Abstract
Background: Efficient delivery of aerosols to the lungs via the nasal route has been difficult to achieve, but it may offer benefits over the traditional oral route for a range of patient populations. Because slow, continuous delivery of short-acting agents could improve safety, tolerability, compliance, and efficacy when compared with the rapid, intermittent aerosol treatments delivered by mouthpiece or mask, a novel trans-nasal pulmonary aerosol delivery (tPAD) device was developed. The tPAD incorporates an aerosol particle-size selection chamber and a custom nasal cannula that are specifically optimized for aerosol delivery to the lung via the nasal route. The tPAD device produced a steady aerosol output (∼2 mL/h) from an optimized nasal cannula with negligible rainout in the cannula for up to 8 hours. The generated aerosol particles were small enough to minimize nasal deposition [volume median diameter (VMD) = 1.4 μm].
Methods: In this proof-of-concept study, gamma scintigraphy was used to quantitate deposition efficiency of 99mTc-labeled DTPA in 7% NaCl (hypertonic saline) in healthy human subjects (n = 6) during a short dosing period (15 minutes). A comparison was made with a standard oral jet nebulizer in the same subjects.
Results: The tPAD device achieved high pulmonary deposition (39% ± 8%), based on emitted dose, and matched that of the oral jet nebulizer (36% ± 9%). Low fractions of aerosol deposition in the head and nose region were observed for tPAD (6% ± 6%) and jet nebulizer deliver (1% ± 1%) as well.
Conclusions: A profile of high pulmonary deposition efficiency and low nasal dose may enable the sustained use of the tPAD platform with a variety of therapeutic agents for a range of pulmonary disorders.
Keywords: : aerosol deposition, hypertonic saline, nasal delivery
Introduction
Most aerosol drug delivery efforts have been focused on the development of nebulizers that rapidly deliver therapeutics to reduce treatment times and encourage treatment adherence. When considering short-acting compounds, this approach typically requires repeated dosing throughout the day and can result in suboptimal treatment responses due to short drug-exposure times and user fatigue, leading to poor adherence. Use of an overnight delivery device, if efficacious and well tolerated, could provide prolonged drug exposure times and improved treatment adherence rates by reducing or eliminating the need for daytime treatments. For selected compounds, this approach could translate into improved clinical outcomes. As an initial exploration of this concept, we explored the ability to deliver hypertonic saline (HS) to the lower airways with the trans-nasal pulmonary aerosol delivery (tPAD) device (Parion Sciences, Inc., Durham, NC).
The tPAD device delivers a continuous aerosol via a specialized nasal cannula that is similar in appearance to those commonly used for supplemental oxygen (tPAD; Parion Sciences, Inc.). Key design features of the tPAD device include utilization of the Aeroneb Pro vibrating mesh aerosol generator (Aerogen, Galway, Ireland) with a modified duty cycle to reduce aerosol output. A proprietary “spacer” was also incorporated to selectively filter out aerosol particles >4 μm in size, thus avoiding excessive nasal deposition. The associated nasal cannula was also engineered to optimize laminar flow, essentially eliminating aerosol impaction and rainout in the cannula, which would lead to obstruction and liquid sputter from the prongs.
Prior studies demonstrated the importance of aerosol particle size and flow rate in nasal/nasopharynx deposition.(1) Generation of a typical therapeutic aerosol [4.23 μm mass median aerodynamic diameter (MMAD) particle size] that was delivered at a continuous inspiratory nasal flow rate of 20 L/min led to 44% of the aerosol being deposited in the nose. However, by reducing particle size to 1.78 μm MMAD, nasal deposition was reduced to 12% under the same flow conditions. Delivery of the smaller aerosol particle at a lower flow rate (10 L/min) essentially eliminated nasal deposition altogether (0.3%).
Similarly, Heyder and Rudolf examined nasal deposition of a monodisperse aerosol of bis(2-ethylhexyl) sebacate at varying flow rates, particle sizes, and inhaled volumes.(2) At 15 L/min constant flow, nasal deposition was 5% with a 1 μm particle and rose to 23% with a 2 μm particle. Counterbalancing the goal to minimize nasal deposition by reducing aerosol particle size is the impact that this maneuver will have on the efficiency of lower airway deposition. As aerosol particle sizes are reduced, pulmonary deposition is diminished due to reduced impaction and gravitational settling.(3) Further, the relative deposition of the delivered aerosol between central and peripheral lung regions will be significantly influenced by particle size, and this also must be considered in the context of the specific medication and treatment goals.
