Abstract
We explored the duration of immune responses and the effect of a late third HIV-modified vaccinia virus Ankara (MVA) boost in HIV-DNA primed and HIV-MVA boosted Tanzanian volunteers. Twenty volunteers who had previously received three HIV-DNA and two HIV-MVA immunizations were given a third HIV-MVA immunization 3 years after the second HIV-MVA boost. At the time of the third HIV-MVA, 90% of the vaccinees had antibodies to HIV-1 subtype C gp140 (median titer 200) and 85% to subtype B gp160 (median titer 100). The majority of vaccinees had detectable antibody-dependent cellular cytotoxicity (ADCC)–mediating antibodies, 70% against CRF01_AE virus–infected cells (median titer 239) and 84% against CRF01_AE gp120–coated cells (median titer 499). A high proportion (74%) of vaccinees had IFN-γ ELISpot responses, 63% to Gag and 42% to Env, 3 years after the second HIV-MVA boost. After the third HIV-MVA, there was an increase in Env-binding antibodies and ADCC-mediating antibodies relative to the response seen at the time of the third HIV-MVA vaccination, p < .0001 and p < .05, respectively. The frequency of IFN-γ ELISpot responses increased to 95% against Gag or Env and 90% to both Gag and Env, p = .064 and p = .002, respectively. In conclusion, the HIV-DNA prime/HIV-MVA boost regimen elicited potent antibody and cellular immune responses with remarkable durability, and a third HIV-MVA immunization significantly boosted both antibody and cellular immune responses relative to the levels detected at the time of the third HIV-MVA, but not to higher levels than after the second HIV-MVA.
Keywords: : HIV, vaccine, DNA, MVA, immune response
Introduction
The development of a safe and efficacious preventive HIV vaccine capable of inducing long-lasting humoral and cellular immune responses remains a global health priority.1,2 Several HIV vaccine trials at various phases have been conducted worldwide.2–4 Only one phase III trial, RV144, has shown a modest efficacy of 31.2% at 42 months. RV144 evaluated the protective effect of a prime–boost regimen consisting of a canarypox-vectored vaccine (ALVAC-HIV vCP1521) and an HIV envelope protein (AIDSVAX® B/E) among HIV-uninfected individuals who were at low risk of acquiring HIV infection.5 In the analysis of immune correlates of risk of HIV infection in the RV144 trial, it was found that antibodies against the V1/V2 region of HIV-1 Env inversely correlated with the risk of HIV infection, while the presence of IgA Env-binding antibodies was associated with a lack of protection.6 Furthermore, antibody-dependent cellular cytotoxicity (ADCC)–mediating antibodies and antibodies to the V3 region correlated with reduced risk of HIV infection in vaccinees with low IgA Env binding antibody titers.6,7 Analysis of cellular immune responses confirmed the specificity of anti-V2 reactivity.8 The efficacy of an HIV vaccine may depend on the longevity of immune responses following vaccination. The parent for the modified vaccinia virus Ankara (MVA) vector, a smallpox vaccine, has the ability to establish long-lived antibody responses.9 In RV144, the majority (95%) of vaccinees had binding antibodies to gp120 HIV antigens 2 weeks postfinal vaccination, which waned significantly (to 19%) by 6 months and had reached almost undetectable levels by 28 weeks after the last immunization.10
In our previous HIVIS03 trial, we demonstrated that priming of healthy Tanzanian volunteers with three HIV-DNA and boosting with two HIV-MVA immunizations elicited high levels of neutralizing antibodies (NAb) in up to 83% of vaccinees using a peripheral blood mononuclear cell (PBMC)/Infectious Molecular Clone (IMC) neutralization assay.11 However, depletion of natural killer cells from the PBMC targets abrogated the NAb activity, indicating that the neutralizing activity was due to Fc receptor–mediated antibody functions. ADCC-mediating antibodies were detected in the majority (97%) of the vaccinees 1 month after the second HIV-MVA immunization.12 Twenty-eight (97%) of 29 vaccinees exhibited HIV-specific IFN-γ ELISpot responses, 27 (93%) to Gag and 23 (79%) to Env after the second HIV-MVA vaccination. In the present study, we explored the duration of immune responses and the effect of a third HIV-MVA boost in the HIVIS03 vaccinees 3 years after receipt of three HIV-DNA and two HIV-MVA vaccinations.
Materials and Methods
Participants
Participants were recruited among volunteers who participated in the previous HIVIS03 trial.11 They had previously received three HIV-DNA immunizations at weeks 0, 4, and 12 and two HIV-MVA immunizations at months 9 and 21. The DNA prime included HIV-1 gp160 subtypes A, B, and C; Rev B; Gag A and B, as well as RTmut B, and the MVA Chiang Mai double recombinant (CMDR, HIV-MVA) boost expressed CRF01_AE HIV-1 Env subtype E and Gag-Pol subtype A. Out of 40 volunteers who had received the active immunizations, 30 had received all five immunizations. During unblinding of the HIVIS03 study, the volunteers were asked if they would participate in a follow-up study in which they would receive a third HIV-MVA immunization. The HIVIS03 volunteers who had accepted to participate in the follow-up study were then called for further information regarding the study plan and 28 volunteers indicated their willingness to participate in this study (HIVIS06). Volunteers were then invited for screening on a first come, first served basis so as to obtain our target number of 20 volunteers. HIV negative status was confirmed by Roche HIV-1 DNA PCR version 1.5 assay. All volunteers provided written informed consent before enrollment into the present study.
