Abstract
The microvasculature within the neural stem cell (NSC) niche promotes self-renewal and regulates lineage progression. Previous work identified endothelial-produced soluble factors as key regulators of neural progenitor cell (NPC) fate and proliferation; however, endothelial cells (ECs) are sensitive to local hemodynamics, and the effect of this key physiological process has not been defined. In this study, we evaluated adult mouse NPC response to soluble factors isolated from static or dynamic (flow) EC cultures. Endothelial factors generated under dynamic conditions significantly increased neuronal differentiation, while those released under static conditions stimulated oligodendrocyte differentiation. Flow increases EC release of neurogenic factors and of heparin sulfate glycosaminoglycans that increase their bioactivity, likely underlying the enhanced neuronal differentiation. Additionally, endothelial factors, especially from static conditions, promoted adherent growth. Together, our data suggest that blood flow may impact proliferation, adhesion, and the neuron-glial fate choice of adult NPCs, with implications for diseases and aging that reduce flow.
Keywords: : neural stem cells, vascular niche, shear stress, neurogenesis, oligodendrocytes
Introduction
Disease and injuries to the central nervous system (CNS) affect millions worldwide, resulting in reduced quality of life due to loss of sensory and motor functions. Currently, there are limited treatment options and the majority of those with CNS damage never regain full function. Resident neural stem cells (NSCs) offer great potential to aid in regeneration due to their ability to self-renew or give rise to neural progenitor cells (NPCs) that differentiate into neurons or glia. Controlled differentiation can be challenging within heterogeneous NSC and NPC populations; thus, a more complete understanding of the niche in which these cells reside will facilitate greater control in their differentiation.
NSCs are found in several adult neural regions, but active neurogenesis occurs in distinct niches, the dentate gyrus [DG] of the hippocampus and the subventricular zone [SVZ]), both of which are adjacent to a rich vasculature [1–5]. ECs within the neurogenic niche regulate NSC and NPC proliferation and fate by providing a basement membrane for stem cell attachment, releasing growth factors and cytokines, and providing direct signaling through stem cell–vascular cell contact [1,2]. Following EC-induced expansion, removal of EC factors resulted in increased neuronal differentiation, demonstrating an impact on both proliferation and neurogenesis [6,7]. While EC-released factors enhance NPC proliferation and differentiation [6,7], a comprehensive understanding of the molecular basis of this key niche interaction remains unknown.
Control over NPC fate using purified growth factors has been studied extensively in vitro and in vivo. While both NSC and NPC populations are present in vitro, within this work, we will refer to cells as NPCs, a blanket term that includes NSCs and more restricted progenitor cells. Individual soluble factors such as basic fibroblast growth factor (FGF2; [8–12]), epidermal growth factor (EGF; [9,10]), insulin-like growth factor (IGF; [13–17]), and sonic hedgehog (Shh; [18–20]) promote NSC self-renewal and/or proliferation, as well as direct neurogenesis or gliogenesis. Conversely, transforming growth factor-β (TGF-β) promotes quiescence and may inhibit neurogenesis of NPCs [21–23].
In some instances, the NPC response is dependent on the soluble factor concentration, for example, Shh promotes neural differentiation at higher concentrations, while oligodendrocyte formation is supported at lower concentrations [18,20,24]. Similarly, EGF promotes self-renewal, but high concentrations increase astroglial lineage [25]. The impact of single factors on NSC fate can be investigated; however, within the SVZ, NPCs are exposed to a milieu of soluble factors and ECs represent a key source of multifaceted cytokine release profile affecting these progenitor cells, and changes in more than a single cytokine may be instructive in the niche.
To recapitulate some of the complexity of the niche, current in vitro coculture models use statically cultured ECs; however, ECs are responsive to fluid shear stress, exhibiting profound changes in bulk soluble factor and matrix production in response to local hemodynamics, including flow type (laminar or pulsatile), rate, and duration [26–28]. Traditional static EC cultures release numerous cytokines, including VEGF, FGF2, EGF, Shh, IGF-1, and TGF-β, but following the application of fluid flow, cytokine production and release are altered, resulting in distinct cytokine profiles that are dependent on the EC source (eg, species and location) in addition to local hemodynamics [29–34].
Endothelial production of proteoglycans has also been shown to increase with the application of shear stress [34–37]. Many cytokines are known to degrade rapidly, on the order of hours, but can be stabilized by proteoglycans. Heparin-binding growth factors (HBGFs) are a class of cytokines (including, but not limited to, EGF, FGF2, IGF, and TGF-β) that bind to heparan sulfate proteoglycans (HSPGs). HSPGs bind growth factors resulting in stabilization and protection from degradation, as well as enhanced growth factor binding to their receptors [36,38–40]. Similarly, chondroitin sulfate proteoglycans (CSPGs) bind growth factors and increase bioavailability to NPCs [38,41,42].
While it is well established that shear stress causes an upregulation of soluble factors by ECs, the impact on NPCs has not yet been elucidated and can have far-reaching indications within the aging brain as cerebral hemodynamics change with aging and disease [43]. Significantly, ischemic small vessels in elderly individuals correlate directly with white matter loss and are abundant proximal to the ventricles [44], suggesting that poor vascular endothelial health contributes to reduced neurogenesis in the SVZ niche. Furthermore, transplantation of aged NSCs into a young NSC niche leads to healthy and prolific NSC colonies, but transplantation of young NSCs into the aged niche leads to a loss of Wnt signaling and reduced proliferation characteristic of aged NSCs [45]. Development of an in vitro model that can delve into the mechanisms by which ECs contribute to maintenance of a regenerative niche may lead to the development of preventative or treatment therapies for the aging population.
In this work, we expanded on previous static coculture models by adding the novel inclusion of dynamically stimulated ECs into our niche model. We subjected mouse brain microvascular ECs to a physiological shear stress of 10 dynes/cm2 or to traditional static conditions for 24 h and collected the EC-conditioned medium for analysis or treatment of NPCs, examining survival, proliferation, neurosphere formation capacity, and differentiation profile. We found that exposure to EC factors exposed to flow dramatically impacts adult NPC behavior.
Experimental Procedures
Dynamic stimulation of ECs
Polystyrene sheets were laser cut to specifications (Boston Lasers, Haverhill, MA) to be compatible with a modified cone and plate viscometer [27]. The polystyrene disks were plasma treated (PDC-001 plasma cleaner; Harrick Plasma, Ithaca, NY) to increase hydrophilicity and then dip-coated in a 0.5% w/v gelatin solution prepared by dissolving laboratory grade 275 bloom gelatin powder (Fisher, Hanover Park, IL) and the cross-linking agent chromium (III) potassium sulfate dodecahydrate (Sigma-Aldrich, St. Louis, MO) into DI water. Gelatin-coated disks were assembled into a cone and plate viscometer chamber, which created the dynamic culture device. For static culture, gelatin-coated disks were placed in a 150-mm nontreated Petri dish (Celltreat, Shirley, MA).
Mouse brain microvascular ECs (mBend.3; ATCC, Manassas, VA) were seeded at 1.8 × 104 cells/cm2 onto the gelatin-coated polystyrene disks in growth medium containing Dulbecco's modified Eagle's medium (DMEM; Cellgro, Manassas, VA) with 4.5 g/L glucose, 2 mM l-glutamine, and 4.5 g/L sodium pyruvate (Gibco, Grand Island, NY) supplemented with 50 U/mL penicillin/streptomycin (P/S; Mediatech, Herndon, VA) and 10% v/v fetal bovine serum (FBS; Gibco). A mouse brain EC line was chosen as it has been used in previous studies under dynamic culture [46] as well as to ensure a reproducible EC source. ECs were cultured for 5 days to develop a confluent monolayer in static culture and allow for complete remodeling of the thin gelatin layer. EC monolayers were then transferred to phenol red-free DMEM supplemented with 2% w/v dextran (MW = 500 kg/mol; Spectrum Chemical, New Brunswick, NJ).
The flow device (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/scd) incorporates a computer interface to control shear stress based on input system parameters: medium viscosity, cone dimensions, and plate dimensions. Unlike the more commonly used parallel perfusion plates, shear stress is generated by rotation of the cone above the polystyrene disk, resulting in fluid flow. Due to the circumferential movement of the culture medium, dextran was used to increase culture medium viscosity to 2 cP, thereby preventing turbulent flow. The endothelial monolayers were either exposed to a shear stress of 10 dynes/cm2 in the flow device or statically cultured for 24 h, at which time the EC-conditioned medium was collected and stored at −20°C for future analysis up to 2 weeks. No significant difference in EC number was quantified (Supplementary Fig. S1) and thus any resulting changes are a direct result of soluble factor expression. Shear stress of 10 dynes/cm2 was chosen as it falls within the reported physiological range of 1–60 dynes/cm2 measured within small vessels from humans, canines, felines, and rodents [47–49], although the exact range of the brain microvasculature is unknown. Further information on the experimental procedures used to assess EC monolayer health can be found in the Supplementary Data.
Isolation and culture of NPCs
Mice were treated according to the Institutional Animal Care and Use Committee guidelines at Rensselaer Polytechnic Institute and all animal procedures were pre-approved by the Committee for collection of tissue. The SVZ was isolated from the brains of adult (5–10 weeks old) female Swiss Webster mice (Taconic Farms, Hudson, NY). The tissue was enzymatically digested as previously described [46]. Dissociated cells were resuspended at 1.5 × 104 cells/mL in nonconditioned DMEM (control) or EC-conditioned DMEM (static or dynamic) supplemented with 1 mM sodium pyruvate, 2 mM l- glutamine, 1 mM NAC, 1 mM N2 supplement (Gibco), 1 mM B-27 supplement (Gibco), and 20 ng/mL FGF2 (Gibco) and EGF (Gibco). Growth factors were added to all expansion conditions as the NPCs would not otherwise survive or expand in control medium.
