Abstract
Given the rising HIV incidence in men who have sex with men (MSM) despite repeatedly proven effectiveness of oral HIV pre-exposure prophylaxis, behaviorally congruent periodic dosing strategies, such as dosing microbicides as lubricants, are now in demand. Rectal microbicide gel studies largely administer gels using vaginal applicators, which have not been well received and do not mimic lubricant use. We compared rectal gel manually dosed as lubricant with applicator dosing in five healthy, HIV-negative MSM who received 10 or 3.5 ml of 99mTc-DTPA-radiolabeled hydroxyethyl cellulose universal placebo gel intrarectally. After washout, participants received 10 ml of radiolabeled Wet® Original® lubricant to apply to the anus with fingers and/or a phallus in a manner typical of sexual lubricant use with a partner, followed by simulated receptive anal intercourse. Single-photon emission computed tomography with transmission computed tomography was performed 4 h after each gel administration. Manual dosing was associated with more variable rectosigmoid distribution, 4.4–15.3 cm from the anorectal junction, compared with more uniform distribution, 5.9–7.4 and 5.3–7.6 cm after 10 and 3.5 ml applicator dosing, respectively. A significantly smaller fraction of the initial 10 ml dose was retained within the colon after manual dosing, 3.4%, compared with 94.9% and 88.4% after 10 and 3.5 ml applicator dosing, respectively (both p < .001). Manual dosing of a sexual lubricant delivered a small, variable fraction of the dose with variable rectosigmoid distribution compared with applicator dosing. These results raise concern that dosing a rectal microbicide gel as a sexual lubricant may not provide adequate or predictable mucosal coverage for HIV protection.
Keywords: : microbicide, pre-exposure prophylaxis, human immunodeficiency virus, gastrointestinal distribution, pharmacokinetics
The rising incidence of HIV infection in men who have sex with men (MSM) in many regions highlights the need for pre-exposure prophylaxis (PrEP). Recognizing that effectiveness of oral PrEP hinges on high levels of adherence and that MSM practicing receptive anal intercourse (RAI) already commonly use sexual lubricants,1 behaviorally congruent PrEP in the form of anal lubricant is of great interest.
Studies of rectal microbicide gels largely employ vaginal applicators that neither align with RAI practices2 nor mimic real-world lubricant use.3 Trial participants voice concerns about applicator comfort, size, transportability, and visual appeal, potentially limiting acceptability and future adherence.2,4,5 Participants and advocates desire a microbicide gel that can be applied as anal lubricant without an applicator.
Rectal microbicide surrogates dosed with an applicator reach colorectal distributions overlapping that of HIV surrogates and achieve excellent retention.6 To assess whether similar distribution and retention occur when rectal gels are applied as sexual lubricants without applicators, we performed an open-label cross-over study comparing colorectal distribution, percentage dose retained, and volume retained, among manual and applicator dosing methods.
This study was approved by the Johns Hopkins Medicine Institutional Review Board. Participants provided informed consent before screening. Eligible participants were healthy, HIV-seronegative MSM who had participated in a sequence-randomized cross-over study of a 10 and 3.5 ml hydroxyethyl cellulose (HEC) universal placebo gel administered intrarectally by a study investigator utilizing a 4 cm plastic applicator attached to a syringe.7
We enrolled five participants from the prior study to evaluate manual dosing of gel as an anal lubricant without an applicator. The lubricant gel, Wet® Original®, is sold over-the-counter and is an aqueous gel like HEC (Table 1). Wet Original was selected based on brand popularity in an International Rectal Microbicide Advocates survey (M. LeBlanc, Pers. Comm., September 21, 2015).
Table 1.
Ingredients of Gels Used for Applicator and Manual Dosing
| Dosing method | Applicator | Manual |
|---|---|---|
| Gel | Wet® Original® | HEC universal placebo |
| Ingredients | Water | Water |
| GlycerinCarboxymethylcellulose | Natrosol 250 HX Pharm HECSodium chloride | |
| Pentylene glycol | Sorbic acid | |
| Potassium sorbate | Caramel color | |
| Sodium hydroxide |
HEC, hydroxyethylcellulose.
Participants received a 10 ml lubricant pillow, which is a plastic pouch filled with gel, radiolabeled with 1,000 μCi of 99mTc (technetium)-DTPA (diethylenetriamine pentaacetate). Participants were asked to apply the gel as they would apply anal lubricant with a sexual partner, using a phallic device as a surrogate for a penis. This was followed by simulated unprotected RAI (suRAI), gamma emission measurements of study materials, and radiolabeled gel imaging with single-photon emission computed tomography with transmission computed tomography (SPECT/CT) 4 h after dosing, as previously described.8,9
Colon SPECT data were fit using a three-dimensional curve-fitting algorithm. Colorectal distribution parameters, Dmax and Dmin (maximal and minimal colorectal distances of radiosignal), DCmax (distance associated with maximum concentration), and Dave (mean resident distance), were determined as previously described.9
Region of interest (ROI) analysis of SPECT/CT images utilized previously described software.8 For each axial slice on attenuation-corrected SPECT scans, ROIs were drawn around areas of signal intensity to quantify differences between intraluminal and extracorporeal signal. ROIs proximal to the anorectal junction, approximated as the axial CT slice inferior to air visualization within the rectal ampulla, were labeled intraluminal. For manual dosing, percentage dose retained was estimated as the product of three variables: percentage of gel removed from the pillow, percentage of removed gel applied to the body, and percentage of applied gel that was intraluminal. Percentage removed, applied, and intraluminal were determined by weights, dosimetry, and ROIs, respectively.
