Abstract
Background: Plasminogen activator inhibitor-1 (PAI-1) is implicated in the pathophysiology of cardiovascular disease (CVD) and increased in individuals with type 2 diabetes mellitus (T2DM). Adipose tissue produces PAI-1, and pericardial fat is a CVD risk factor. We sought to determine the relationship between PAI-1 and pericardial fat in males and females with well-controlled T2DM.
Methods: The study population consisted of 32 males and 19 females, aged 35–70 years with T2DM, without clinical evidence of CVD or other active medical problems except for hypertension. Subjects were studied under good cardiometabolic control. Study procedures included fasting blood work and cardiovascular imaging. Cardiac magnetic resonance imaging of the heart was used to identify and quantify pericardial fat from the bifurcation of the pulmonary trunk to the last slice containing cardiac tissue.
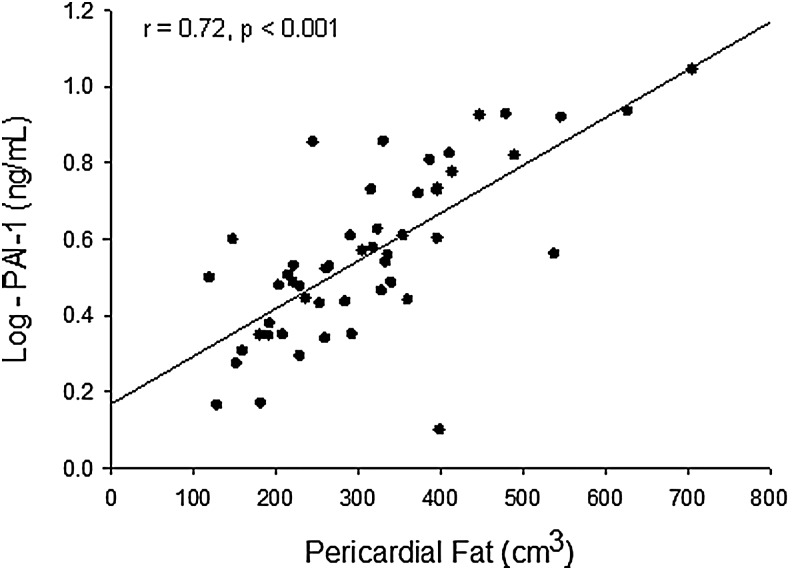
Results: PAI-1 was positively correlated with pericardial fat (β = 0.72, r = 0.72, P < 0.001) as well as with homeostatic model assessment of insulin resistance (r = 0.31, P = 0.03) and serum triglycerides (r = 0.27, P = 0.05). In a multivariable regression model, controlling for insulin sensitivity, triglycerides, and body mass index, pericardial fat was independently associated with PAI-1 (β = 0.80, P < 0.001).
Conclusions: PAI-1 is positively associated with pericardial fat in individuals with T2DM.
Keywords: : type 2 diabetes, plasminogen activator inhibitor-1, pericardial fat, adipose tissue
Introduction
Type 2 diabetes mellitus (T2DM) is associated with an increased risk of cardiovascular disease (CVD).1 Individuals with T2DM, compared to age-matched controls, have elevated levels of plasminogen activator inhibitor-1 (PAI-1), which appears to be implicated in the pathophysiology of CVD.2,3 PAI-1 is a serine proteinase inhibitor4 and is the main physiological inhibitor of the endogenous fibrinolytic system primarily through inhibition of tissue plasminogen activator.5 Circulating PAI-1 concentrations are positively associated with incident cardiovascular events in at-risk individuals.6 Furthermore, in individuals with no prior history of CVD, PAI-1 is a predictor of CVD independent of conventional risk factors.7
PAI-1 is produced by multiple tissues, including vascular endothelium and adipose tissue.5 Visceral adipose tissue is metabolically more active than subcutaneous adipose tissue8 and produces more PAI-1.9 Pericardial fat, which includes epicardial and paracardial fat, is an independent predictor of CVD10 and is associated with calcified coronary plaque.11 One study showed an association between epicardial fat, a form of visceral adipose tissue,12 and PAI-1 in 27 females with morbid obesity without diabetes.13 To our knowledge, the association between PAI-1 and pericardial fat, or its components, has not been studied in males and females with T2DM.