Cells were plated in nontreated six-well plates (Celltreat) and expanded as neurospheres for 14 days with daily feedings of concentrated growth factors, N2, NAC, and B27, unless otherwise noted, at 37°C and 5% CO2. As media are not exchanged for 14 days, it is important to resupplement not only the growth factors that have half-lives on the order of a few hours but also the nutrient mixes that contain factors such as insulin with limited half-lives. Plates were moved minimally during feedings in an effort to prevent aggregation that may occur with cell concentrations greater than 10 cells/μL [50]. EC-conditioned medium contained 2% w/v dextran to prevent turbulent flow, and as such, DMEM supplemented with 2% w/v dextran was also tested for NPC viability, sphere formation, and phenotype and no differences were noted with or without dextran (Supplementary Fig. S2). Given that no differences were noted, the nonconditioned control media were not supplemented with dextran to recapitulate the nonconditioned controls utilized in other NPC-EC studies [6,7].
After 14 days, neurosphere number and size were quantified using an Olympus CKX41 inverted light microscope (Olympus, Center Valley, PA). ImageJ was used to assess neurosphere number and diameter. Neurosphere diameter was binned into groups by 0.05 mm to visualize size distribution. There were six experimental replicates (n = 6) with 3–4 sample replicates (m = 3) for each condition. The sum of the colony data from each well was averaged for each sample replicate, which was then averaged across the experimental replicates.
Neurospheres were collected and adherent cells were lifted from the culture surface with trypsin-EDTA (Cellgro). All cells within the cultures from each medium condition (control, static, or dynamic) were dissociated into a single-cell suspension. Using a hemocytometer, the cell number was quantified for each condition and the presence of only singlet cells was validated. Only cells from the primary expansion were evaluated or cultured for immature phenotype, differentiation, or potency, as described below.
Immature phenotype evaluation
NPC phenotype was assessed within cultures expanded initially in EC-conditioned or control medium for 14 days, after which the cells were collected, dissociated, and seeded at 3 × 104 cells/cm2 in 96-well plates coated with poly-l-ornithine (PLO) in nonconditioned DMEM (control) or EC-conditioned DMEM (static or dynamic) supplemented with 1 mM sodium pyruvate, 2 mM l- glutamine, 1 mM NAC, 1 mM N2 supplement, 1 mM B-27 supplement, and 5 ng/mL FGF2 and EGF. After 24 h, the cells were fixed with 4% w/v paraformaldehyde.
Differentiation
NPCs expanded for 14 days in control or EC-conditioned (dynamic or static) medium were seeded at a density of 3 × 104 cells/cm2 in 96-well plates on 10% Matrigel (BD Biosciences, San Jose, CA)-coated tissue culture plastic in Neurocult® basal medium (Stem Cell Technologies, Vancouver, BC) with Neurocult differentiation supplement (1% fetal bovine serum) and 50 U/mL P/S. The differentiation medium was replaced every other day for 3 weeks. Cells were then fixed with 4% w/v paraformaldehyde in PHEM buffer and then immunostained.
Evaluation of NPC survival, proliferation, and potency
NPCs expanded for 14 days in nonconditioned (control) or endothelial-conditioned (static or dynamic) medium were dissociated into a single-cell suspension. Only cells from the primary expansion were used in the Neurocult neural colony-forming cell (NCFC; Stem Cell Technologies) assay. The NCFC assay was used to assess NPC survival following removal from EC factors and to assess stem and progenitor potency, as determined by neurosphere size. In this 3D assay, single cells grow into colonies, while cell aggregation is prevented within the semisolid hydrogel. Dissociated cells originally expanded in control or endothelial-conditioned medium were seeded according to manufacturer instructions at a final concentration of 1.6 × 103 cells/mL in a semisolid collagen gel devoid of EC-derived factors and cultured for 21 days, with feedings every 7 days of 10 ng/mL FGF2, 20 ng/mL EGF, and 100 μg/mL heparin. As cells are fed every 7 days based on manufacturer instructions and due to the short half-life of FGF2 and EGF, as per the assay instructions, heparin is included to prevent rapid degradation of growth factors.
At 21 days, colony number and size were quantified using an Olympus CKX41 inverted light microscope (Olympus, Center Valley, PA). ImageJ was used to assess neurosphere number and diameter. Neurosphere diameter was binned into groups by 0.5 mm to visualize size distribution. There were six experimental replicates (n = 6) with 3–4 sample replicates (m = 3) for each condition. Colony size is a measure of NSC or NPC proliferation, while colony number is an indicator of the number of NPCs or NSCs that survive and are capable of nonadherent cell growth.
Immunocytochemistry
For surface staining, fixed cells were blocked overnight in 10% goat serum (Sigma-Aldrich) at 4°C, then stained with 1:4 mouse anti-O4 (ATCC) and 1:400 rabbit anti-NG2 (Millipore, Billerica, MA) antibodies, followed by goat anti-mouse IgM and goat anti-rabbit IgG secondary antibodies (Invitrogen, Carlsbad, CA). For intracellular differentiation staining, fixed cells were permeabilized with 0.01% Triton-X 100 in PBS, then blocked overnight in 10% goat serum at 4°C. Cells were stained with 1:1,000 rabbit anti-GFAP (Dako, Carpenteria, CA), 1:500 mouse anti-NeuN (Millipore), or 1:10 mouse anti-Nestin (DSHB, Iowa City, IA), followed by goat anti-rabbit IgG and goat anti-mouse IgG1 antibodies (Invitrogen). For immature phenotype assessment, cells were stained with 1:1,000 rabbit anti-GFAP (Dako), 1:500 mouse anti-EGFR (Millipore), and 1:300 rat anti-CD133, followed by goat anti-rabbit IgG, goat anti-mouse IgG1, and goat anti-rat IgG antibodies (Invitrogen). Other samples were sequentially stained with 1:300 mouse anti-PSA-NCAM (Millipore), followed by goat anti-mouse IgM (Invitrogen), then 1:1,000 rabbit anti-Mash1 (Millipore) and 1:500 mouse anti-EGFR with appropriate secondary antibodies. Hoechst 33342 (1:2,000) was used as a counterstain to identify nuclei in all samples. Samples were imaged using an Olympus IX81 inverted microscope (Olympus) with a 20 × dry objective for all samples.
NIH ImageJ toolkit (National Institutes of Health, Bethesda, MD) was used to quantify total and phenotype-specific cell numbers. To reduce variability in cell number due to cell seeding, cells of a given phenotype in each image are represented as a percentage of the total cells in each image. Three images were taken per sample to obtain a sample average, with a minimum of three sample replicates of each condition in each of the four experimental replicates of independent animal isolations (n = 4, m = 3).
Cytokine degradation and quantification
Mouse G2000 cytokine arrays (Raybiotech, Norcross, GA) were used according to the manufacturer's instructions to quantify cytokines in EC-conditioned medium collected after 24 h of dynamic versus static culture. Enzyme-linked immunosorbent assays (ELISAs) were used to quantify changes in EC-produced cytokines in the conditioned medium, specifically for cytokines known to impact NPC fate: FGF2 (Raybiotech) and EGF (Raybiotech). ELISAs were used according to manufacturer instructions to analyze the endothelial-conditioned medium. For analysis, four experimental replicates (n = 4, m = 2) were analyzed with two well replicates to generate an average cytokine concentration for each experimental condition.
Glycosaminoglycan content analysis
EC-conditioned medium was frozen at −80°C and lyophilized. Samples were digested and evaluated using ultrahigh-performance liquid chromatography–mass spectrometry (UPLC-MS) [51]. Briefly, the samples were proteolyzed with actinase E solution, and glycosaminoglycans (GAGs) were purified with anion exchange spin columns, followed by digestion with heparin lyases (I, II, and III) and chondroitinase (ACII and ABC). The recovered disaccharides were then tagged with 2-aminoacridone and analyzed using LC-MS on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Inc., Wilmington, DE). Total GAG content is reported as μg present in 16 mL of conditioned medium, which is equivalent to what is collected in total for one static or dynamic culture experiment. GAG content was not normalized to cell number as the cell numbers were not found to be statistically different (Supplementary Fig. S1).
Statistical analysis
A one-way ANOVA was performed to determine statistical significance between conditions (P-value <0.05) for all datasets using Prism (GraphPad Software, La Jolla, CA). Analysis of cytokine array data was performed according to manufacturer instructions. Briefly, multiple replicates on each array are averaged for each EC culture condition. Each sample culture condition (dynamic or static) is normalized to respective positive and negative controls on each array, then a fold change (p1/py) is calculated based on the ratio of positive controls for static (p1, reference array) divided by dynamic arrays (py). This ratio is specific to each chip and the experimental dynamic intensity values (xy) are multiplied by the ratio of the positive controls (p1/py) to generate a scaled dynamic intensity value (xNy = xy*p1/py) that corresponds to the scaling of the reference static array as specified by the manufacturer. The average for each factor in the dynamic condition is then normalized to the average for each factor in the static condition to generate the reported fold change. Based on manufacturer instructions, a fold change greater than or equal to 1.5-fold or less than or equal to 0.65-fold in signal intensity is considered measurable and significantly different in expression (P < 0.05) after complete analysis. More information regarding quantification of the cytokine levels can be found in the Supplemental Data.
