Abstract
Culex flavivirus (CxFV) is an insect-specific flavivirus infecting Culex mosquitoes, which are important vectors of West Nile virus (WNV). CxFV and WNV cocirculate in nature and coinfect Culex mosquitoes, including in a WNV “hotspot” in suburban Chicago. We previously identified a positive association between CxFV and WNV in mosquito pools collected from suburban Chicago in 2006. To further investigate this phenomenon, we compared the spatial and temporal distribution of CxFV during an interepidemic year (2011) and an epidemic year (2012) for WNV. Both viruses were more prevalent in mosquito pools in 2012 compared to 2011. During both years, the CxFV infection status of mosquito pools was associated with environmental factors such as habitat type and precipitation frequency rather than coinfection with WNV. These results support the idea that WNV and CxFV are ecologically associated, perhaps because both viruses respond to similar environmental drivers of mosquito populations.
Keywords: : Culex flavivirus, epidemiology, virus ecology, West Nile virus
Introduction
West Nile virus (WNV) is a mosquito-borne flavivirus in the Japanese encephalitis virus serocomplex, which also includes the closely related subtype Kunjin virus, St. Louis encephalitis virus (SLEV), Usutu virus, and others. WNV is maintained in nature in an enzootic transmission cycle between competent mosquito vectors and avian hosts, in particular, passerines (order Passeriformes) (Hayes et al. 2005, Turell et al. 2005, Kilpatrick et al. 2006). Following its emergence in New York State in 1999, WNV spread steadily westward across the continental United States causing epidemic and epizootic disease over the subsequent decade (Hayes et al. 2005, Hayes and Gubler 2006). During 2012, the United States experienced a large WNV outbreak with over 5000 human cases and more than 22,000 positive mosquito pools reported (Beasley et al. 2013, USGS 2015). In contrast, during 2011, there were only ∼700 human cases and fewer than 10,000 positive mosquito pools reported (USGS 2015).
Geographic foci of transmission, or “hotspots,” have been identified for WNV in the United States. One of these is the city of Chicago and its suburbs in Cook County (Illinois) (Bertolotti et al. 2008, Hamer et al. 2008b, 2009). The southwestern suburbs of Chicago have experienced enzootic WNV transmission in all years since it emerged in the region in 2001, and epizootic and epidemic transmission in many years (Bertolotti et al. 2008). Following the same temporal pattern as rest of the United States, Cook County experienced a large WNV outbreak during 2012, with 174 human cases identified and 2766 positive mosquito pools collected, while only 22 human cases and 852 positive mosquito pools were identified in 2011 (USGS 2015).
The primary vectors of WNV are Culex species mosquitoes, including Culex pipiens, the northern house mosquito. In urban and suburban areas of the upper Midwest, including Chicago, C. pipiens is ornithophilic, preferentially feeding on avian hosts, but has the potential to act as a WNV bridge vector between birds and humans (Kilpatrick et al. 2005, Hamer et al. 2008a). Culex mosquitoes can also carry Culex flavivirus (CxFV). CxFV is an insect-specific flavivirus (ISFV) along with cell fusing agent virus, and Calbertado virus, which are related to, but phylogenetically distinct from, encephalitic or hemorrhagic arboviruses such as WNV, SLEV, and dengue viruses (DENV1-4) that also belong to the genus Flavivirus (Moureau et al. 2015). CxFV was first isolated in Culex mosquito populations in Japan in 2007 and has since been identified in mosquitoes globally (Hoshino et al. 2007, Morales-Betoulle et al. 2008, Cook et al. 2009, Kim et al. 2009, Bolling et al. 2011, Newman et al. 2011). Like other ISFVs, CxFV does not infect vertebrate cells and is primarily maintained in mosquito populations through vertical transmission (Hoshino et al. 2007, Bolling et al. 2011, Saiyasombat et al. 2011).