Patients with cystic fibrosis (CF) often spend several hours per day inhaling various medications, including HS. HS has been shown to stimulate mucociliary clearance and to reduce pulmonary exacerbations in these patients.(4,5) Because the effect of HS on mucociliary clearance is proportional to the mass of sodium chloride delivered to airways over time, yet can be poorly tolerated when high concentrations are delivered rapidly,(6) this therapy represents an attractive candidate for tPAD. In the current study, we used gamma scintigraphy to assess the pulmonary and extra-pulmonary deposition of 7% NaCl by using 99mTc-DTPA (diethylene triamine pentaacetic acid labeled with 99mTechnetium) as the radiolabeled marker. For comparison, the same methods were used to assess regional deposition after oral inhalation from a commonly used jet nebulizer (PARI LC® Star).
Materials and Methods
Study design
We performed a randomized, open-label, crossover study to measure pulmonary deposition of 7% NaCl/99mTc-DTPA delivered via the tPAD device and the PARI LC Star (PARI, Midlothian, VA). Six healthy, nonsmoking adults with normal pulmonary function were enrolled and completed all study procedures. Two separate study visits were typically conducted 2 days apart (range 2–12 days; median 2 days). Spirometry was performed before and 45 minutes after each inhalation procedure to assess safety. The study was approved by the Biomedical Institutional Review Board of the University of North Carolina at Chapel Hill, and all subjects provided informed consent before their participation.
Study solutions
99mTc-DTPA (diethylene triamine pentaacetic acid labeled with 99mTechnetium) was obtained from the North Carolina Memorial Hospital Radiopharmacy and added to 7% NaCl (HyperSal; PARI Respiratory Equipment, Inc., Midlothian, VA) to formulate the test solutions. We performed cascade impaction (Sierra eight-stage impaction plates) separation of particle sizes to confirm that the radiolabel accurately tracked the “drug” (NaCl) during aerosolization. There was a tight association of % salt mass and % 99mTc-DTPA activity across all plates during three separate runs with each device. For the tPAD, the line describing this correlation had a slope of correlation 1.2, a y-intercept of −2.3%, and a Pearson R value of 0.98. For the LC Star, the line describing the % salt mass versus % 99mTc activity had a slope of 1.0, an intercept of −0.3%, and a Pearson R value of 0.99.
Study nebulizers
The tPAD device (Parion Sciences, Inc., Durham, NC) utilized in this study is depicted in Figure 1. Key components include a 250 mL medication reservoir that feeds the 510-k-approved and commercially available Aerogen Pro aerosol generator. The Aerogen electronic controller (Aerogen) controls the duty cycle of the nebulizer. A custom-manufactured integrated nebulization chamber (INC) is fitted downstream of the nebulizer to remove aerosol particles >4 μm in diameter by virtual impaction. A filtered aerosol that is unable to pass through the INC is sequestered in a conservation chamber within the device. The resulting fine aerosol is entrained and directed through the custom-designed nasal cannula via a 2 L/min air pump. Prior studies to characterize the aerosol leaving the assembled tPAD device revealed emitted aerosol particles with a volume mean diameter of 1.38 μm via laser diffraction (Sympatec HELOS; Sympatec GmbH, Clausthal-Zellerfeld, Germany). Device output during bench top testing was measured at 30.7 ± 4.3 (SD) μL/min.
FIG. 1.
A schematic of the tPAD device. tPAD, trans-nasal pulmonary aerosol delivery.
The PARI LC Star (PARI Respiratory Equipment, Inc., Midlothian, VA) breath-enhanced jet nebulizer was driven with 10 L/min airflow by using a PARI Ultra compressor. The manufacturer reports that the aerosol particle size emitted from this device is 3.1 μm MMAD, with an output of 400 μg NaCl/min during laboratory testing (continuous nebulization of 0.9% NaCl; 20 L/min inspiratory flow; 1.2 bar compressor at 23°C).