Results
Conditioned medium collected from dynamic, but not static, EC cultures supports neurosphere formation
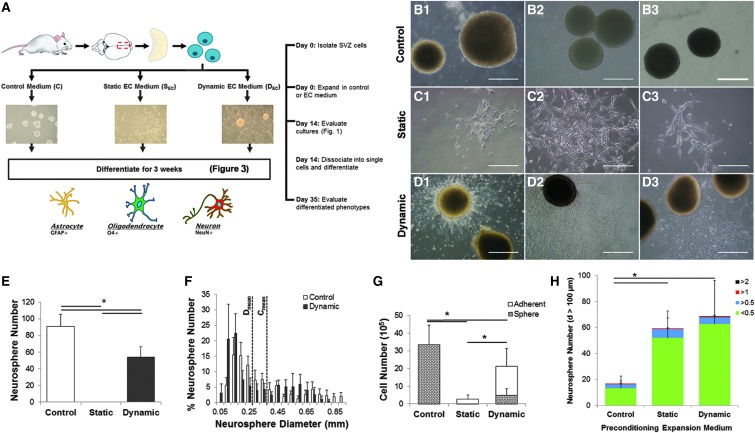
EC monolayers cultured under dynamic fluid flow or static conditions exhibited healthy morphologies and low/absent caspase activity (Supplementary Fig. S1). Additionally, EC numbers were not significantly different when cultured under dynamic or static regimes, thus any phenotypic differences seen among NPCs can be attributed to variable factor release profiles and not EC cell number (Supplementary Fig. S1). NPCs isolated from the adult SVZ were cultured in control (nonconditioned/vehicle) medium (Fig. 1A, B) or in EC-conditioned medium collected from either static (Fig. 1A, C) or dynamic cultures (Fig. 1A, D). Both the control medium and dynamic EC-conditioned medium support neurosphere formation and growth (Fig. 1 B, D). However, unlike the control medium where all cells grow as nonadherent neurospheres, approximately half of the NPCs cultured in the dynamic EC-conditioned medium adhered to the uncoated nontissue culture-treated plastic, and in static EC-conditioned medium, all cells were adherent (Fig. 1C).
FIG. 1.
EC-conditioned medium reduces neurosphere formation in favor of adherent NPC cultures. Schematic representation of the experimental design (A) for cells isolated from the adult SVZ and expanded in neurosphere cultures using a base medium of DMEM (control) (B1-3), static EC-conditioned (C1-3), or dynamic EC-conditioned (D1-3) medium for 14 days. Neurospheres developed in control and dynamic EC-conditioned medium-treated cultures; however, the static EC-conditioned medium formed only adherent cultures. Dynamic EC-conditioned medium also supported adherent cultures. Control medium supports greater neurosphere numbers (E) compared with dynamic EC-conditioned medium. The average neurosphere size (F) was not significantly different for cells expanded in either the control or dynamic EC-conditioned medium. (G) Evaluation of the total cell number shows that control medium generated significantly (*P < 0.05, n = 6) more cells than those cultured in EC-conditioned medium. Mixed cultures in dynamic EC-conditioned medium generated significantly more cells than those expanded as adherent cells in static EC-conditioned medium (*P < 0.05, n = 6). (H) Cells expanded for 14 days in experimental and control conditions were dissociated and subsequently cultured for an additional 21 days in an NCFC assay to assess potency. Cells preconditioned in EC-conditioned medium (dynamic and static, P < 0.05, n = 6) generate larger and more numerous colonies. Colonies greater than 2 mm are considered to be indicative of NSC colonies, rather than NPC colonies. Only SVZ cells preconditioned with EC-conditioned medium collected under dynamic culture generated the largest >2-mm neurospheres at the end of 21 days; however, this result was not statistically significant as so few colonies were detected. Data are represented as mean ± standard deviation. Scale bar = 0.5 mm. EC, endothelial cell; NCFC, neural colony-forming cell; NPC, neural progenitor cell; SVZ, subventricular zone.
The control medium supported the greatest number of neurospheres even with potential aggregation of colonies that may arise due to seeding density in the control medium, resulting in a 1.7-fold increase in number compared with dynamic EC-conditioned medium (Fig. 1E). For those cultures that did support neurosphere formation, there was no significant difference in average neurosphere diameter with both supporting a wide distribution of neurosphere diameters ranging up to 1 mm after 14 days of expansion (Fig. 1F). Neurosphere number increases by increasing the feeding frequency to daily growth factor additions versus feeding every 3 days (Supplementary Fig. S3A), but the neurosphere diameter or ability of cells in static EC-conditioned media to form spheres did not vary with increased feeding frequency (data not shown). This suggests that cell survival may be sensitive to cytokine concentration and composition.
Given that the EC-conditioned and control media produce significant differences in neurosphere and adherent culture formation, all the cells (adherent and nonadherent) within a treatment group were collected, dissociated, and quantified at the single-cell level. As expected, greater cell numbers were present in colonies capable of neurosphere formation (control and dynamic EC-conditioned media) compared with adherent colonies (static EC-conditioned medium; Fig. 1G). Cell number was proportional to feeding frequency with a three-fold increase in cell number for cultures fed daily compared with every third day (Supplementary Fig. S3B). Irrespective of feeding frequency, there were significantly greater cell numbers present in control medium relative to EC-conditioned medium with greater expansion occurring in dynamic compared with static EC-conditioned medium (Fig. 1 and Supplementary Fig. S3).
SVZ phenotype was assessed in the expanded cell populations to evaluate whether differences in phenotype were detected between cells that proliferated as neurospheres compared with adherent cultures as a result of the culture media. Multiple SVZ phenotypes were evaluated to capture the full range of maturity from stem cells to more mature neuroblasts, including quiescent type B cells (NSCs; GFAP+CD133+), active type B cells (NSCs; GFAP+EGFR+), transit-amplifying type C cells (NPCs; Mash1+EGFR+), and type A neuroblasts (PSA-NCAM+). Quiescent NSCs (GFAP+CD133+) were not detected in any of the cultures, likely due to the longer duration of expansion that is reported to be unfavorable for quiescent NSCs [50]. All other SVZ subtypes were present; however, no significant differences existed in the distribution of SVZ phenotypes between control and EC-conditioned media (static and dynamic) (Fig. 2).
FIG. 2.
NPCs expanded in control or EC-conditioned medium maintain SVZ precursor phenotypes. NPCs cultured in (A) control, (B) static EC-conditioned, or (C) dynamic EC-conditioned medium were stained for Mash1+ (type C cells, magenta, indicated by *), EGFR (type C cells, red), or PSA-NCAM+ (type A neuroblasts, indicated by ^). Similarly, NPCs cultured in (D) control, (E) static EC-conditioned, or (F) dynamic EC-conditioned medium were stained for GFAP+ (yellow, indicated by caret [<]), EGFR+ (red), or CD133+ (green). Cells immunoreactive for GFAP+CD133+ (quiescent type B cells) were not detected in any culture conditions. (G) No statistically significant (n = 4) differences were observed in phenotype across any of the control or EC-conditioned media. Data are represented as mean ± standard deviation. Scale bar = 50 μm.
To evaluate proliferation and survival upon removal of EC-derived factors, a hydrogel-based NCFC assay was used to evaluate colony size (proliferation) and number (survival) in an environment preventing neurosphere aggregation. NPCs were expanded in either EC-conditioned medium (static or dynamic) or nonconditioned control medium. The preconditioned dissociated cells were seeded in a semisolid collagen hydrogel, devoid of EC factors, but all cultures were supplemented with heparin to prevent proteolytic degradation of FGF2 and EGF based on manufacturer instructions. After 3 weeks of culture, colonies greater than 100 μm were counted and those greater than 2 mm in diameter are thought to have arisen from less mature NSCs, whereas colonies less than 1.5 mm in diameter are thought to have been generated from NPCs [25,52,53]. Cells initially expanded in the EC-conditioned medium (static or dynamic) generate larger and more numerous neurospheres than control-expanded NPCs (P < 0.05, Fig. 1H) even after the absence of EC-derived factors for 3 weeks. This suggests enhanced survival and proliferation of NSCs primed with EC factors compared with traditional expansion methods and that these characteristics are maintained following removal of EC factors. Neurospheres with diameters greater than 2 mm were only detected in cultures initially expanded in dynamic EC-conditioned medium, suggesting that these dynamically derived EC factors can prime NSCs for subsequent greater proliferation. Few NSCs are typically expected in adult SVZ cultures as there are few NSCs in the niche and these cells are difficult to maintain in vitro. Moreover, neurosphere assays are unable to support quiescent NSCs (CD133+GFAP+) as seen in Fig. 2 and described in detail by Pastrana et al. [25].
