Abstract
Introduction: Nonalcoholic fatty liver disease (NAFLD), characterized by hepatocyte dysfunction, fat accumulation, and fibrosis, is the most common cause of chronic liver disease in children. Elevated levels of serum alanine aminotransferase (ALT) are used clinically to identify potential liver dysfunction. Our goal was to assess for changes in the national prevalence of elevated ALT over time and potential relationship to trends in the metabolic syndrome (MetS) severity and elevated body mass index (BMI).
Materials and Methods: We studied 5411 non-Hispanic white, non-Hispanic black, and Hispanic adolescents aged 12–19 with complete MetS Z-score and ALT data from the National Health and Nutrition Examination Survey 1999–2014. Elevated ALT levels were defined by two different cutoffs: one for both sexes (30 U/L) and another that was sex specific (22 U/L girls; 25 U/L boys). MetS severity was assessed using a sex- and race-/ethnicity-specific MetS Z-score.
Results: We did not find a statistically significant linear increase in either mean ALT or the prevalence of elevated ALT differed over time. As expected, ALT levels were significantly correlated with BMI Z-score and MetS Z-score (P < 0.0001). Over time, BMI Z-scores increased and MetS severity Z-score decreased.
Conclusion: Prevalence of elevated ALT did not exhibit a linear change between 1999 and 2014 in U.S. adolescents, potentially due to divergent trends regarding BMI and MetS severity. Continued vigilance in monitoring BMI and ALT levels is advised for the U.S. adolescent population. MetS Z-score could act as an additional tool to monitor risk of elevated ALT and subsequent development of NAFLD.
Keywords: : nonalcoholic fatty liver disease, alanine aminotransferase, obesity, metabolic syndrome, adolescent
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a condition characterized by pathological changes to liver structure and function, including hepatocyte dysfunction, fat accumulation, and fibrosis.1,2 If left undiagnosed or untreated in the pediatric population, NAFLD could yield poor health outcomes in adulthood, including cirrhosis and cancer.3,4 As NAFLD is now the most common liver disease in children and adolescents, being found in ∼7.6% of the U.S. adolescent population,5 it is of utmost importance to understand the pathophysiology of NAFLD for earlier prevention. The gold standard for diagnosis of NAFLD is liver biopsy; however, the invasiveness of this procedure has led to the use of other approaches to identify risk for NAFLD.6
Serum alanine aminotransferase (ALT) is a liver enzyme used to evaluate liver dysfunction and serves as a screening tool for the detection of possible chronic liver disease.7–9 While ALT has limitations in accurately identifying NAFLD,10–13 there are numerous studies validating its use to identify fatty liver disease in adolescents, and serve as a warning sign for cardiometabolic risk factors.14–20 It has even been shown that lowering ALT thresholds below levels commonly used in United States hospitals yielded better sensitivities in detecting chronic liver disease in children, with little compromise in specificity.19 In surveys of healthy U.S. adolescents, the prevalence of increased serum ALT, which differs significantly by age, gender, and race/ethnicity,21 ranges between 2% and 8%, depending on gender and ALT cutoff used.19
The metabolic syndrome (MetS) is a cluster of multiple cardiovascular risk factors, including elevated waist circumference (WC), hypertension, elevated triglycerides, low high-density lipoprotein cholesterol (HDL-C), and elevated fasting glucose. Features of MetS have been strongly associated with NAFLD4 and elevated ALT.22 There is also evidence that conditions subsequent to MetS, like hypercholesterolemia and type 2 diabetes mellitus (T2DM), may also have a relationship to NAFLD.3,23 Individual MetS components or related factors like increased body mass index (BMI), WC, hypertriglyceridemia, and insulin resistance are associated with the development of NAFLD.7,18,24
Previously, we found that among U.S. adolescents over the period of 1999–2012, the severity of MetS decreased, while BMI Z-scores increased.25 The goal of this study was to assess trends in ALT over time in the U.S. adolescent population and evaluate potential upstream factors related to ALT elevations, including MetS severity and elevated BMI. We hypothesized that over time, ALT levels and ALT elevations would be found to increase to mirror the trends of increasing BMI Z-scores. We also hypothesize that in the adolescent population, ALT would be significantly correlated with the BMI Z-score, MetS Severity Score (MetS-Z), and individual components of MetS. Such knowledge may help in addressing NAFLD-related needs among adolescents.