Previously, we identified CxFV in Culex mosquitoes from the Chicago area and also observed a positive association between WNV and CxFV in Culex mosquito pools collected during 2006; WNV-positive mosquito pools were approximately four times more likely to also be CxFV positive than WNV-negative mosquito pools (Newman et al. 2011). Similarly, we identified WNV and CxFV coinfections in 6 of 15 individual Culex mosquitoes collected between 2005 and 2009 (Newman et al. 2011). In this study, we examine the occurrence and co-occurrence of WNV and CxFV in suburban Chicago between 2011 and 2012. Comparing infection patterns during 2 years with very different intensities of WNV transmission offers a unique “natural experiment” for investigating the association between these two viruses in nature. We also describe additional individual Culex species mosquitoes coinfected with CxFV and WNV, which were collected individually from the study site between 2010 and 2012.
Materials and Methods
Field and laboratory methods
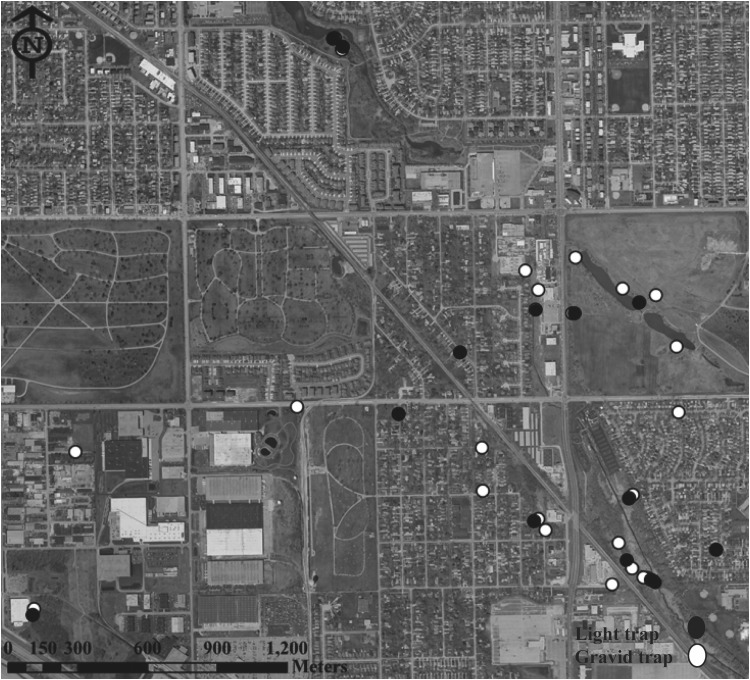
We collected mosquitoes from southwest suburban Chicago (Cook County), using both CO2-baited CDC miniature light traps and gravid traps baited with rabbit pellet infusion water. We deployed traps weekly across the study area at 37 fixed locations between 2011 and 2012 (Fig. 1). Traps included 23 CO2-baited CDC miniature light traps and 14 infusion-baited gravid traps and were grouped for analyses based on habitat type: 15 (5 gravid traps, 10 light traps) in residential neighborhood sites, including yards and commercial properties; and 22 (9 gravid traps, 13 light traps) in urban green space sites, including parks and cemeteries. Mosquitoes were identified and pooled into groups of 50 or fewer by collection date and trap location. Culex mosquito pools (combined C. pipiens and Culex restuans) were collected from the beginning of June through the end of September (MMWR week 23 through MMWR week 39) in 2011 and 2012. Pools were collected from locations in the Village of Oak Lawn, Illinois (41°42′54″N 87°45′12″W), and the Village of Alsip, Illinois (41°40′14″N 87°43′56″W). Mosquito pools were processed for RNA extraction using the MagMAX RNA Isolation kit (Life Technologies, Grand Island, New York) and tested for WNV RNA using real-time RT-PCR as described previously (Hamer et al. 2008b). Extracted RNA was tested for the presence of CxFV using a previously described CxFV-specific PCR (Newman et al. 2011).
FIG. 1.
