Abstract
The objective of this study was to characterize cervicovaginal (CV) mucosal factors modulating susceptibility to human immunodeficiency virus (HIV) acquisition in healthy premenopausal (PRE) and postmenopausal (POST) women before and after treatment with estradiol (E2). We compared CV mucosal epithelial histology and immune cells, vaginal microbiota, antimicrobial activity of and soluble mucosal protein concentrations in the CV fluid lavage (CVL), and p24 antigen production after ex vivo infection of ectocervical tissues with HIV-1BaL among PRE women (n = 20) in the follicular and luteal phases of the menstrual cycle and POST women (n = 17) at baseline and after ∼1 month of treatment with 0.01% vaginal E2 cream. Compared to PRE women, we measured higher levels of p24 antigen after ex vivo infection in tissues from POST women. POST women had a significantly thinner vaginal epithelium with decreased tight junction proteins and a higher density of mucosal immune T cells and lower levels of CD1a antigen-presenting cells, antimicrobial peptides, and inflammatory cytokines in the CVL (p values <.05). POST women had higher vaginal pH and lower vaginal Lactobacilli (p values <.05) than PRE women. After vaginal E2 therapy, CV endpoints and ex vivo HIV replication in POST tissues were similar to those observed in PRE tissues. The CV mucosa in POST women is thinned and compromised, with increased HIV-target immune cells and decreased antimicrobial factors, being more susceptible to HIV infection. After POST women receive topical E2 treatment, mucosal endpoints are similar to PRE levels.
Keywords: : HIV, menopause, cervicovaginal, mucosal, hormones
Introduction
In regions of the world where human immunodeficiency virus type 1 (HIV-1) incidence is highest, women are disproportionately infected with HIV-1.1 While social, behavioral, and economic conditions are factors in the increased vulnerability of women to HIV-1, the endogenous hormonal milieu may be a cofactor in HIV-1 acquisition, transmission, and disease progression. Levels of serum estradiol (E2) and progesterone (P4) are significantly different among premenopausal (PRE) versus postmenopausal (POST) women2 and offer an opportunity to study the potential effects of endogenous hormone levels on biologic endpoints associated with mucosal HIV-1 acquisition.
Although most new HIV-1 infections worldwide in women occur in adolescents and young women,1 between 2010 and 2014, ∼35% of new HIV-1 diagnoses in the United States were in persons aged 50 years or older.3 An early case series and observational studies among serodiscordant couples suggested that male-to-female transmission of HIV-1 was more efficient in older women.4–6 Women who are anovulatory due to breastfeeding have a hormonal milieu similar to POST women2,7 and have been shown to be more susceptible to HIV-1 than nonpregnant, nonlactating women.8–10 Use of depot medroxyprogesterone acetate (DMPA), which lowers serum E2 levels,11–13 may increase susceptibility to HIV-1.14
There are limited data on the effect of menopause, where both E2 and P4 levels are low, on several biologic endpoints potentially related to mucosal HIV-1 acquisition. The primary objective of this study was to compare vaginal immune cells and microbiota, antimicrobial activity of and soluble mucosal protein concentrations in cervicovaginal (CV) fluid lavage (CVL), and p24 antigen production after ex vivo HIV challenge of ectocervical tissues among healthy, HIV-negative PRE and POST women before and after treatment with vaginal E2 cream. Our hypothesis was that POST women at baseline would have significant differences in CV mucosal factors related to HIV-1 acquisition compared to PRE women, and that vaginal E2 supplementation in POST women would alter some of the protective factors to levels seen in the PRE cohort.
Methods
Clinical study
The CONRAD A12-124 protocol was approved by the Eastern Virginia Medical School (EVMS) Institutional Review Board (IRB) (13-02-FB-0017) and registered with ClinicalTrials.gov (#NCT01810315). We enrolled healthy, HIV-1-negative, nonpregnant PRE (n = 20) and POST (n = 20) women, who were not on exogenous hormones by self-report, had no evidence of reproductive tract infections such as bacterial vaginosis (Nugent score 7–10) or yeast vaginitis or sexually transmitted infections, and did not smoke or use vaginal products (e.g., douches, nonoxynol-9 spermicides). PRE participants reported a history of regular menstrual cycles and had a luteal phase P4 level of 3 ng/ml or higher. POST women ceased menstruation for 12 months or longer, had no contraindications to vaginal E2 therapy, and had a follicle stimulating hormone (FSH) level of 20 mIU/ml or higher.
We obtained baseline samples among PRE women during menstrual cycle days 5–10 for follicular (FOL) phase samples and menstrual cycle days 20–25 for luteal (LUT) phase samples. POST women had baseline genital sampling obtained and then started on vaginal E2 0.01% cream (Estrace; Allergan, Rockaway, NJ) in the approved dosing regimen for the treatment of vulvo-vaginal atrophy ∼1 month after sampling to allow the biopsy sites to heal. We obtained post-treatment samples after the POST cohort used vaginal E2 cream for ∼1 month. All participants were required to refrain from vaginal intercourse 48 h before each genital sampling procedure.
At each visit, we performed point-of-care testing for prostate-specific antigen to exclude recent vaginal semen exposure (ABAcard; Abacus Diagnostics, West Hills, CA). After confirming the absence of recent semen exposure, we took the following samples: CV swabs, a CVL using 4 cc of normal saline, and ectocervical and vaginal biopsies. All laboratory personnel assessing study endpoints were blinded to PRE or POST cohort assignment and visit type.
Serum E2 and P4 levels
At all visits, we measured E2 and P4 levels in serum by solid-phase, enzyme-labeled chemiluminescent competitive immune assay (Siemens Immulite 1000 System, Ehrlanger, Germany).
Vaginal pH and Nugent score
We measured the Nugent score15 from vaginal secretions and epithelial cells and the vaginal pH using pH paper with a range of 4.0–7.0 (MColorpHast ph-Indicator strips; EMD Millipore, Billerica, MA).
Semiquantitative vaginal cultures
Two Dacron swabs containing CV fluid were placed in a Port-A-Cul transport tube (Becton, Dickinson, Sparks, MD) and transported on ice within 24 h of collection for cultivation of cultivable microbiota as previously described.16
Antimicrobial activity of the CVL
Within 30 min of collection, the CVL was centrifuged at 4°C for 10 min at 500 g. Aliquots of CVL supernatant were stored at −80°C for testing of the antimicrobial activity of the CVL. The activity of CVL supernatant against HIV-1, herpes simplex virus type 2 (HSV-2), and Escherichia coli was measured within 12 months of collection as previously described17 by the laboratory of B Herold MD at Albert Einstein College of Medicine.
Soluble mucosal proteins in CV secretions
Aliquots of CVL supernatant were stored at −80°C and shipped to the laboratory of B. Herold MD at Albert Einstein College of Medicine for testing of secreted mucosal proteins18 (assay kits listed in Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/aid). Total protein concentrations in CVL specimens were quantified by microBCA assay and CVL specimens were diluted to enable interpolation from a standard curve. Concentrations below the lower limit of detection (LLOD) were set to the midpoint between 0 and the LLOD and corrected for dilution. Mucosal protein concentrations were normalized to total protein content, in mg, of the CVL.
Analysis of vaginal immune cell populations and histology
One vaginal biopsy from each visit was placed in 10% neutral buffered formalin for 24–48 h, transferred to an embedding cassette, and processed as per our existing protocol.19 The antigens were detected using the AEC chromogen–substrate kit (ScyTek Labs, Mississauga, Canada) and mounted with Accergyl mounting media (Accurate Chemicals, Westbury, NY). Cell phenotype was identified using specific monoclonal antibodies against CD45, CD3, CD8, CD1a, CCR5, CD4, and HLA-DR. Positive stained cells were counted under the microscope (Nikon E-800). In brief, 5–6 fields were randomly selected using a Nikon E800 microscope from each section and these images were captured using a CCD camera (Spot Camera; Diagnostic Instruments, MI). Cell density was expressed as the mean of the counts in 5–6 fields in cells/mm2.