The vaccination schedule for the HIVIS03/06 trial is shown in Table 1. HIVIS06 was an open labeled study whereby all volunteers received a third immunization of 1 ml containing 108 plaque forming units (pfu) of recombinant HIV-MVA vaccine administered intramuscularly (i.m.) in the left deltoid muscle. The interval between the second HIV-MVA (fifth immunization at months 21) and the third HIV-MVA (sixth immunization at months 53–59) was ∼3 years (median 35 months, range 32–38). Of the 20 vaccinees, 9 had received 1.0 mg HIV-DNA intradermally (i.d.) by the Bioject device and 11 had been given 3.8 mg HIV-DNA i.m. by needle.
Table 1.
Vaccination Schedule for HIVIS03 and HIVIS06 Trials
| HIVIS03 trial [detailed in Bakari et al.11] | HIVIS06 trial | ||||
|---|---|---|---|---|---|
| Vaccine | N | HIV-DNA immunization | HIV-MVA immunization | N | HIV-MVA immunization |
| Time point | Months 0, 1, and 3 | Months 9 and 21 | Month 53–59 | ||
| Group I | 20 | 3.8 mg i.m. | 108 pfu i.m. | 11 | 108 pfu i.m. |
| Group II | 20 | 1 mg i.d. | 108 pfu i.m. | 9 | 108 pfu i.m. |
| Group IIIa | 10 | Saline i.m. | Saline i.m. | ||
| Group IIIb | 10 | Saline i.d. | Saline i.m. | ||
All DNA immunizations were given by a needle-free injection device (Biojector 2000; Bioject Medical Technologies). At each i.d. immunization at months 0, 1, and 3, three injections of 0.1 ml were given in the skin over the left deltoid and two injections of 0.1 ml over the right deltoid for a total of 1.0 mg per immunization. The total i.d. dose was thus 3 mg. The i.m. immunization was given in the deltoid muscles for a total of 3.8 mg per immunization. The total i.d. dose was thus 11.4 mg. The immunogens were divided so that the env/rev plasmids were given in the left arm and the gag/pol plasmids in the right arm. All HIV-MVA vaccinations were delivered using needle and syringe, and 1 ml containing 108 pfu of recombinant HIV-MVA vaccine was administered i.m. in the left deltoid muscle.
MVA, modified vaccinia virus Ankara; i.d., intradermally; i.m., intramuscularly; pfu, plaque forming units.
The HIVIS06 was registered with Clinical trials.gov registry with a clinical trial Registration number NCT01461447.
Safety assessments
Reactogenicity was evaluated by solicited local and systemic events recorded in diary cards that were filled out for the week following the vaccination and complemented with interviews at 2 weeks after the immunization. Blood samples were collected at screening and every 2 weeks for complete blood count and chemistry (alanine aminotransferase, total and direct bilirubin, random blood glucose, and creatinine). Urinalysis and pregnancy tests were performed at screening, before immunization and on the final visit. Twelve lead electrocardiographs and troponin testing were performed before HIV-MVA vaccination and 2 weeks postvaccination to monitor for peri-myocarditis.
HIV-specific binding antibodies
Testing of binding antibodies to HIV-1 IIIB subtype B gp160 (Advanced Biotechnologies, Inc., Columbia, MD) or recombinant HIV-196ZM651 subtype C gp140 (kindly provided by Programme EVA, Centre for AIDS Reagents, NIBSC, Potters Bar, United Kingdom) was performed by enzyme-linked immunosorbent assays (ELISA) as previously described.13 Data were reported as reciprocal endpoint titers.
ADCC GranToxiLux assay
ADCC-mediating antibodies were detected according to the previously described flow cytometry GranToxiLux (GTL) based assay using gp120-coated target cells.14 The CEM.NKRCCR5 target cells were coated with recombinant gp120 HIV-1 protein subtype E from CM243 CRF01_AE (GenBank Accession No. AY214109; Protein Sciences Corporation). The results were expressed as percentage of Granzyme B (GzB) activity positive cells.14
ADCC luciferase assay
An ADCC assay, which uses Env.IMC.LucR virus–infected cells as targets, was used as previously described.12,15 Subtype CRF01_AE HIV-CM235-2-LucR.T2A.ecto/293T (IMCCM235) (GenBank Accession No. AF259954.1) and SF162.LucR.T2A.ecto/293T (IMCSF162) (GenBank Accession No. EU123924) were used in this assay following transfection of 293T/17 cells with proviral IMC plasmid DNA. ADCC activity was measured as the percentage of loss of luciferase activity observed in the presence of serum.
Neutralization assay
NAb in sera were measured against subtype B SF162 and CRF01_AE CM 244 virus isolates in a PBMC-based assay using a p24 read out and against SF162 and subtype C 93MW965.26 pseudotyped viruses in a TZM-bl assay as described previously.16
IFN-γ ELISpot assay
IFN-γ ELISpot assay was performed on freshly isolated PBMCs using the h-IFN-γ ELISpot PLUS Kit in a two-step detection system (Mabtech, Nacka, Sweden) as previously detailed.11 Results were expressed as spot-forming cell (SFC)/106 PBMCs. ELISpot responses were considered positive if the number of SFC/106 PBMCs was >4 times the background and baseline value and >55 SFC/106 PBMCs. Data were excluded from analyses if the background responses in medium wells exceeded 60 SFC/106 PBMCs.
