prosthesis uncomfortable or even impossible, thus reducing an amputee's functional abilities and quality of life.
Figure 1.

Right lower extremity amputee, ambulating with her prosthesis post-TMR surgery. TMR, targeted muscle reinnervation.
In addition to neuroma pain, amputees often also experience phantom limb pain (PLP) and phantom limb sensation (PLS) within their residual limb(s). PLP describes painful sensations referred to the absent limb, which is attributed to a nerve injury followed by chemical, physiological, and morphological changes.7 Although the exact mechanism of PLP and PLS is poorly understood, it is surmised that spontaneous and abnormal peripheral nerve signals along with central changes including cortical reorganization and gray matter changes may play a role.8–11,13
Despite recent advancements in prostheses to help amputees regain functionality, the aforementioned causation of pain can often impede the tolerance of the devices to the interface of the residual limb. There have been multiple studies that evaluate the prevalence of symptomatic neuroma and phantom limb pain (NPLP) in amputees to denote the magnitude of this morbidity. These studies ultimately present varying rates of symptomatic PLP and neuroma pain in the amputee patient as early as 3 to 6 months postoperatively at 9–67% and 2–25%, respectively.2–4,6,14 In the military population, Carlen et al. found that 67% of soldiers with traumatic amputation had pain in the phantom limb in the first few months after surgery.15
Treatment options
Before targeted muscle reinnervation (TMR) was introduced within the past decade, numerous therapies for symptomatic NPLP provided inconsistent resolution of symptoms. Furthermore, neuromas are often missed on diagnosis, which leads to multiple surgical interventions, stump revisions, and poor outcomes.5 Currently, there are three categories of methods to treat chronic stump pain: pharmacological, psychological, and physical.5 The pharmacological agents include narcotics, nonsteroidal anti-inflammatory drugs, antidepressants, anticonvulsants, and nerve modulators, as well as local nerve blocks, steroid injections, and lidocaine patches. The psychological methods include mirror box therapy and antidepressant therapy.16 Physical methods include transcutaneous electrical nerve stimulation to the limbs, spinal electrical stimulators, rehabilitation with exercise or massage, and surgery.5
The surgical treatments for symptomatic NPLP are vast. In a majority of amputations, traction neurectomy has been used to address the peripheral nerves. For this procedure, the nerve is released from the soft tissues and pulled under tension before transecting the nerve more proximally and allowing it to retract into a better soft tissue bed. Although this option is very popular, it often leads to neuroma within the transected nerve stump. In 1984, Dellon et al. demonstrated that excision of the neuroma and burial of the cut nerve in muscle improved neuroma pain in a large number of patients.17 Other options described include crushing the distal nerve stump,18 burying the nerve stump within a cortical window cut into a nearby bone,19 and sewing two distal nerve stumps directly together into a loop.20
Despite numerous methods and advances, none have proven consistent enough to set a standard of management for both preventing and treating NPLP in amputee populations. During this millennium, however, researchers continue to make significant advancements in understanding and treating symptomatic NPLP. TMR is one of the most promising and fascinating methods, and is be discussed further in the following sections.
Translational Relevance
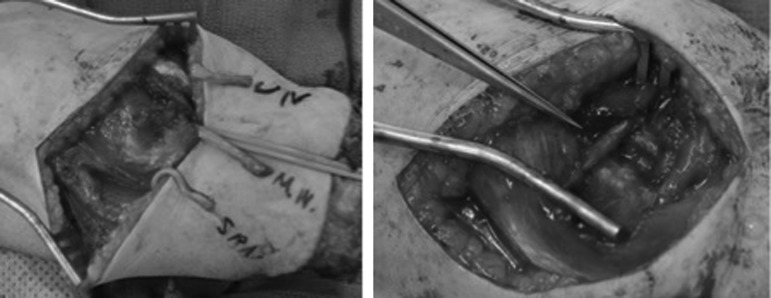
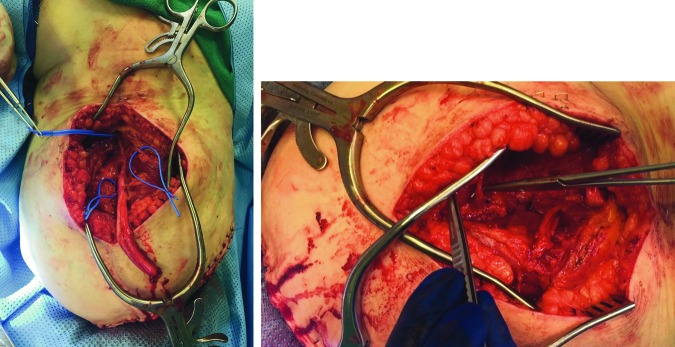
TMR consists of a nerve transfer of residual peripheral nerves to otherwise redundant target muscle motor nerves.21–26 When TMR is performed on an amputee, the residual peripheral nerve is mobilized and any neuroma is excised. The native motor nerve of the target muscle is then located through nerve stimulators and transected near the muscle. Lastly, the residual peripheral nerve is coapted to the motor nerve, close to its point of entry into the muscle (Figs. 2 and 3).