E-cadherin was stained with the rabbit monoclonal antibody (Abcam, Cambridge, MA), and the immunolabeling of E cadherin was analyzed semiquantitatively using ImageJ software (NIH, Bethesda, MD). Five to six fields were randomly selected using a Nikon E800 microscope from each section and these images were captured using a CCD camera (Spot Camera; Diagnostic Instruments). The integrated optical density (IOD) in each area was calculated for the positive staining color. The IOD of the negative control (no primary antibody) was subtracted from the IOD values for each tissue and the mean value was calculated for the areas of each tissue sample.
HIV-1 p24 antigen production from ectocervical tissues after ex vivo infection
Ectocervical tissue biopsies were placed in a microcentrifuge tube containing complete Leibovitz 15 (cL15) tissue culture media (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (Gibco). Explants and biopsies were kept on ice and shipped via overnight courier to the laboratory of Dr. Susana Asin (V.A. Medical Center, White River Junction, VT). The following day, the biopsy was stabilized for 4 h at 37°C, infected with 104 TCID50 of HIVBaL in a final volume of 100 μl per well in a 96-well plate, and processed as previously described.20
Explant tissues obtained for p24 antigen production subset study
Genital tissue explants were obtained from HIV-negative PRE and POST women, who were not using exogenous hormones and had no preoperative evidence of gynecologic dysplasia or neoplasia, undergoing elective gynecologic surgeries for benign indications at EVMS for a substudy, under an EVMS IRB-approved protocol (09-09-FB-0175). Women were categorized as PRE or POST based on their age, menstrual history, surgical history, and relevant laboratory data, such as serum FSH. Tissue explants were obtained fresh from the operating room and taken immediately to our laboratory in chilled RPMI 1640 media (Life Technologies, Carlsbad, CA) containing 10% FBS (ATCC, Manassas, VA), 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher Scientific, Waltham, MA). Surgical explant tissues were processed for ex vivo HIV-1BaL infection as previously described.20
Statistical analyses were performed with SAS version 9.3 (Cary, NC). Descriptive statistics included mean, median, standard deviation for continuous variables and frequencies and percentages for categorical variables. Continuous endpoints from PRE and POST cohorts were compared using an independent samples t-test for normally distributed data (serum E2 and P4 concentrations, vaginal epithelial thickness, number of vaginal cell layers) or Wilcoxon–Mann–Whitney test for non-normally distributed data (vaginal pH, Nugent score, soluble protein mediators, antimicrobial activity of the CVL supernatant, p24 antigen production). For categorical variables, chi-square statistic or Fisher's exact tests were used as indicated by expected cell size. Paired comparisons using a paired t test or Wilcoxon signed rank sum test were performed on difference variables to compare PRE samples obtained in the FOL versus LUT phase and POST samples obtained before and after vaginal E2 therapy. To achieve normality, data were log transformed for the variables p24 production, soluble mucosal proteins, and immune cell types and confirmed with parametric methods.21,22 For the analyses of p24 antigen production, categorization of infected versus not infected samples was done using 0.1 log steps and a Boolean method, followed by comparison of infected versus not infected samples by Fisher's exact test or McNemar's test as appropriate. For the correlations between serum E2 and P4, a Pearson correlation coefficient was calculated for normally distributed data and a Spearman correlation coefficient was calculated for non-normal data. Multiple linear regression to predict serum E2 and P4 levels was used, where dependent variables included endpoints that were significant in the univariate correlations. Statistical significance was determined at the level of α = 0.05.
Results
Demographics and study design
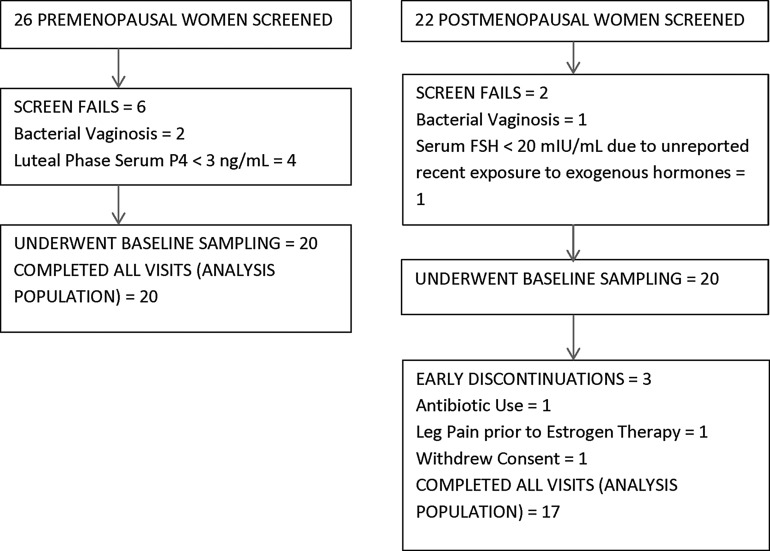
We consented and screened 26 PRE and 22 POST women for the study. Among the women enrolled, 20 PRE and 17 POST women attended all visits and represented the analysis population (Fig. 1). Table 1 demonstrates that at screening (visit 1, V1), there were expected differences between PRE and POST women.
FIG. 1.
Pre- and postmenopausal populations.
Table 1.
Analysis Populations
| Premenopausal cohort (n = 20) | Postmenopausal cohort (n = 17) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | N | Mean | SD | N | Mean | SD | p Value |
| Age | 20 | 35.2 | 6 | 17 | 60.6 | 9.3 | <.01 |
| Years of education | 20 | 14 | 1.5 | 17 | 13.8 | 2.2 | .89 |
| Gravidity | 20 | 3.1 | 1.6 | 17 | 3.2 | 3.2 | .40 |
| Parity | 20 | 2.5 | 1.4 | 17 | 2.4 | 1.9 | .70 |
| Body mass index (kg/m2) | 20 | 31.9 | 9.8 | 17 | 29.3 | 6.2 | .53 |
| Systolic blood pressure (mm Hg) | 20 | 115.3 | 18 | 17 | 129.8 | 20.1 | .02 |
| Diastolic blood pressure (mm Hg) | 20 | 71.9 | 9.7 | 17 | 76.4 | 13.4 | .09 |
| Vaginal pH at visit 1 | 20 | 4.3 | 0.3 | 17 | 5.3 | 0.7 | <.01 |
| Vaginal Nugent score at visit 1 | 20 | 1.3 | 1.4 | 17 | 4.1 | 1.9 | <.01 |
| N | % of group | N | % of group | ||
|---|---|---|---|---|---|
| Ethnicity | |||||
| Non-Hispanic | 17 | 85 | 17 | 100 | .15 |
| Hispanic | 3 | 15 | 0 | 0 | |
| Race | |||||
| American Indian or Alaskan Native | 1 | 5 | 0 | 0 | .11 |
| White | 13 | 65 | 14 | 82 | |
| Black or African American | 6 | 30 | 3 | 18 | |
| Partner information | |||||
| Living with sexual partner | 12 | 60 | 6 | 35 | .02 |
| Not living with sexual partner | 5 | 25 | 1 | 6 | |
| No sexual partner | 3 | 15 | 10 | 59 | |
Bold numbers indicate significant p value <0.05.
Differences in pharmacokinetic parameters in FOL versus LUT phases in PRE women
For PRE participants, FOL phase sampling occurred on menstrual cycle day 7.7 ± 1.9, while LUT phase sampling was on menstrual cycle day 22.3 ± 3.1. Among the PRE cohort, the mean serum E2 and P4 levels in the LUT phase (121.3 ± 75.7 and 7.2 ± 5.2 ng/ml, respectively) were significantly higher than in the FOL phase (76.1 ± 63.3 and 0.4 ± 0.2 ng/ml, respectively) (all p-values <.04).