For applicator dosing, percentage dose retained was estimated as previously described.7 Volume retained was estimated as the product of percentage dose retained and volume of the original dose unit. Continuous measures were described as medians and ranges. Differences among dosing arms were tested with Friedman repeated measures analysis of variance (ANOVA), followed by Tukey's test for multiple comparisons, with p-values < .05 considered statistically significant.
Five healthy HIV-negative MSM were enrolled. Median age was 48 years (23–57). For manual dosing, four participants used fingers and phallus to administer lubricant, whereas one used only the phallus. Similar colorectal distribution was observed among dosing arms, with no statistically significant differences in Dmax, DCmax, or Dave. Dmin was 2.8 and 3.2 cm greater for manual than 10 and 3.5 ml applicator dosing, respectively (p = .01, p < .01). Dmax was more variable for manual than 10 and 3.5 ml applicator dosing, with ranges of 4.4–15.3, 5.9–7.4, and 5.3–7.6, respectively.
For manual dosing, participants removed a median of 3.2 ml (1.8, 6.6) from the 10 ml pillow (32%). Then 20.8% (17.1, 39.9) of removed lubricant was applied to the body, and 51% (20, 89) of applied lubricant was intraluminal based on imaging (Fig. 1). Thus, of the initial 10 ml dose contained in the pillow, 3.4% (0.01, 23.4) was delivered intraluminally.
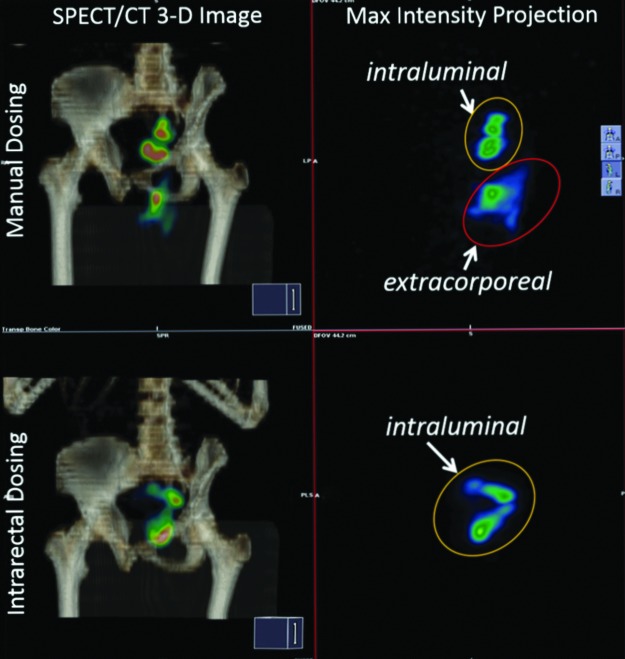
FIG. 1.
Manual dosing images (top panels) indicate a substantial fraction of the dose falling outside the body (extracorporeal), posteroinferior to the pelvis, mostly in the midline gluteal folds in addition to a fraction within the colonic lumen. The intrarectal dosing images (bottom panels) indicate all visible signals within the colonic lumen within the pelvis. Fused SPECT and CT images (left panels) indicate bone in amber scale with lumbosacral spine, pelvis, and humerus (top to bottom); color in images indicate radiolabel intensity. Right panels are maximum intensity projections of SPECT image (color scale signal intensity) at similar angle of rotation without bony landmarks; labels indicate intraluminal and extracorporeal radiolabel. SPECT, single-photon emission computed tomography; CT, transmission computed tomography.
For applicator dosing, 94.9% (94.3, 95.6) and 88.4% (86.4, 89.5) of the 10 and 3.5 ml dose contained in the syringe were ejected, respectively. For both arms, 100% (100, 100) of ejected gel was delivered intraluminally. Thus, of the 10 and 3.5 ml doses contained in the syringe, 94.9% (94.3, 95.6) and 88.4% (86.4, 89.5) of the full original dose, respectively, were delivered intraluminally. Overall, percentage dose retained for manual dosing was 32- and 29-fold less than 10 and 3.5 ml applicator dosing, respectively (both p < .001). The median intracolonic volume delivered was 0.3, 9.5, and 3.1 ml for manual and 10 and 3.5 ml for applicator dosing, respectively.
The number of participants was too small to statistically test for participant variables, like age, which might correlate with measured parameters, although no clear trends were noted in the data. However, the participant with the greatest percentage of retained gel and the greatest luminal distribution after manual dosing was the only participant who did not use fingers for gel application and only used the phallus.