Anti-vaccinia neutralization assay
Vaccinia neutralizing antibodies were measured using inhibition of plaque-forming virus vaccinia (strain Elstree) by serum dilutions as described previously.17 Antibody titers more than four were considered positive.
Statistical methods
The analysis of immunological data was performed using GraphPad PRISM version 6. For pairwise analysis, the Wilcoxon matched-pair signed rank test was used to compare the magnitudes of humoral and cellular immune responses before and after the vaccinations. For analysis of ADCC data, any samples with a value of 0 were arbitrarily given a value of 10. The Mann–Whitney test was used for comparison of IFN-γ ELISpot responses in i.d. versus i.m HIV-DNA vaccine recipients. Fischer's exact test was used for comparisons of frequencies. A two-sided p-value of <.05 was considered statistically significant.
Ethics statement
The HIVIS06 trial protocol was approved by Tanzania's National Health Research Ethics Committee, as well as the Senate Research and Publications Committee [local Institutional Review Board (IRB)] of the Muhimbili University of Health and Allied Sciences (MUHAS). The Tanzania Food and Drugs Authority approved use of the vaccine candidate products for humans in Tanzania. The HIVIS06 trial was also approved by the Regional Ethics Committee, Stockholm, Sweden. The HIVIS06 study was conducted in accordance with the International Conference on Harmonization and Good Clinical Practice guidelines. Written informed consent was obtained from all clinical trial participants.
Results
Enrollment
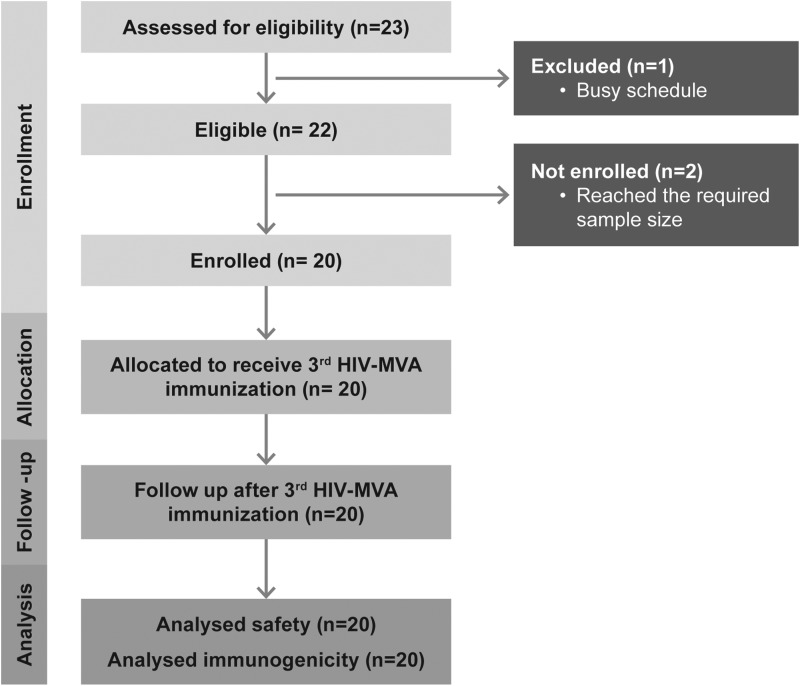
Screening of the participants began in March 2012 and the last participants were followed up in June 2012. Out of the 28 who had indicated their willingness to be screened, we screened 23 HIVIS03 volunteers. At screening, one participant informed that he had a busy schedule. Twenty-two participants were screened, and we ultimately enrolled 20 healthy HIV-noninfected volunteers who had participated in the HIVIS03 study.11 Two volunteers were not enrolled as the number of required volunteers was reached (Fig. 1). Of the 20 volunteers, 18 were males and 2 were females. The median age of the participants was 34 years (range 27–51 years). Eleven volunteers had a scar indicating earlier smallpox vaccination.
FIG. 1.
The number of volunteers screened, enrolled, allocated, and analyzed in the trial.
Safety and tolerability
The HIV-MVA vaccine delivered i.m. with needle was well tolerated. Eighteen reactogenicity events were recorded in seven volunteers of whom 15 were considered as mild (all definitely related) and 2 were graded as moderate (chills and local swelling definitely/probably related in the same volunteer). These clinical events occurred within a week from the time of immunization. Two further events of mild conjunctivitis and one event of severe abdominal pain were recorded 2 weeks after vaccination. These events were not considered to be related to the immunizations. None of the volunteers had a serious adverse event. There were no clinically significant changes in ECG readings or troponin levels from baseline to 2 weeks after the third HIV-MVA injection.
Binding antibody responses
Binding antibodies to HIV-1 subtype C gp140 and subtype B gp160 were detected in 20/20 (100%) and 17/19 (89%) vaccinees, respectively, 4 weeks after the second HIV-MVA and in 18/20 (90%) and 17/20 (85%) vaccinees, respectively, 3 years after the second HIV-MVA, at the time of the third HIV-MVA vaccination. Four weeks after the third HIV-MVA vaccination, all 20 (100%) vaccinees had antibodies to gp140, whereas 17/20 (85%) exhibited antibodies to gp160 (Table 2).
Table 2.