The main purpose of this study was to determine the relationship between the two cardiovascular risk factors, PAI-1 and pericardial fat, in individuals with T2DM and no prior history of CVD. In addition, we evaluated the relationship between PAI-1 and additional cardiometabolic risk factors in T2DM, including body mass index (BMI), lipids, blood pressure, insulin sensitivity measures and two early CVD markers, coronary flow reserve (CFR), and myocardial extracellular volume (ECV), a marker of extracellular matrix expansion. Reductions of CFR and increases in ECV are known to be associated with increased cardiovascular morbidity and mortality in individuals with T2DM.14,15
Materials and Methods
Study population
This is a post-hoc analysis of baseline data obtained before drug randomization from a previously published study (ClinicalTrials.gov identifier: NCT00865124).16 The study included males and females with T2DM without clinical evidence of coronary artery disease or other active medical problems except for hypertension. Exclusion criteria were clinical evidence of coronary, cerebrovascular, or peripheral vascular disease; asthma; estimated glomerular filtration rate <60 mL/min/1.73 m2; cigarette smoking; pregnancy; illicit drug use; contraindications to cardiac magnetic resonance or adenosine; uncontrolled hypertension (systolic blood pressure >160 mmHg); and active major medical illness. Fifty-one participants (32 males and 19 females) with T2DM had PAI-1 levels and pericardial fat assessed at baseline and were included in the primary analysis. Partners HealthCare Institutional Review Board approved the protocol, and all participants provided written informed consent.
Study procedures
Before the baseline assessment, all participants completed a 3-month run-in period to establish good metabolic control. Each participant was placed on enalapril 20 mg daily with tapering of all other antihypertensive agents except amlodipine, which was added if systolic blood pressure ≥140 mmHg; was started on simvastatin if low-density lipoprotein was >100 mg/dL; and had antidiabetic medications adjusted to target a glycosylated hemoglobin (HbA1c) level ≤7%, as previously described.16 Study procedures were conducted after admitting the study participants to the inpatient Center for Clinical Investigation at Brigham and Women's Hospital.
Assessment protocol
Four days before and during the 2-day inpatient admission, participants consumed a caffeine-free, isocaloric diet (250 mmol/day of sodium, 100 mmol/day of potassium, 1000 mg/day of calcium, 300 mg/day of magnesium, and at least 30% carbohydrate by calories). During the diet, doses of antidiabetic medications were reduced as needed to avoid hypoglycemia. After an overnight fast, blood samples were collected and supine systolic and diastolic blood pressures were measured every 5 min for 30 min by automated blood pressure cuff (GE Healthcare, Marlborough, MA), and the average blood pressures (systolic and diastolic, respectively) were used for analysis. Subjects underwent cardiac positron emission tomography imaging for the determination of CFR (ratio of adenosine stimulated to rest myocardial blood flow corrected for the baseline pulse-pressure product), and cardiac magnetic resonance imaging (MRI) for the determination of myocardial ECV, allowing us to quantify extracellular matrix expansion, as previously described.16 Cardiac MRI was also used to determine pericardial fat (see below).
Serum PAI-1 was analyzed using an ELISA assay (R&D Systems, Minneapolis, MN; intra-assay variation 5.8%–7.4% and inter-assay variation 5.6%–7.2%). Insulin was measured using an Access Chemiluminescent Immunoassay (Beckman Coulter, Fullerton, CA; intra-assay variation 2.0%–4.2% and inter-assay variation 3.1%–5.6%). Blood levels of electrolytes, HbA1c, and lipids were assessed using routine clinical assays from the Laboratory Corporation of America (Raritan, NJ).
Pericardial adipose tissue quantification
Cardiac MRI was performed using a 3-T magnet (Magnetom Tim Trio or Verio; Siemens Healthcare, Erlangen, Germany) with an 8-element phased-array coil. Axial T1 turbo spin echo images were used for pericardial adipose tissue quantification. QMass MR Version 7.6 software (Medis Medical Imaging Systems, Raleigh, NC) was used to trace and quantify adipose tissue on a dedicated offline workstation. Axial slices (8 mm thickness) were analyzed from the bifurcation of the pulmonary trunk (superior border) to the last slice containing cardiac tissue (inferior border). The anterior and posterior borders of analysis were the anterior edge of the thoracic cavity and the posterior segment of the descending aorta and periaortic fat, respectively (see Fig. 1). We traced an outer border containing all pericardial adipose tissue (consisting of both epicardial and paracardial adipose tissues) and an inner border within the myocardium to exclude nonadipose tissue. Periaortic fat is contiguous with pericardial fat at multiple axial levels of the heart. Accordingly, periaortic fat was included across all slices in the analysis for consistency. Near the inferior border of analysis, abdominal structures and the diaphragm become apparent. Thus, to assure the tissue analyzed was cardiac in nature, sagittal cross-section images were used simultaneously with axial images to identify the tissue in two planes and accurately trace it. A reference region was drawn within posterior skeletal muscle for each imaging slice. Pericardial adipose tissue was defined as tissue within the two borders traced with pixel intensity more than four standard deviations (SDs) above the mean pixel intensity for the reference posterior skeletal muscle.