Consistent with our published data,20 among the PRE cohort, we found no significant differences in paired comparisons of endpoints obtained in the FOL versus LUT phases: antimicrobial (E. coli, HSV-2, and HIV) activity of the CVL, soluble mucosal immune protein concentrations in the CVL supernatant, p24 HIV antigen production from ex vivo infected ectocervical tissues, vaginal immune cell density and phenotype, semiquantitative levels of vaginal bacterial species, vaginal pH, and Nugent score (all p-values >.10, data not shown). Therefore, when comparing endpoints among independent PRE and POST cohorts, we combined the baseline FOL (n = 20) and LUT (n = 20) samples in PRE women.
Endpoint comparison in PRE versus POST women
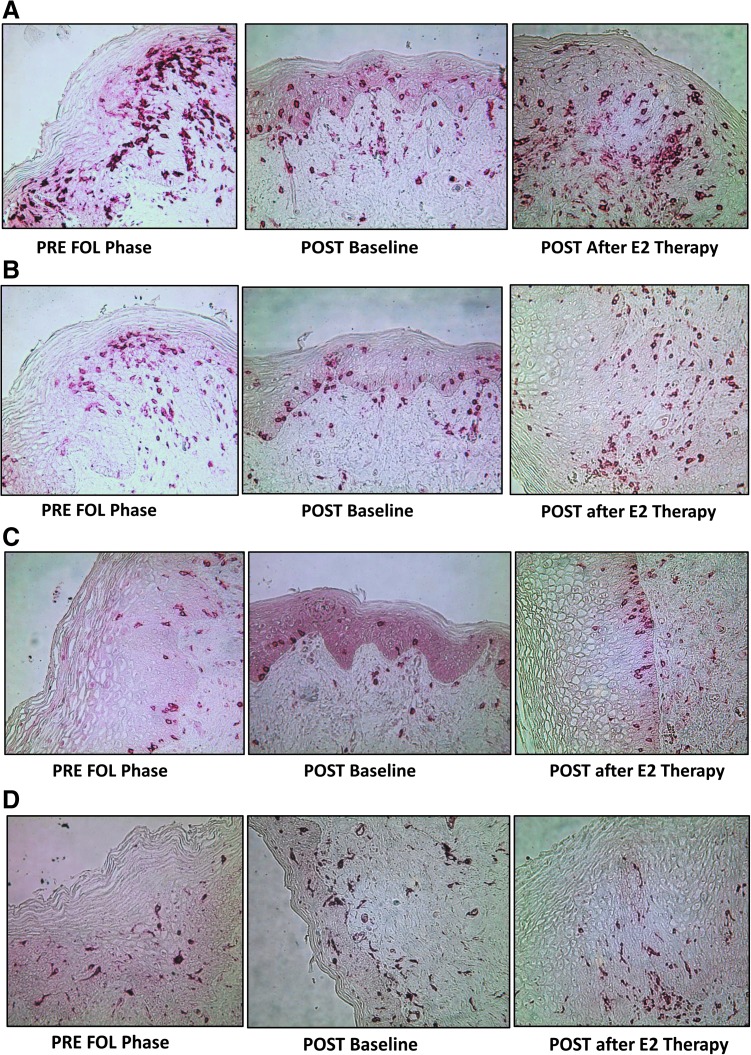
Serum E2 (28.9 ± 11.9 pg/mL) and serum P4 (0.2 ± 0.1 ng/mL) were significantly lower in POST women compared to PRE in either the FOL or LUT phases (all p-values <.01). At baseline (Tables 2–4), compared to PRE women, POST women had a significantly thinner epithelium and reduced density of E cadherin (Table 2). POST women also had a significantly higher density of immune cells, except for CD1a cells, in the vaginal epithelium and lamina propria (Table 2 and Fig. 2a–d).
Table 2.
Comparisons of Vaginal Histology, Immune Cell Populations, and Epithelial Adhesion Protein Between Premenopausal Participants in the Follicular and Luteal Phases Combined Versus Postmenopausal Participants at Baseline and After Topical Vaginal Estradiol Therapy
| Premenopausal cohort at baseline in FOL and LUT phases combined (n = 40 samples) | Postmenopausal cohort at baseline (n = 17 samples) | Postmenopausal cohort after 1 month of vaginal E2 cream therapy (n = 17 samples) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p Value PRE vs. POST baseline | Mean | SD | p Value POST baseline vs. s/p E2 | p Value PRE vs. POST s/p E2 |
| Vaginal epithelium | |||||||||
| Epithelial thickness (μm) | 314.3 | 116.8 | 115.2 | 54.2 | <.01 | 276.3 | 66.9 | <.01 | .25 |
| Number of cell layers | 21.4 | 4.6 | 10.7 | 3.6 | <.01 | 18.2 | 6.2 | <.01 | .04 |
| Vaginal immune cell populations/phenotype (cells/mm2) in the epithelium | |||||||||
| CD45 | 93.2 | 37.3 | 231.1 | 116.1 | <.01 | 82.8 | 39.9 | <.01 | .42 |
| CD3 | 70.8 | 33.2 | 187.4 | 97.3 | <.01 | 63.6 | 32 | <.01 | .64 |
| HLADR | 31.2 | 16.4 | 113.6 | 46.3 | <.01 | 52.4 | 20.3 | <.01 | <.01 |
| CCR5 | 0 | 0 | 0.2 | 0.8 | .15 | 0.1 | 0.6 | .83 | .15 |
| CD4 | 0 | 0.2 | 0.8 | 2.7 | .17 | 0.6 | 1.4 | .74 | .05 |
| Estimated CD4 (CD3–CD8) | 19.0 | 18.0 | 52.0 | 37.8 | <.01 | 18.5 | 15.2 | .05 | .83 |
| CD8 | 51.8 | 25.2 | 135.4 | 75.6 | <.01 | 45.1 | 24.9 | <.01 | .60 |
| CD1a | 39.4 | 15.2 | 23.9 | 24.1 | <.01 | 31.8 | 11.8 | .15 | .10 |
| Vaginal immune cell populations/phenotype (cells/mm2) in the lamina propria | |||||||||
| CD45 | 72.5 | 42 | 79.9 | 25.3 | .12 | 103 | 38.6 | .06 | <.01 |
| CD3 | 47.8 | 32.1 | 63 | 25.1 | .01 | 80.2 | 35.1 | .13 | <.01 |
| HLADR | 26.9 | 16.2 | 48 | 16.7 | <.01 | 57.1 | 24.9 | .18 | <.01 |
| CCR5 | 3.2 | 3.7 | 1.8 | 2.4 | .22 | 2.8 | 3.7 | .41 | .74 |
| CD4 | 2 | 2.9 | 3.3 | 3.7 | .19 | 3.5 | 4.4 | .97 | .31 |
| Estimated CD4 (CD3–CD8) | 16.4 | 14.4 | 24.0 | 14.4 | .05 | 42.2 | 24.8 | .10 | <.01 |
| CD8 | 31.3 | 21.8 | 38.9 | 17.6 | .05 | 38 | 19.3 | .89 | .09 |
| CD1a | 0.9 | 2.3 | 0.4 | 1.1 | .57 | 2.8 | 4.6 | .13 | .03 |
| E-cadherin in the vaginal epithelium ( × 106 integrated optical density of positively stained area) | |||||||||
| E-cadherin | 8.8 | 3.0 | 6.8 | 2.6 | .01 | 9.9 | 2.8 | .01 | .19 |
Bold numbers indicate significant p value <0.05.
FOL, follicular; LUT, luteal; E2, estradiol; PRE, premenopausal; POST, postmenopausal.