Frequency of Immune Responses After the Second, at the Time of Third, and After the Third HIV-MVA Vaccination
| After the second HIV-MVA | At the time of the third HIV-MVA | After the third HIV-MVA | |
|---|---|---|---|
| Assay and antigen | Positive/total number tested (%) | Positive/total number tested (%) | Positive/total number tested (%) |
| ELISA binding antibody | |||
| gp140 96ZM651 C | 20/20 (100)a | 18/20 (90) | 20/20 (100)a |
| gp160 IIIB | 17/19 (89)a | 17/20 (85) | 17/20 (85)a |
| ADCC-GTL | |||
| gp120 CM243 AE | 16/18 (88)a | 16/19 (84) | 18/19 (95)a |
| ADCC-luciferase | |||
| IMCCM235 AE | 18/19 (95)a | 14/20 (70) | 14/20 (70)a |
| IMCSF162 B | 13/18 (72)a | 9/19 (47) | 14/19 (74)a |
| Neutralization | |||
| PBMC assay (SF162, CM244) | 0/20a | ND | 0/20a |
| TZM-bl assay (SF162, MW965.26) | 0/20a | ND | 0/20a |
| ELISpot | |||
| Gag SMIc | ND | 13/19 (68) | 17/20 (85)b |
| Gag CMDRd | 16/18 (89)b | 12/19 (63) | 18/20 (90)b |
| Env CMDRe | 16/18 (89)b | 8/19 (42) | 18/20 (90)b |
| Gag or Env | 16/18 (89)b | 14/19 (74) | 19/20 (95)b |
| Anti-vaccinia neutralization | |||
| Strain Elstree | 19/20 (95)a | 20/20 (100) | 20/20 (100)a |
Testing performed 4 weeks after the HIV-MVA vaccination.
Testing performed 2 weeks after the HIV-MVA vaccination.
Gag-specific peptide pool corresponding to the DNA vaccine.
Gag-specific peptide pool corresponding to the MVA vaccine.
Env-specific peptide pool corresponding to the MVA vaccine.
ADCC-GTL, antibody-dependent cellular cytotoxicity-GranToxiLux assay; IMC, infectious molecular clone; ND, testing not done; PBMC, peripheral blood mononuclear cell.
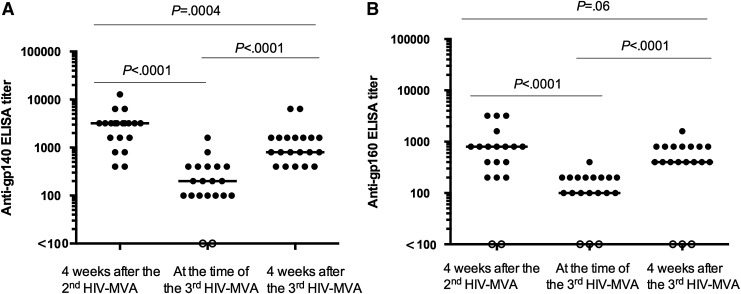
The anti-gp140 titers were significantly higher after the third HIV-MVA [median 800, interquartile range (IQR) 500–1,600] compared to the titers at the time of the third HIV-MVA (median 200, IQR 100–400), p < .0001 (Fig. 2A). Similarly, anti-gp160 titers were also significantly higher 4 weeks after the third HIV-MVA (median 400, IQR 400–800) compared to the titers at the time of the third HIV-MVA (median 100, IQR 100–200), p < .0001 (Fig. 2B). There were no significant differences in anti-gp160 titers of the same individuals tested 4 weeks after the third HIV-MVA (median 400, IQR 400–800) compared to the previously reported anti-gp160 titers tested 4 weeks after the second HIV-MVA (median 800, IQR 200–800), p = .06. However, the anti-gp140 titers were significantly higher after the second HIV-MVA (median 3,200, IQR 1,600–3,200) compared to the titers after the third HIV-MVA (median 800, IQR 500–1,600), p = .0004. Nonetheless, the subtype C gp140 antibody titers correlated with subtype B gp160 antibody titers before and after the third HIV-MVA vaccination r = 0.83, p < .0001 and r = 0.64, p = .002, respectively (data not shown).
FIG. 2.
The magnitude of binding antibodies to (A) gp140 HIV-1 subtype C (n = 20) and (B) gp160 subtype B (n = 19) among the volunteers 4 weeks after the second HIV-MVA and at the time of and 4 weeks after the third HIV-MVA boost. The Wilcoxon matched-pair signed rank test was used to compare the magnitude of responses at different time points. Bars illustrate median values. Open circles indicate negative values.
There was no significant difference in magnitude of binding antibodies to subtype C gp140 or subtype B gp160 between i.d. and i.m DNA-primed vaccinees at the time of the third HIV-MVA, p = .145 and p = .384, respectively, or after the third HIV-MVA boost, p = .486 and p = .516, respectively.
ADCC-mediating antibody responses
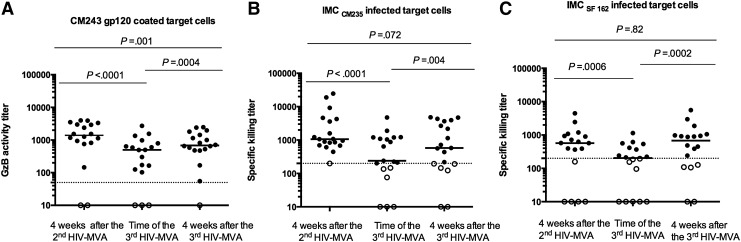
Sera from the 20 vaccinees were tested for ADCC-mediating antibody responses using both gp120-coated target cells and IMC-infected target cells. ADCC responses to subtype E CM243 gp120–coated cells were detected in 16/18 (88%) evaluable vaccinees 4 weeks after the second HIV-MVA and in 16/19 (84%) vaccinees at the time of the third HIV-MVA. The number of vaccinees exhibiting ADCC activity to CM243 increased to 18/19 (95%) 4 weeks after the third HIV-MVA (Table 2), and the magnitude of ADCC responses also increased from a median titer of 499 (IQR 120–839) to a median titer of 683 (IQR 480–1,530), p = .0004 (Fig. 3A). However, the magnitude of ADCC-mediating antibody responses was significantly lower 4 weeks after the third HIV-MVA boost compared to 4 weeks after the second HIV-MVA boost, median titer of 1401 (IQR 806–3085), p = .001 (Fig. 3A).