Aerosol delivery
The six subjects were assigned a randomized two-visit cross-over schedule to inhale the test aerosol delivered from either the LC Star nebulizer or the tPAD while seated upright for 15 minutes. Subjects were asked to breathe normally through the nose with the tPAD. Breathing patterns were recorded. Inhalation was from room air; exhaled gases were exhausted through a filter and a pneumotachograph along with excess aerosol produced from the nebulizers during exhalation. A visual feedback display of expiratory flow was used to encourage mean expiratory flows of 250 mL/s. A 15-minute acclimatization period, while breathing clean air (no aerosol) through the tPAD and a radiation containment mask, was utilized to encourage a normal, tidal breathing pattern before proceeding with the tPAD deposition study.
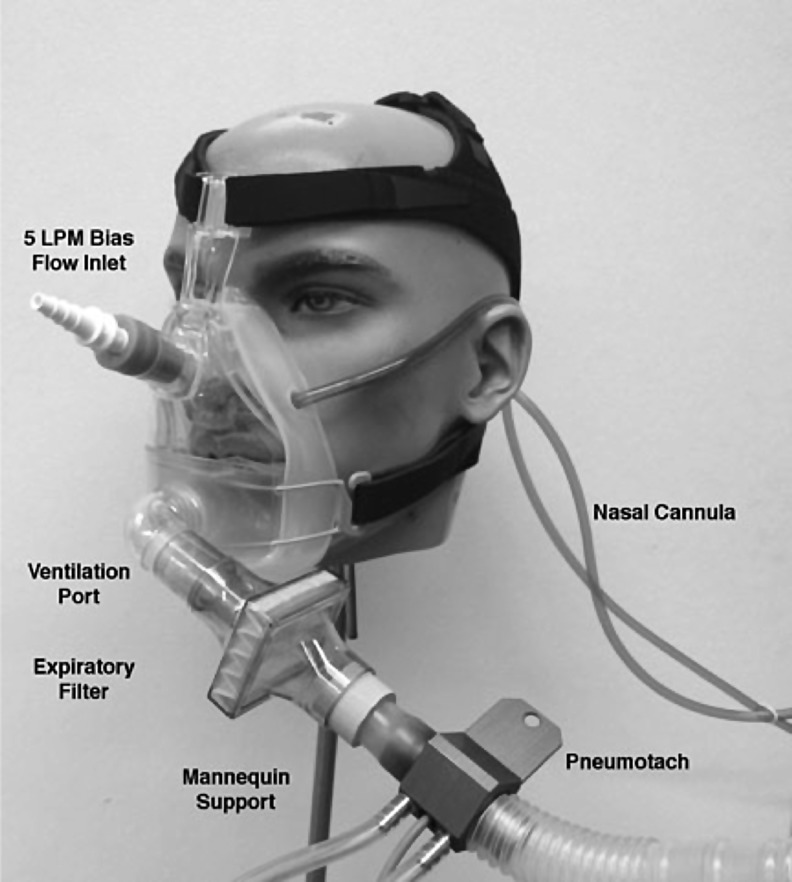
A customized radiation containment mask was constructed to safely study radioaerosol deposition from the nasal tPAD cannula, thus preventing contamination of the subject's face/body surfaces and the laboratory environment (Fig. 2). This modified continuous positive airway pressure mask covered the nose and mouth and was fitted with a single exhalation port, leading to a pneumotachometer and a filter. The tPAD cannula entered the mask through installed ports and delivered a continuous airflow of ∼2 L/min. A separate port was used to provide continuous clean room air (5 L/min), thus preventing rebreathing of CO2 or radiotracer. When studying the LC Star nebulizer, an adapter connected a pneumotachometer and exhaust filter to the nebulizer's exhaust port.
FIG. 2.
Radiation containment mask used for tPAD delivery of 99mTc-DTPA-labeled HS. HS, hypertonic saline.
The aerosol devices were filled with ∼8 mL for the LC Star and 10 mL for the tPAD to allow 15 minutes of inhalation of 99mTc-DTPA in 7% NaCl. The specific activity of the test solution (0.5 mCi/mL with tPAD; 0.05 mCi/mL with the LC Star) was adjusted so that the resulting deposition image would contain adequate signals for similar inhalation times for both devices, despite anticipated differences in device output and possible differences in deposition efficiency.