Methods
Data were sourced from the Center for Disease Control National Health and Nutrition Examination Survey (NHANES), a nationally representative sample of the U.S. population. This study was approved by the National Center for Health Statistics Research Ethics Review Board and all participants gave informed consent or assent. We analyzed data between 1999 and 2014 in adolescents 12–19 years of age. Laboratory assessments used in our analyses have been described previously.22 We included participants with complete data regarding MetS factors and ALT measurements. Out of 14,775 adolescents evaluated in NHANES between 1999 and 2014, 5019 met all inclusion criteria for this study. The majority of participants were excluded as a result of nonfasting status for laboratory measures, leading to missing data for MetS Z-score (n = 1925). Participants were also excluded if they were pregnant (n = 181), diagnosed with diabetes (n = 73), had missing ALT values (n = 1925), had the Hepatitis B surface antigen (n = 11), or had missing weight. In total, 9364 participants were noneligible, with many participants meeting multiple exclusion criteria (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/met). The yearly breakdown of our participant pool was similar to prior analyses.25 Our final pool of participants (n = 5411) was weighted to be nationally representative of U.S. adolescents based on survey design and fasting status.
ALT and cutoffs
We used two different sets of ALT cutoffs to identify elevated ALT values, as no consensus cutoff exists. The first, 30 U/L, has been commonly used in the literature as the upper limit for ALT.7,26 The other set was derived from the SAFETY Study, which determined cutoff values of 22 U/L for females and 25 U/L for males. Using these values to detect NAFLD resulted in higher sensitivity with relatively little reduction in specificity.19,21
An elevated BMI Z-Score was defined as greater than or equal to the 85th percentile, also classified as “overweight”. An elevated MetS Z-score was defined as greater than Z-score = 0.75.27
MetS classification and severity
The MetS severity Z-score used in our sample accounts for race-/ethnic- and sex-specific differences in MetS27,28 that may be important in determining racial/ethnic differences in how MetS relates to ALT elevations.22,29,30 To derive this set of scores, confirmatory factor analysis was performed using data from NHANES 1999–2010 separately for male and female non-Hispanic-white, non-Hispanic-black, and Hispanic adolescents aged 12–19 years, yielding differential loading factors for each MetS component among these six sex- and race-/ethnicity-specific subgroups. These loading factors were then used to generate equations for each sex and racial/ethnic subgroup with weights for each MetS component based on how MetS was manifested in that subgroup. These equations31 were used to calculate the MetS Z-scores. This MetS Z-score linked with long-term risk of developing MetS-related conditions in adulthood.25,27
In addition, MetS was defined using a commonly used pediatric/adolescent adaptation of traditional adult criteria.32,33 Participants had to meet ≥3 of the following five criteria: concentration of triglycerides ≥110 mg/dL, HDL-C ≤40 mg/dL, WC ≥90th percentile for age/sex34 glucose concentration ≥100 mg/dL, and systolic or diastolic blood pressure ≥90th percentile (age, height, and sex specific).35
Statistical analysis
Statistical analysis was performed using SAS (SAS 9.4, Cary, NC) survey procedures to account for the complex survey design of NHANES. Values for fasting triglycerides and serum ALT were log-transformed to yield a normal distribution. Frequency procedures, chi-square testing, and regression analysis were used to assess prevalence, correlations, and significant differences between groups. Logistic regression was used to estimate odds ratios (OR). Significance was determined at a 95% confidence level, P < 0.05. In our analyses, we looked at trends of ALT, BMI-Z, and MetS-Z; this included prevalence of elevated values as well as mean values over time. In addition, the relationships of ALT with BMI-Z and MetS-Z were assessed.
Results
Our final sample consisted of 5019 participants; 51.5% were male and the mean age, 15.5 years. Overall, the mean ALT value was 19.4 U/L; mean ALT values varied across racial ethnic groups and were 19.1 U/L, among non-Hispanic whites, 17.5 U/L, among non-Hispanic blacks, and 21.8 U/L, among Hispanics (Table 1). In our weighted sample between 1999 and 2014, only 481 individuals (9.14%) had elevated ALT levels as defined by a cutoff of 30 U/L. In contrast, 842 individuals (16.25%) had elevated ALT levels as defined by the gender-specific cutoffs.