FIG. 1.
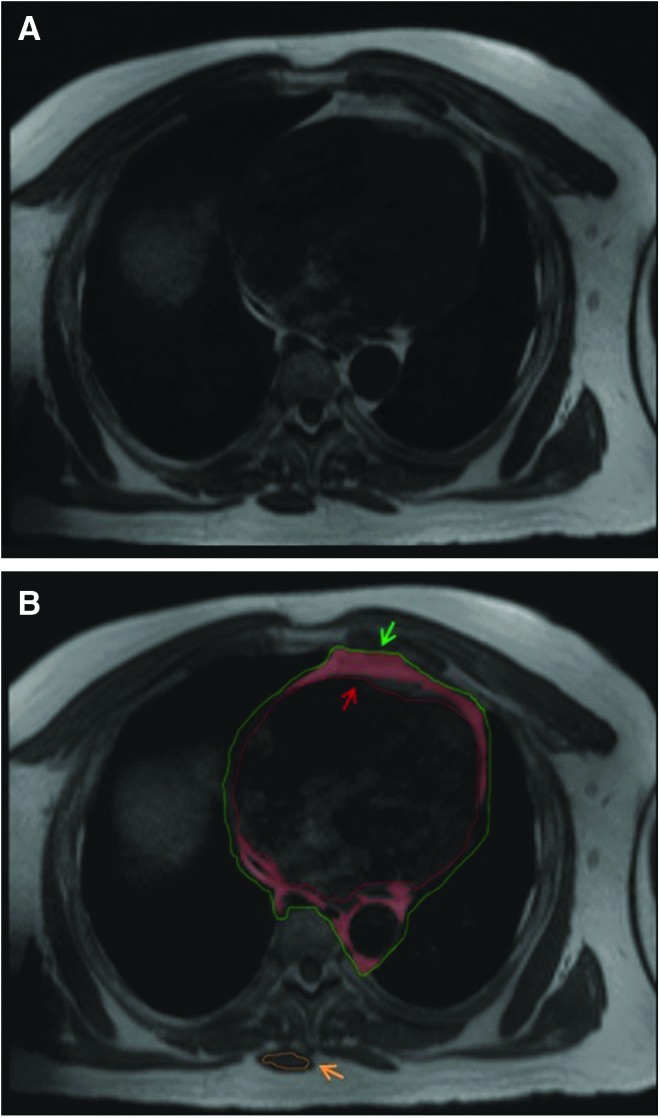
Example of axial T1 turbo spin echo slice. (A) Unmarked axial slice. (B) Axial slice after cardiac contours drawn. The pericardial fat is highlighted in red and is located between the green (outer paracardial fat and posterior periaortic fat borders) and red (myoepicardial border) lines as indicated by the green and red arrows. Fat is defined as tissue with pixel intensity more than four SDs above the mean pixel intensity for the reference posterior skeletal muscle (orange circle and orange arrow). SDs, standard deviations.
The QMass MR Version 7.6 software quantified pericardial adipose tissue across all analyzed slices for a given participant and output a total volume. Intrareader and interreader reproducibility assessed on a random sample of 20% of participants were excellent: intraclass correlation coefficients were 0.99 and 0.98 for intrareader and interreader analyses, respectively.
Statistical methods
Normally distributed data are expressed as mean ± SD. Data not normally distributed are expressed as median [interquartile range]. Our primary goal was to test the association between PAI-1 levels and pericardial fat volume. Secondary analyses included the relationships between PAI-1 and biochemical markers of diabetes, as well as CFR and ECV. Pearson's correlations were used to assess relationships between PAI-1 and CVD risk factors. Power calculations were performed for the parent study.16 For this post-hoc analysis, a sample size of 51 provides at least 80% power with 5% alpha for a Pearson correlation coefficient of 0.40 or greater. Data not normally distributed were log-transformed for Pearson's correlations. Below- and above-median comparisons for demographic and other variables were performed using Wilcoxon signed-rank tests. Categorical data analyses were performed using Fisher's exact test. Given pericardial fat was our main exposure, we constructed multivariable regression models to assess the association between PAI-1 and pericardial fat, while adjusting for other factors (age, sex, BMI, and any other variable with a P ≤ 0.05 on univariate correlation with PAI-1). A value of P ≤ 0.05 was deemed statistically significant. Data more than four SDs from the mean of all participants were removed for analysis. All statistical analyses were performed with SPSS version 24 (IBM Corporation, Armonk, NY).