Map of suburban Chicago study site with 37 locations where traps were deployed in both 2011 and 2012. CO2-baited CDC light traps are shown as white circles, infusion-baited gravid traps are shown as black circles.
Infection rates for WNV and CxFV were calculated using the bias-corrected maximum likelihood estimation method to account for variation in pool sizes in the Microsoft Excel add-in (Microsoft, Inc., Redmond, Washington) Pooled Infection Rate version 4.0 (Biggerstaff 2009). Both CxFV and WNV infection rates were compared weekly between years and trap sites using Wilcoxon signed rank tests for nonparametric datasets and Student's t-tests for parametric datasets. To evaluate the correlation between WNV infection rate and CxFV infection rate at WNV-positive traps during both years, we calculated Pearson's product-moment correlation coefficient. To account for potential bias in the number of pools tested by trap type, we compared numbers of mosquito pools collected from gravid traps with the number collected from light traps using an unpaired t-test. To account for variation in mosquito pool size by trap type, we compared pool sizes collected from gravid traps and pool sizes collected from light traps for both years using unpaired t-tests. All statistical analyses were performed in R version 3.0.3 (R Core Team 2014). In all cases, we considered results statistically significant when p values were less than 0.05.
Average seasonal and weekly nighttime temperatures
Nighttime (1700-0800) temperature data were collected from trap locations between 2011 and 2012 using HOBO data loggers (Onset Computer Corporation: Bourne, Massachusetts). Because data loggers were deployed in full sun locations (and were thus susceptible to solar heat effects) we included only nighttime temperatures in our analyses. In addition, nighttime temperatures correspond with increased periods of host and oviposition seeking activity of Culex mosquitoes (Reddy et al. 2007). Temperatures were averaged weekly from the beginning of June (MMWR week 23) through the end of September (MMWR week 39). Differences in average nighttime temperatures were compared between 2011 and 2012 using a paired t-test.
Average seasonal and weekly precipitation amount and precipitation frequency
Precipitation data were obtained from the nearest National Oceanic and Atmospheric Administration (NOAA) weather station, located at Midway International Airport (KMDW, 41°47′10″N 87°45′09″W) for 2011 and 2012. Total and average weekly precipitation in centimeters were determined for each week in 2011 and 2012 from MMWR week 23 through MMWR week 39. Precipitation frequency was quantified as the number of recorded precipitation events by week from the beginning of June (MMWR week 23) through the end of September (MMWR week 39). Differences in average precipitation were compared between 2011 and 2012 using a Wilcoxon signed-rank test.
Logistic regression
We used logistic regression to evaluate associations between CxFV and WNV status (positive or negative) of mosquito pools and average nighttime temperature and precipitation frequency during the week of collection, habitat type from which a pool was collected, and the trap type. For all models evaluated, we converted CxFV infection status and WNV infection status to dichotomous outcomes (positive = 1, negative = 0). In addition, we included year as a random effect, and we included mosquito pool size as an offset to account for the possible confounding effects of trap type. We based model selection on the minimization of Akaike information criterion (AIC) and the Akaike weight for each model. We evaluated models using the lme4 and AICcmodavg packages (Bates et al. 2015, Mazerolle 2016).
Results
Mosquito collections and virus infection rates
Culex mosquito pool sizes in 2011 and 2012 ranged from 1 to 50 mosquitoes and averaged 8.2 and 9.0 mosquitoes, respectively. There was no significant difference in the number of pools by trap type (t = 1.62, df = 35, p > 0.1). During 2012, mosquito pool sizes collected from gravid traps (average = 14.3) were larger than those collected from light traps (average = 6.3) (t = 6.54, df = 470, p < 0.001). However, there was no such difference in the sizes of pools collected from gravid versus light traps during 2011 (average for gravid traps and light traps were 8.98 and 6.29, respectively; t = 1.09, df = 544, p > 0.1).