Figure 2.
Forearm amputation pre- (left) and post- (right) targeted reinnervation. Left: Ulnar, median, and superficial radial nerves are identified. Right: Superficial radial nerve is coapted to the anterior interosseous nerve; median nerve is divided with one segment coapted to the motor branch of the FDS (index and middle finger), the other coapted to the motor branch of the FDS (ring and little finger); ulnar nerve to the FCU motor branch. FCU, flexor carpi ulnaris muscle; FDS, flexor digitorum superficialis muscle.
Figure 3.
Right AKA pre- (left) and post- (right) targeted reinnervation. Left: The common peroneal and tibial nerve components of sciatic nerve as well as motor nerve targets (blue loops around target motor nerves) were identified. The posterior nerve of the thigh was also identified and length preserved but not visible in this image. Right: The following nerve coaptations were performed: (1) common peroneal nerve component to a motor branch of the biceps femoris; (2) the tibial nerve component to a motor branch of the semitendinosis; (3) the posterior cutaneous nerve of the thigh to another motor branch to the biceps femoris. AKA, above knee amputation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
TMR was originally designed to provide amputees better intuitive control of upper limb myoelectric and bioprostheses.21–26 The central principle underlying the nerve transfers in TMR surgery is to reestablish some of the functions of the amputated nerve by creating multiple bioamplifiers. These bioamplifiers effectively increase the number of signal generators to enhance the degrees of freedom for prosthetic motor function outlays, allowing for more definitive and specific signals to enhance bioprosthetic control. In 2004, Kuiken et al. described the novel concept of TMR in a bilateral shoulder disarticulation amputee who underwent brachial plexus nerve transfers to divided regions of his pectoralis major and minor.21 By transferring the transected brachial plexus nerves to proximal “myoneurosomes,” or segments of muscle with distinct nerve supplies and signals,22 the muscles could be used as “bioamplifiers” for amputated nerve signals, which were then successfully detected by electromyogram (EMG) to enhance prosthesis control. In the case of shoulder amputees who previously had to lock one joint (shoulder, wrist, or elbow) before moving the next, TMR provided simultaneous control in two degrees of movement. These TMR patients exhibited improvements in prosthetic speed and control, but they also reported increased comfort and showed regains in sensory input.23 Subsequent studies confirmed increased prosthesis functions including speed and coordination, especially as refinements in TMR surgery proceeded. Although the size mismatch between the residual amputated nerve and the more proximal nerve was initially a concern, it was determined that this mismatch creates favorable hyperinnervation, which improves the odds of reinnervating those target muscles proximal to the amputation.25
Beyond its success in improving prosthesis control, TMR has also had notable effects on preventing and/or reducing the formation of neuromas. Although neuroma pain often makes prosthesis wear uncomfortable and sometimes impossible, it was noted in an early series of TMR procedures by Souza et al. that although many of the amputees complained of neuroma pain before TMR surgery, the vast majority of these patients reported resolution of their neuroma pain symptoms for the 10 years after TMR.12 In contrast to previously described neuroma treatments, TMR represents a controlled and predictable procedure with the added benefit of improved prosthesis control. The coaptation of the amputated nerve stumps to recipient motor nerve branches encourages organized nerve regeneration into the denervated target muscles, thus preventing the chaotic and misdirected nerve growth that leads to neuroma formation. Simply put, TMR gives the nerves somewhere to go and something to do by restoring an appropriate physiological continuity. This critical element is lacking in other neuroma treatments.21,27–29 Understandably, its effect on neuroma formation became the interest of many studies, which ultimately proved its benefit even on a microscopic scale. Kim et al. examined the histological findings of neuromas in rabbits after amputation, and noted several unique characteristics, including increased amounts of myelinated fibers, increased overall cross-sectional diameter, a decreased size of individual fibers, and an increased amount of stromal tissue suggestive of inflammation and increased collagen deposition.29 In later studies, Kim et al. compared the histology of nerves after TMR and found that they more closely resembled preamputation nerves than the neuromas they previously described.28 These findings provided histological evidence of the efficacy of TMR as a treatment for neuromas, evidence which was lacking for other neuroma surgical options.