Table 1.
Participant Characteristics, Weighted to Be Nationally Representative
| Non-Hispanic white | Non-Hispanic black | Hispanic | Total | |
|---|---|---|---|---|
| Number | 1472 | 1532 | 2015 | 5019 |
| % Malea | 51.56 (48.67, 54.44) | 50.57 (47.40, 53.74) | 52.02 (49.03, 55.01) | 51.50 (49.42, 53.57) |
| Mean age in yearsb | 15.51 (15.38, 15.65) | 15.45 (15.32, 15.58) | 15.35 (15.22,15.49) | 15.47 (15.38, 15.57) |
| Mean BMI-Zb | 0.53 (0.47, 0.59) | 0.79 (0.71, 0.86) | 0.73 (0.65, 0.81) | 0.61 (0.56, 0.65) |
| Mean ALT in U/Lb | 19.14 (18.66, 19.63) | 17.52 (17.02, 18.02) | 21.75 (20.79, 22.72) | 19.41 (19.02, 19.79) |
| % Elevated ALTa | 8.74 (6.94, 10.54) | 5.49 (3.98, 7.00) | 13.33 (11.45, 15.20) | 9.14 (7.86, 10.43) |
| Mean MetS Z-scoreb | −0.02 (−0.06, 0.03) | −0.14 (−0.19, −0.09) | 0.09 (0.03, 0.14) | −0.02 (−0.05, 0.02) |
| % ATP III MetS prevalencea | 7.89 (6.25, 9.53) | 4.41 (3.23, 5.59) | 9.90 (8.25, 11.55) | 7.77 (6.60, 8.90) |
| % Elevated BPa | 6.75 (5.18, 8.33) | 12.05 (10.29, 13.80) | 6.56 (4.99, 8.13) | 7.52 (6.34, 8.71) |
| % Elevated fasting glucosea | 14.04 (11.87, 16.21) | 9.78 (7.67, 11.89) | 19.31 (16.89, 21.73) | 14.42 (13.02, 15.82) |
| Mean fasting glucosea | 93.01 (92.55, 93.48) | 91.22 (90.63, 91.81) | 94.19 (93.60, 94.79) | 92.97 (92.60, 93.34) |
| % Low HDLa | 15.85 (13.87, 17.83) | 8.96 (7.05, 10.86) | 15.07 (13.05, 17.10) | 14.63 (13.22, 16.04) |
| % Elevated fasting triglycerides | 23.08 (20.72, 25.45) | 8.99 (7.42, 10.57) | 24.89 (22.52, 27.26) | 21.27 (19.62, 22.92) |
| Mean HbA1Cb | 5.12 (5.11, 5.14) | 5.26 (5.23, 5.29) | 5.17 (5.15, 5.19) | 5.15 (5.14, 5.17) |
Weighted percentage; 95% confidence interval.
Weighted mean; 95% confidence interval.
BMI, body mass index; ALT, alanine aminotransferase; HDL, high-density lipoprotein; BP, blood pressure; MetS, metabolic syndrome; HbA1C, glycosylated hemoglobin.
ALT and MetS severity Z-score, BMI Z-score
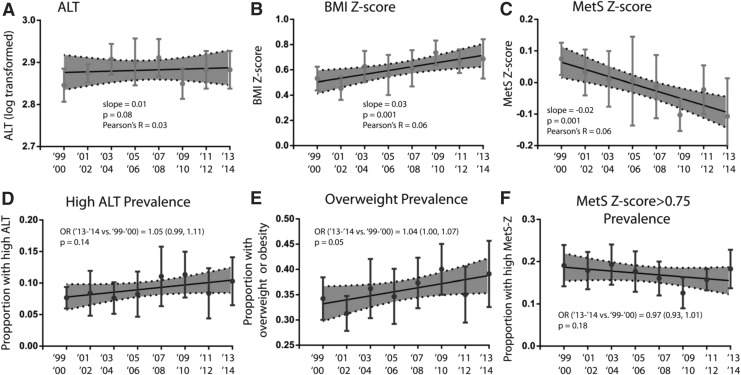
While there appeared to be a gradual increase in ALT over time, this increase was nonsignificant (P = 0.08) (Fig. 1A). There was no change in prevalence of an elevated ALT over time, assessed as odds of higher prevalence in 2013–2014 compared to 1999–2000; this was true using both the cut-off of 30 U/L [OR: 1.05, confidence interval (CI): 0.99–1.12) (Fig. 1D) and the gender-specific cutoff values (OR: 1.04, CI: 0.99–1.10).