Neurospheres expanded in endothelial-conditioned medium have distinct differentiation profiles
To evaluate the influence of EC-derived factors on NPC fate, isolated SVZ cells expanded in either EC-conditioned (dynamic or static) or control medium were dissociated and differentiated for 3 weeks. Presumably, differences arose within NPCs during this initial expansion phase, albeit not in the overall distribution of SVZ phenotype (Fig. 2) as all NPCs were exposed to the same differentiation regime after expansion. Prior to differentiation, >95% of the cells were Nestin+, and after differentiation, none of the cells stained positive for Nestin (data not shown). Regardless of the primary expansion medium, the majority of NPCs differentiate into GFAP+ astrocytes (Fig. 3A, C, E, G) and were absent of Nestin+ immature cell phenotypes (Fig. 3G). Supplementary Figure S4 displays an extended panel of the differentiated cell immunostaining. NPCs expanded in control medium resulted in approximately 20% NeuN+ neurons, no O4+ oligodendrocytes, and approximately 10% NG2+ cells presumed to be glial progenitor cells (Fig. 3B, G). NPCs expanded in static EC-conditioned medium resulted in 10% O4+ oligodendrocytes, which were not present in either the control or dynamic EC-conditioned medium conditions (P < 0.05, Fig. 3D, G), with similar astrocyte and neuron production as control culture (Fig. 3C, G). NPCs expanded in dynamic EC-conditioned medium exhibited significantly increased neuronal differentiation (approximately 30% of total cells) compared with both control and static EC-conditioned media (P < 0.05, Fig. 3E, G). Taken together, these results provide evidence that EC-conditioned medium produced under static or dynamic conditions can promote NPC lineage-specific differentiation.
FIG. 3.

EC-conditioned medium enhances neuronal and oligodendrocyte differentiation. Isolated SVZ cells were cultured in either the control (nonconditioned) or EC-conditioned (static or dynamic) medium, dissociated, and subsequently differentiated in 1% FBS for 3 weeks. At 3 weeks, cells initially cultured in control (A, B), static EC-conditioned (C, D), or dynamic EC-conditioned (E, F) medium were stained either for astrocytes (GFAP; red) and neurons (NeuN; green) (A, C, E) or oligodendrocytes (O4, green) and oligodendrocyte precursor cells (OPCs: NG2; red) (B, D, F). All cells were visualized with DAPI (blue). (G) Cells expanded in EC-conditioned medium from static cultures resulted in O4+ oligodendrocyte populations that were not present in dynamic EC-conditioned or control medium. Expansion in dynamic EC-conditioned medium resulted in a significant increase (*P < 0.05, n = 4) in NeuN+ cells compared with the static and control-expanded populations. Nestin+ precursor cells were not detected after 3 weeks. See Supplementary Fig. S3 for individual channels of this figure. Scale bar = 50 μm. Data are represented as mean ± standard deviation.
Dynamically stimulated ECs exhibit differential cytokine level
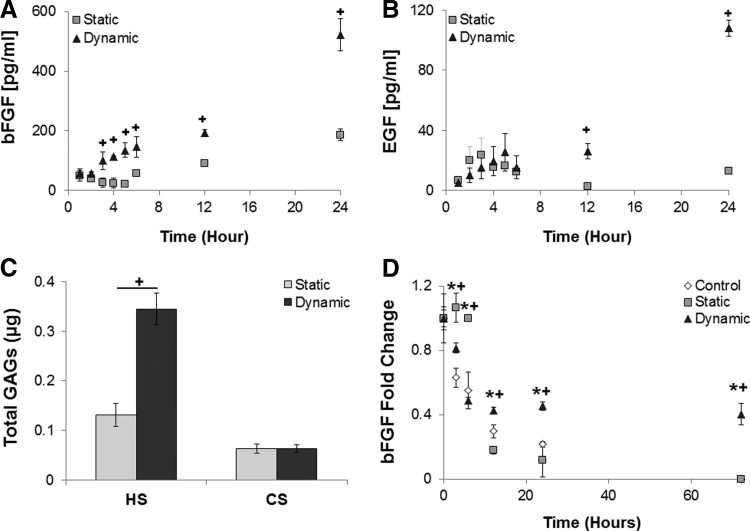
Differences in NPC survival, proliferation, and differentiation are observed following culture in EC-conditioned medium collected from static versus dynamic cultures, suggesting the differential production of bulk soluble factors under these conditions. Given that the endothelial phenotype is altered due to shear stress, we expect to see differences in the EC-conditioned medium that would contribute to the varied NPC response. As FGF2 and EGF are well known to promote NPC expansion [8,9,54], their levels were quantified by ELISA for conditioned medium collected following 24 h of dynamic or static culture as an internal control to verify expected differences due to fluid flow. There was a significant increase in the EC release of FGF2 (Fig. 4A) and EGF (Fig. 4B) at 3, 12 and 24 h, respectively, in dynamic culture (10 dynes/cm2).
FIG. 4.
Increased cytokine production and stabilization are observed in dynamic EC-conditioned medium. EC-produced FGF2 (A) and EGF (B) increase in response to dynamic culture (10 dynes/cm2), resulting in a significant 2.8-fold and 8.7-fold increase over 24 h relative to statically cultured ECs. (C) Heparan sulfate (HS) and chondroitin sulfate (CS) GAGs are present in both the static and dynamic EC-conditioned media. There is a significant 2.6-fold increase in HS within the medium collected from dynamic culture compared with the static EC-conditioned medium, while no statistically significant difference was detected in CS content. GAG content is known to stabilize growth factors and the buffering capacity of the EC-conditioned medium for growth factor stabilization was evaluated. (D) Degradation of FGF2 in EC-conditioned medium (dynamic or static) or in control medium supplemented with 100 pg/mL mouse FGF2 at 37°C was evaluated using ELISA. Model HBGF factor (FGF2) degrades rapidly for all culture media over the first 12 h. In control and static EC-conditioned media, FGF2 continues to degrade below ELISA detection limits (8 pg/mL) by 72 h, while FGF2 levels plateau in dynamic EC-conditioned medium between 6 and 72 h at 40% of the original FGF2 concentration. * indicates dynamic is significantly (P < 0.05, n = 4) higher than control; + indicates dynamic is significantly (P < 0.05, n = 4) higher than static medium. Data are represented as mean ± standard deviation. GAG, glycosaminoglycan.
Evaluation of FGF2 and EGF confirms that the biochemical milieu is altered and a cytokine array was subsequently used to identify differences in levels of other soluble factors. A 144-target cytokine array identified differences in the secretome between the dynamic and static EC-conditioned media (Supplementary Table S1) that were not present in the control medium. Over 100 different factors were identified in the EC-conditioned media, including growth factors (FGF2, EGF, IGF, and Shh), and secreted cell adhesion molecules (CAMs; such as VCAM, ICAM, selectins, and cadherins). The presence of secreted CAMs may contribute to cell–substrate attachment seen in Fig. 1.
Dynamic EC-conditioned medium contains higher concentrations of several cytokines known to promote NPC proliferation compared with the static EC-conditioned medium [FGF2 [8,9,54], EGF [9], IGF-1 [14,55], IGF-2 [13], interleukin (IL)-1α [56], IL-15 [57], leptin [58,59], receptor for advanced glycation end products (RAGE) [60], stromal-derived factor-1 (SDF-1) [61], and tumor necrosis factor-α (TNF-α) [62]] (Table 1). IL-1β was only present in static EC-conditioned medium, while interferon (IFN) and IL-10 were present in both EC-conditioned media, all of which have been shown to decrease NPC proliferation [63]. Together, these results may underlie the increased NPC expansion seen in dynamic versus static medium. Additionally, differences in cell fate seen in cultures with the dynamic and static media may be attributed to differences in the conditioned medium. IGF-1 [16], SDF-1 [64], VEGF-D [64], amphiregulin [65], and Shh [18,20] promote neural differentiation, while IGF-1 [66] and Shh [18,24] can promote oligodendrocyte differentiation in a concentration-dependent manner.
Table 1.