Our current research seeks to prove that TMR, both alone and in conjunction with other surgical advancements such as regenerative peripheral nerve interface (RPNI) and implantable myoelectric electrode systems (IMESs), is a consistent and reliable treatment of symptomatic NPLP that also benefits use of advanced prosthetic systems.
Clinical Relevance
Thus far, the success of TMR in symptomatic improvement and potential resolution of NPLP in a significant number of residual limb patients has been promising, but has not been the study outcome of a large trial until recently. A current prospective multicenter, randomized study seeks to specifically analyze the long-term success of TMR in resolving symptomatic NPLP compared with the current standard of care surgical treatments described within this work.
Early results from our work and supported by studies performed elsewhere suggest that symptoms of neuroma pain and PLP may persist for a short period after surgery; by 3–6 months postoperation, patients experience considerable reduction in pain scores and symptoms, less inhibition from PLP, as well as improvement in function and prosthetic use with improved tolerance rates. These gains continue over time. PLSs may persist after TMR, but are not usually associated with significant inhibition of function or prosthetic use.
Although these studies examine the impact of TMR for the treatment of symptomatic NPLP after amputation, current studies taking place at the authors' institution look to examine the use of TMR performed at the time of amputation as a preventative measure for symptomatic NPLP—“primary TMR.” A large series of 20 patients undergoing primary TMR at our institution show trends toward early and persistent reduction and/or resolution of PLP, early use of prosthetics, and earlier discontinuation of narcotics as compared with a non-TMR amputee cohort when compared for same level amputation matching controls. Furthermore, this primary TMR cohort has an extremely low incidence of symptomatic neuroma with follow-up exceeding 12 months. We hypothesize that primary TMR has the advantage of decreased muscle atrophy, improved nerve in-growth, less muscle denervation, and greater neuroplasticity.
If successful, primary TMR will reduce the total number of surgeries, thus eliminating recovery time and other risks associated with additional operations. It is our hope that prevention of NPLP symptoms will lead to earlier, more consistent, and comfortable prosthesis use and improved health outcomes overall. The results of primary TMR will continue to be examined through close patient follow-up to determine its long-term effects on NPLP prevention.
Additional Considerations
Additional recent efforts have focused on improving prosthetic control and providing afferent feedback to allow for sensory capable prosthetics. These advances include targeted sensory reinnervation (TSR), IMESs, and RPNI. Each seeks to amplify the myoelectric signal to the advanced prosthetic system.
TSR follows the premise of TMR. However, rather than a motor nerve coaptation, an amputated sensory nerve is transferred to a motor nerve branch. This nerve transfer allows sensory nerve ingrowth through the muscle and into the overlying skin. Studies have demonstrated regained sensation to touch, proprioception, and temperature in patients after this procedure.30 IMES utilizes implantable EMG systems that provide a more robust signal to the prosthesis by alleviating the problems of surface EMG electrode, therefore, stabilizing the EMG control of advanced prosthesis.31–33 RPNI creates a localized myoelectric signal through a free muscle neurotized with the transected end of a nerve fascicle.30,34 Through an insulated electrode placed on the muscle surface, there is stimulation of the muscle's afferent nerve that then provides sensory feedback. The sensory regenerative peripheral nerve interface has the ability to transduce distinguishable and graded sensory signals across the peripheral nerve when electrically stimulated.34 These efforts will not only allow sensory input to prosthetic limbs but will also aid in control and dexterity, contributing to the overall goal of more realistic prostheses.
Take-Home Messages.
• There are currently 2 million amputees in the United States; ∼25% suffer from chronic neuroma pain that can make prosthesis wear impossible.
• Despite >150 surgical treatments for neuroma, no treatment has been shown to lead to consistent resolution of symptoms.
• TMR, although originally developed for improved prosthesis control, has shown promising results in the treatment of neuromas.
• Unlike previous neuroma treatments, TMR is a controlled and predictable procedure that restores physiological continuity to nerves involved in amputation.
• Primary TMR takes place at the time of amputation and has shown promising results for the prevention of NPLP.