Infection rates for both WNV and CxFV at the suburban Chicago study site were higher in 2012 than in 2011 (Table 1). In 2011, the WNV infection rate was 1.1 per 1000 mosquitoes (5 of 546 mosquito pools WNV positive), whereas in 2012, the WNV infection rate was 6.2 per 1000 mosquitoes (25 of 472 mosquito pools WNV positive). CxFV infection rates were approximately two orders of magnitude higher, but followed the same pattern, being 102.1 per 1000 mosquitoes (275 of 546 mosquito pools CxFV positive) in 2011 and 170.2 per 1000 mosquitoes (296 of 472 mosquito pools CxFV positive) in 2012. WNV was identified at 5 of 37 trap locations in 2011 and 16 of 37 trap locations in 2012, while CxFV was identified at all 37 trap locations during both years.
Table 1.
Comparison of Maximum Likelihood-Based Estimates of Culex Flavivirus and West Nile Virus Infection Rates (Shown per 1000 Mosquitoes)
| Virus | 2011 infection rate (95% CI) | 2012 infection rate (95% CI) | Test statistic (V) | p |
|---|---|---|---|---|
| CxFV | 102 (93–113) | 170 (154–189) | 164 | <0.001** |
| WNV | 1.1 (0.4–2.5) | 6.2 (4.2–9.0) | 41 | <0.02* |
MIL-based infection rates are based on all Culex species mosquito pools collected and tested (2011: N = 546, 2012: N = 472) from June through September, using Wilcoxon signed-rank tests between 2011 WNV interepidemic and 2012 WNV epidemic years in suburban Chicago (95% confidence intervals in parentheses).
= < 0.05; ** = < 0.01.
CxFV, Culex flavivirus; WNV, West Nile virus.
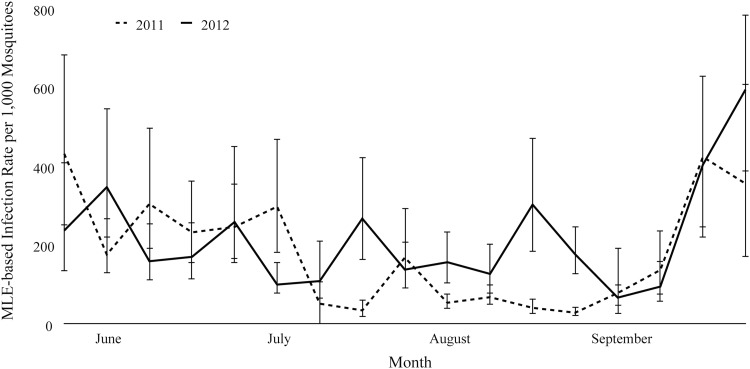
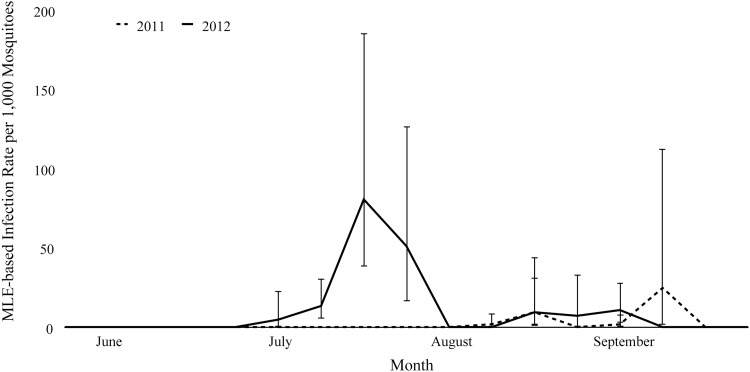
The odds of a mosquito pool testing positive for WNV in 2012 was approximately six times higher than in 2011 (OR: 6.1, 95% confidence interval [CI]: 2.3–15.9, p < 0.001). The CxFV infection rate differed between 2011 and 2012 (V = 164, p < 0.001) and varied from week to week during both years (Fig. 2). In addition, the infection rate of CxFV varied significantly among trap locations (t = 2.75, df = 36, p < 0.01). Similarly, WNV infection rate differed between 2011 and 2012 (V = 41, p < 0.02) and varied from week to week within and between years (Fig. 3). WNV infection rate also varied significantly between individual traps (V = 41, p < 0.05).