We describe the first study evaluating distribution and retention of a rectal gel administered as a sexual lubricant. Compared with applicator dosing, manual dosing delivered a small, variable dose with variable rectosigmoid distribution. Although highly variable, similar median colorectal distribution estimates of the manually applied gel, when compared with the applicator applied gel, were unanticipated in light of the far smaller percentage of dose retained with manual dosing. This distribution similarity may be explained by the gel vehicles having different osmolalities (3,679 and 304 mOsm/kg for Wet Original and HEC gels, respectively). The lubricant, with 10-fold greater osmolality due largely to the glycerin content (Table 1), likely drew additional fluid intraluminally, increasing volume and colonic spread. For manual dosing, the larger Dmin was likely related to the radiosignal being below the limit of quantitation because of the small dose retained in the rectum. Although high osmolality gel may provide the better option for increased luminal distribution, it is also associated with significant epithelial toxicity that might increase HIV risk.10
The highly variable rectosigmoid distribution of lubricant among participants could be attributed to diverse dosing practices, resulting in heterogeneous application methods and dosing volumes. For example, the finger-free dosing method of one participant achieved nearly a 10-fold greater amount of retained lubricant within the colorectal lumen. However, adapting new methods of gel dosing might also introduce a requirement for behavioral change and our intent was to see how well manual gel dosing fared with existing behaviors.
The study had several limitations including a small sample size. suRAI only occurred with manual dosing; however, based on CHARM-02, suRAI is unlikely to alter colorectal distribution or retention.8 Investigators administered the gel volume using a syringe/applicator, whereas participants performed manual dosing, removing as much gel from the pillow as needed for lubrication based on personal preference; this contributed to the greater efficiency of applicator dosing. In addition, HEC gel and a lubricant were used, rather than a rectal microbicide gel in development, so no information could be gleaned about the distribution of an active pharmaceutical ingredient. Finally, manual dosing was performed with a restricted maximum gel volume without a sexual partner, diverging from real-world lubricant practices.
This study suggests that, without applicators, manual dosing of a rectal microbicide gel as lubricant may not provide adequate or predictable mucosal coverage, with possible negative impact on HIV protection. A critically complementary study, MTN-033/IPM-044, will address the impact of manual dosing of dapivirine rectal gel on tissue concentration and ex vivo antiviral effect in a larger population. Ultimately, manual dosing of rectal microbicide gel as lubricant may require modifying microbicide formulations in development to achieve adequate antiretroviral delivery. Should rectal gels require applicators to reach HIV-protective colorectal distribution, wide acceptance may be challenging.
Acknowledgments
The authors acknowledge the time of research participants, the Drug Development Unit, Jeff Leal from the Image Response Assessment Team (IRAT) Lab at Johns Hopkins University and Jim Turpin of the Integrated Preclinical/Clinical HIV Topical Microbicide Program. E.C.S. is supported by a training grant from the NIH Institutional National Research Service Award T32 GM066691.
Author Disclosure Statement
E.D.W., E.J.F., and C.W.H. receive funds for clinical research from ViiV/GSK and NIH managed through Johns Hopkins University. E.C.S. receives funds from NIH T32. For the remaining authors, no conflicts of interest were declared. This work was supported by the John Hopkins Drug Development Unit, ImQuest BioSciences, and NIH U19 AI101961 and T32 GM066691.
References
- 1.Carballo-Dieguez A, et al. : Frequent use of lubricants for anal sex among men who have sex with men: The HIV prevention potential of a microbicidal gel. Am J Public Health 2000;90:1117–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carballo-Dieguez A, et al. : Rectal-specific microbicide applicator: Evaluation and comparison with a vaginal applicator used rectally. AIDS Behav 2014;18:1734–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pines HA, et al. : Commercial lubricant use among HIV-negative men who have sex with men in Los Angeles: Implications for the development of rectal microbicides for HIV prevention. AIDS Care 2014;26:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross M, et al. : Acceptability of a bioadhesive nonoxynol-9 gel delivered by an applicator as a rectal microbicide. Sex Transm Dis 1999;26:572–578 [DOI] [PubMed] [Google Scholar]
- 5.Ventuneac A, et al. : Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav 2010;14:618–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louissaint NA, et al. : Distribution of cell-free and cell-associated HIV surrogates in the colon after simulated receptive anal intercourse in men who have sex with men. J Acquir Immune Defic Syndr 2012;59:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weld ED, et al. : A comparative pre-phase I study of the impact of gel vehicle volume on distal colon distribution, user experience, and acceptability. AIDS Res Hum Retroviruses 2017; 33:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiruy H, et al. : A phase 1 randomized, blinded comparison of the pharmacokinetics and colonic distribution of three candidate rectal microbicide formulations of Tenofovir 1% gel with simulated unprotected sex (CHARM-02). AIDS Res Hum Retroviruses 2015;31:1098–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao YJ, et al. : Quantification of the spatial distribution of rectally applied surrogates for microbicide and semen in colon with SPECT and magnetic resonance imaging. Br J Clin Pharmacol 2012;74:1013–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs EJ, et al. : Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: Potential implication for HIV transmission. J Infect Dis 2007;195:703–710 [DOI] [PubMed] [Google Scholar]