Differential Cytokine Expression in Endothelial Cell Cultures
| Cytokine | D/S | Cytokine | D/S | Cytokine | D/S | Cytokine | D/S |
|---|---|---|---|---|---|---|---|
| Flt-3 L | >1 | IL-1rα | 2.73 | Amphiregulin | 1.39 | IL-17F | 0.67 |
| TIMP-2 | >1 | Resistin | 2.67 | IL-7 | 1.38 | Chordin | 0.65 |
| P-Selectin | >1 | sIL-6R | 2.57 | CD30 | 1.32 | HGF | 0.64 |
| TROY | >1 | IL-11 | 2.55 | IFN | 1.25 | MMP-3 | 0.63 |
| CX3CL1 | >1 | BLC | 2.49 | IL-10 | 1.24 | IL-20 | 0.58 |
| Leptin R | >1 | GITF | 2.35 | IL-3Rβ | 1.23 | IGFBP2 | 0.54 |
| Lungkine | >1 | SDF-1α | 2.31 | CD36 | 1.21 | TACI | 0.48 |
| M-CSF | >1 | IL-17B R | 2.26 | TIMP-1 | 1.19 | Galectin-1 | 0.46 |
| MIP-1γ | >1 | IGF-1 | 2.25 | IL-17A | 1.16 | MadCAM-1 | 0.45 |
| Leptin OB | >1 | IL-9 | 2.22 | IL-21 | 1.13 | MCP-1 | 0.44 |
| RAGE | 10.1 | IL-12 p70 | 2.20 | TCA-3 | 1.13 | Osteopontin | 0.37 |
| EGF | 8.45 | TNFR1 | 2.18 | CTACK | 1.13 | TWEAK R | 0.36 |
| VCAM | 7.09 | NPTX2 | 2.16 | TARC | 1.12 | TECK | 0.33 |
| MCP-5 | 7.07 | 6Ckine | 2.13 | GM-CSF | 1.11 | VEGF-D | 0.32 |
| FGF-2 | 6.35 | Prolactin | 2.09 | Epigen | 1.11 | Neprilysin | 0.32 |
| IL-1α | 5.70 | VEGF R1 | 2.07 | LIX (CXCL5) | 1.11 | Dkk-1 | 0.31 |
| TCK-1 | 5.17 | TNF-α | 2.05 | sTNFR2 | 1.07 | CXCL16 | 0.20 |
| E-Cadherin | 5.15 | JAM-A | 1.95 | MIP-3β | 1.06 | MIP-2 | 0.17 |
| ICAM1 | 3.88 | I-TAC | 1.90 | TWEAK | 1.05 | L-Selectin | 0.10 |
| IL-15 | 3.60 | IL-5 | 1.89 | FAS-L | 1.04 | RANTES | 0.10 |
| E-Selectin | 3.49 | IL-28 | 1.76 | CD40 L | 0.97 | CD27 | 0.08 |
| CD27L | 3.40 | IGF-2 | 1.73 | IL-2 | 0.96 | IGFBP3 | 0.07 |
| Decorin | 3.27 | IL-17B | 1.63 | TREM1 | 0.95 | Eotaxin | 0.07 |
| IGFBP5 | 3.18 | MIG | 1.61 | MDC | 0.89 | GRO-α | 0.03 |
| IL-12 p40/p70 | 3.17 | MMP-9 | 1.61 | ALK-1 | 0.87 | VEGF R3 | 0.01 |
| VEGF R2 | 3.06 | Fc-γ RIIB | 1.56 | CTLA4 | 0.87 | IL-1β | → 0 |
| Eotaxin-2 | 2.96 | IL-4 | 1.51 | Epiregulin | 0.85 | ShhN | → 0 |
| PF4 (CXCL4) | 2.87 | IL-1R4 | 1.50 | Endoglin | 0.82 | CD40 | → 0 |
| 4-1 BB | 2.84 | GAS6 | 1.47 | GZMB | 0.81 | CTF-1 | → 0 |
| Lymphotactin | 2.80 | HGFR | 1.43 | IL-17E | 0.77 | MGF-EB | → 0 |
| Dtk | 2.79 | TRANCE | 1.40 | Thrombopoietin | 0.75 |
Cytokine expression is represented as a fold change for each cytokine produced in dynamic EC-conditioned medium over static EC-conditioned medium. Cytokines that were absent from both the static and dynamic conditioned media were removed from the table. Numerous cytokines experienced a significantly increased (D/S > 1.5, dark gray) or decreased (D/S < 0.65, light gray) expression within the dynamically stimulated EC cultures compared with statically stimulated EC cultures (P < 0.05, n = 4). The fold change for factors that were not above background for the static array is represented as >1 and has been organized from highest to lowest intensity values within the dynamic conditioned medium. Fold change for factors absent in the dynamic medium could not be determined as they approach zero (→ 0) and has been ranked as least to most prevalent in static medium.
EC, endothelial cell.
FGF2 stabilization increases in dynamically stimulated EC culture medium
In addition to soluble factor production, ECs produce GAG-rich proteoglycans that can enhance soluble factor stability, regulate soluble factor receptor availability on the cell surface, and bind to their receptors [38,67,68]. EC-conditioned medium and extracellular matrix were analyzed using UPLC-MS to quantify bulk differences in CS and HS GAG production. An increased HS concentration was detected in dynamic compared with static EC-conditioned medium, while no significant differences in CS concentration were measured (Fig. 4C). These results suggest that EC-conditioned medium from dynamic cultures would have a greater ability to stabilize cytokines through the HS GAGs.
To test increased cytokine stability in conditioned medium, FGF2 degradation in three medium types in the absence of cells was evaluated using ELISA; each sample was normalized to the 0-h sample. FGF2 was used an example of an HBGF as it is exogenously supplemented in all culture conditions with cells. There are numerous HBGFs secreted by ECs at different concentrations identified in the cytokine array that would be absent in nonconditioned controls used in NPC cultures within this study. Control samples lacking EC-produced FGF2 were supplemented with 100 pg/mL mouse FGF2, while EC-conditioned samples did not receive additional FGF2.
Significant differences were detected in FGF2 concentration between experimental and control cultures after 6 h (Fig. 4D). After 6 h, FGF2 concentration in dynamic EC-conditioned medium was reduced by 60% and appeared to plateau. This is indicative of stabilization by the HS GAGs even at the extended 72-h time point, perhaps representative of the buffering capacity of the EC-produced HS GAGs. In contrast, EC-conditioned medium from static cultures and control medium with supplemented FGF2 continued to experience significant degradation throughout the study and was undetectable at 72 h, likely due to the low or absent HS GAGs. While the concentrations of FGF2 used for stabilization were lower than those seen within NPC cultures that were supplemented with exogenous FGF2, the percent decrease would remain consistent as it is based off a proteolytic degradation half-life. FGF2 is only one example of an HBGF stabilized by HS GAGs present instead of degrading rapidly in culture media [69,70] and differences in stabilization due to EC-produced factors may contribute to the observed differences in proliferation and fate. Other HBGFs would see similar trends in stabilization by HS GAGs [36,38,71]; however, the time course would vary based on the protein half-life.
Discussion
This work expands prior studies of EC-NPC interactions by considering changes to NPC proliferation and fate in response to flow, a key element of any vascularized stem cell niche, through the novel inclusion of fluid flow for EC cultures. We observed differences in fate after NPC expansion in EC-conditioned medium collected from dynamic or static culture, influencing both neural and oligodendrocyte differentiation. Similarly, striking differences in cell adhesion and expansion capacity were evident. This work provides the first evidence that EC exposure to flow is a key influential niche factor.
Dynamic EC-conditioned medium supported greater proliferation relative to cells in static EC-conditioned medium; however, control medium supported the greatest overall cell number. Increasing the feeding frequency resulted in a similar increase across all medium conditions, but did not equalize the disparity in neurosphere formation. Interestingly, neurosphere formation through aggregation is said to occur above a clonal density of 10 cells/μL [50]; however, cells in EC-conditioned media exhibit reduced or absence of neurosphere formation at these high clonal densities since the majority of cells were attached to the culture plates rather than being available for colony aggregation. This would suggest that aggregation is not a significant contributor for cellular expansion in EC-conditioned medium. Distribution of neurosphere diameter was similar for dynamic EC-conditioned and nonconditioned cultures, suggesting that aggregation at this seeding density may not significantly impact colony expansion in nonconditioned controls. However, aggregation of colonies in the nonconditioned control medium cannot be entirely dismissed, both neurosphere counts/size and cell number are reported as metrics of cell growth.
Presumably, differences in neurosphere formation were due to EC-produced soluble factors influencing different proliferation and attachment pathways initiated by the distinct biochemical milieu present in each culture condition. While not evaluated, neurotrophin-3 and bone morphogenetic proteins have also been shown to reduce neurosphere formation. Similarly, a number of factors found in the EC-conditioned medium can reduce proliferation, such as IL-1β, IFN, and IL-10, albeit an evaluation on neurosphere formation capacity is unknown. Shen et al. demonstrate that static ECs cocultured with adherent NSCs increase NSC self-renewal compared with control cultures [6], which was not evident in neurosphere cultures done in this work presumably due to the difference in NSC source (adult vs. embryonic), NSC culture (adherent vs. neurosphere), and inclusion of ECs through conditioned medium instead of transwell inserts.
The increased release of factors known to enhance integrin or cell–cell adhesion molecule concentrations on the surface of NPCs would affect cell–substrate and cell–cell binding, respectively, and may lead to different modes of proliferation (adherent or neurosphere cultures) in each culture condition. The interplay of these pathways can be quite complex as there are a number of factors working in combination to regulate NPC proliferation and fate, particularly in the EC-conditioned medium that contains both growth promoting and inhibitory factors. SDF-1 and IGF-1 are present in EC-conditioned medium and are known to upregulate the α6 integrin subunit [61,72] that can be used to attach to matrix or laminin produced by cells within the neurospheres [73]. Similarly, FGF2 and EGF are present in all culture regimes and promote upregulation of the β1 integrin subunit involved in both cell–substrate attachment and neurosphere formation [8,73]. It is likely that there is a convergence of many signaling cascades that are activated in the presence of EC-conditioned medium compared with the well-characterized control medium and these differences dictate the formation of neurospheres or adherent cultures. Isolating potential candidate factors responsible for these differences in integrin upregulation in the EC-conditioned medium provides an opportunity for future study that may help improve proliferation of NPCs in EC-conditioned medium as neurospheres rather than adherent or mixed cultures.
Differences in the affinity of cell–cell and cell–substrate interactions may not be entirely due to the presence or absence of growth factors. Secreted CAMs in the EC-conditioned medium may have settled to the bottom of the culture plates, fostering cell attachment directly to soluble CAMs rather than CAMs expressed by cells in culture. Dynamic EC-conditioned medium exhibited elevated levels of E-cadherin, selectins (E, L, and P), intercellular CAM 1 (ICAM1), and vascular CAM (VCAM). Several of these proteins were also present in static EC-conditioned medium, in addition to elevated levels of galectin, which can modulate integrin-mediated adhesion. Within the SVZ, VCAM is highly expressed by NPCs and required for establishment of a neurogenic niche [74].