FIG. 2.
Maximum likelihood-based estimates of the infection rates of CxFV during each week from June through September in 2011 and 2012. Error bars represent the bias-corrected 95% confidence intervals. CxFV, Culex flavivirus.
FIG. 3.
Maximum likelihood-based estimates of the infection rates of WNV during each week from June through September in 2011 and 2012. Error bars represent the bias-corrected 95% confidence intervals. WNV, West Nile virus.
During 2012, the trap location with the highest WNV infection rate (28 per 1000 mosquitoes, 95% CI: 6–97) also had the highest CxFV infection rate (799 per 1000 mosquitoes, 95% CI: 429–988). During 2011, the trap location with the highest WNV infection rate (29.8 per 1000 mosquitoes, 95% CI: 2–215) had a CxFV infection rate of 359 per 1000 mosquitoes (95% CI: 147–657), which was intermediate. During 2012, WNV-positive trap locations had higher CxFV infection rates than WNV-negative trap locations (t = 2.47, df = 21.5, p < 0.05). During 2011, the CxFV infection rate at WNV-positive trap locations and WNV-negative trap locations did not differ significantly (W = 65, p = 0.52). There was a positive and approximately linear correlation between the WNV infection rate and the CxFV infection rate at WNV-positive traps during 2011 (t = 4.31, df = 3, p = 0.023, R2 = 0.93), but not during 2012 (t = 1.61, df = 14, p = 0.13, R2 = 0.40) when the infection rates of both viruses were higher and more variable over time.
Average seasonal and weekly nighttime temperatures and precipitation
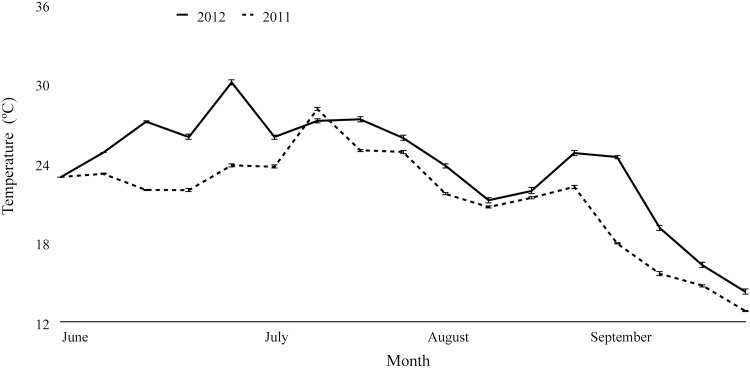
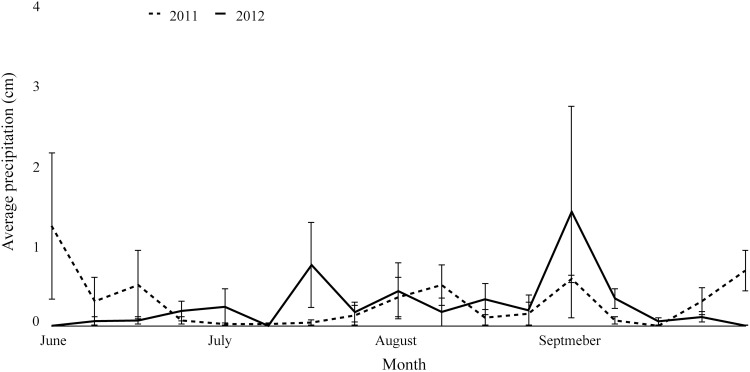
For each week in 2011 and 2012, we collected between 2000 and 10,500 temperature readings. The average nighttime temperature during 2011 (June to September) was 21.3 (±0.96)°C, while the average nighttime temperature during 2012 was 23.8 (±0.99)°C (Fig. 4), and this difference was statistically significant (t = 4.65, df = 16, p < 0.001). We identified 118 precipitation readings from the beginning of June through the end of September in 2011 and 109 precipitation readings for the same period of time in 2012. The average precipitation for June through September was 0.26 (±0.07) centimeters in 2011 and 0.27 (±0.10) centimeters in 2012 (Fig. 5). Average precipitation did not differ significantly between years (V = 74, p > 0.1).