Results
Subject characteristics
We studied 51 individuals (19 females and 32 males) aged 54.5 ± 7.5 years and BMI 32.2 ± 4.8 kg/m2, with no differences in age and BMI between sexes. Mean pericardial fat volume was significantly higher in males versus females (345.6 ± 136.7 vs. 262.1 ± 86.9 cm3, P = 0.02) and in Caucasian participants versus non-Caucasian participants (358.1 ± 124.7 vs. 241 ± 92.1 cm3, P < 0.001).
The baseline characteristics of the 51 individuals are stratified by below- and above-median pericardial fat and are displayed in Table 1. Individuals with below-median pericardial fat had significantly higher HbA1c values than individuals with above-median pericardial fat (P = 0.04, see Table 1). However, the use of antidiabetic medications, including use of insulin and/or insulin secretagogues did not differ between below and above-median pericardial fat categories. The most common antidiabetic medications used in this cohort were metformin (84.3% of participants), sulfonylureas (31.4%), and insulin (17.6%). All other classes of antidiabetic medications were used in <10% of the cohort and were in combination with a class of antidiabetic medication listed above.
Table 1.
Study Sample Characteristics
| Stratification by pericardial fat | ≤Median pericardial fat (n = 26) | >Median pericardial fat (n = 25) |
|---|---|---|
| Age (years) | 54 ± 8.3 | 55 ± 6.7 |
| Sex | ||
| Male (n, %total) | 13, 25.5 | 19, 37.2 |
| Female (n, %total) | 13, 25.5 | 6, 11.8 |
| BMI (kg/m2) | 31.4 ± 4 | 32.9 ± 5.4 |
| Racea | ||
| Non-Caucasian (n, %total) | 12, 23.5 | 5, 9.8 |
| Caucasian (n, %total) | 14, 27.5 | 20, 39.2 |
| Pericardial fat (cm3) | 218.6 ± 51.4 | 414.2 ± 100.9 a |
| PAI-1 (ng/mL) | 2.8 [1.1] | 5.4 [3.3]a |
| C-reactive protein (mg/L) | 2 [2.4] | 1.9 [2] |
| Duration of diabetes (years) | 7.5 [12.3] | 6 [8] |
| Diabetic medications | ||
| Diet controlled (n, % total) | 2, 3.9 | 3, 5.9 |
| Monotherapy (n, % total) | 11, 21.6 | 9, 17.6 |
| ≥Two medications (n,% total) | 13, 25.5 | 13, 25.5 |
| Insulin use (n,% total) | 7, 13.7 | 2, 3.9 |
| Hemoglobin A1c (%) | 7 [1.1] | 6.6 [1.1]b |
| HOMA-IR [(glucose (mg/dL) × insulin (μIU/mL)/405)] | 3 ± 2.3 | 2.7 ± 1.8 |
| Blood pressure | ||
| Systolic blood pressure (mmHg) | 123.5 ± 12.1 | 126.2 ± 14.2 |
| Diastolic blood pressure (mmHg) | 72.2 [11.7] | 74.3 [8.7] |
| Amlodipine use (n, % total) | 8, 15.7 | 9, 17.6% |
| Lipids | ||
| Total cholesterol (mg/dL) | 142.5 [34] | 149 [35.5] |
| Triglycerides (mg/dL) | 102.5 [86] | 110 [54.5] |
| High-density lipoprotein (mg/dL) | 43.5 ± 10.1 | 42.3 ± 14.5 |
| Low-density lipoprotein (mg/dL) | 79 ± 29.2 | 80 ± 23 |
| Statin use (n, % total) | 20 39.2 | 20, 39.2% |
| 24-hr urine creatinine (mg/total volume) | 1435.5 ± 446 | 1667 ± 337.6b |
| 24-hr urine sodium (mEq/total volume) | 272.5 [98] | 275 [85.3] |
| 24-hr urine aldosterone (μg/total volume) | 4 [6.8] | 7.5 [8] |
| Aldosterone, baseline (ng/dL) | 2.5 [0.7] | 2.5 [1.6] |
| Aldosterone, stimulated (ng/dL) | 8.3 [6] | 9.7 [3.9] |
| Myocardial ECV | 0.4 ± 0.1 | 0.4 ± 0.1 |
| CFR | 2.32 [0.8] | 2.3 [1] |
| ASCVD 10-year risk score (%) | 7 [7] | 11.1 [10.9] |
| ASCVD lifetime risk score (%) | 50 [19] | 69 [9.5]a |
P ≤ 0.01 between groups.