As indicated using the NCFC assay, primary expansion with EC-conditioned medium supported larger and more numerous neurosphere colonies during a second expansion phase in the absence of the EC-conditioned medium, indicating that the EC factors can prime them for greater growth in the future. This may potentially be due to increased bioavailability of growth factors due to HS GAGs in the endothelial-conditioned medium. HS GAGs in EC-conditioned medium as well as heparin in the NCFC assay increase bioavailability by binding growth factors to prevent degradation, modulate ligand–receptor binding, and serve as a cofactor to initiate signaling [38].
With the cells, the elevated growth factor bioavailability would mean that the growth factor concentration would decrease at a slower rate and increase the maintenance of a more stem-like phenotype due to consistent growth factor bioavailability throughout the culture duration [69], particularly evident in dynamic EC-conditioned medium cultures that had substantially more stabilizers (HS-GAGs, heparin) throughout expansion (14 days) and the NCFC assay (21 days). Conversely, cells expanded in nonconditioned or static EC-conditioned medium did not produce stem-like phenotypes, presumably due to their inability to maintain this phenotype in the presence of little to no HS-GAG-mediated growth factor stabilization during expansion (14 days), and were incapable of rescuing a stem-like phenotype in the presence of heparin-mediated growth factor stabilization in the NCFC assay. Together, these findings suggests that cells initially expanded in EC-conditioned medium, and more importantly dynamic EC-conditioned medium, may be more likely to maintain viability and potency long after the removal of EC-derived factors, provided stable growth factor concentrations continue to exist. To illustrate this, Lowry et al. describe in vivo transplantation of embryonic spinal cord NSCs cocultured with static ECs and found that the coculture led to enhanced survival and differentiation potential [19], supporting the idea that priming adult SVZ NSCs with EC factors provides lasting beneficial effects.
Nondirected differentiation capacity is equally important to transplantation survival and proliferation as we do not want to expand NPCs that are primed toward an astroglial lineage. Cells expanded in control medium generated primarily astrocytes with some neurons and oligodendrocyte precursor cells. In contrast, cells initially expanded in static EC-conditioned medium were the only treatment to result in differentiated oligodendrocytes, while cells in dynamic EC-conditioned medium exhibited a significant increase in neuron differentiation. Increased neural differentiation in static EC-NSC differentiation has previously been demonstrated by Shen et al. [6]; however, in this culture regime, we saw a significant increase due to dynamic EC culture with an overall higher percentage of neurons from adult NSCs (20–30% compared with 2%). These results are exciting as they suggest that there are profound differences in the EC phenotype through exposure to shear stress, resulting in distinct differentiation profiles of adult NPCs that may be beneficial for translational applications. Prior studies showed that embryonic spinal cord stem cells expanded with static EC cocultures and then transplanted into a dorsal hemisection spinal cord injury model showed increased survival and oligodendrocyte formation [19], supporting the fate choices demonstrated in this work. By coculturing with ECs exposed to flow, NPCs may demonstrate enhanced neurogenesis following transplantation.
The synthesis and release of many cytokines present in EC-conditioned medium were responsive to dynamic versus static EC culture and may be responsible for differences in fate choice. NPC sensitivity to Shh is concentration dependent, as demonstrated by the role Shh plays in ventral patterning during development, resulting in neurogenesis at higher concentrations and oligodendrocyte differentiation at lower concentrations [18,20,24]. The static EC-conditioned medium contained Shh that may be sufficient for enhanced oligodendrocyte differentiation, but insufficient to encourage enhanced neurogenesis. Monocyte chemoattractant protein-1 (MCP-1) has also been shown to promote oligodendrocyte differentiation [62] and was 2.27-fold higher in the static compared with dynamic EC-conditioned medium, thus supporting the increased oligodendrocyte population following NPC culture in static EC-conditioned medium.
IGF-1 has been shown to support both oligodendrocyte and neuronal differentiation through the ERK1/2 and PI3K/Akt pathways, respectively [16,66]. Dynamic EC-conditioned medium contains 2.25-times the IGF-1 compared with static EC-conditioned medium, thus, while not evaluated, the difference in IGF-1 concentration could have activated PI3K/Akt and ERK1/2 pathways contributing to differences in neuron and oligodendrocyte differentiation, respectively. It is unclear whether activation of these neuro- or oligospecific pathways is selected through concentration dependence or through other cofactors that work in combination with IGF-1 as many soluble factors can activate the highly utilized ERK1/2 and PI3K/Akt pathways.
Enhanced neurogenesis associated with dynamic EC-conditioned medium may be in response to elevated IGF-1 [16], SDF-1α [64], amphiregulin [65], prolactin [75], and RAGE [60], which have been shown to promote neuronal differentiation in culture. Furthermore, several cytokines known to inhibit neurogenesis (IL-1β [76], MCP-1 [62], and eotaxin [77]) are only present in the static EC-conditioned medium. Neurogenesis is not completely eliminated in static conditioned medium, perhaps, in part, due to the presence of the proneurogenic factors VEGF-D [64,78], GRO-α [62], granulocyte macrophage colony-stimulating factor (GM-CSF) [79], and interferon [80] identified in the static EC-conditioned medium. Future analyses will include cytokines not present on the array and cytokines that are present, but not previously studied for their effects on NPC differentiation.
Conditioned medium from static or dynamic EC cultures presents an opportunity to demonstrate the importance of fluid flow within the niche. ECs and shear stress are only two important factors within the niche. Within the niche, there are complex multicellular interactions between NSCs, NPCs, ECs, pericytes, and astrocytes. This study focuses primarily on the EC effects on NPCs, but pericytes also are present within the microvasculature and would be impacted by fluid flow. It is also well established that gradients within the niche are influential in lineage progression as well as migration out of the niche. This work utilizes bulk factors present in the conditioned medium that can be depleted over time and negates the influence of NPCs on EC phenotype [81,82]. Within the niche, HSPG-rich fractones are also influential through regulation of growth factor presentation to cells within the niche [11,67,83]; however, the cellular source of fractones is unknown. Increased production of HS GAGs in this study in the presence of fluid flow was able to similarly increase the bioactivity of growth factors for NPCs, suggesting ECs as a potential source.
The focus of this work was on NPCs as we were unable to maintain quiescent NSC populations that have been reported in EC-NSC cocultures through direct cell–cell contact [84] or neurotrophin-3 release [85]. Within the niche, type B NSCs become responsive to an EGF gradient that gives rise to transient-amplifying type C NPCs that were abundant in the presence of EC-conditioned medium. These cells differentiate into neuroblasts that migrate out of the niche along the rostral migratory stream to the olfactory bulb whether they terminally differentiate into neurons. Within this study, neurogenesis is highest in the presence of a dynamic EC phenotype rather than physiologically aberrant static ECs. As we wanted to evaluate whether fluid flow is influential within the niche and not develop a pseudoniche, we did not evaluate the role of fluid flow on neuroblast migration. Presumably, neurotrophic gradients promote neuroblast migration that were absent in this model.
Future work aims to include ECs cultured under fluid flow with statically cultured NPCs to build a neurogenic niche model that allows for constant replenishment of diffused factors in real time, including cross talk between the two cell populations. Taking cues from the niche cytoarchitecture, NPCs would likely need to be within close apposition of dynamic ECs as they are within 10 μm of the laminin-rich vascular that comprised primarily ECs [1,2]. This is likely attributed to the presence of basement membrane and fractones that facilitate NPC attachment, play a role in neuroblast migration out of the niche [86], and provide a reservoir of growth factors through HSPG-mediated sequestration [11]. The results of the presented work provide a basis for including fluid flow in future EC-NPC niche models, and from here, the niche model can be developed further as a tool to study NSC potential with aging and disease.
Conclusions
Profound compositional differences occur in EC-conditioned medium collected from static and dynamic ECs due to shear stress and duration of flow. NPCs are sensitive to these changes in the two biochemically distinct EC-conditioned media, resulting in altered proliferation, survival, and differentiation. Current in vitro EC-NPC models utilize only static ECs, while ECs within the niche are exposed to shear stress generated by blood flow. Moving forward, we propose that in vitro models of the NPC niche incorporate the more physiologically relevant dynamic EC phenotype to study EC-NPC interactions and that the EC phenotype can be varied through dynamic culture. This work focused on a single shear stress regime as an approximation of physiological hemodynamic shear stress [47,48]; however, shear stress magnitude, duration, and type (laminar or pulsatile) can be varied to generate other distinct EC-conditioned media that may further enhance neuronal or oligodendrocyte differentiation or lead to an improved understanding of the aging niche. Further investigation and characterization of EC-cytokine cocktails using a variety of shear stress parameters could further enrich desired NSC-based cell populations or precursors.
This work could prove instrumental in not only improving current in vitro models but also in directing in vivo NPC expansion through the use of EC-derived factors. In Alzheimer's disease and vascular dementia, vascular flow is reduced and may become more turbulent due to ischemia and plaque aggregation of the diseased vasculature [43,87–90]. It will be worthwhile exploring whether altered hemodynamics contribute to the devastating loss of adult NSCs and the decline in neurogenesis in patients with this disease, as is implicated by increased ischemia of vessels near the subventricular niche [44]. Significant changes of flow due to disease or injury could be modulated or compensated by factor supplementation to improve regeneration and tissue repair. Further investigation into this niche model will be fundamental in providing the groundwork to develop therapeutics to target the vasculature leading to NSC-mediated regeneration after injury or prevention of age-related degeneration.
Supplementary Material
Acknowledgments
Funding was provided by the New York State Department of Health NYSTEM (C026419- DMT), National Institutes of Health (RO1AG041861-ST), and the National Science Foundation (CBET-1350240- GD).