FIG. 4.
Average nighttime temperature measured in degrees Celsius (°C) by week (June to September) in 2011 and 2012, collected from 37 fixed mosquito trap locations at the suburban Chicago study site. Error bars represent ±SEM for each week. Average nighttime temperatures were significantly higher in 2012 than in 2011 (t = 4.65, df = 16, p < 0.0001).
FIG. 5.
Average precipitation (cm) by week from June through September in 2011 and 2012 at Midway Airport (KMDW). Error bars represent ±SEM for each week. Overall, there was no difference in average precipitation between years for the time period examined.
Environmental predictors of CxFV infection status
We evaluated associations between CxFV infection status, WNV infection status, and environmental factors by examining all combinations of five variables (WNV infection status, average nighttime temperature, precipitation frequency, habitat type, and trap type), resulting in 30 competing models (Table 2). All models included year as a random effect and pool size as an offset to account for differences between gravid traps and light traps. The data were best fit (highest Akaike weight) by a model that included nighttime temperature, precipitation frequency, habitat type, and trap type (Table 3). Akaike weights are a conditional probability, representing the relative likelihood of a given model; highest weight corresponds to highest relative likelihood (Wagenmakers and Farrell, 2004). Overall, nighttime temperature had a negative, but not statistically significant, association with the CxFV infection status of a mosquito pool. CxFV infection status was positively associated with precipitation frequency and collection from residential habitats. CxFV infection status was negatively associated with collection from a CDC miniature light trap. Infusion-baited gravid traps are specifically designed to attract Culex mosquitoes, which may partially explain this result. WNV infection status was not associated with the CxFV infection status in either 2011 or 2012.
Table 2.
Candidate Models for Examining the Culex Flavivirus Infection Status of Culex Mosquito Pools Collected During the 2011 Interepidemic and 2012 Epidemic Years at the Suburban Chicago Study Site
| Model | AICc | ΔAIC | Akaike weight |
|---|---|---|---|
| tempb + precipc + habitatd + trap_typee | 1193.39 | 0 | 0.38 |
| WNVa + precip + habitat + trap_type | 1194.29 | 0.9 | 0.24 |
| WNV + temp + precip + habitat + trap_type | 1195.42 | 2.03 | 0.14 |
| habitat + trap_type | 1196.26 | 2.87 | 0.09 |
| temp + habitat + trap_type | 1196.4 | 3.02 | 0.08 |
| WNV + temp + habitat + trap_type | 1198.41 | 5.03 | 0.03 |
| WNV + habitat + trap_type | 1198.27 | 4.88 | 0.03 |
| WNV + temp + precip + trap_type | 1233.1 | 39.71 | 0 |
| WNV + temp + precip + habitat | 1276.53 | 83.14 | 0 |
| WNV + temp + precip | 1308.91 | 115.52 | 0 |
| WNV + temp + habitat | 1277.97 | 84.59 | 0 |
| WNV + precip + habitat | 1275.18 | 81.79 | 0 |
| temp + precip + habitat | 1274.88 | 81.49 | 0 |
| WNV + temp + trap_type | 1234.46 | 41.08 | 0 |
| WNV + precip + trap_type | 1232.09 | 38.7 | 0 |
| temp + precip + trap_type | 1231.09 | 37.7 | 0 |
| WNV + temp | 1309.13 | 115.75 | 0 |
| WNV + precip | 1307.69 | 114.31 | 0 |
| WNV + trap_type | 1234.31 | 40.92 | 0 |
| WNV + habitat | 1277.34 | 83.95 | 0 |
| temp + precip | 1307.49 | 114.1 | 0 |
| temp + trap_type | 1232.49 | 39.1 | 0 |
| temp + habitat | 1276.46 | 83.08 | 0 |
| precip + trap_type | 1230.07 | 36.68 | 0 |
| precip + habitat | 1273.48 | 80.09 | 0 |
| WNV | 1308.57 | 115.18 | 0 |
| Temp | 1307.88 | 114.49 | 0 |
| Precip | 1306.21 | 112.82 | 0 |
| trap_type | 1232.33 | 38.94 | 0 |
| Habitat | 1275.75 | 82.36 | 0 |
Year was included as a random effect and pool size was included as an offset in all models to account for differences in average pool sizes between light and gravid traps. Models are arranged by Akaike weights with the model that has the highest weight listed first.