Table 3.
Comparisons of Antimicrobial Activity of Cervicovaginal Secretions, Soluble Immune Mediators, and Vaginal Flora Between Premenopausal Participants in the Follicular and Luteal Phases Combined Versus Postmenopausal Participants at Baseline and After Topical Vaginal Estradiol Therapy
| Premenopausal cohort at baseline in FOL and LUT phases combined (n = 40 samples) | Postmenopausal cohort at baseline (n = 17 samples) | Postmenopausal cohort after 1 month of vaginal E2 cream therapy (n = 17 samples) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p Value PRE vs. POST baseline | Mean | SD | p Value POST baseline vs. s/p E2 | p Value PRE vs. POST s/p E2 |
| Antimicrobial activity of the cervicovaginal fluid lavage (CVL) % inhibition | |||||||||
| HSV2 | 49.8 | 32.5 | 48.1 | 29.8 | .70 | 48.3 | 26.7 | 0.57 | 0.86 |
| E. coli | 48.1 | 41.1 | 36.9 | 35.3 | .25 | 9.3 | 27.3 | 0.45 | <.01 |
| HIV | 26.4 | 47.8 | 24 | 55.1 | .45 | 17.8 | 55.3 | 0.80 | .71 |
| Cytokines, chemokines, and other soluble mucosal proteins in the CVL (unless otherwise specified pg/mL · mg total protein in CVL) | |||||||||
| HD5 (ng/mL · mg protein) | 3397.1 | 2011.6 | 5060.0 | 3722.1 | .09 | 2263.7 | 1596.8 | .03 | .05 |
| Lactoferrin (ng/mL · mg protein) | 3172.5 | 2347.6 | 1862.4 | 1049.4 | .05 | 1262.1 | 1076.9 | .10 | <.01 |
| SLPI | 962533.2 | 776159.9 | 215966.8 | 314031.9 | <.01 | 748736.6 | 787560.9 | <.01 | .26 |
| Elafin | 253247.1 | 516250.6 | 804246.9 | 1288862.0 | .08 | 644075.9 | 668506.8 | .24 | <.01 |
| HNP 1–3 | 583173.3 | 586520.8 | 549448.2 | 412557.9 | .77 | 483556.4 | 496821.5 | .28 | .52 |
| HBD2 | 5026.3 | 7585.9 | 2510.1 | 4645.9 | .02 | 8086.1 | 10979.2 | .03 | .21 |
| HBD3 | 4869.6 | 6833.6 | 662.5 | 989.1 | <.01 | 6838.0 | 10776.8 | <.01 | .89 |
| IL-1B | 87.0 | 318.5 | 17.5 | 20.4 | .55 | 17.9 | 26.2 | .55 | .93 |
| TNF-α | 50.1 | 257.5 | 12.4 | 20.8 | <.01 | 4.5 | 3.0 | .02 | <.01 |
| GRO-α | 6153.5 | 8241.7 | 2068.3 | 2330.1 | .18 | 2412.0 | 2785.6 | .97 | .15 |
| MIP-1α | 97.4 | 369.7 | 48.7 | 50.5 | .01 | 15.2 | 11.1 | <.01 | .13 |
| MIP-1β | 146.5 | 503.4 | 69.3 | 100.7 | .02 | 11.7 | 15.0 | .03 | .97 |
| IP-10 | 931.7 | 1264.1 | 215.5 | 244.5 | <.01 | 313.1 | 315.2 | .32 | .06 |
| ICAM-1 | 75.8 | 411.4 | 92.9 | 263.3 | .03 | 4.8 | 11.1 | .04 | .55 |
| Assessment of vaginal microflora: vaginal pH, Nugent score, and semiquantitative levels of select vaginal bacteria (0–4 scale) | |||||||||
| Vaginal pH | 4.3 | 0.4 | 5.2 | 0.7 | <.01 | 4.6 | 0.4 | <.01 | .01 |
| Nugent score | 2.2 | 2.2 | 3.6 | 2.1 | <.01 | 3.2 | 2.4 | .48 | .06 |
| Lactobacillus H2O2+ | 2.6 | 1.3 | 1.2 | 1.2 | <.01 | 1.6 | 1.1 | .31 | .06 |
| Gardnerella | 0.5 | 1.2 | 0.8 | 1.4 | .52 | 0.6 | 1.1 | .41 | .41 |
Bold numbers indicate significant p value <0.05.
SLPI, secretory leukocyte protease inhibitor; HNP 1–3, human neutrophil peptides 1–3; HD5, human alpha defensin 5; HBD-2, human beta defensin 2; HBD-3, human beta defensin 3; IL-1β, interleukin 1β; MIP-1α, macrophage inflammatory protein 1α; MIP-1β, macrophage inflammatory protein 1β; TNF-α, tumor necrosis factor α; IP-10, interferon gamma inducible protein 10; GRO-α, growth-regulated protein α; ICAM-1, intercellular adhesion molecule 1.
Table 4.
Comparisons of p24 Antigen Production from Ectocervical Tissue Biopsies After Ex Vivo HIV-1BaL Infection Between Premenopausal Participants in the Follicular and Luteal Phases Combined Versus Postmenopausal Participants at Baseline and After Topical Vaginal E2 Therapy
| Premenopausal cohort at baseline in FOL and LUT phases combined (n = 40 samples) | Postmenopausal cohort at baseline (n = 17 samples) | Postmenopausal cohort after 1 month of vaginal E2 cream therapy (n = 17 samples) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | p Value PRE vs. POST baseline | Mean | SD | p Value POST baseline vs. s/p E2 | p Value PRE vs. POST s/p E2 |
| p24 antigen production by ectocervical tissues after ex vivo infection with HIV-1BaL (pg/mL · mg tissue). | |||||||||
| P24 day 0 | 0.34 | 0.3 | 0.2 | 0.09 | .06 | 0.2 | 0.09 | NA | .06 |
| P24 day 7 | 13.98 | 14.33 | 10.1 | 6.69 | .66 | 14.72 | 11.41 | .25 | .70 |
| p24 day 14 | 15.97 | 13.42 | 35.88 | 81.79 | .32 | 25.22 | 34.71 | .73 | .15 |
| p24 day 21 | 20.22 | 19.15 | 126.73 | 314.55 | .25a | 48.27 | 141.02 | .47 | .80 |
| P24 soft endpoint | 20.46 | 19.79 | 126.61 | 314.62 | .27a | 48.61 | 140.92 | .58 | .61 |
| P24 maximum | 22.82 | 20.8 | 128.51 | 313.91 | .75 | 52.06 | 140.29 | .39 | .87 |
| P24 cumulative | 50.51 | 40.34 | 172.91 | 362.95 | .31b | 88.41 | 179.34 | .72 | .50 |
| P24 area under curve | 281.63 | 230.47 | 766.14 | 1480.23 | .42 | 449.24 | 766.91 | .83 | .26 |
Boolean categorical analysis revealed more infected tissues in postmenopausal women (statistically significant for log p24 values of 2.0 or higher considered as infected).
Boolean categorical analysis revealed more infected tissues in postmenopausal women (statistically significant for log p24 values of 2.3 or higher considered as infected).
HIV-1, human immunodeficiency virus type 1; NA, not applicable.
FIG. 2.
(A) CD45+ cells in the vaginal mucosa. (B) CD3+ cells in the vaginal mucosa. (C) CD8+ cells in the vaginal mucosa. (D) HLADR+ cells in the vaginal mucosa.
PRE and POST cohorts at baseline had significant differences in vaginal microbiota and soluble antimicrobial and immune factors in CV fluids (Table 3). Although there were no significant differences in the whole antimicrobial activity of the CVL, POST women showed a significant decrease in several antimicrobial polypeptides (e.g., SLPI). The POST cohort also showed a decrease in several inflammatory mediators [e.g., tumor necrosis factor-alpha (TNF-α) and macrophage inflammatory protein 1α (MIP-1α)] and in intercellular adhesion molecule 1 (ICAM-1) (Table 3).