P ≤ 0.05 between groups.
Data are presented as mean ± standard deviation or as median [interquartile range].
ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CFR, coronary flow reserve; ECV, extracellular volume; HOMA-IR, homeostatic model assessment of insulin resistance; PAI-1, plasminogen activator inhibitor-1.
PAI-1, pericardial fat, and cardiometabolic risk factors
PAI-1 levels were similar between males and females (4.4 ± 2.5 vs. 3.7 ± 1.7 ng/mL, P = 0.32) and between Caucasians and non-Caucasians (4.5 ± 2.4 vs. 3.6 ± 1.8 ng/mL, P = 0.16). Subjects with above-median pericardial fat had significantly higher PAI-1 levels (P < 0.001) and lifetime atherosclerotic cardiovascular disease (ASCVD) risk scores (P < 0.01) compared to those with below-median pericardial fat. However, there were no differences between below- and above-median pericardial fat in other cardiovascular risk factors, including blood pressure, lipid levels, C-reactive protein (CRP), homeostatic model assessment of insulin resistance (HOMA-IR), CFR, or myocardial ECV. In addition, there was no difference between below- and above-median pericardial fat with amlodipine or statin medication use.
PAI-1 was positively correlated with pericardial fat (β = 0.72, r = 0.72, P < 0.001) (Fig. 2), HOMA-IR (r = 0.31, P = 0.03), and triglycerides (r = 0.27, P = 0.05). The correlation between PAI-1 and BMI was not statistically significant (r = 0.25, P = 0.08). In addition, PAI-1 did not correlate with CFR and myocardial ECV (r = 0.25, P = 0.08; r = 0.02, P = 0.91, respectively), ASCVD, or the other factors, including CRP, displayed in Table 2.
FIG. 2.
Pericardial fat is positively correlated with PAI-1; (n = 51; equation of regression line: y = 0.0013x + 0.1675). PAI-1, plasminogen activator inhibitor-1.
Table 2.
Pearson's Correlations with Plasminogen Activator Inhibitor-1
| Variable | r | p | n |
|---|---|---|---|
| Age | 0.10 | 0.50 | 51 |
| Body mass index | 0.25 | 0.08 | 51 |
| Pericardial fata | 0.72 | <0.001 | 51 |
| C-reactive proteinc | 0.00 | 0.98 | 50 |
| Duration of diabetesc | 0.10 | 0.49 | 51 |
| Hemoglobin A1cc | 0.03 | 0.86 | 50 |
| HOMA-IR [(glucose × insulin/405)]b,c | 0.31 | 0.03 | 51 |
| Systolic blood pressure | 0.22 | 0.12 | 51 |
| Diastolic blood pressurec | 0.05 | 0.74 | 51 |
| Triglyceridesb,c | 0.27 | 0.05 | 51 |
| Cholesterolc | 0.09 | 0.55 | 51 |
| High-density lipoproteinc | 0.16 | 0.25 | 51 |
| 24-hr urine creatinine | 0.17 | 0.22 | 51 |
| 24-hr urine sodium | 0.07 | 0.62 | 51 |
| 24-hr urine aldosteronec | 0.25 | 0.07 | 51 |
| Aldosterone, baselinec | 0.10 | 0.49 | 51 |
| Angiotensin II-stimulated aldosteronec | 0.01 | 0.95 | 44 |
| Myocardial ECV | 0.02 | 0.91 | 51 |
| CFRc | 0.25 | 0.08 | 50 |
| ASCVD 10-year riskc | 0.16 | 0.26 | 51 |
| ASCVD lifetime riskc | 0.24 | 0.09 | 51 |
P ≤ 0.01.
P ≤ 0.05.
Represents a variable log-transformed for Pearson's correlation.
ASCVD, atherosclerotic cardiovascular disease; CFR, cornoary flow reserve; HOMA-IR, homeostatic model assessment of insulin resistance.