Author Disclosure Statement
The authors declare no competing interests.
References
- 1.Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B. and Temple S. (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3:289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM. and Doetsch F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3:279–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louissaint A, Jr., Rao S, Leventhal C. and Goldman SA. (2002). Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron 34:945–960 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds BA. and Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710 [DOI] [PubMed] [Google Scholar]
- 5.Palmer TD, Willhoite AR. and Gage FH. (2000). Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494 [DOI] [PubMed] [Google Scholar]
- 6.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, Vincent P, Pumiglia K. and Temple S. (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304:1338–1340 [DOI] [PubMed] [Google Scholar]
- 7.Gama Sosa MA, De Gasperi R, Rocher AB, Perez GM, Simons K, Cruz DE, Hof PR. and Elder GA. (2007). Interactions of primary neuroepithelial progenitor and brain endothelial cells: distinct effect on neural progenitor maintenance and differentiation by soluble factors and direct contact. Cell Res 17:619–626 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Yanagisawa M, Yagi H, Nakatani Y. and Yu RK. (2010). Involvement of beta1-integrin up-regulation in basic fibroblast growth factor- and epidermal growth factor-induced proliferation of mouse neuroepithelial cells. J Biol Chem 285:18443–18451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF. and van der Kooy D. (1999). Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol 208:166–188 [DOI] [PubMed] [Google Scholar]
- 10.Silva-Vargas V, Maldonado-Soto AR, Mizrak D, Codega P. and Doetsch F. (2016). Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19:643–652 [DOI] [PubMed] [Google Scholar]
- 11.Kerever A, Mercier F, Nonaka R, de Vega S, Oda Y, Zalc B, Okada Y, Hattori N, Yamada Y. and Arikawa-Hirasawa E. (2014). Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res 12:492–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergano-Vera E, Diaz-Guerra E, Rodriguez-Traver E, Mendez-Gomez HR, Solis O, Pignatelli J, Pickel J, Lee SH, Moratalla R. and Vicario-Abejon C. (2015). Nurr1 blocks the mitogenic effect of FGF-2 and EGF, inducing olfactory bulb neural stem cells to adopt dopaminergic and dopaminergic-GABAergic neuronal phenotypes. Dev Neurobiol 75:823–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, Maynard T, Gonzalez D, Kim S, et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh J, Aimone JB, Kaspar BK, Kuwabara T, Nakashima K. and Gage FH. (2004). IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J Cell Biol 164:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaker Z, Aid S, Berry H. and Holzenberger M. (2015). Suppression of IGF-I signals in neural stem cells enhances neurogenesis and olfactory function during aging. Aging Cell 14:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang L, Cheng X, Guo Y, Sun X, Chen G, Li H, Li P, Lu X, et al. (2014). IGF-1 Promotes Brn-4 Expression and Neuronal Differentiation of Neural Stem Cells via the PI3K/Akt Pathway. PLoS One 9:e113801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler AN, Chidambaram S, Forbes BE, Wood TL. and Levison SW. (2014). Insulin-like growth factor-II (IGF-II) and IGF-II analogs with enhanced insulin receptor-a binding affinity promote neural stem cell expansion. J Biol Chem 289:4626–4633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gritli-Linde A, Lewis P, McMahon AP. and Linde A. (2001). The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol 236:364–386 [DOI] [PubMed] [Google Scholar]
- 19.Lowry N, Goderie SK, Adamo M, Lederman P, Charniga C, Gill J, Silver J. and Temple S. (2008). Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol 209:510–522 [DOI] [PubMed] [Google Scholar]
- 20.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, Gitton Y, Carleton A, Alvarez-Buylla A. and Ruiz i Altaba A. (2005). Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 132:335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aigner L. and Bogdahn U. (2008). TGF-beta in neural stem cells and in tumors of the central nervous system. Cell Tissue Res 331:225–241 [DOI] [PubMed] [Google Scholar]
- 22.Pineda JR, Daynac M, Chicheportiche A, Cebrian-Silla A, Sii Felice K, Garcia-Verdugo JM, Boussin FD. and Mouthon MA. (2013). Vascular-derived TGF-beta increases in the stem cell niche and perturbs neurogenesis during aging and following irradiation in the adult mouse brain. EMBO Mol Med 5:548–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandasamy M, Lehner B, Kraus S, Sander PR, Marschallinger J, Rivera FJ, Trumbach D, Ueberham U, Reitsamer HA, et al. (2014). TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J Cell Mol Med 18:1444–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tekki-Kessaris N, Woodruff R, Hall AC, Gaffield W, Kimura S, Stiles CD, Rowitch DH. and Richardson WD. (2001). Hedgehog-dependent oligodendrocyte lineage specification in the telencephalon. Development 128:2545–2554 [DOI] [PubMed] [Google Scholar]
- 25.Pastrana E, Silva-Vargas V. and Doetsch F. (2011). Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell 8:486–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies PF. (1995). Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena and G.Gimbrone MA., Jr. (2004). Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A 101:14871–14876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ando J. and Yamamoto K. (2009). Vascular mechanobiology: endothelial cell responses to fluid shear stress. Circ J 73:1983–1992 [DOI] [PubMed] [Google Scholar]
- 29.Malek AM, Gibbons GH, Dzau VJ. and Izumo S. (1993). Fluid shear stress differentially modulates expression of genes encoding basic fibroblast growth factor and platelet-derived growth factor B chain in vascular endothelium. J Clin Invest 92:2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morita T, Yoshizumi M, Kurihara H, Maemura K, Nagai R. and Yazaki Y. (1993). Shear stress increases heparin-binding epidermal growth factor-like growth factor mRNA levels in human vascular endothelial cells. Biochem Biophys Res Commun 197:256–262 [DOI] [PubMed] [Google Scholar]
- 31.Ohno M, Cooke JP, Dzau VJ. and Gibbons GH. (1995). Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest 95:1363–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walshe TE, Connell P, Cryan L, Ferguson G, Gardiner T, Morrow D, Redmond EM, O'Brien C. and Cahill PA. (2011). Microvascular retinal endothelial and pericyte cell apoptosis in vitro: role of hedgehog and Notch signaling. Invest Ophthalmol Vis Sci 52:4472–4483 [DOI] [PubMed] [Google Scholar]
- 33.Gloe T, Sohn HY, Meininger GA. and Pohl U. (2002). Shear stress-induced release of basic fibroblast growth factor from endothelial cells is mediated by matrix interaction via integrin alpha(v)beta3. J Biol Chem 277:23453–23458 [DOI] [PubMed] [Google Scholar]
- 34.Reisig K. and Clyne AM. (2010). Fibroblast growth factor-2 binding to the endothelial basement membrane peaks at a physiologically relevant shear stress. Matrix Biol 29:586–593 [DOI] [PubMed] [Google Scholar]
- 35.Arisaka T, Mitsumata M, Kawasumi M, Tohjima T, Hirose S. and Yoshida Y. (1995). Effects of shear stress on glycosaminoglycan synthesis in vascular endothelial cells. Ann N Y Acad Sci 748:543–554 [DOI] [PubMed] [Google Scholar]
- 36.Saksela O, Moscatelli D, Sommer A. and Rifkin DB. (1988). Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol 107:743–751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, et al. (2010). Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature 468:310–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandtlow CE. and Zimmermann DR. (2000). Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev 80:1267–1290 [DOI] [PubMed] [Google Scholar]
- 39.Aviezer D, Levy E, Safran M, Svahn C, Buddecke E, Schmidt A, David G, Vlodavsky I. and Yayon A. (1994). Differential structural requirements of heparin and heparan sulfate proteoglycans that promote binding of basic fibroblast growth factor to its receptor. J Biol Chem 269:114–121 [PubMed] [Google Scholar]
- 40.Nugent MA. and Edelman ER. (1992). Kinetics of basic fibroblast growth factor binding to its receptor and heparan sulfate proteoglycan: a mechanism for cooperactivity. Biochemistry 31:8876–8883 [DOI] [PubMed] [Google Scholar]
- 41.Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T. and Oohira A. (2006). Identification and functions of chondroitin sulfate in the milieu of neural stem cells. J Biol Chem 281:5982–5991 [DOI] [PubMed] [Google Scholar]
- 42.Sirko S, von Holst A, Wizenmann A, Gotz M. and Faissner A. (2007). Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development 134:2727–2738 [DOI] [PubMed] [Google Scholar]
- 43.Ajmani RS, Metter EJ, Jaykumar R, Ingram DK, Spangler EL, Abugo OO. and Rifkind JM. (2000). Hemodynamic changes during aging associated with cerebral blood flow and impaired cognitive function. Neurobiol Aging 21:257–269 [DOI] [PubMed] [Google Scholar]
- 44.Purkayastha S, Fadar O, Mehregan A, Salat DH, Moscufo N, Meier DS, Guttmann CR, Fisher ND, Lipsitz LA. and Sorond FA. (2014). Impaired cerebrovascular hemodynamics are associated with cerebral white matter damage. J Cereb Blood Flow Metab 34:228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piccin D, Tufford A. and Morshead CM. (2014). Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol Aging 35:1669–1679 [DOI] [PubMed] [Google Scholar]