Subjects were seated in front of a single-crystal Nuclear Data detector during inhalation to monitor estimated activity deposited in the nose/lungs to assure adequate deposition for scintigraphy analysis while not exceeding estimated radiation doses to the subjects. The total output of each device was determined by measuring the loaded volume, total 99mTc activity loaded into the device before dosing, and the total activity that left the device (depositing in the subject, expiratory filter, and containment mask, if applicable) during the 15-minute inhalation period. Wasted aerosol (i.e., not deposited in the body) was quantitated by measuring 99mTc activity in the exhalation filter and, in the case of the tPAD, in the radiation containment mask and cannula. All radioactive activity measurements were corrected for decay over time to the mid-inhalation time point.
Gamma scintigraphy
Before aerosol inhalation, a 15-minute background image was acquired with the subject seated upright in front of a gamma camera fitted with a low-energy collimator positioned posteriorly (BodyScan/Scintron; MiE America, Grove Village, IL). A 5-minute 57Co transmission image of the subject, using a planar source, was then acquired to delineate lung boundaries and to define regions of interest (ROI). Next, a 1-minute image using the same 57Co source placed in the same position, but without the seated subject, was acquired to allow calculation of a regional matrix of tissue attenuation factors for each subject (see Regions of Interest). Finally, the loaded nebulizer was counted for 1 minute on the camera just before the start of inhalation to quantitate its initial activity.
After the 15-minute aerosol inhalation period, the subject was quickly seated in front of the gamma camera (within 4 minutes) to obtain a dynamic series of 30, 1-minute images (128 × 128 pixel). Only the first image was used for analyses of lung deposition. After removing the subject, the 99mTc activity contained in various nebulizer components, exhaust filter, containment mask, and cannula (with the tPAD) was counted on the gamma camera for 1 minute.
Image processing and statistical analysis
Images were processed and analyzed by using ImageJ, v1.45s (National Institute of Health). Further numerical analysis and statistical analysis were performed by using functions in Microsoft® Excel® for Mac 2011, v14.3.8 and STATA 11.0 for Mac 2009. Values are given as mean and standard deviation (SD), unless otherwise indicated. Comparisons between the two study inhalations were made by the Wilcoxon matched-pair signed-rank test, and significance in difference was set at p < 0.05 (STATA 11.0 for Mac). Not significant (NS) indicates p > 0.05.
Regions of interest
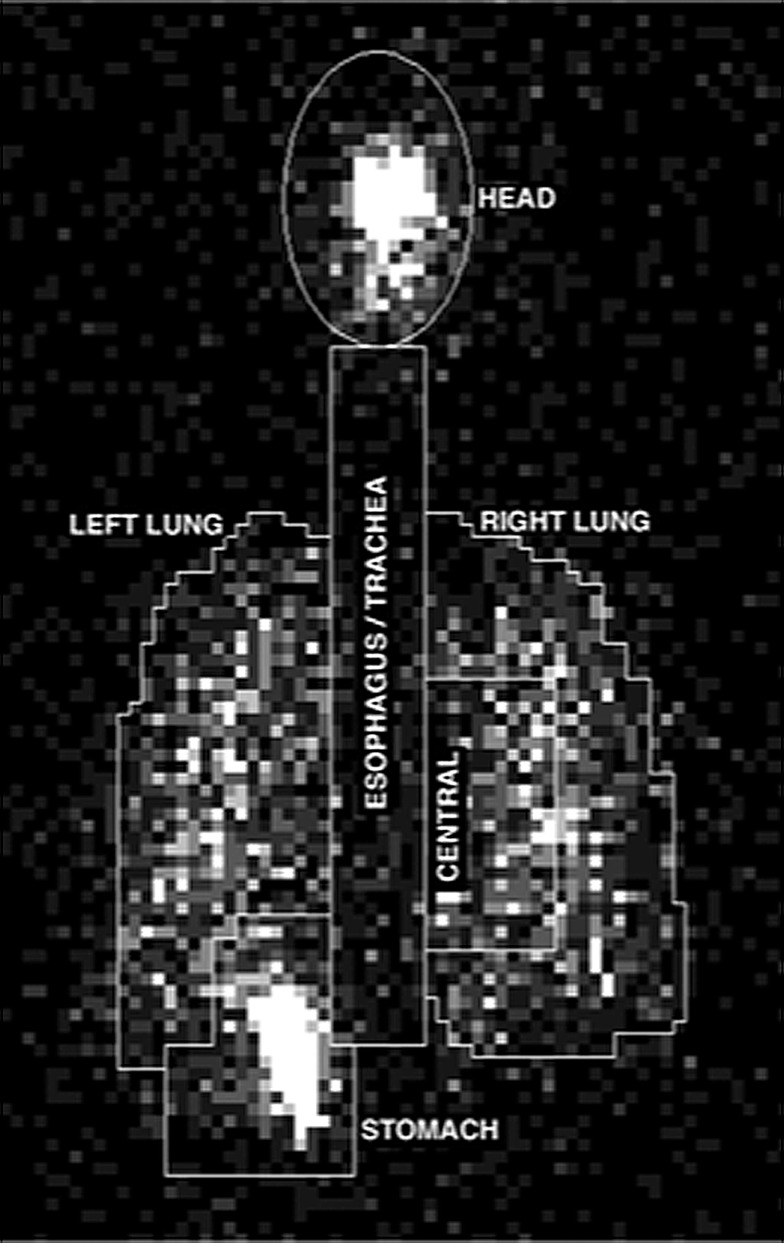
Using a previously described planar isocontour technique,(7) the 57Co transmission image was used to define lung ROI (central, peripheral, whole lung) for each subject. Figure 3 illustrates the ROIs used for the analysis. The medial boundary of each lung ROI was truncated at 4 pixels from centerline. An oval-shaped ROI was drawn over the naso/oropharyngeal area of the head to circumscribe the counts in the deposition image that were above the maximum of the range of background counts of the adjacent tissue. This ROI was also used to determine the attenuation factors from the transmission image for this region. The esophageal/tracheal ROI was drawn between the lung ROIs, spanning the region between the stomach and the head region.