In a multivariable regression model including age, sex, BMI, HOMA-IR, and triglycerides, pericardial fat was independently associated with PAI-1 (β = 0.80, P < 0.001) (displayed in Table 3). The removal of five participants without clinical evidence of CVD, but with evidence of coronary artery disease on imaging analysis did not affect the significance of the results.
Table 3.
Multivariable Regression Analysis of Predictors of Plasminogen Activator Inhibitor-1
| Model | R-squared (r2) | Effect estimatea | P |
|---|---|---|---|
| 1b | 0.52 | 0.72 | <0.001 |
| 2c | 0.57 | 0.79 | <0.001 |
| 3d | 0.58 | 0.83 | <0.001 |
| 4e | 0.59 | 0.81 | <0.001 |
| 5f | 0.60 | 0.81 | <0.001 |
| 6g | 0.60 | 0.80 | <0.001 |
Effect estimate (β coefficient) for pericardial fat in each model.
Pericardial fat unadjusted.
Pericardial fat adjusted for age and sex.
Pericardial fat adjusted for age, sex, and BMI.
Pericardial fat adjusted for age, sex, BMI, and triglycerides.
Pericardial fat adjusted for age, sex, BMI, and HOMA-IR.
Pericardial fat adjusted for age, sex, BMI, triglycerides, and HOMA-IR.
BMI, body mass index; HOMA-IR, homeostatic model assessment of insulin resistance.
To assess whether antidiabetic medications had an impact on PAI-1 levels in our participants, we performed an exploratory analysis focused on the most frequently used antidiabetic medications in the study. PAI-1 levels were similar in those on and off metformin (4.2 ± 2.3 vs. 3.8 ± 1.7 ng/mL; P = 0.68), on and off sulfonylureas (4.8 ± 2.9 vs. 3.8 ± 1.7 ng/mL; P = 0.13), and on and off insulin (3.2 ± 0.9 vs. 4.3 ± 2.4 ng/mL; P = 0.19).
Discussion
To our knowledge, this study is the first to investigate the association between PAI-1 and pericardial fat in individuals with diabetes. We demonstrate that PAI-1 levels positively correlate with pericardial fat volume in males and females with T2DM, no prior history of CVD, and good cardiometabolic control. The association between PAI-1 and pericardial fat was independent of other factors known to be associated with PAI-1 (e.g., BMI, triglycerides, and insulin sensitivity).
Despite ongoing efforts to improve control of glycemia, blood pressure, and lipids, individuals with T2DM have an increased risk of CVD.17 PAI-1 is a cardiovascular risk factor and is thought to have a role in thrombotic vascular disease.18 An increased PAI-1 gene expression has been observed in atherosclerotic plaque.19 Relative to individuals without diabetes, individuals with T2DM have increased PAI-1 in comparably obstructive excised segments of diseased coronary arteries.20 Furthermore, PAI-1 levels are increased in diabetes.21 Consistent with this finding, PAI-1 levels were elevated in our study population compared with published values of PAI-1 in healthy subjects (∼1.5 ng/mL) assessed at a similar time of day.22
Prior studies have shown that PAI-1 levels are positively associated with BMI,23 triglycerides,24 and measures of insulin sensitivity.2 We found similar associations in our cohort of individuals with T2DM. However, these associations were no longer apparent when pericardial fat was included in the multivariable regression model. This raises the possibility that pericardial fat may have unique characteristics. Pericardial fat is composed of epicardial fat (fat present between the myoepicardium and the visceral pericardium) and paracardial fat (fat situated external to the parietal pericardium).25 Epicardial fat is supplied by branches of the coronary arteries and shares a microcirculation with the myocardium. Thus, it has been suggested that there may be a direct influence of epicardial fat on coronary vasculature and myocardium, perhaps through paracrine or vasocrine secretion of bioactive molecules.26 Indeed, epicardial fat is associated with fatal and nonfatal coronary events in the general population, independent of traditional cardiovascular risk factors.27
When primary adult rat cardiomyocytes are incubated in conditioned media generated from explants of epicardial adipose tissue biopsies obtained from humans with T2DM, contractile function is markedly impaired and insulin-stimulated signaling is significantly blunted.28 The authors found that the secretory profile (PAI-1 was not assessed) of epicardial fat was considerably different from paracardial fat and the secretion patterns differed between individuals with and without diabetes. In addition, they showed that the increased secretory profile from epicardial fat in individuals with T2DM (compared to individuals without diabetes) contributed to cardiomyocyte dysfunction in rat cells. In total, the above evidence suggests that diabetes-specific secretory changes from epicardial fat could contribute to the pathogenesis of T2DM-related cardiovascular dysfunction.