- 46.Zwolinski CM, Ellison KS, Depaola N. and Thompson DM. (2011). Generation of cell-derived three dimensional extracellular matrix substrates from two dimensional endothelial cell cultures. Tissue Eng Part C Methods 17:589–595 [DOI] [PubMed] [Google Scholar]
- 47.Stepp DW, Nishikawa Y. and Chilian WM. (1999). Regulation of shear stress in the canine coronary microcirculation. Circulation 100:1555–1561 [DOI] [PubMed] [Google Scholar]
- 48.Lipowsky HH. (2005). Microvascular rheology and hemodynamics. Microcirculation 12:5–15 [DOI] [PubMed] [Google Scholar]
- 49.Reneman RS. and Hoeks AP. (2008). Wall shear stress as measured in vivo: consequences for the design of the arterial system. Med Biol Eng Comput 46:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coles-Takabe BL, Brain I, Purpura KA, Karpowicz P, Zandstra PW, Morshead CM. and van der Kooy D. (2008). Don't look: growing clonal versus nonclonal neural stem cell colonies. Stem Cells 26:2938–2944 [DOI] [PubMed] [Google Scholar]
- 51.Yang B, Chang Y, Weyers AM, Sterner E. and Linhardt RJ. (2012). Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography-mass spectrometry. J Chromatogr A 1225:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golmohammadi MG, Blackmore DG, Large B, Azari H, Esfandiary E, Paxinos G, Franklin KB, Reynolds BA. and Rietze RL. (2008). Comparative analysis of the frequency and distribution of stem and progenitor cells in the adult mouse brain. Stem Cells 26:979–987 [DOI] [PubMed] [Google Scholar]
- 53.Louis SA, Rietze RL, Deleyrolle L, Wagey RE, Thomas TE, Eaves AC. and Reynolds BA. (2008). Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells 26:988–996 [DOI] [PubMed] [Google Scholar]
- 54.Gensburger C, Labourdette G. and Sensenbrenner M. (1987). Brain basic fibroblast growth factor stimulates the proliferation of rat neuronal precursor cells in vitro. FEBS Lett 217:1–5 [DOI] [PubMed] [Google Scholar]
- 55.Erickson RI, Paucar AA, Jackson RL, Visnyei K. and Kornblum H. (2008). Roles of insulin and transferrin in neural progenitor survival and proliferation. J Neurosci Res 86:1884–1894 [DOI] [PubMed] [Google Scholar]
- 56.McPherson CA, Aoyama M. and Harry GJ. (2011). Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun 25:850–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez-Nicola D, Valle-Argos B, Pallas-Bazarra N. and Nieto-Sampedro M. (2011). Interleukin-15 regulates proliferation and self-renewal of adult neural stem cells. Mol Biol Cell 22:1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Desai M, Li T. and Ross MG. (2011). Fetal hypothalamic neuroprogenitor cell culture: preferential differentiation paths induced by leptin and insulin. Endocrinology 152:3192–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Udagawa J, Ono A, Kawamoto M. and Otani H. (2010). Leptin and its intracellular signaling pathway maintains the neurosphere. Neuroreport 21:1140–1145 [DOI] [PubMed] [Google Scholar]
- 60.Meneghini V, Francese MT, Carraro L. and Grilli M. (2010). A novel role for the Receptor for Advanced Glycation End-products in neural progenitor cells derived from adult SubVentricular Zone. Mol Cell Neurosci 45:139–150 [DOI] [PubMed] [Google Scholar]
- 61.Kokovay E, Goderie S, Wang Y, Lotz S, Lin G, Sun Y, Roysam B, Shen Q. and Temple S. (2010). Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 7:163–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez-Perez O, Gutierrez-Fernandez F, Lopez-Virgen V, Collas-Aguilar J, Quinones-Hinojosa A. and Garcia-Verdugo JM. (2012). Immunological regulation of neurogenic niches in the adult brain. Neuroscience 226:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borsini A, Zunszain PA, Thuret S. and Pariante CM. (2015). The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci 38:145–157 [DOI] [PubMed] [Google Scholar]
- 64.Schwartz CM, Tavakoli T, Jamias C, Park SS, Maudsley S, Martin B, Phillips TM, Yao PJ, Itoh K, et al. (2012). Stromal factors SDF1alpha, sFRP1, and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res 90:1367–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Falk A. and Frisen J. (2002). Amphiregulin is a mitogen for adult neural stem cells. J Neurosci Res 69:757–762 [DOI] [PubMed] [Google Scholar]
- 66.Shi B, Ding J, Liu Y, Zhuang X, Zhuang X, Chen X. and Fu C. (2014). ERK1/2 pathway-mediated differentiation of IGF-1-transfected spinal cord-derived neural stem cells into oligodendrocytes. PLoS One 9:e106038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kerever A, Schnack J, Vellinga D, Ichikawa N, Moon C, Arikawa-Hirasawa E, Efird JT. and Mercier F. (2007). Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25:2146–2157 [DOI] [PubMed] [Google Scholar]
- 68.Nissen NN, Shankar R, Gamelli RL, Singh A. and DiPietro LA. (1999). Heparin and heparan sulphate protect basic fibroblast growth factor from non-enzymic glycosylation. Biochem J 338 (Pt 3):637–642 [PMC free article] [PubMed] [Google Scholar]
- 69.Lotz S, Goderie S, Tokas N, Hirsch SE, Ahmad F, Corneo B, Le S, Banerjee A, Kane RS, et al. (2013). Sustained levels of FGF2 maintain undifferentiated stem cell cultures with biweekly feeding. PLoS One 8:e56289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nieto-Estevez V, Pignatelli J, Arauzo-Bravo MJ, Hurtado-Chong A. and Vicario-Abejon C. (2013). A global transcriptome analysis reveals molecular hallmarks of neural stem cell death, survival, and differentiation in response to partial FGF-2 and EGF deprivation. PLoS One 8:e53594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tumova S, Woods A. and Couchman JR. (2000). Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int J Biochem Cell Biol 32:269–288 [DOI] [PubMed] [Google Scholar]
- 72.Frade JM, Marti E, Bovolenta P, Rodriguez-Pena MA, Perez-Garcia D, Rohrer H, Edgar D. and Rodriguez-Tebar A. (1996). Insulin-like growth factor-I stimulates neurogenesis in chick retina by regulating expression of the alpha 6 integrin subunit. Development 122:2497–2506 [DOI] [PubMed] [Google Scholar]
- 73.Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U. and ffrench-Constant C. (2004). Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131:3433–3444 [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Kane AW, Lee C. and Ahn S. (2014). Gli3 repressor controls cell fates and cell adhesion for proper establishment of neurogenic niche. Cell Rep 8:1093–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC. and Weiss S. (2003). Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299:117–120 [DOI] [PubMed] [Google Scholar]
- 76.Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, et al. (2010). Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse 64:721–728 [DOI] [PubMed] [Google Scholar]
- 77.Mendelsohn AR. and Larrick JW. (2011). Overcoming the aging systemic milieu to restore neural stem cell function. Rejuvenation Res 14:681–684 [DOI] [PubMed] [Google Scholar]
- 78.Shin YJ, Choi JS, Choi JY, Cha JH, Chun MH. and Lee MY. (2010). Enhanced expression of vascular endothelial growth factor receptor-3 in the subventricular zone of stroke-lesioned rats. Neurosci Lett 469:194–198 [DOI] [PubMed] [Google Scholar]
- 79.Kruger C, Laage R, Pitzer C, Schabitz WR. and Schneider A. (2007). The hematopoietic factor GM-CSF (granulocyte-macrophage colony-stimulating factor) promotes neuronal differentiation of adult neural stem cells in vitro. BMC Neurosci 8:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wong G, Goldshmit Y. and Turnley AM. (2004). Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol 187:171–177 [DOI] [PubMed] [Google Scholar]
- 81.Chou CH. and Modo M. (2016). Human neural stem cell-induced endothelial morphogenesis requires autocrine/paracrine and juxtacrine signaling. Sci Rep 6:29029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chou CH, Sinden JD, Couraud PO. and Modo M. (2014). In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. PLoS One 9:e106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mercier F, Kitasako JT. and Hatton GI. (2002). Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188 [DOI] [PubMed] [Google Scholar]
- 84.Ottone C, Krusche B, Whitby A, Clements M, Quadrato G, Pitulescu ME, Adams RH. and Parrinello S. (2014). Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat Cell Biol 16:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delgado AC, Ferron SR, Vicente D, Porlan E, Perez-Villalba A, Trujillo CM, D'Ocon P. and Farinas I. (2014). Endothelial NT-3 delivered by vasculature and CSF promotes quiescence of subependymal neural stem cells through nitric oxide induction. Neuron 83:572–585 [DOI] [PubMed] [Google Scholar]
- 86.Bovetti S, Hsieh YC, Bovolin P, Perroteau I, Kazunori T. and Puche AC. (2007). Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J Neurosci 27:5976–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mazza M, Marano G, Traversi G, Bria P. and Mazza S. (2011). Primary cerebral blood flow deficiency and Alzheimer's disease: shadows and lights. J Alzheimers Dis 23:375–389 [DOI] [PubMed] [Google Scholar]
- 88.de la Torre JC. (2013). Vascular risk factors: a ticking time bomb to Alzheimer's disease. Am J Alzheimers Dis Other Demen 28:551–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pimentel-Coelho PM. and Rivest S. (2012). The early contribution of cerebrovascular factors to the pathogenesis of Alzheimer's disease. Eur J Neurosci 35:1917–1937 [DOI] [PubMed] [Google Scholar]
- 90.Grammas P, Martinez J. and Miller B. (2011). Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med 13:e19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.