FIG. 3.
A typical aerosol deposition image with analyzed regions of interest indicated on the image.
A stomach ROI was drawn to encompass counts above background in the expected anatomical region of the stomach and was enlarged to include duodenal counts. The overlapping counts in the stomach region were subtracted from the left lung counts. Because the left lung and stomach overlap within the “stomach ROI,” counts in a small neighboring ROI in the left lung periphery away from the stomach were measured, corrected for relative ROI size to the overlapping region, and used to estimate and correct for the left lung contribution to the stomach ROI in the overlapping region. Subtraction of the left lung contribution from the total stomach ROI counts yielded a corrected stomach ROI count value. The same correction counts were added to the left lung counts, yielding a corrected lung count value.
Tissue attenuation correction
A tissue attenuation factor for each ROI region was calculated by using the smoothed flood (no subject) and transmission (subject positioned) images. Attenuation factors were developed on a pixel-by-pixel basis by dividing the 57Co flood image (1-minute image) by its respective transmission image (5-minute image) collected with the same position and orientation. The longer acquisition time during the transmission image was used so that counts over soft-tissue areas approximated those obtained during the 1-minute, unattenuated image. Counts collected during the transmission image were divided by five to normalize for the image time difference. The square root of the ratio of attenuated to unattenuated pixel values produced the attenuation factors for each pixel location.(8) Scattering and camera efficiency correction were not included. Attenuation for each ROI was then calculated from the mean of the attenuation values within that region. Nebulizer and associated equipment counts, measured at the camera face, were not corrected for attenuation, as these were considered insignificant.
Calculation of regional deposition fractions
After aligning particle deposition images with the transmission image, the counts in each ROI were background subtracted, multiplied by the respective attenuation factor, and corrected for isotopic decay. All counts, from the initial 99mTc-DTPA load through subject imaging and postinhalation equipment counts, were decay corrected to the time of mid-inhalation. This time correction was 4 minutes or less from the end of 99mTc-DTPA inhalation to the camera start. The total sum of counts measured in the residual solution remaining in the nebulizer after inhalation, tubing, exhalation filter, mask (if applicable), mouthpieces, and the subject was compared with the counts in the initial nebulizer load to calculate the recovered fraction. The fraction of counts in each ROI (head, trachea/esophagus, right lung, left lung, stomach, and exhaled/nondeposited fraction in filters or devices) was related to the total counts that left the nebulizer mouthpiece (LC Star) or cannula (tPAD) (emitted dose). The first deposition image acquired by gamma camera immediately after particle inhalation was analyzed for regional deposition.