In this study, CFR and ECV did not significantly correlate with PAI-1 on univariate analysis. CFR is reduced in individuals with diabetes versus nondiabetic controls.29 CFR in our study population was consistent with previously published values for individuals with T2DM.30 Myocardial ECV for our study population was elevated compared with published values of myocardial ECV in healthy subjects,31 consistent with studies showing increased myocardial ECV in individuals with diabetes versus nondiabetic controls.15 The lack of significant correlation between PAI-1 and CFR, and ECV may suggest that these factors, all of which are affected in diabetes, may be regulated through distinct mechanisms.
Our study has some important limitations to address. The study was cross-sectional and does not demonstrate cause and effect. The number of participants studied may not allow us to determine whether specific antidiabetic medications affect PAI-1 levels. The sensitivity of our imaging was not sufficient to reliably identify the pericardial lining with each axial slice, likely due to the potential temporal motion artifact inherent to the cardiovascular MRI technique.12 Consequently, we quantified pericardial adipose tissue in total and did not separate the volume into its components of epicardial and paracardial adipose tissues. We did not assess abdominal visceral adipose tissue, which is known to significantly correlate with PAI-1,32 so we cannot determine the relative importance of pericardial and abdominal visceral fat in predicting PAI-1 levels. The one study examining the relationship between epicardial fat and inflammatory markers reported, in 27 females with morbid obesity, but not diabetes, that epicardial fat thickness correlated with PAI-1 independent of abdominal visceral adipose tissue.13 This suggests a unique relationship between epicardial fat and PAI-1 levels.
Our study has multiple advantages. MRI technique is noninvasive, without radiation, and able to calculate a total fat volume. We performed our assessments in participants under good cardiometabolic control in our Center for Clinical Investigation to control for and accurately assess factors that have been associated with PAI-1 such as BMI,23 triglycerides,24 aldosterone,33 glycemia,2 circadian rhythm,22 and blood pressure.34 Given our control of cardiometabolic factors, our population may not be representative of the average population with T2DM, and this may explain why our PAI-1 levels, although elevated compared to healthy controls, were not as high as some reports of individuals with T2DM.21
Conclusion
Our study suggests that in individuals with well-controlled diabetes, PAI-1 is positively associated with pericardial fat. Future studies are needed to determine whether, in T2DM, pericardial fat affects the coronary arterial system, whether the effects of its components (epicardial and paracardial fat) differ, and whether these effects impact cardiac function in humans with T2DM.
Acknowledgments
The authors would like to thank all of the study participants as well as the nursing and administrative staff at the Center for Clinical Investigation at Brigham and Women's Hospital and the Harvard Clinical and Translational Science Center who made this study possible.
Funding
This work was supported by the National Institutes of Health [award number UL-1TR-000170 (Harvard Catalyst, Harvard Clinical and Translational Science Center)]; by the National Heart, Lung, and Blood Institute of the National Institutes of Health [award numbers K24-HL-103845 (G.K.A.); R01-HL-087060 (G.K.A.); T32-HL-007609 (A.D.R.); K23-HL-111771 (A.V.)]; by the National Institutes of Diabetes and Digestive and Kidney Disease of the National Institutes of Health [award number R01-DK-107407 (A.V.)]; and by the Doris Duke Charitable Foundation [Grant Number 2015085 (A.V.)].