WNV infection status (positive = 1, negative = 0).
Nighttime temperature.
Precipitation frequency.
Habitat (residential or urban green space).
Gravid or light trap.
Table 3.
Model Parameters for the Top Ranked Models Predicting the Culex Flavivirus Infection Status of Mosquito Pools Collected in 2012 and 2011
| Variable | Estimate | SE | OR | 95% CI | p |
|---|---|---|---|---|---|
| Temp | −0.01 | 0.01 | 0.99 | 0.96–1.01 | 0.34 |
| Precip | 0.14 | 0.06 | 1.15 | 1.02–1.30 | 0.03* |
| habitat (residential) | 1.01 | 0.16 | 2.73 | 1.99–3.75 | <0.0001*** |
| trap_type (light) | −1.51 | 0.17 | 0.22 | 0.16–0.31 | <0.0001*** |
= < 0.05; *** = < 0.001.
CxFV and WNV coinfection of Culex species mosquitoes
In addition to pooled mosquito samples, we also collected and identified 21 individual Culex mosquitoes (species not determined) that were positive for WNV viral RNA (vRNA) between 2010 and 2012, and tested them for coinfection with CxFV. Fourteen of these WNV-positive individual mosquitoes also tested positive for CxFV. Combined with individual Culex mosquitoes previously collected from the area between 2005 and 2009 (Newman et al. 2011), 20 out of 36 mosquitoes tested positive for both viruses between 2005 and 2012.
Discussion/Conclusions
At our study site in suburban Chicago, Culex mosquito pools were six times more likely to test positive for WNV during an epidemic year (2012) than during an interepidemic year (2011). Similarly, CxFV was 7% more prevalent across the study site in 2012 than 2011. Overall, for both years, the CxFV infection status of a mosquito pool was positively associated with precipitation frequency and collection from residential habitats, and negatively associated with collection from a CDC miniature light trap.
Our results suggest that a correlation between CxFV and WNV at WNV-positive traps during 2011 is not directly causal, but rather reflects common environmental drivers. At traps where WNV-positive pools were collected, CxFV infection rate increased approximately linearly with WNV infection rate. This finding is consistent with our previous identification of a positive ecological association between CxFV and WNV in mosquito pools collected in 2006 (Newman et al. 2011). However, our overall finding that CxFV infection status is primarily associated with environmental factors is more consistent with that reported for Culex quinquefasciatus from the Southeastern United States (Kent Crockett et al. 2012). Kent Crockett et al. (2012) found no evidence of an association between CxFV and WNV in C. quinquefasciatus populations from sites in Georgia, Louisiana, and Mississippi during 2009.