FIG. 3.
The magnitude of ADCC-mediating antibodies 4 weeks after the second HIV-MVA, at the time of the third, and 4 weeks after the third HIV-MVA boost against (A) CM243 CRF01_AE gp120–coated cells (n = 18), (B) CM235 CRF01_AE–target cells (n = 19), and (C) SF162 subtype B–infected target cells (n = 18). The Wilcoxon matched-pair signed rank test was used for statistics. Bars illustrate median values. Open circles indicate negative values. The dotted line indicates the cutoff for positive values. For the ADCC GranToxiLux assay, the cutoff was at a GzB activity titer of 50, while for the ADCC luciferase assay the cutoff was a specific killing titer of 200. ADCC, antibody-dependent cellular cytotoxicity; GzB, Granzyme B.
ADCC activity to CM235 CRF01_AE–infected target cells was detected in 18/19 (95%) vaccinees 4 weeks after the second HIV-MVA and in 14/20 (70%) of vaccinees both at the time of and 4 weeks after the third HIV-MVA (Table 2). The ADCC-mediating antibody titer to CM235 CRF01_AE–infected target cells was significantly higher after the third HIV-MVA (median 578, IQR 192–3,663) compared to the titer at the time of the third HIV-MVA (median 239, IQR 136–1,198), p = .004, but not significantly different from the titer 4 weeks after the second HIV-MVA (median 1,064, IQR 698–4,258), p = .072 (Fig. 3B).
The frequency of ADCC responses to SF162 subtype B–infected cells was 13/18 (72%) 4 weeks after the second HIV-MVA and 9/19 (47%) before the third HIV-MVA, which increased to 14/19 (74%) 4 weeks after the third HIV-MVA boost (Table 2). The ADCC-mediating antibody titer to SF162 subtype B was significantly higher after the third HIV-MVA (median 681, IQR 123–992), compared to the titer at the time of the third HIV-MVA (median 204, IQR 10–445), p = .0002, but not significantly different from the titer 4 weeks after the second HIV-MVA (median 571, IQR 121–975), p = .82 (Fig. 3C).
There was no significant difference in magnitude of ADCC-mediating antibody responses to subtype E CM243 gp120–coated cells between i.d. and i.m. DNA-primed vaccinees at the time of the third HIV-MVA or after the third HIV-MVA boost, p = .219 and p = .108, respectively. Furthermore, there was no significant difference in magnitude of ADCC-mediating antibody responses to either CM235 CRF01_AE–infected cells or SF162 subtype B–infected cells between i.d. and i.m. DNA-primed vaccinees at the time of the third HIV-MVA, p = .330 and p = .111, respectively, or after the third HIV-MVA boost, p = .268 and p = .450, respectively.
Neutralizing antibody responses
NAb activity was determined in the sera of 20 individuals at baseline before any vaccination, 4 weeks after the second HIV-MVA and 4 weeks after the third HIV-MVA vaccination. There was no demonstrable NAb activity in either the PBMC assay using a p24-read out or the TZM-bl based neutralizing assay (Table 2).
IFN-γ ELISpot responses
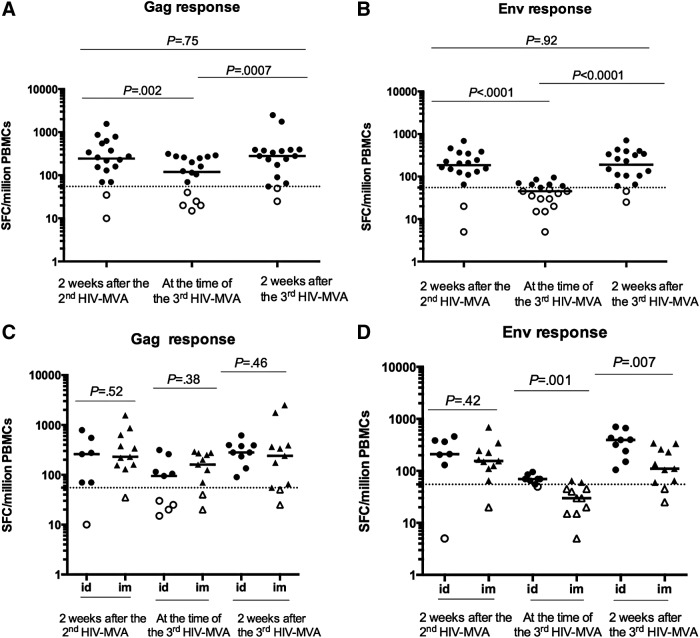
The frequency of IFN-γ ELISpot responses to Gag and Env in vaccinees before and after the third HIV-MVA vaccination is summarized in Table 2. At the time of the third HIV-MVA, 3 years after the second HIV-MVA, an overall 14/19 (79%) vaccinees had IFN-γ ELISpot responses. The response rate to Gag was reduced from 89% to 63% (p = .12) and to Env from 89% to 42% (p = .005) compared to the response rates after the second HIV-MVA vaccination 3 years earlier. Two weeks after the third HIV-MVA, 19/20 (95%) vaccinees had IFN-γ ELISpot responses, 18 (90%) to both Gag and Env. The increase of IFN-γ ELISpot responses was statistically significant to Env (from 8/19 to 18/20, p = .002) but not to Gag (from 12/19 to 18/20, p = .064).