Finally, although the absolute level of p24 antigen released from ectocervical tissue biopsies was highly variable, POST ectocervical tissue biopsies produced approximately double the mean level of p24 antigen compared to PRE ectocervical tissue biopsies. When infected versus noninfected tissues were categorized based on a standard stepwise process (Boolean Method),22,23 there were significantly more tissues from POST women categorized as infected compared to those of PRE women (Table 4), supporting higher production of p24 antigen by POST tissues.
To confirm the finding of increased p24 antigen production in POST tissue biopsies at baseline compared to PRE, we performed an ex vivo infection substudy using ectocervical explants obtained from gynecologic surgical specimens (e.g., explants from hysterectomies). Similar to what we observed in tissue biopsy samples, ectocervical explants from POST women produced significantly higher levels of p24 antigen and, compared to PRE ectocervical explants, a significantly higher proportion of the POST ectocervical explants were considered infected using the stepwise categorization method (Table 5).
Table 5.
Comparison of p24 Antigen Production (pg/mL · mg Tissue) from Premenopausal Versus Postmenopausal Ectocervical Explants Obtained from Surgical Specimens
| P24 production from ectocervical explants treated with growth media and 1 × 104 HIV-1 BaL (pg/mL · mg tissue) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PRE (n = 24) | POST (n = 12) | |||||||
| Variable | N | Mean | SD | N | Mean | SD | p Value continuous variables | p Value proportion of infected samples |
| P24 day 0 | 24 | 0.06 | 0.06 | 12 | 0.07 | 0.03 | .17 | .25 |
| P24 day 7 | 24 | 6.76 | 8.79 | 12 | 16.91 | 22.27 | <.01 | <.01 |
| p24 day 14 | 24 | 25.3 | 39.96 | 12 | 50.19 | 57.04 | <.01 | <.01 |
| p24 day 21 | 24 | 25.53 | 39.87 | 12 | 49.97 | 56.69 | <.05 | <.05 |
| P24 soft endpoint | 24 | 25.82 | 39.79 | 12 | 51.26 | 56.6 | .04 | <.05 |
| P24 maximum | 24 | 32.12 | 46.85 | 12 | 67.17 | 66.18 | <.01 | <.01 |
| P24 cumulative | 24 | 213.83 | 298.44 | 12 | 462.47 | 443.32 | <.01 | <.01 |
Bold numbers indicate significant p value <0.05.
Paired samples from POST women at baseline and after topical vaginal E2 therapy
Vaginal E2 therapy did not alter serum E2 (28.7 ± 8.8 pg/ml) or serum P4 (0.2 ± 0.1 ng/ml) from baseline in POST women (p values .27 and .93, respectively). Vaginal E2 therapy resulted in a significant increase in epithelial layers and E cadherin expression, and a reduction in epithelial immune cell density compared to baseline in POST women (Table 2). The density of vaginal immune cells in the lamina propria was unaffected or showed a nonsignificant increase after vaginal E2 treatment. Compared to baseline in POST women, E2 treatment induced a significant increase in antimicrobial factors while decreasing some proinflammatory mediators (Table 3). Vaginal E2 treatment resulted in decreased p24 antigen production by ectocervical tissues compared to POST women at baseline, however, this change was not statistically significant.
Comparison of PRE women to POST women after vaginal E2 therapy
In general, vaginal E2 therapy in POST women altered several, although not all, mucosal parameters in the CV tissue resulting in levels similar to those measured in the separate PRE cohort (Tables 2–4). After vaginal E2 therapy, POST women's vaginal epithelial thickness increased, vaginal immune cell density decreased, CD1a cells increased, and antimicrobial factor concentrations were similar to those measured in PRE women. Select vaginal immune cells in the lamina propria, including estimated CD4 density, however, remained elevated compared to PRE women, even after E2 treatment. At baseline, POST women produced more p24 antigen from ectocervical tissues infected ex vivo with HIV-1BaL than PRE women (Table 4). After topical E2 therapy, p24 antigen production decreased in POST women to levels similar to those observed in PRE women (Table 4).
Correlations between serum hormone concentrations and mucosal endpoints
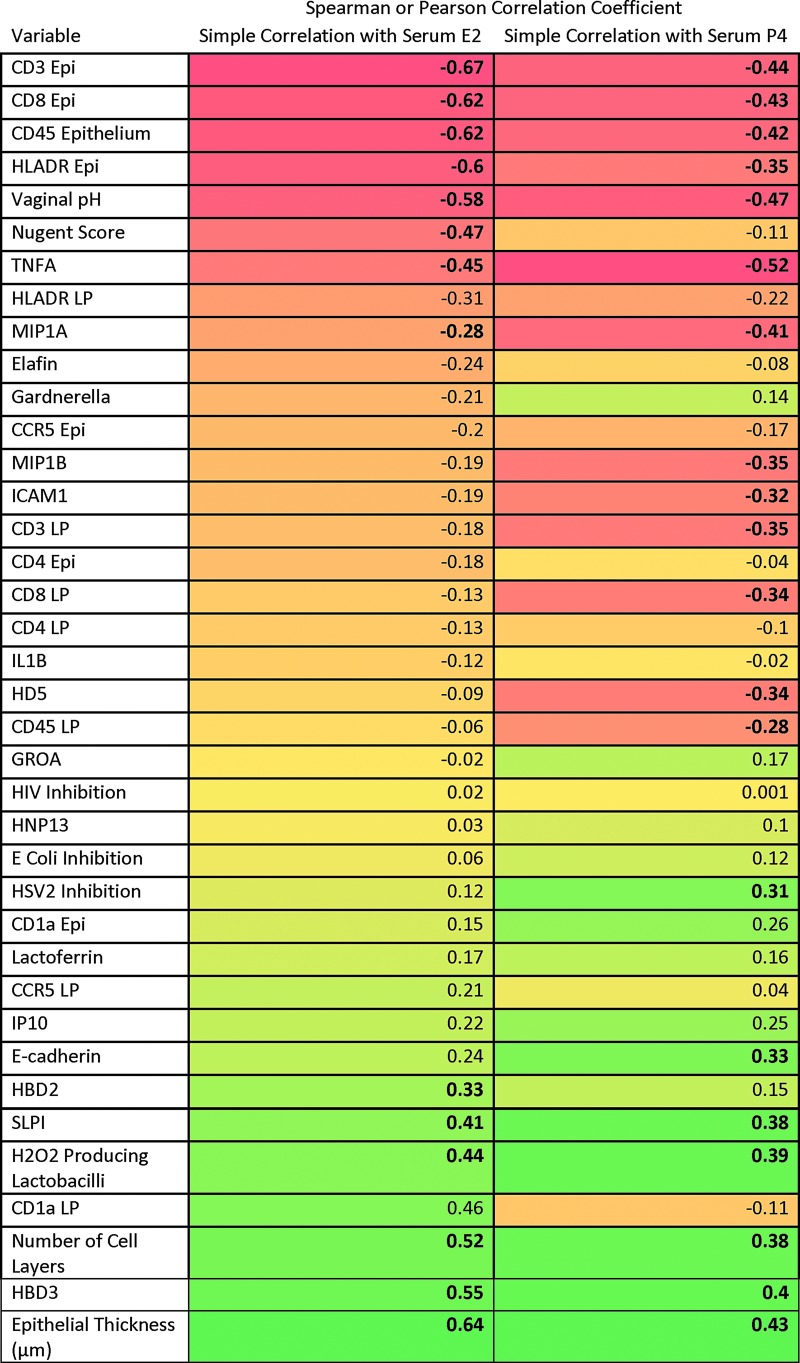
Serum levels of E2 and P4 from all visits from PRE and POST women were correlated with CV endpoints. We found that increases in serum E2 were inversely correlated with microbiota markers (Fig. 3), while increases in serum E2 were positively correlated with vaginal epithelial thickness and numbers of vaginal cell layers. Increases in serum E2 were negatively correlated with densities of several vaginal epithelial immune cells (CD45, CD3, HLADR, and CD8). With increases in serum E2, CVL antiviral factors such as SLPI, HBD2, and HBD3 increased, while inflammatory cytokines TNF-α and MIP-1α decreased significantly. Serum P4 concentrations showed similar correlations to CV endpoints. Increasing serum P4 levels were also positively and significantly associated with HSV-2 inhibition in the CVL.