The ratio of central (C) to peripheral (P) deposition was used to assess the depth of aerosol penetration into the lung. To accomplish this, the central to peripheral (C/P) ratio of 99mTc-DTPA activity was normalized to the C/P ratio of 57Co activity on the transmission scan for each subject. This normalization accounts for differences in lung thickness/volume between the central and peripheral regions that would otherwise not be accounted for when analyzing a planar deposition image in isolation.(7) For a uniformly deposited aerosol, the C/P ratio of particle deposition normalized to its 57Co transmission image would be near 1.0. Both the central and peripheral regions contain alveoli and small airways, and the central region also incorporates large, bronchial airways that are not present in the peripheral region. Therefore, increases in C/P to values greater than 1.0 reflect increased large airway deposition.
Results
Safety, tolerability, and subject ventilation parameters
Six healthy subjects were enrolled and completed all study procedures. There were no instances of intolerability or adverse events during study procedures. Spirometry testing revealed no change from predose values in FEV1 after dosing with either device (not shown).
Subject ventilation was measured for the two arms during the 15 minutes of inhalation. The mean (SD) expiratory ventilation flow rate was not different when measured during use of each device [261 (30) and 235 (26) mL/s for the tPAD and LC Star, respectively (NS)]. Tidal volumes were significantly smaller during tPAD use, however, when compared with the LC Star [0.65 (0.27) L vs. 1.1 (0.4) L, respectively (p = 0.028)], likely reflecting, in part, the additional ventilation dead space introduced by an oral nebulizer.
Device performance
A description of the aerosol fate with each device is described in Table 1. The fraction of the emitted dose that deposited in the lungs was surprisingly similar for the tPAD (39% ± 8%) and LC Star (36% ± 9%) devices, despite the tPAD utilizing the nasal route of delivery. Right and left lung deposition was symmetrical in each case, but the C/P ratio of aerosol deposition was significantly lower with the tPAD [1.12 (0.14) vs. 1.36 (0.24); p = 0.028], signifying deeper lung penetration with the tPAD. A nonsignificant increase in head deposition (including nose and mouth) was observed with the tPAD, versus the LC Star [6 (6)% vs. 1 (1)%; p = NS], although head deposition was low in both instances. No differences in stomach or trachea/esophagus deposition were noted. Importantly, an accounting of all radioactive material recovered after each nebulization, as a fraction of activity loaded into the nebulizer revealed no missing material [% of starting material: 102 (2)% and 100 (1)% for the tPAD and LC Star, respectively (NS)].
Table 1.
Aerosol Deposition Comparison
| Device | Right lung (%) | Left lung (%) | Head (%) | Total extra-pulmonary (%) | Waste (%) | C/Pa |
|---|---|---|---|---|---|---|
| tPAD | 20 (4) | 19 (5) | 6 (6) | 13 (13) | 48 (13) | 1.12 (0.14) |
| LC Star | 18 (5) | 18 (4) | 1 (1) | 8 (5) | 56 (7) | 1.36 (0.24) |
The mean (SD) values for regional ex-device deposition percentage and C/P ratio after inhalation of radiolabeled 7% NaCl via the tPAD and PARI LC Star. “Head” designation includes all the deposition above the chin (nose and oropharynx). Deposition onto the nasal containment mask and cannula (tPAD), and the exhaled fraction collected on the exhaust filter are included in the “waste” fraction.
p = 0.028 comparing the C/P ratio between the two devices.
C/P, central to peripheral; tPAD, trans-nasal pulmonary aerosol delivery; SD, standard deviation.
By measuring radioactive counts in each device before nebulization, and in the patient and each nebulizer component after dosing, a precise determination of the fate of the emitted dose from each nebulizer was made. These calculations were corrected for radioactive decay over time and corrected for tissue attenuation factors. The LC Star nebulizer was observed to have an output rate of 121 ± 13 μL/min, and the tPAD device emitted 24 ± 9 μL/min (Table 2). This estimate of tPAD output was insignificantly different from that measured during bench-top testing, 30.7 ± 4.3 μL/min.
Table 2.
Device Output and Delivery Comparison
| Device | Emitted rate (μL/min) | Expected lung deposition rate (μL/min)a | NaCl deposition rate (mg/min)b | NaCl deposited per day (mg) |
|---|---|---|---|---|
| tPAD | 24 (9) | 9.4 | 0.67 | 384c |
| PARI LC Star | 121 (13) | 43.6 | 3.1 | 151d |
Mean values (SD) for output and anticipated lung delivery with tPAD and LC Star devices.