Figure 4 shows the magnitude of the IFN-γ ELISpot responses among the vaccinees at three time points. At the time of the third HIV-MVA boost, the magnitude of responses had waned significantly since the second HIV-MVA, from a median of 245 (IQR 115–570) SFC/million PBMCs to a median of 120 (IQR 24–263) SFC/million PBMCs to Gag (p = .002) and from a median of 185 (IQR 121–350) SFC/million PBMCs to a median of 45 (IQR 28–65) SFC/million PBMCs to Env (p < .0001). Two weeks after the third HIV-MVA immunization, the median responses increased significantly to 280 SFC/million PBMCs (IQR 84–390) against Gag CMDR (p = .0007) and 190 SFC/million PBMCs (IQR, 95–353) against Env CMDR (p < .0001). There was no significant difference in the magnitude of HIV-specific IFN-γ ELISpot responses 2 weeks after the second HIV-MVA compared to 2 weeks after the third HIV-MVA boost (Fig. 4A, B). Notably, the magnitude of IFN-γ ELISpot responses to Env was significantly higher among the volunteers previously HIV-DNA primed i.d. compared to i.m., both at the time of and 2 weeks after the third HIV-MVA boost, p = .001 and p = .007, respectively (Fig. 4D).
FIG. 4.
The magnitude of IFN-γ ELISpot responses to Gag (A) and Env (B) 2 weeks after the second HIV-MVA, at the time of the third, and 2 weeks after the third HIV-MVA boost. HIV-specific IFN-γ ELISpot responses to Gag (C) and Env (D) in the DNA i.d. and i.m. primed vaccinees. ELISpot responses were considered positive if the number of SFCs was >55 SFC/million PBMCs and four times the background value. The dotted line is placed at 55 SFC/million PBMC. Bars illustrate median values. The Wilcoxon matched-pair signed rank test was used to compare the magnitude of responses in 18 paired samples (A, B) and the Mann–Whitney test in (C, D). Open symbols indicate negative values. i.m., intramuscularly; i.d., intradermally; PBMCs, peripheral blood mononuclear cells; SFCs, spot-forming cells.
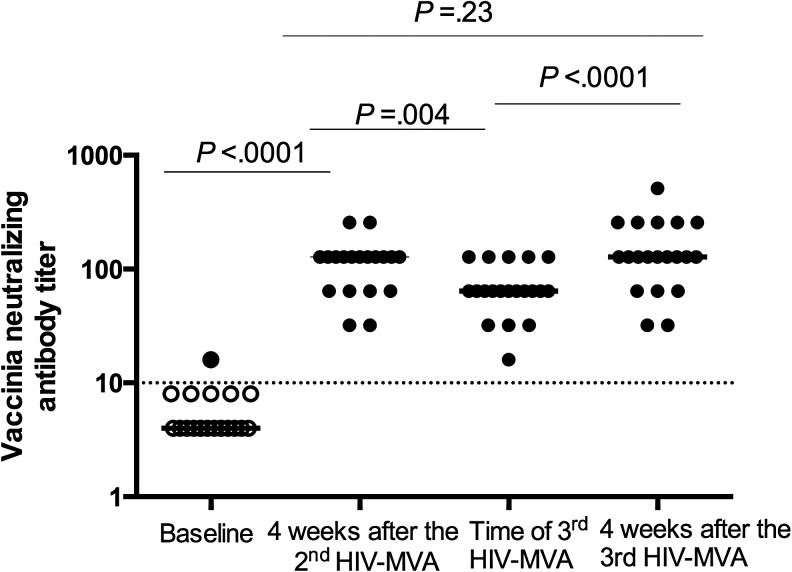
Vaccinia antibody responses
Vaccinia NAb testing was performed on sera collected at baseline, 4 weeks after the second HIV-MVA, and at the time of and 4 weeks after the third HIV-MVA boost (Fig. 5). Only one individual had detectable vaccinia NAb before any HIV-DNA/MVA immunizations. All MVA-immunized individuals (100%) had developed vaccinia-NAb responses 4 weeks after the second HIV-MVA. At the time of the third HIV-MVA immunization, vaccinia-NAb were still detectable in all vaccinees. One month after the third HIV-MVA immunization, the magnitude of vaccinia NAb was significantly increased compared to the titer at the time of the third HIV-MVA from a median of 64 to 128 pfu, p < .0001 (Fig. 5).
FIG. 5.
Vaccinia neutralizing antibody titers in vaccinees tested before any HIV vaccination and after the second and third HIV-MVA boosts. The bars illustrate the median titers. The Wilcoxon matched-pair signed rank test was used to compare the magnitudes at different time points. Open circles indicate negative values. The dotted line indicates cutoff for positive values.