FIG. 3.
Heat map of Pearson or Spearman correlation coefficients. Correlation of cervicovaginal endpoints with serum E2 or P4 (bolded correlation coefficients have p-value <.05, red boxes show negative/inverse correlation, yellow to orange boxes show intermediate correlation, and green boxes show positive/direct correlation). E2, estradiol; P4, progesterone.
Significant endpoints (p values <.05) from simple linear correlations (bolded in Fig. 2) were introduced into multiple linear regression models. These models supported increases in SLPI [parameter estimate 0.08, standard error (SE) 0.04, p = .05], decreases in CD8 cells in the epithelium (parameter estimate −0.61, SE 0.11, p ≤ .0001), and decreases in Nugent score (parameter estimate −0.36, SE 0.11, p ≤ .01), which were significantly associated with increases in serum E2. Increases in serum P4 were correlated with decreases in HD5 (parameter estimate −0.48, SE 0.24, p = .05), decreases in CD3 in the lamina propria (parameter estimate −0.77, SE 0.32, p = .02), increases in HBD3 (parameter estimate 0.31, SE 0.11, p = .01), and increases in E-cadherin (parameter estimate 1.17, SE 0.44, p = .01).
Discussion
The findings of this study show that compared to PRE women, the CV mucosa of POST women had significant differences in epithelial structure and function, immune cells, and antimicrobial and proinflammatory factors, ultimately producing higher levels of p24 antigen after ex vivo HIV-1BaL infection, a reflection of higher susceptibility to acquire HIV-1. This greater ability to be infected by, and allow for replication of, HIV-1 in CV tissues of POST women compared to those of PRE women was supported by using explanted tissues derived from gynecologic surgeries. Topical E2 treatment in POST women returned several mucosal parameters, including mucosal p24 antigen production, to levels observed in PRE women. Our data on p24 antigen production from both tissue biopsies and ectocervical explants are consistent with a previous report based on surgical explants.24 Furthermore, we found several differences in PRE and POST women's CV mucosa, which may explain mechanisms associated with increased susceptibility of the CV mucosa of POST women.
POST women showed a structurally reduced CV epithelium, with fewer cell layers and decreased thickness. Due to a reduction in the area of the epithelium, most epithelial immune cells (CD45, CD3, and CD8) increased in density in POST women compared to PRE. CD4 and CCR5 antigens were sensitive to paraffin embedding and could not be properly quantified directly. By estimating CD4 cell numbers by subtracting CD8 cells from CD3 cells,25,26 we showed an increase of epithelial CD4 cells in POST women. There was also an increase in the number of HLA-DR cells, reflecting an increased activation status.27–29 The only cells that were not increased in the CV epithelium of POST women were CD1a antigen-presenting cells. These cells, likely Langerhans cells (LC), are intimately related to the epithelium and when the epithelium is reduced, so are the numbers of LC.30,31 In the mucosal lamina propria, there was an increase in CD3 T cells and HLA-DR expressing cells in POST women.
Serum E2 and P4 levels were inversely correlated with vaginal epithelial and lamina propria immune cell concentrations, consistent with previous data from our laboratory showing that vaginal immune cells increase in healthy PRE women after treatment with DMPA.19 Others reported that POST women (n = 24) had significantly higher percentages of CCR5+CD4+ T cells and CCR5+DR +38+CD4+ T cells in the blood and endocervix compared with PRE women (n = 22) and age was linearly related to the percent of CCR5+CD4+ and CCR5+DR +38+CD4+ cells in the cervix.32
Compared to PRE women, POST women have significantly reduced levels of vaginal epithelial E-cadherin, a tight junction protein, which mediates epithelial cell-to-cell adhesion and has been reported to be a good marker of vaginal epithelial integrity.33,34 Disruption of tight junctions has been shown to facilitate HIV infection in an ex vivo infection model.33,35 While other in vitro models show that HIV infection of cervical and colonic cells is accompanied by increases in inflammatory cytokines without effect on tight junctions.36 Increases in P4 were associated with significantly higher epithelial E-cadherin levels in multiple linear regression analyses, supporting that ovulatory PRE women have increased vaginal epithelial integrity, which represents a mechanism of defense against mucosal infection. In addition, we confirmed that E2 supplementation increased vaginal epithelial thickness and the number of cell layers significantly among POST women.37,38 E2 therapy also increased E-cadherin density, strengthening the epithelial junction barrier. Although the effect of isoflavonoids and other estrogenic compounds on epithelial tight junctions has been previously reported,39,40 our data on the effect of in vivo topical E2 application on the CV mucosal expression of E-cadherin are original. Decreased vaginal epithelial thickness and tight junction proteins and increased density of vaginal immune cells per mm2 of epithelium provide a biological mechanism underlying increased susceptibility to HIV infection in vivo in POST compared to PRE women.
The mucosal immune mediator profiles of the CVLs support the hypothesis that POST women may be more susceptible to viral and bacterial infections, with significantly lower concentrations of antimicrobial polypeptides (SLPI, HBD2, HBD3, and lactoferrin) compared to healthy PRE women. PRE women had higher concentrations of some CVL inflammatory mediators [TNF-α, MIP-1α, macrophage inflammatory protein 1β (MIP-1β), interferon gamma inducible protein 10] compared to POST women. While an increased local inflammatory milieu has been associated with incident HIV41–44 and higher viral load and lower CD4 counts postinfection,42,45,46 other in vitro modeling studies show that a mix of inflammatory and antimicrobial mucosal mediators is optimal for anti-HIV activity.47 These inflammatory mediators also attract immune cells to mount an antiviral response. Others confirm that alterations in endogenous hormones, such as seen in pregnancy, are associated with suppression of mucosal immune mediators.48
Compared to baseline samples in POST women, we found that vaginal E2 therapy in POST women resulted in significant increases in protective molecules such as SLPI, HBD2, and HBD3 and reductions in some inflammatory mediators (TNF-α, MIP-1α, and MIP-1β). ICAM-1 was also decreased by E2 therapy. It has been shown that soluble ICAM-1 levels produced by human vaginal, ectocervical, and endocervical epithelial cells are increased by proinflammatory cytokines as well as by infection with sexually transmitted infection (STI) pathogens.49–51 This shift in the soluble mucosal protein profile after vaginal E2 therapy supports the hypothesis that restoring the CV mucosa's structure and function to levels measured in PRE women may enhance mechanisms of protection against HIV in POST women.44,52 After receiving topical E2 therapy, the levels of some, but not all soluble, mediators in POST women were similar to those measured in PRE women. The duration of E2 treatment in POST women was chosen due to known beneficial effects of E2 treatment on vaginal histology and microflora.37 It is not known whether longer durations of E2 treatment would result in additional similarities of measured mucosal endpoints to PRE women or if some mediators are more responsive to exogenous hormones than others.