Calculation utilizes the deposition fraction for each device that was measured in this study (39% of emitted dose from tPAD and 36% of emitted dose from LC Star).
Calculation made for 7% NaCl solution.
Presumes tPAD is used for 8 hours (e.g., overnight).
For PARI LC Star, presume that 3 mL of the 4 mL loaded dose is nebulized (i.e., 1.0 mL dead volume), that the device is used twice daily, and that 36% of this emitted dose deposits in the lung.
Discussion
This study assessed the ability of a novel aerosol delivery device to efficiently target the lung via the nasal route. The driver for this effort is to provide an alternate means of therapeutic delivery that could, in some cases, improve efficacy, tolerability, and treatment adherence. As an initial proof of concept, our data clearly support the notion that the tPAD is able to efficiently deposit drug in the lung, and that use of the device while awake, over a short time interval, is well tolerated. The low level of nasal deposition was also encouraging, given the prospect of exploring prolonged use during sleep.
Achieving a degree of pulmonary deposition that equaled that from an oral jet nebulizer exceeded prestudy expectations and appears to be very reproducible. The ability to fully account for all radioactivity that entered the study, with little deviation at the end of the study from starting quantities, suggests that our procedures provide an accurate quantification of pulmonary and extra-pulmonary deposition. Although this study does not answer the question whether this novel mode of aerosol delivery will be as efficacious as the traditional oral route, it does suggest that the creative engineering of a device can produce a highly refined aerosol that largely avoids nasal deposition while efficiently targeting the lung in an awake, upright subject. Clearly, though, a number of questions must be addressed before this device could be considered for clinical use.
Perhaps the most pressing question to answer is whether tPAD of a therapeutic agent provides at least equivalent, or superior efficacy to traditional delivery. There are a number of factors that are likely pertinent that should be considered. The first is how a slow, prolonged delivery of a given therapy may compare with relatively rapid and intermittent treatments. For HS, one might see advantages related to slower delivery, including reduced airway irritation, and the ability to deliver a larger total daily dose of NaCl if pursuing overnight treatment. In fact, considering the measured device output rates and deposition efficiencies measured in this study, we estimate that 8 hours of tPAD use would be able to deliver more than twice that achieved with the usual clinical dose (4 mL BID) delivered via LC Star (Table 2). Because the device output rate is ∼20% that of the comparator jet nebulizer, tolerability to HS might also be improved (Table 2). Unknown, however, is whether the airway epithelia would respond to HS in a delivery rate-dependent fashion—that is, would mucociliary clearance be similarly accelerated with slow/continuous delivery, versus a “bolus” of intermittent HS?
Also unknown is whether a sustained period of mucus hydration and accelerated mucociliary clearance during sleep would be as effective as intermittent treatment while awake, active, and coupled to other forms of airway clearance. The complexity of how the airway may respond to HS administration makes any prediction uncertain at this point in time. Although it is very unlikely that the lung could be volume overloaded with this prolonged delivery approach, reductions in nebulizer duty cycle or total time used per night would easily correct for this issue. When considering other therapeutics, similar questions will need to be addressed.
For example, would slow/continuous use of a beta-agonist lead to more or less receptor desensitization? Could specific antibiotics be more effective with continuous delivery? A longer time above minimum inhibitory concentration (MIC) could be advantageous with a beta-lactam, whereas lower peak levels might be less effective with an agent that relies on concentration-dependent killing (e.g., aminoglycosides). Finally, does the regional lung deposition of drugs have a major effect on efficacy, as discussed in more detail later?
If one envisions the tPAD to be a device utilized during sleep, other factors that impact efficacy could come into play. Clearly, body position affects ventilation patterns within the lung, and the typical supine or recumbent position assumed during sleep could lead to altered drug deposition compared with that achieved when upright. Ventilation during sleep is also variable. Generally speaking, ventilation is reduced and pCO2 is elevated compared with waking levels due to smaller tidal volumes during non-rapid eye movement (REM) sleep. Ventilation may be erratic during REM sleep. As upper airway resistance increases during sleep, typically at the level of the oropharynx, one could also imagine this impacting upper airway aerosol deposition, even in patients without clinically apparent sleep disorders. Finally, the simple effect of wearing a nasal cannula during sleep could negatively impact patient comfort and, therefore, normal sleep patterns and the likelihood of maintaining the device in place throughout the night. Besides comfort, acceptability of using the tPAD during sleep may also relate to general ease of use, medication loading, and cleaning procedures.