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fox CS, Golden SH, Anderson C, et al. . Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in ight of recent evidence: A scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2015;132:691–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider DJ, Sobel BE. PAI-1 and diabetes: A journey from the bench to the bedside. Diabetes Care 2012;35:1961–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimomura I, Funahashi T, Takahashi M, et al. . Enhanced expression of PAI-1 in visceral fat: Possible contributor to vascular disease in obesity. Nat Med 1996;2:800–803 [DOI] [PubMed] [Google Scholar]
- 4.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 2005;3:35–45 [DOI] [PubMed] [Google Scholar]
- 5.Vaughan DE. PAI-1 and atherothrombosis. J Thromb Haemost 2005;3:1879–1883 [DOI] [PubMed] [Google Scholar]
- 6.Xanthakis V, Enserro DM, Murabito JM, et al. . Ideal cardiovascular health: Associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014;130:1676–1683 [DOI] [PubMed] [Google Scholar]
- 7.Tofler GH, Massaro J, O'Donnell CJ, et al. . Plasminogen activator inhibitor and the risk of cardiovascular disease: The Framingham Heart Study. Thromb Res 2016;140:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim MM. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes Rev 2010;11:11–18 [DOI] [PubMed] [Google Scholar]
- 9.Fain JN, Madan AK, Hiler ML, et al. . Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004;145:2273–2282 [DOI] [PubMed] [Google Scholar]
- 10.Fox CS, Gona P, Hoffmann U, et al. . Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: The Framingham Heart Study. Circulation 2009;119:1586–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding J, Kritchevsky SB, Harris TB, et al. . The association of pericardial fat with calcified coronary plaque. Obesity (Silver Spring) 2008;16:1914–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaushik M, Reddy YM. Distinction of “fat around the heart”. J Am Coll Cardiol 2011;58:1640.; author reply 1640–1641. [DOI] [PubMed] [Google Scholar]
- 13.Malavazos AE, Ermetici F, Cereda E, et al. . Epicardial fat thickness: Relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr Metab Cardiovasc Dis 2008;18:523–530 [DOI] [PubMed] [Google Scholar]
- 14.Murthy VL, Naya M, Foster CR, et al. . Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TC, Piehler KM, Kang IA, et al. . Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J 2014;35:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg R, Rao AD, Baimas-George M, et al. . Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannucci E, Dicembrini I, Lauria A, and Pozzilli P. Is glucose control important for prevention of cardiovascular disease in diabetes? Diabetes Care 2013;36Suppl 2:S259–S263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med 2000;342:1792–1801 [DOI] [PubMed] [Google Scholar]
- 19.Schneiderman J, Sawdey MS, Keeton MR, et al. . Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proc Natl Acad Sci U S A 1992;89:6998–7002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sobel BE, Woodcock-Mitchell J, Schneider DJ, et al. . Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy specimens from type 2 diabetic compared with nondiabetic patients: A potential factor predisposing to thrombosis and its persistence. Circulation 1998;97:2213–2221 [DOI] [PubMed] [Google Scholar]
- 21.Yarmolinsky J, Bordin Barbieri N, Weinmann T, et al. . Plasminogen activator inhibitor-1 and type 2 diabetes: A systematic review and meta-analysis of observational studies. Sci Rep 2016;6:17714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheer FA, Shea SA. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014;123:590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vague P, Juhan-Vague I, Chabert V, et al. . Fat distribution and plasminogen activator inhibitor activity in nondiabetic obese women. Metabolism 1989;38:913–915 [DOI] [PubMed] [Google Scholar]
- 24.Hamsten A, Wiman B, de Faire U, et al. . Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. N Engl J Med 1985;313:1557–1563 [DOI] [PubMed] [Google Scholar]
- 25.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: A review of research and clinical applications. J Am Soc Echocardiogr 2009;22:1311–1319; quiz 1417–1418. [DOI] [PubMed] [Google Scholar]
- 26.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nat Rev Endocrinol 2015;11:363–371 [DOI] [PubMed] [Google Scholar]
- 27.Mahabadi AA, Berg MH, Lehmann N, et al. . Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf recall study. J Am Coll Cardiol 2013;61:1388–1395 [DOI] [PubMed] [Google Scholar]
- 28.Greulich S, Maxhera B, Vandenplas G, et al. . Secretory products from epicardial adipose tissue of patients with type 2 diabetes mellitus induce cardiomyocyte dysfunction. Circulation 2012;126:2324–2334 [DOI] [PubMed] [Google Scholar]
- 29.Nahser PJ, Jr., Brown RE, Oskarsson H, et al. . Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation 1995;91:635–640 [DOI] [PubMed] [Google Scholar]
- 30.Di Carli MF, Janisse J, Grunberger G, et al. . Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003;41:1387–1393 [DOI] [PubMed] [Google Scholar]
- 31.Ugander M, Oki AJ, Hsu LY, et al. . Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J 2012;33:1268–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sam S, Haffner S, Davidson MH, et al. . Relation of abdominal fat depots to systemic markers of inflammation in type 2 diabetes. Diabetes Care 2009;32:932–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown NJ, Kim KS, Chen YQ, et al. . Synergistic effect of adrenal steroids and angiotensin II on plasminogen activator inhibitor-1 production. J Clin Endocrinol Metab 2000;85:336–344 [DOI] [PubMed] [Google Scholar]
- 34.Landin K, Tengborn L, Smith U. Elevated fibrinogen and plasminogen activator inhibitor (PAI-1) in hypertension are related to metabolic risk factors for cardiovascular disease. J Intern Med 1990;227:273–278 [DOI] [PubMed] [Google Scholar]