At baseline, we found no significant differences in the antimicrobial (HIV, E. coli, and HSV-2) activity of the CVL between PRE and POST cohorts. Our data are consistent with a recent study showing no difference in the CVL supernatant activity against HIVBaL among PRE women compared to pregnant women,48 who have an altered endogenous hormonal mileu.53 A previous cross-sectional analysis of the antimicrobial activity of the CVL among 28 POST women versus 136 PRE women found that POST women had significantly less anti-HIV activity of the CVL compared to PRE women.54 However, 61% of PRE women in this cohort were using exogenous contraceptive hormones.54 Once women using exogenous hormones were removed, there was a nonsignificant trend toward lower antimicrobial activity of the CVL in POST women compared to PRE women. Another recent study reports similar ranges of antimicrobial activity of the CVL supernatant among PRE and POST women.55 The inhibitory activity of the CVL supernatant against HIVBaL was significantly increased in POST women, after adjusting for vaginal pH, the presence of reproductive tract infections, and total protein content.55
Increases in E. coli inhibitory activity of the CVL were previously found to be associated with HIV seroconversion in the CAPRISA 004 and HPTN 035 trials and may be a functional marker of heightened genital tract inflammation.41,56 After E2 therapy, the in vitro inhibitory activity of the CVL against E. coli in POST women was significantly reduced, which is a functional marker of reduced genital tract inflammation and potentially decreased susceptibility to HIV-1.41,46,56 Along this line, topical E2 therapy in POST women led to reduced HIV p24 antigen production, supporting a protective effect of vaginal E2 supplementation. We are not aware of other studies in which POST women were treated in vivo with vaginal E2 therapy and lower genital tract biopsies were infected ex vivo with HIV-1.
This study has several strengths in that well-screened groups of healthy PRE and POST women were followed longitudinally and underwent detailed genital tract samplings, along with endogenous serum hormone measurements, and multiple mucosal endpoints were analyzed, including epithelial, immune, antimicrobial, and inflammatory factors, and susceptibility to ex vivo HIV infection. POST women were treated in vivo with an FDA-approved treatment for urogenital atrophy, vaginal E2 cream, to see the impact of restoring epithelial structure and function.
We hypothesized that there would be less variability in p24 antigen production by obtaining accurately timed tissue biopsies from well screened, healthy PRE and POST women. However, we found considerable variability of p24 antigen production from both tissue biopsies and from surgical explants, after ex vivo HIV-1BaL infection. A limitation of this study is the difficulty of directly assessing CD4+ cells in the CV epithelium using paraffin-embedded tissues. To address this issue, we estimated CD4 numbers by subtracting the number of CD8 cells from total CD3 cells,25,26 as has previously been described. There is also considerable variability of p24 antigen production from tissues after ex vivo infection of biopsies,22,57 but we confirmed the tissue biopsy findings with data obtained from hysterectomy-derived explant samples.
Our main measure of adherence to the vaginal E2 therapy among POST women was participant report and returned unused product, which can be biased; however, the fact that established markers of E2 treatment (vaginal epithelial thickness, number of vaginal cell layers, and vaginal pH) changed significantly, in an expected manner, after E2 treatment,58 supports that this cohort was exposed to vaginal E2.
Non-Hispanic white women comprised the majority of PRE and POST women in this cohort and the study was conducted in the United States. The sample size could introduce the possibility of a type II error for some endpoints.
Although we report differences in p24 antigen production between PRE and POST tissues, we found no difference in the antimicrobial inhibitory activity of the CVL. The antimicrobial activity of the CVL is thought to be a functional assay of immune protection, which is likely influenced by several endogenous (e.g., microflora, hormones) and exogenous (e.g., semen, contraceptives) factors, which may not be controlled for exploratory studies. We used multiple models to test our hypothesis that POST women have an altered mucosal environment, which may make them more susceptible to HIV-1.
In conclusion, we found that the CV mucosa of POST women at baseline differed in many respects from that of PRE women, showing a decreased epithelial barrier together with increased immune HIV target cell density and reduced antiviral factors, all mechanisms that would facilitate HIV-1 infection and replication in vivo. Ex vivo, we found that POST tissue biopsies produced, on average, at least double the levels of p24 antigen compared to PRE tissues. This increased susceptibility agrees with small observational studies and epidemiological data.4–6 Treatment with vaginal E2 in POST women altered the structure and functionality of CV tissues and decreased HIV p24 antigen production from ectocervical tissues back to the levels measured in PRE women. These data are important in understanding how changes in the endogenous hormonal milieu, which all women experience, may alter mucosal susceptibility to HIV-1.
Source of Funding
This work was funded by an RO1 from the National Institutes of Child Health and Human Development (NICHD) (5R01HD072705). The views expressed by the authors do not necessarily reflect those of the funding agency or CONRAD.
Supplementary Material
Author Disclosure Statement
No competing financial interests exist.
References
- 1.UNAIDS: The gap report. Available at www.unaids.org/sites/default/files/media_asset/UNAIDS_Gap_report_en.pdf (2014), accessed May4, 2017
- 2.Speroff L, Glass RH, Kase NG: Steroid metabolism. In: Clinical Endocrinology and Infertility, Vol 4th (Speroff L, ed.) Philadelphia, PA: Lippincott Williams and Wilkins, 1989, p. 629 [Google Scholar]
- 3.Centers for Disease Control and Prevention: HIV surveillance report 2014. Available at www.cdc.gov/hiv/library/reports/surveillance/ (2014), accessed May4, 2017
- 4.Dwyer JM, Penny R, Gatenby PA, Learmont J: Susceptibility of postmenopausal women to infection with HIV during vaginal intercourse. Med J Aust 1990;153:299. [DOI] [PubMed] [Google Scholar]
- 5.HIV ESGoHTo: Comparison of female to male and male to female transmission of HIV in 563 stable couples. European Study Group on heterosexual transmission of HIV. BMJ 1992;304:809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaby P, Ariyoshi K, Buckner M, et al. : Age of wife as a major determinant of male-to-female transmission of HIV-2 infection: A community study from rural West Africa. AIDS 1996;10:1585–1590 [DOI] [PubMed] [Google Scholar]
- 7.Dorgan JF, Baer DJ, Albert PS, et al. : Serum hormones and the alcohol-breast cancer association in postmenopausal women. J Natl Cancer Inst 2001;93:710–715 [DOI] [PubMed] [Google Scholar]
- 8.Leroy V, Van de Perre P, Lepage P, et al. : Seroincidence of HIV-1 infection in African women of reproductive age: A prospective cohort study in Kigali, Rwanda, 1988–1992. AIDS 1994;8:983–986 [DOI] [PubMed] [Google Scholar]
- 9.Taha TE, Dallabetta GA, Hoover DR, et al. : Trends of HIV-1 and sexually transmitted diseases among pregnant and postpartum women in urban Malawi. AIDS 1998;12:197–203 [DOI] [PubMed] [Google Scholar]
- 10.Gray RH, Li X, Kigozi G, et al. : Increased risk of incident HIV during pregnancy in Rakai, Uganda: A prospective study. Lancet 2005;366:1182–1188 [DOI] [PubMed] [Google Scholar]
- 11.Mishell DR, Jr.: Pharmacokinetics of depot medroxyprogesterone acetate contraception. J Reprod Med 1996;41(5 Suppl):381–390 [PubMed] [Google Scholar]
- 12.Clark MK, Sowers M, Levy BT, Tenhundfeld P: Magnitude and variability of sequential estradiol and progesterone concentrations in women using depot medroxyprogesterone acetate for contraception. Fertil Steril 2001;75:871–877 [DOI] [PubMed] [Google Scholar]
- 13.Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR: Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab 1977;44:32–38 [DOI] [PubMed] [Google Scholar]