FIG. 1.
ALT, BMI-z and MetS severity over time. Data shown reflect mean levels of (A) ALT, (B) BMI Z-score, and (C) MetS Z-score and proportion of adolescents with elevated of (D) ALT, (E) BMI, and (F) MetS Z by NHANES wave weighted to be nationally representative. In (D–F), the odds ratio for the abnormality are provided for last wave vs. first wave. BMI, body mass index; ALT, alanine aminotransferase; MetS, metabolic syndrome.
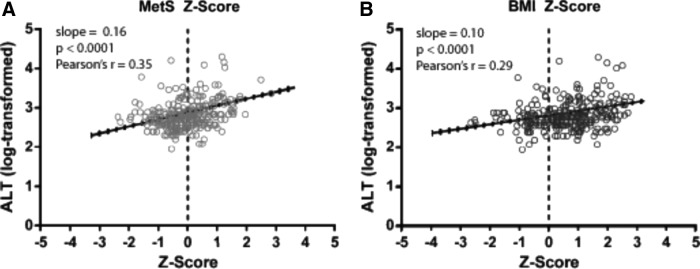
To assess for potential influences on the prevalence of elevated ALT, we next evaluated the correlation of BMI and MetS-Z with ALT levels. ALT was significantly correlated with MetS-Z (r = 0.35, P < 0.0001) (Fig. 2A) and BMI-Z (r = 0.29, P < 0.0001) (Fig. 2B) (Table 2). This relationship appeared to differ by sex and race/ethnicity, seen by a three-way interaction between MetS-Z, race/ethnicity, and gender. Correlations were evaluated by race and gender and remained significant in each subgroup (all P < 0.0001) (Table 2). Non-Hispanic blacks had generally lower correlations between ALT and MetS-Z and BMI-Z, when compared to non-Hispanic whites and Hispanics. Males in each racial/ethnic group appeared to have stronger correlations between ALT and MetS-Z/BMI-Z than females. There were significant correlations between ALT and Z-scores of the individual components of MetS, which included blood pressure, fasting triglycerides, fasting glucose, and HDL (Supplementary Table S1).
FIG. 2.
Linear regression of ALT (log-transformed) with (A) MetS Z score and (B) BMI Z score.
Table 2.
Linear Regression of log-Alanine Aminotransferase (as the Outcome), Modeling Separately as a Function of Metabolic Syndrome-Z and Body Mass Index-Z (as Predictors), Overall and by Race/Ethnicity And Gender
| Predictor: MetS-Z | Predictor: BMI-Z | ||||
|---|---|---|---|---|---|
| n | Slope estimate (CI) | r | Slope estimate (CI) | r | |
| All | |||||
| Overall | 5019 | 0.16 (0.14–0.18)*,a | 0.35 | 0.10 (0.09–0.12)*,a | 0.29 |
| Males | 2635 | 0.18 (0.15–0.20)* | 0.37 | 0.13 (0.11–0.15)* | 0.37 |
| Females | 2384 | 0.10 (0.07–0.12)* | 0.24 | 0.07 (0.05–0.08)* | 0.22 |
| NHW | |||||
| Overall | 1472 | 0.16 (0.13–0.19)* | 0.36 | 0.10 (0.08–0.12)* | 0.30 |
| Males | 781 | 0.17 (0.13–0.21)* | 0.38 | 0.12 (0.10–0.15)* | 0.37 |
| Females | 691 | 0.08 (0.04–0.12)* | 0.02 | 0.07 (0.04–0.09)* | 0.22 |
| NHB | |||||
| Overall | 1532 | 0.11 (0.08–0.14)* | 0.25 | 0.08 (0.06–0.10)* | 0.24 |
| Males | 844 | 0.16 (0.12–0.19)* | 0.35 | 0.12 (0.10–0.15)* | 0.40 |
| Females | 688 | 0.08 (0.04–0.12)* | 0.21 | 0.05 (0.02–0.07)* | 0.17 |
| Hispanic | |||||
| Overall | 2015 | 0.20 (0.16–0.23)* | 0.37 | 0.13 (0.11–0.15)* | 0.32 |
| Males | 1010 | 0.21 (0.16–0.25)* | 0.37 | 0.15 (0.12–0.18)* | 0.38 |
| Females | 1005 | 0.15 (0.11–0.20)* | 0.34 | 0.10 (0.07–0.13)* | 0.29 |
Significant interactions between MetS-Z/BMI-Z and race and gender.