Summary
Because of modern medicine, patients are living longer and more active lives after the diagnosis of cancer or the unfortunate circumstance of traumatic amputation. In this modern era, the amputee patient demands an active life. TMR offers promising results to enable amputee patients to return to their life as it was before loss of a limb. Our work with primary TMR sets out to achieve these goals with optimal efficacy and efficiency; preliminary results demonstrate great potential for establishing a new standard of care for amputees. Current and future studies will examine the use of TMR in a variety of populations, such as oncological, trauma, and burn patients to determine its effectiveness across diverse patient populations.
Future Directions
Future research will continue to focus on TMR's effect on prosthesis wear, including decreased time to wear, increased comfort, and improved control with advanced prostheses. We seek to contribute to the field of advanced prostheses by exploring the potential of TMR alongside other innovations. The future will be in the merger of biological strategies that aim to improve nerve and muscle interactions as bioamplifiers intertwined with engineering advances in biosensors and signal generators such as IMESs and RPNI. This merger aims to improve upon aspects such as fine bioprosthetic movements and sensation by optimizing the residual limb interface with the external environment. In addition, such strategies must account for sensory integration and pain control to provide for continued use and integration of prosthetic to the patient. The future results of this work will impact not only the field of plastic surgery but also the fields of orthopedic trauma, orthopedic oncology, and surgical oncology, potentially changing the surgical treatment of amputees.
Abbreviations and Acronyms
- IMESs
implantable myoelectric electrode systems
- NPLP
neuroma and phantom limb pain
- PLP
phantom limb pain
- PLS
phantom limb sensation
- RPNI
regenerative peripheral nerve interface
- TMR
targeted muscle reinnervation
- TSR
targeted sensory reinnervation
Acknowledgments and Funding Sources
The authors would like to acknowledge our research colleagues from our partner institutions. From the Division of Plastic Surgery at Northwestern University, Drs. Dumanian, Ko, and Souza. From Walter-Reed National Military Medical Center, Drs. Potter, Nanos, and Tintle. From San Antonio Military Medical Center, Dr. Cho. We have no funding acknowledgments to disclose.
Author Disclosure and Ghostwriting
The authors have no affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of Defense, the U.S. Government, or the State Government.
About the Authors
J. Byers Bowen, MD, MS, is an independent plastic surgery resident at The Ohio State University Wexner Medical Center. He completed his general surgery residency at Eastern Virginia Medical School in Norfolk, VA. His interests include general reconstruction, complex abdominal wall reconstruction, migraine headache surgery, and the surgical management of NPLP through targeted reinnervation. Corinne E. Wee, BA, is a fourth year medical student at the Ohio State University College of Medicine who is applying to plastic and reconstructive surgery residency programs. Her research interests include traumatic and oncological reconstruction and amputee care. Jaclyn Kalik, BS, is a 2014 graduate of the United States Military Academy at West Point. She is currently a third year medical student at the Ohio State University College of Medicine. Her research interests include traumatic reconstruction. Ian L. Valerio, MD, MS, MBA, FACS, is the Director of Burn, Wound, and Trauma with Associate Professor appointments in the Departments of Plastic, Orthopedic, and General Surgery at the Ohio State Wexner Medical Center, Columbus, OH, and serves as a Commander in the Medical Corps for the Expeditionary Medical Forces—Bethesda and Great Lakes. He is a plastic surgeon who specializes in traumatic, oncological, craniofacial, burn, wound, and pediatric reconstructive surgery, amputee care, peripheral nerve repair, targeted reinnervation, vascularized composite tissue allotransplantation, and aesthetic surgery. In addition, he has experience in wounded warrior care and deployment experience as a surgeon who operated in the U.S. NATO-affiliated countries and Afghanistan.