- 14.Polis CB, Curtis KM, Hannaford PC, et al. : Update on hormonal contraceptive methods and risk of HIV acquisition in women: A systematic review of epidemiological evidence, 2016. AIDS 2016;30:2665–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nugent RP, Krohn MA, Hillier SL: Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol 1991;29:297–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurman AR, Kimble T, Herold B, et al. : Bacterial vaginosis and subclinical markers of genital tract inflammation and mucosal immunity. AIDS Res Hum Retroviruses 2015;31:1139–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller MJ, Madan RP, Torres NM, et al. : A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011;6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy K, Richardson BA, Dezzutti CS, et al. : Levels of genital tract defensins and cytokines differ between HIV-uninfected US and African women. Am J Reprod Immunol 2015;74:313–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra N, Thurman AR, Anderson S, et al. : Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses 2013;29:592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurman AR, Chandra N, Yousefieh N, et al. : Comparison of follicular and luteal phase mucosal markers of HIV susceptibility in healthy women. AIDS Res Hum Retroviruses 2016;32:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson-Harman N, Mauck C, McGowan I, Anton P: Dose–response relationship between tissue concentrations of UC781 and explant infectibility with HIV type 1 in the RMP-01 rectal safety study. AIDS Res Hum Retroviruses 2012;28:1422–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson-Harman N, Lackman-Smith C, Fletcher PS, et al. : Multisite comparison of anti-human immunodeficiency virus microbicide activity in explant assays using a novel endpoint analysis. J Clin Microbiol 2009;47:3530–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson-Harman N, Hendrix CW, Bumpus NN, et al. : Correlation between compartmental tenofovir concentrations and an ex vivo rectal biopsy model of tissue infectibility in the RMP-02/MTN-006 phase 1 study. PLoS One 2014;9:e111507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollenhagen C, Asin SN: Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol 2011;4:671–681 [DOI] [PubMed] [Google Scholar]
- 25.Yeaman GR, White HD, Howell A, Prabhala R, Wira CR: The mucosal immune system in the human female reproductive tract: Potential insights into the heterosexual transmission of HIV. AIDS Res Hum Retroviruses 1998;14 Suppl 1:S57–S62 [PubMed] [Google Scholar]
- 26.Saba E, Grivel JC, Vanpouille C, et al. : HIV-1 sexual transmission: Early events of HIV-1 infection of human cervico-vaginal tissue in an optimized ex vivo model. Mucosal Immunol 2010;3:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veazey RS, Tham IC, Mansfield KG, et al. : Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: Highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol 2000;74:57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore AC, Bixler SL, Lewis MG, Verthelyi D, Mattapallil JJ: Mucosal and peripheral Lin- HLA-DR+ CD11c/123− CD13+ CD14− mononuclear cells are preferentially infected during acute simian immunodeficiency virus infection. J Virol 2012;86:1069–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaspan HB, Liebenberg L, Hanekom W, et al. : Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis 2011;204:1550–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoopat L, Eiangleng L, Rugpao S, et al. : In vivo identification of Langerhans and related dendritic cells infected with HIV-1 subtype E in vaginal mucosa of asymptomatic patients. Mod Pathol 2001;14:1263–1269 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed SM, Al-Doujaily H, Johnson MA, Kitchen V, Reid WM, Poulter LW: Immunity in the female lower genital tract and the impact of HIV infection. Scand J Immunol 2001;54:225–238 [DOI] [PubMed] [Google Scholar]
- 32.Meditz AL, Moreau KL, MaWhinney S, et al. : CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr 2012;59:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesquita PM, Cheshenko N, Wilson SS, et al. : Disruption of tight junctions by cellulose sulfate facilitates HIV infection: Model of microbicide safety. J Infect Dis 2009;200:599–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue M, Ogawa H, Miyata M, Shiozaki H, Tanizawa O: Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. Am J Clin Pathol 1992;98:76–80 [DOI] [PubMed] [Google Scholar]
- 35.Kaushic C, Nazli A, Ferreira VH, Kafka JK: Primary human epithelial cell culture system for studying interactions between female upper genital tract and sexually transmitted viruses, HSV-2 and HIV-1. Methods 2011;55:114–121 [DOI] [PubMed] [Google Scholar]
- 36.Sankapal S, Gupta P, Ratner D, et al. : HIV Exposure to the epithelia in ectocervical and colon tissues induces inflammatory cytokines without tight junction disruption. AIDS Res Hum Retroviruses 2016;32:1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molander U, Milsom I, Ekelund P, Mellstrom D, Eriksson O: Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post-menopause. Maturitas 1990;12:113–120 [DOI] [PubMed] [Google Scholar]
- 38.Gupta S, Kumar N, Singhal N, Manektala U, Jain S, Sodhani P: Cytohormonal and morphological alterations in cervicovaginal smears of postmenopausal women on hormone replacement therapy. Diagn Cytopathol 2006;34:676–681 [DOI] [PubMed] [Google Scholar]
- 39.Someya M, Kojima T, Ogawa M, et al. : Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res 2013;354:481–494 [DOI] [PubMed] [Google Scholar]
- 40.Kiatprasert P, Deachapunya C, Benjanirat C, Poonyachoti S: Soy isoflavones improves endometrial barrier through tight junction gene expression. Reproduction 2015;149:269–280 [DOI] [PubMed] [Google Scholar]
- 41.Pellett Madan R, Masson L, Tugetman J, et al. : Innate antibacterial activity in female genital tract secretions is associated with increased risk of HIV acquisition. AIDS Res Hum Retroviruses 2015;31:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bebell LM, Passmore JA, Williamson C, et al. : Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis 2008;198:710–714 [DOI] [PubMed] [Google Scholar]
- 43.Levinson P, Kaul R, Kimani J, et al. : Levels of innate immune factors in genital fluids: Association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS 2009;23:309–317 [DOI] [PubMed] [Google Scholar]
- 44.Morrison C, Fichorova RN, Mauck C, et al. : Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014;66:109–117 [DOI] [PubMed] [Google Scholar]
- 45.Roberts L, Passmore JA, Mlisana K, et al. : Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis 2012;205:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Herold BC, Keller MJ, Shi Q, et al. : Plasma and mucosal HIV viral loads are associated with genital tract inflammation in HIV-infected women. J Acquir Immune Defic Syndr 2013;63:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM: Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol 2005;175:7560–7567 [DOI] [PubMed] [Google Scholar]
- 48.Hughes BL, Dutt R, Raker C, et al. : The impact of pregnancy on anti-HIV activity of cervicovaginal secretions. Am J Obstet Gynecol 2016;215:748.e1–748.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fichorova RN, Desai PJ, Gibson FC, 3rd, Genco CA: Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun 2001;69:5840–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fichorova RN, Anderson DJ: Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 1999;60:508–514 [DOI] [PubMed] [Google Scholar]
- 51.Fichorova RN, Lee Y, Yamamoto HS, et al. : Endobiont viruses sensed by the human host—beyond conventional antiparasitic therapy. PloS One 2012;7:e48418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iqbal SM, Ball TB, Levinson P, et al. : Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS 2009;23:1669–1677 [DOI] [PubMed] [Google Scholar]
- 53.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ: Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril 2005;84:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chappell CA, Isaacs CE, Xu W, et al. : The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol 2015;213:204.e1–204.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M: Reduced levels of genital tract immune biomarkers in postmenopausal women: Implications for HIV acquisition. Am J Obstet Gynecol 2016;215:324.e1–324.e10 [DOI] [PubMed] [Google Scholar]
- 56.Dezzutti CS, Richardson BA, Marrazzo JM, et al. : Mucosal Escherichia coli bactericidal activity and immune mediators are associated with HIV-1 seroconversion in women participating in the HPTN 035 trial. J Infect Dis 2012;206:1931–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dezzutti CS, Uranker K, Bunge KE, Richardson-Harman N, Macio I, Hillier SL: HIV-1 infection of female genital tract tissue for use in prevention studies. J Acquir Immune Defic Syndr 2013;63:548–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suckling J, Lethaby A, Kennedy R: Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev 2006:CD001500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.