P < 0.001.
CI, confidence interval; NHW, non-Hispanic white; NHB, non-Hispanic black.
In addition, the odds of having ALT levels over 30 U/L were significantly heightened in the presence of either elevated MetS-Z (Z-Score >0.75) or BMI >85th percentile (i.e., overweight) with respective OR of 6.99 and 7.04 (Table 3). Using categories of both elevated MetS-Z and overweight BMI, odds of having elevated ALT were highest in non-Hispanic whites, followed by Hispanic participants and non-Hispanic blacks. Similar findings were found when using gender-specific cutoffs (Table 3). These findings did not change after adjustment for total calorie and saturated fat intake, variables available in NHANES that we found to be associated with ALT (results not shown).
Table 3.
Odds Ratios (95% Confidence Interval) of Having High Alanine Aminotransferase Measures (Using Two Different Cutoffs), for High Metabolic Syndrome-Z (>0.75 vs. ≤0.75) and High Body Mass Index Percentile (>85th vs. ≤85th), Adjusted for Age and Sex
| Elevated ALT (30 U/L cutoff) | Elevated ALT gender-specific cutoff (Girls 22 U/L, Boys 25 U/L) | |||
|---|---|---|---|---|
| MetS-Z > 0.75 | BMI percentile > 85th | MetS-Z > 0.75 | BMI percentile > 85th | |
| Overall | 6.99 (5.00–9.77) | 7.04 (5.40–9.16) | 5.67 (4.4–7.3) | 4.62 (3.62–5.91) |
| Non-Hispanic white | 7.77 (4.61–13.09) | 9.43 (6.17–14.41) | 6.31 (4.30–9.25) | 5.54 (3.89–7.87) |
| Non-Hispanic black | 5.13 (2.76–9.52) | 3.87 (2.10–7.17) | 4.66 (3.21–6.76) | 3.28 (2.20–4.90) |
| Hispanic | 5.52 (3.81–8.00) | 4.83 (3.31–7.04) | 4.62 (3.38–6.30) | 3.70 (2.76–4.96) |
Laboratory values and prevalence of elevated ALT over time
Having established links between ALT and BMI and MetS, we assessed for changes in BMI and MetS over time to evaluate whether these trends may have related to a lack of change in ALT. The mean BMI Z-score increased over time (P = 0.0011) (Fig. 1B) and the mean MetS Z-score decreased over time (P = 0.0013) (Fig. 1C) as reported previously for a different NHANES sample of U.S. adolescents.25 There was a statistically significant increase in the prevalence of elevated BMI-Z from the 1999–2000 to the 2013–2014 NHANES cycles (OR: 1.04, CI: 1.0–1.07) (Fig. 1E); when stratified by race/ethnicity, only Hispanic adolescents displayed this increasing trend over time (OR 1.05, CI: 1.01–1.09). The prevalence of elevated MetS-Z did not differ significantly over time (OR: 0.97, CI: 0.92–1.02) (Fig. 1F).
Discussion
Our data demonstrated that there was no significant linear increase in prevalence of elevated ALT using two different cutoffs in U.S. adolescents over a recent 16-year time frame. This was in some ways surprising, given that, (1) there were higher odds of having elevated ALT in the presence of an overweight BMI (or elevated MetS-Z) as noted previously7,36,37 and (2) the prevalence of overweight in this sample increased over this time frame.25 While not certain, the reason that ALT elevations did not parallel BMI Z-score elevations may have been because of declining MetS severity Z-score over time. Regardless of the cause, we found the lack of increase in elevated ALT reassuring in the setting of the current obesity epidemic.