Discussion
In the present study, we demonstrate that priming with three HIV-DNA and boosting with two HIV-MVA immunizations in Tanzanian volunteers elicited high frequencies of potent immune responses lasting >3 years. Furthermore, a third HIV-MVA immunization given to the same vaccinees 3 years after the second HIV-MVA vaccination was safe, well tolerated, and generated potent HIV-specific humoral and cellular immune responses indicating that HIV-DNA/MVA-induced immune responses can be maintained by regular boosting with HIV-MVA despite increased vector induced anti-vaccinia antibodies.
The HIV antibody responses in the vaccinees showed excellent durability with 90% having detectable binding antibodies to subtype C gp140 and 85% to subtype B gp160 3 years after the second HIV-MVA immunization. The response rate had decreased by only 5%–10% indicating that this prime–boost regimen induced long-lasting anti-HIV memory responses. In a study by Goepfert et al., in which two immunizations with JS7 DNA vaccine followed by two immunizations with MVA/HIV62B elicited binding antibody responses to gp140 in 100% of vaccinees 2 weeks after the last vaccination, the proportion of responders had decreased by 15.6% 24 weeks after the final vaccination,18 indicating a shorter durability of HIV antibody responses than in our study. However, they reported that the proportion of responders had not decreased 24 weeks after three immunizations with MVA/HIV62B.18 Following the third HIV-MVA boost in the present study, the frequency of binding antibody responses to HIV-1 subtype C gp140 increased to 100% and the magnitude of antibodies both to subtype C gp140 and subtype B gp160 increased significantly. In a study with the same HIV-MVA-CMDR vaccine without DNA priming, binding antibodies against gp120 were generated in up to 90% of vaccinees after three doses of 108 pfu i.m.19
In the present study, we also found an impressive durability of ADCC-mediating antibodies. Three years after the second HIV-MVA vaccination, 84% of the vaccinees had ADCC-mediating antibodies against CRF01_AE gp120–coated cells and 70% against CRF01_AE-infected cells. The proportion of vaccinees having ADCC-mediating antibody responses to CRF01_AE gp120–coated cells increased to 95% after the third HIV-MVA immunization in the ADCC-GTL assay. Although the ADCC response rates to CM235 CRF01_AE–infected cells were similar at the time of and 4 weeks after the third HIV-MVA boost, the magnitude increased significantly (from a median titer of 239 to 578) after the third HIV-MVA vaccination. These data indicate that three HIV-DNA primes and two or more HIV-MVA boosts can maintain functional ADCC antibodies. Using an assay employing 51Cr labeled CEM.Nkr cells coated with CRF01_AE gp120 (CM243) or subtype B gp120 (MN), Currier et al. reported a lower frequency of ADCC activity in RV158 vaccinees who had received 108 pfu of MVA-CMDR i.m. thrice without HIV-DNA priming.19 In the RV158 study, 40% of the vaccinees showed ADCC reactivity to CRF01_AE gp120 and 30% to subtype B gp120 2 weeks after the third MVA-CMDR.19
In the present study, no HIV NAb were detected at the time of and/or after the third HIV-MVA boost using either PBMC or TZM-bl based neutralization assays. We have previously reported that NAb responses against CM235 CRF01_AE were detected in 83% of vaccinees when a PBMC/IMC assay was used, while no NAb were detected using a TZM-bl/pseudovirus assay.11 The apparent discrepancy can be explained by the fact that in the present study a standard PBMC-based assay with virus grown in PBMC and an HIV p24 antigen-based assay were used instead of the PBMC/IMC assay. In the former assay, serum and virus inocula are washed out during the experiment, whereas in the PBMC/IMC assay there is no washing of cells, and thus, antibodies remain with the cells and virus for the entire duration of the assay allowing other effector cells present in the PBMC population, like natural killer cells and/or monocytes, to exert functional activity.20
The cellular immune responses induced by three HIV-DNA and two HIV-MVA immunizations in our study were also durable. Three years after the second HIV-MVA immunization, 74% of vaccinees still had IFN-γ ELISpot responses, including 63% with responses to Gag and 43% to Env. After the third HIV-MVA immunization, 95% of the volunteers exhibited IFN-γ ELISpot responses, 90% to both Gag and Env, and the magnitude of Gag and Env specific responses increased significantly from the time before the third HIV-MVA vaccination. In a study by Currier et al. of vaccinees who had received three immunizations of 108 pfu of MVA-CMDR i.m. without DNA priming, the frequency of IFN-γ ELISpot responders was lower, 60% to Env and 30% to Gag.19
In this study, we also determined the impact of anti-vaccinia responses before and after MVA vaccinations. Only one vaccinee had detectable vaccinia antibodies before any vaccination most probably persisting from childhood vaccination against smallpox. All vaccinees developed significant levels of vaccinia neutralizing antibodies 2 weeks after the second HIV-MVA, as well as at the time of and 4 weeks after the third HIV-MVA immunization. Nonetheless, neutralizing vaccinia antibody responses induced after the second HIV-MVA vaccination did not prevent the elicitation of cellular or humoral anti-HIV-specific immune responses following the third HIV-MVA boost. We have previously reported that preexisting vaccinia immunity did not affect the number of immune responders following a late second HIV-MVA vaccination in a Swedish trial using the same vaccines as used in this study.13 Yang et al. reported that pre-existing vaccinia immunity affected T cell responses in a vaccine study in mice of an Ebola virus glycoprotein expressed by vaccinia. The inhibition was largely overcome by priming with a DNA expression vector.21 Our observations show that poxvirus-vectored vaccines can be used efficiently even in vaccinia preexposed populations following DNA priming.