An important observation from our data is that the aerosol generated by the tPAD deposited more deeply in the lung than with the LC Star, as reflected by the statistically significant reduction in C/P ratio measured within the lung compartment. The impact of this difference is unknown. It is possible that for disease states such as CF, which are characterized by small airways involvement and significant foci of upstream obstruction as well, that the finer aerosol will improve drug deposition in the diseased small airways and improve efficacy.
It is also possible, however, that a greater fraction of aerosol could deposit in deep lung compartments that are distal to those we would like to target, that is, alveoli. For HS, the hygroscopic nature of this aerosol could increase deposition in conducting airways, if aerosol particles have sufficient time to grow in size. The strategy of utilizing a hypertonic excipient for other drug formulations used in the tPAD could also turn out to be important. However, because one might anticipate that excessively peripheral aerosol deposition could minimize the beneficial effects of HS on mucociliary clearance (MCC), comparative studies that directly measure HS effects on MCC (tPAD vs. oral nebulizer) will be useful to determine the impact of this deeper pattern of drug deposition on therapeutic efficacy.
Another observation from our data is that ventilatory rates during delivery by both devices were nearly similar but tidal volumes were larger with the LC Star. This difference could reflect the fact that the oral nebulizer adds dead space to the respiratory system, which was compensated for through deeper tidal volumes. In contrast, no dead space was added with the nasal cannula. It is also known that spontaneous inhalation via a mouthpiece (as is the case with the LC Star) induces deeper breathing (larger tidal volumes) than spontaneous breathing without a mouthpiece,(9) which may have influenced breathing patterns.
In summary, this study has shown that the pulmonary deposition of a nasally delivered therapy is similar to that achieved with an oral jet nebulizer, that is, ∼39% of aerosol leaving the nosepiece is deposited in the lung, while minimizing deposition in the nasal cavity. The slower delivery rate by the nasal device could lower irritation and peak doses, reducing side effects of some aerosols. Further studies are needed to determine whether this approach is well tolerated over longer time periods/overnight, and whether similar physiologic and clinical effects can be realized with this novel delivery strategy.
Acknowledgments
The authors would like to thank Dr. Tomas Navratil and Jihong Wu for their invaluable assistance with these studies. Funding source: NHLBI (P01 HL108808).
Author Disclosure Statement
K.H.D. is an employee of Parion Sciences, Inc., and holds equity interests. R.C.B. is the founder of Parion Sciences, Inc., and holds equity interests.
References
- 1.Hounam RF, Black A, and Walsh M: The deposition of aerosol particles in the nasopharyngeal region of the human respiratory tract. Inhaled Part. 1970;1:71–80 [PubMed] [Google Scholar]
- 2.Heyder J, and Rudolf G: Deposition of aerosol particles in the human nose. In: Walton WH, (ed). Inhaled Particles IV. Pergamon Press, Oxford; pp. 107–126, 1977 [PubMed] [Google Scholar]
- 3.Darquenne C: Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, and Boucher RC: Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250 [DOI] [PubMed] [Google Scholar]
- 5.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, and Bye PT: A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240 [DOI] [PubMed] [Google Scholar]
- 6.Robinson M, Hemming AL, Regnis JA, Wong AG, Bailey DL, Bautovich GJ, King M, and Bye PT: Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax. 1997;52:900–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeman KL, Wu J, Donaldson SH, and Bennett WD: Comparison of 133 xenon ventilation equilibrium scan (XV) and 99 m technetium transmission (TT) scan for use in regional lung analysis by 2D gamma scintigraphy in healthy and cystic fibrosis lungs. J Aerosol Med Pulm Drug Deliv. 2013;26:94–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macey DJ, and Marshall R: Absolute quantitation of radiotracer uptake in the lungs using a gamma camera. J Nucl Med. 1982;23:731–734 [PubMed] [Google Scholar]
- 9.Bennett WD, Zeman KL, and Kim C: Variability of fine particle deposition in healthy adults: effect of age and gender. Am J Respir Crit Care Med. 1996;153:1641–1647 [DOI] [PubMed] [Google Scholar]