In assessing the odds of elevated ALT (>30 U/L) in relationship to BMI Z-score and MetS Z-score, we found higher odds if elevated MetS-Z was present (OR 3.52), compared to if elevated BMI was present (OR 2.789); similar findings were found with the gender-specific cutoffs. There is already a common consensus that NAFLD is associated with MetS components,1,4 as well as with chronic diseases associated with MetS, like T2DM, cardiovascular disease, and cardiometabolic abnormalities.1,3,23 Moreover, the dangerous consequences of uncontrolled NAFLD, which include cirrhosis and hepatocellular carcinoma, warrant its early detection and prevention in adolescents.1–3 While BMI has clear merit as an indicator of risk for elevated ALT levels, the MetS severity Z-score in adolescents may have value as a novel tool for identifying patients with risk of NAFLD as well.
The relationship between ALT and MetS raises the question of what led to this trend of declining MetS during this time period in U.S. adolescents. In a prior analysis, this appeared to be due to improvements in lipid abnormalities related to changes in dietary practices during this time period.25 Prior work with a different sample of NHANES adolescents showed that adolescents in this time period engaged in healthier food consumption, including a decrease in consumption of total calorie and carbohydrate consumption, as well as an increase in unsaturated fat consumption.25 During the time frame of this study, there was also an increased push for reduced consumption of unhealthy beverages through efforts like the Dietary Guidelines of America.38 Between 2001 and 2010, an NHANES analysis found a significant decrease in the purchase and consumption of sugar-sweetened soda, whole milk, fruit juice with sugar added, and fruit-flavored drinks.39,40 There was also an increased consumption of healthier drink options, including unsweetened juices and lowfat/nonfat milk.40 Overall, these dietary changes may have contributed to both less MetS and less NAFLD. However, adjustments for diet did not alter our observed associations between MetS and elevated ALT.
In studying the relationship between ALT and MetS and BMI, we found differences that appeared to depend on race/ethnicity and gender. Previous research highlighted similar trends of decreased high ALT prevalence or decreased association with cardiometabolic abnormalities in non-Hispanic blacks, similar to what we found in our analyses.22,37,41 This appears to support a racial difference in ALT levels and the impact of race/ethnicity on measures of obesity and the MetS. Furthermore, other studies have found no difference among races regarding the upper limit normal ALT level.36 So, while non-Hispanic black adolescents appear to have a lesser tendency toward cardiometabolism-related ALT abnormalities, it is still sound clinical advice to screen all obese children for elevated ALT, to provide necessary interventions against fatty liver disease. Regarding gender differences, males appear to have stronger correlations than females for MetS-Z and BMI, generally seen in each racial/ethnic group. This finding is in concordance with established evidence of males having an increased prevalence of fatty liver diseases.5 This also underscores the potential for a gender-specific ALT cutoff to more accurately identify youth at risk for fatty liver.19
The gender-specific cutoffs identify almost twice more adolescents with an elevated ALT. Other studies have shown that ALT values lower than the clinically accepted cutoff of 30 U/L can predict fatty liver disease and identification of NAFLD has a gender-related component.42 Reevaluation of ALT cutoffs and appropriate screening is needed to accurately identify more individuals at risk of chronic liver diseases, especially if lower cutoffs have better sensitivity without compromising the specificity of the screening tool.8,19 ALT levels have been found to decrease with healthier diets;43 so, with earlier identification and intervention, more children may avoid a diagnosis of NAFLD.