In conclusion, the HIV-DNA prime/HIV-MVA boost regimen elicited potent HIV-specific antibody and cellular immune responses with remarkable 3-year durability. A third HIV-MVA immunization given 3 years after the second HIV-MVA immunization significantly boosted both antibody and cellular HIV-specific immune responses relative to the levels detected at the time of the third HIV-MVA immunization, but not to higher levels than after the second HIV-MVA.
Acknowledgments
This work was supported by the European Union [INCO-DEV A4 ICFP501A4PR03], the Swedish International Development Cooperation Agency (Sida), Swedish Embassy Tanzania, the European Developing Countries Clinical Trials Partnership (EDCTP) [grant CT.2006.33111.007], the Regional HIV/AIDS Team for Africa, the Embassy of Sweden in Lusaka, jointly funded by Sweden and Norway [Sida ID 2150012801], the U.S. Military HIV Research program, Walter Reed Army Institute of Research (WRAIR), the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID), and National Institutes of Health (NIH). This study was partially supported by Duke University Center for AIDS Research (CFAR) [grant P30 AI64518]. The authors gratefully acknowledge all the volunteers who participated in this study. The authors thank all the clinical and laboratory personnel at MUHAS. Special thanks to Brandy Ward, Justin Pollara, Faraha Brewer, Christopher Seliga, Jeremy Fitzpatrick, Monica Tolazzi, and Katarina Karlén for their excellent technical support. The authors thank the Centre for AIDS Reagents at NIBSC, Potters Bar, United Kingdom, for providing PSVs, neutralizing control sera, and TZM-bl cells.
The results have been partially presented at the AIDS Vaccine 2013 Conference in Barcelona, Spain, October 7–10, 2013. Abstract P04.43 LB.
Author Disclosure Statement
The authors declare that no competing interests exist. The views and opinions expressed herein do not necessarily reflect those of the U.S. Army or the Department of Defense.
References
- 1.Excler JL, Robb ML, Kim JH: Prospects for a globally effective HIV-1 vaccine. Vaccine 2015;33:D4–D12 [DOI] [PubMed] [Google Scholar]
- 2.Corey L, Gilbert PB, Tomaras GD, Haynes BD, Pantaleo G, Fauci AS: Immune correlates of vaccine protection against HIV-1 acquisition. Sci Transl Med 2015;7:310rv7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Connell RJ, Kim JH, Corey L, Michael NL: Human immunodeficiency virus vaccine trials. Cold Spring Harb Perspect Med 2012;2:a007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim JH, Excler JL, Michael NL: Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection. Annu Rev Med 2015;66:423–437 [DOI] [PubMed] [Google Scholar]
- 5.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 6.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottardo R, Bailer RT, Korber BT, et al. : Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLoS One 2013;8:e75665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza MS, Ratto-Kim S, Chuenarom W, et al. : The Thai phase III Trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol 2012;188:5166–5176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub DD, Ershler WB, Janowski M, et al. : Immunity from smallpox vaccine persists for decades: A longitudinal study. Amer J Med 2008;121:1058–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karasavvas N, Billings E, Rao M, et al. : The Thai phase III HIV Type 1 vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses 2012;28:1444–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakari M, Aboud S, Nilsson C, et al. : Broad and potent immune responses to a low dose intradermal HIV-1 DNA boosted with HIV-1 recombinant MVA among healthy adults in Tanzania. Vaccine 2011;29:8417–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joachim A, Nilsson C, Aboud S, et al. : Potent functional antibody responses elicited by HIV-1 DNA priming and boosting with heterologous HIV-1 recombinant MVA in healthy Tanzanian adults. PLoS One 2015;10:e0118486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson C, Godoy-Ramirez K, Hejdeman B, et al. : Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus Ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS Res Hum Retroviruses 2014;30:299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollara J, Hart L, Brewer F, et al. : High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytometry 2011;A79:603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollara J, Bonsignori M, Moody MA, et al. : HIV-1 vaccine-induced C1 and V2 env-specific antibodies synergize for increased antiviral activities. J Virol 2014;88:7715–7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsson C, Hejdeman B, Godoy-Ramirez K, et al. : HIV-DNA given with or without intradermal electroporation is safe and highly immunogenic in healthy Swedish HIV-1 DNA/MVA vaccinees: A phase I randomized trial. PLoS One 2015;10:e013174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gudmundsdotter L, Nilsson C, Brave A, et al. : Recombinant modified vaccinia Ankara (MVA) efficiently boosts DNA-primed HIV specific immune responses in humans despite pre-existing vaccinia immunity. Vaccine 2009;27:4468–4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goepfert PA, Elizaga ML, Seaton K, et al. : Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2014;210:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Currier JR, Ngauy V, de Souza MS, et al. : Phase I safety and immunogenicity evaluation of MVA-CMDR, a multigenic, recombinant modified vaccinia Ankara-HIV-1 vaccine candidate. PLoS One 2010;5:e13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BK, Wieczorek L, Kijak G, et al. : The role of natural killer (NK) cells and NK cell receptor polymorphisms in the assessment of HIV-1 neutralization. PLoS One 2012;7:e29454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z-Y, Wyatt LS, Kong W-P, Moodie Z, Moss B, Nabel GJ: Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol 2003;77:799–803 [DOI] [PMC free article] [PubMed] [Google Scholar]