References
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89:422–429 [DOI] [PubMed] [Google Scholar]
- 2.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Phantom limb, phantom pain and stump pain in amputees during the first 6 months following limb amputation. Pain 1983;17:243–256 [DOI] [PubMed] [Google Scholar]
- 3.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain 1985;21:267–278 [DOI] [PubMed] [Google Scholar]
- 4.Pierce RO, Jr., Kernek CB, Ambrose TA, 2nd The plight of the traumatic amputee. Orthopedics 1993;16:793–797 [DOI] [PubMed] [Google Scholar]
- 5.Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg 2008;121:908–914; discussion 915–917 [DOI] [PubMed] [Google Scholar]
- 6.Harris AM, Althausen PL, James K, Bosse MJ, Renan C. Complications following limb-threatening lower extremity trauma. J Orthop Trauma 2009;23:1–6 [DOI] [PubMed] [Google Scholar]
- 7.Nikolajsen L, Lone N, Jensen TS. Phantom limb pain. Curr Rev Pain 2000;4:166–170 [DOI] [PubMed] [Google Scholar]
- 8.Bolognini N, et al. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain 2013;154:1274–1280 [DOI] [PubMed] [Google Scholar]
- 9.Elbert T, Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist 2004;10:129–141 [DOI] [PubMed] [Google Scholar]
- 10.Flor H, et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 1995;375:482–484 [DOI] [PubMed] [Google Scholar]
- 11.Preissler S, et al. Gray matter changes following limb amputation with high and low intensities of phantom limb pain. Cereb Cortex 2013;23:1038–1048 [DOI] [PubMed] [Google Scholar]
- 12.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res 2014;472:2984–2990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montoya P, et al. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci 1998;10:1095–1102 [DOI] [PubMed] [Google Scholar]
- 14.Hertel R, Strebel N, Ganz R. Amputation versus reconstruction in traumatic defects of the leg: outcome and costs. J Orthop Trauma 1996;10:223–229 [DOI] [PubMed] [Google Scholar]
- 15.Carlen PL, Wall PD, Nadvorna H, Steinbach T. Phantom limbs and related phenomena in recent traumatic amputations. Neurology 1978;28:211–217 [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc Biol Sci 1996;263:377–386 [DOI] [PubMed] [Google Scholar]
- 17.Dellon AL, Lee Dellon A, Mackinnon SE, Alan P. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg 1984;12:30–40 [DOI] [PubMed] [Google Scholar]
- 18.Kruger H. To prevent development of painful neuromas after amputations. Miinchen Med Wechnsehr 1916;63:368 [Google Scholar]
- 19.Laing T, Tereze L, Aftab S, Manu S. The management of neuropathic pain from neuromas in the upper limb: surgical techniques and future directions. Plast Aesthet Res 2015;2:165 [Google Scholar]
- 20.Belcher HJ, Pandya AN. Centro-central union for the prevention of neuroma formation after finger amputation. J Hand Surg Br 2000;25:154–159 [DOI] [PubMed] [Google Scholar]
- 21.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. Targeted muscle reinnervation for improved myoelectric prosthesis control. In: Conference Proceedings. 2nd International IEEE EMBS Conference on Neural Engineering, 2005. doi: 10.1109/cne.2005.1419642 [DOI] [Google Scholar]
- 22.Hijjawi JB, et al. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg 2006;118:1573–1578 [DOI] [PubMed] [Google Scholar]
- 23.Kuiken TA, et al. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet 2007;369:371–380 [DOI] [PubMed] [Google Scholar]
- 24.Dumanian GA, et al. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg 2009;124:863–869 [DOI] [PubMed] [Google Scholar]
- 25.O'Shaughnessy KD, et al. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am 2008;90:393–400 [DOI] [PubMed] [Google Scholar]
- 26.Kuiken TA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA 2009;301:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal muscle. Brain Res 1995;676:113–123 [DOI] [PubMed] [Google Scholar]
- 28.Kim PS, et al. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am 2012;37:1609–1616 [DOI] [PubMed] [Google Scholar]
- 29.Kim PS, Ko J, O'Shaughnessy KK, Kuiken TA, Dumanian GA. Novel model for end-neuroma formation in the amputated rabbit forelimb. J Brachial Plex Peripher Nerve Inj 2010;5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuiken TA, Marasco PD, Lock BA, Harden RN, Dewald JP. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci U S A 2007;104:20061–20066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller LA, Stubblefield KA, Lipschutz RD, Lock BA, Kuiken T.A. Improved myoelectric prosthesis control using targeted reinnervation surgery: a case series. IEEE Trans Neural Syst Rehabil Eng 2008;16:46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lowery MM, Weir RF, Kuiken T.A. Simulation of intramuscular EMG signals detected using implantable myoelectric sensors (IMES). IEEE Trans Biomed Eng 2006;53:1926–1933 [DOI] [PubMed] [Google Scholar]
- 33.Loeb GE, Peck RA, Moore WH, Kevin H. BION™ system for distributed neural prosthetic interfaces. Med Eng Phys 2001;23:9–18 [DOI] [PubMed] [Google Scholar]
- 34.Nghiem BT, et al. Providing a sense of touch to prosthetic hands. Plast Reconstr Surg 2015;135:1652–1663 [DOI] [PubMed] [Google Scholar]