This study had several limitations. Because NHANES data were gathered through a cross-sectional study, we are unable to determine causality in the relationships we have described. We lacked consideration of other potentially important factors, including, physical activity, sedentary behavior, and living settings (e.g., rural vs. urban). Furthermore, for the current analyses, we did not focus primarily on factors such as food intake, which have clear importance in the etiology of NAFLD, but are limited in the quality of assessment in large-scale surveys such as this.44,45 However, our sensitivity analysis after adjustment for relevant dietary variables revealed our observed associations between MetS and elevated ALT remained. In the future, more work can be done to understand the mechanism behind obesity, MetS, and their potentially causal effects on ALT levels. This study is also limited in the relatively small sample size, due to limitations of fasting status and individuals with missing primary components of the analysis. However, the fasting weights of the complex survey design enabled us to evaluate these as a nationally representative sample. With regard to exclusion criteria, there may be other factors besides diabetes, pregnancy, or Hepatitis B infection that could account for increases in ALT in this population; however, we believe additional excluded participants were a small portion of our sample and would not significantly affect our results. We assessed relationships between multiple variables, raising the potential for identifying associations based on chance alone (i.e., Type 1 error). Finally, there is still some uncertainty if ALT is the best marker for fatty liver disease, since it does not work as a measure of NAFLD severity and may even be normal in individuals with fatty liver disease.10,12 Given the current markers available for fatty liver disease and the invasive nature of the current gold standard, liver biopsy, ALT still appears to be a useful clinical marker to screen for disease.
Conclusion
We did not observe a statistically significant linear increase in elevated ALT among U.S. adolescents between 1999 and 2014. This finding is potentially related to the divergent effects of worsening obesity and improving MetS in this population. The MetS severity Z-score may be a tool helpful in identifying youth with high ALT levels and possible NAFLD. Continued improvement of diet in this population is advised to improve obesity and related cardiometabolic problems.
Funding
This work was supported by National Institutes of Health grant 1R01HL120960 (M.J.G. and M.D.D.).
Supplementary Material
Author Disclosure Statement
No conflicting financial interests exist.
References
- 1.Dowman JK, Tomlinson JW, Newsome PN. Pathogenesis of non-alcoholic fatty liver disease. QJM 2010;103:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641–649 [DOI] [PubMed] [Google Scholar]
- 3.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, et al. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut 2009;58:1538–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwimmer JB, Pardee PE, Lavine JE, et al. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 2008;118:277–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson EL, Howe LD, Jones HE, et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: A systematic review and meta-analysis. PLoS One 2015;10:e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machado MV, Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J Hepatol 2013;58:1007–1019 [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology 2007;133:1814–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwimmer JB, Newton KP, Awai HI, et al. Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected nonalcoholic fatty liver disease. Aliment Pharmacol Therapeut 2013;38:1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkey CS, Rockett HRH, Willett WC, et al. Milk, dairy fat, dietary calcium, and weight gain —a longitudinal study of adolescents. Arch Pediatr Adolesc Med 2005;159:543–550 [DOI] [PubMed] [Google Scholar]
- 10.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: A longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132–138 [DOI] [PubMed] [Google Scholar]
- 11.Sanyal AJ, Brunt EM, Kleiner DE, et al. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology 2011;54:344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S, Jensen D, Hart J, et al. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int 2013;33:1398–1405 [DOI] [PubMed] [Google Scholar]
- 13.Middleton JP, Wiener RC, Barnes BH, et al. Clinical features of pediatric nonalcoholic fatty liver disease: A need for increased awareness and a consensus for screening. Clin Pediatr (Phila) 2014;53:1318–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007;120:S164–S192 [DOI] [PubMed] [Google Scholar]
- 15.Chan DF, Li AM, Chu WC, et al. Hepatic steatosis in obese Chinese children. Int J Obes Relat Metab Disord 2004;28:1257–1263 [DOI] [PubMed] [Google Scholar]
- 16.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children—ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci 1997;42:1428–1432 [DOI] [PubMed] [Google Scholar]
- 17.Porter SA, Pedley A, Massaro JM, et al. Aminotransferase levels are associated with cardiometabolic risk above and beyond visceral fat and insulin resistance: The Framingham Heart Study. Arterioscler Thromb Vasc Biol 2013;33:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwimmer JB, Deutsch R, Rauch JB, et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr 2003;143:500–505 [DOI] [PubMed] [Google Scholar]
- 19.Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: Alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology 2010;138:1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tazawa Y, Noguchi H, Nishinomiya F, et al. Serum alanine aminotransferase activity in obese children. Acta Paediatr 1997;86:238–241 [DOI] [PubMed] [Google Scholar]
- 21.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388–1393 [DOI] [PubMed] [Google Scholar]
- 22.Deboer MD, Wiener RC, Barnes BH, et al. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics 2013;132:e718–e726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadeau KJ, Klingensmith G, Zeitler P. Type 2 diabetes in children is frequently associated with elevated alanine aminotransferase. J Pediatr Gastroenterol Nutr 2005;41:94–98 [DOI] [PubMed] [Google Scholar]
- 24.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 2005;115:1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee AM, Gurka MJ, DeBoer MD. Trends in metabolic syndrome severity and lifestyle factors among adolescents. Pediatrics 2016;137:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr 2000;136:727–733 [PubMed] [Google Scholar]
- 27.Gurka MJ, Ice CL, Sun SS, et al. A confirmatory factor analysis of the metabolic syndrome in adolescents: An examination of sex and racial/ethnic differences. Cardiovasc Diabetol 2012;11:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurka MJ, Lilly CL, Norman OM, et al. An examination of sex and racial/ethnic differences in the metabolic syndrome among adults: A confirmatory factor analysis and a resulting continuous severity score. Metabolism 2014;63:218–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graham RC, Burke A, Stettler N. Ethnic and sex differences in the association between metabolic syndrome and suspected nonalcoholic fatty liver disease in a nationally representative sample of US adolescents. J Pediatr Gastroenterol Nutr 2009;49:442–449 [DOI] [PubMed] [Google Scholar]
- 30.DeBoer MD. Underdiagnosis of metabolic syndrome in non-hispanic black adolescents: A call for ethnic-specific criteria. Curr Cardiovasc Risk Rep 2010;4:302–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: Considerations for practitioners. Diabetes Metab Syndr Obes 2017;10:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford ES, Li C, Cook S, et al. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007;115:2526–2532 [DOI] [PubMed] [Google Scholar]
- 33.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: Data from the national health and nutrition examination survey 1999–2006. Metab Syndr Relat Disord 2010;8:343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez JR, Redden DT, Pietrobelli A, et al. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444 [DOI] [PubMed] [Google Scholar]
- 35.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576 [PubMed] [Google Scholar]
- 36.Kliethermes S, Ma M, Purtell C, et al. An assessment of racial differences in the upper limits of normal ALT levels in children and the effect of obesity on elevated values. Pediatr Obes 2016. [Epub ahead of print]; DOI: 10.1111/ijpo.12152 [DOI] [PubMed] [Google Scholar]
- 37.Louthan MV, Theriot JA, Zimmerman E, et al. Decreased prevalence of nonalcoholic fatty liver disease in black obese children. J Pediatr Gastroenterol Nutr 2005;41:426–429 [DOI] [PubMed] [Google Scholar]
- 38.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, December 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mesirow MS, Welsh JA. Changing beverage consumption patterns have resulted in fewer liquid calories in the diets of US children: National Health and Nutrition Examination Survey 2001–2010. J Acad Nutr Diet 2015;115:559–566.e4 [DOI] [PubMed] [Google Scholar]
- 40.Piernas C, Ng SW, Popkin B. Trends in purchases and intake of foods and beverages containing caloric and low-calorie sweeteners over the last decade in the United States. Pediatr Obes 2013;8:294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwimmer JB, McGreal N, Deutsch R, et al. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics 2005;115:e561–e565 [DOI] [PubMed] [Google Scholar]
- 42.Saad V, Wicklow B, Wittmeier K, et al. A clinically relevant method to screen for hepatic steatosis in overweight adolescents: A cross sectional study. BMC Pediatr 2015;15:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mager DR, Mazurak V, Rodriguez-Dimitrescu C, et al. A meal high in saturated fat evokes postprandial dyslipemia, hyperinsulinemia, and altered lipoprotein expression in obese children with and without nonalcoholic fatty liver disease. JPEN J Parenter Enteral Nutr 2013;37:517–528 [DOI] [PubMed] [Google Scholar]
- 44.Loprinzi PD, Smit E, Cardinal BJ, et al. Valid and invalid accelerometry data among children and adolescents: Comparison across demographic, behavioral, and biological variables. Am J Health Promot 2014;28:155–158 [DOI] [PubMed] [Google Scholar]
- 45.Collins CE, Watson J, Burrows T. Measuring dietary intake in children and adolescents in the context of overweight and obesity. Int J Obes (Lond) 2010;34:1103–1115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.