We detected CxFV in Culex mosquito pools during all weeks from May through October in 2011 and 2012, and although infection rates varied from week to week across the seasons, differences within a given year were not statistically significant (Fig. 2). In a study of CxFV infection in C. pipiens and Culex tarsalis mosquitoes from Colorado, as well as in another study of CxFV infection in C. quinquefasciatus and C. restuans mosquitoes in East Texas, infection rates appeared to be seasonal (Kim et al. 2009, Bolling et al. 2011). In Colorado, the CxFV infection rates of C. pipiens increased gradually from June to September in 2006, while in 2007, the infection rate was highest in June and decreased gradually in September (Bolling et al. 2011). In Texas, CxFV was detected only during February and March; continued surveillance during warmer periods (April to August) resulted in no detection of CxFV-positive mosquito pools (Kim et al. 2009).
Overall, 2012 was significantly warmer than 2011 (Fig. 4). Temperature is an important factor influencing WNV transmission and is known to affect the extrinsic incubation period (EIP) of WNV in mosquitoes (Dohm et al. 2002a, Reisen et al. 2006, Kilpatrick et al. 2008). For example, warmer temperatures have been associated with a shorter EIP for the WN02 genotype of WNV in Culex species mosquitoes in the United States (Kilpatrick et al. 2008). The warmer weather observed at our study site during 2012 (Fig. 4) may partially explain the increased prevalence of WNV when compared with 2011; however, we did not directly examine the influence of temperature on WNV prevalence in this study. Temperature is also an important factor influencing mosquito abundance (Hayes et al. 2005, Reisen et al. 2010, Chaves et al. 2011). For example, at warmer environmental temperatures, mosquito development may be accelerated and influence temporal abundance (Ewing et al. 2016). However, increasing temperature is also associated with a decreased lifespan in adult Culex mosquitoes (Loetti et al. 2011). The effects of temperature on the maintenance of CxFV in natural populations are not known. However, the apparent decrease in CxFV prevalence in some regions during the summer months suggests that temperature may be influencing CxFV prevalence indirectly through mosquito abundance (Kim et al. 2009). Increases or decreases in mosquito abundance on a fine scale could influence the prevalence of a vertically transmitted virus such as CxFV. Culex abundance may also be influenced by intermittent larval control and application of adulticides (Village of Alsip 2015), which we did not measure.
Higher frequency of precipitation events was positively associated with CxFV infection status (Table 3). Increased precipitation can influence mosquito abundance (Koenraadt and Harrington 2008, Gardner et al. 2012). Low precipitation and high mean daily temperatures were associated with high Culex larval abundance in catch basins at our site during 2010 (Gardner et al. 2012). However, whether this is also observed during a particularly hot year like 2012 is not known (NOAA 2012, 2015). Our finding that CxFV infection status was positively associated with precipitation frequency in 2011 and 2012 may be related to the potential for increased availability of oviposition habitat following rainfall events during hot and dry periods, although we did not measure mosquito productivity in catch basins in this study.
Mosquito pools collected from residential habitats (properties of individual homeowners and businesses) were more likely to be CxFV positive than pools collected from urban green spaces (parks and cemeteries). This finding is consistent with our previous findings (Newman et al. 2011). In this study, pools collected from residential habitats were almost thrice more likely to be infected with CxFV compared to pools collected from urban green spaces in both 2011 and 2012. Warmer and drier conditions, such as those that occurred overall during 2012 (NOAA 2012, 2015), might reduce potential oviposition habitat in urban green spaces, whereas in residential areas, human water usage (swimming pools, planters, and bird baths) might maintain a more consistent breeding habitat. In particular, storm water catch basins are abundant in the residential neighborhoods of the suburban Chicago landscape and are important Culex oviposition sites (Gerry and Holub 1989, Gardner et al. 2012).
Overall, our results suggest that WNV and CxFV respond to similar ecological drivers, such as precipitation frequency and habitat type. Weather patterns and climate variability are known to influence mosquito abundance, competence, and arbovirus infection rates (Reisen et al. 2006, 2010, Vaidyanathan and Scott 2007, Chaves et al. 2011, Wang et al. 2011). These factors have historically been important for explaining differences in mosquito infection rates with WNV between years (Ruiz et al. 2010). Our results suggest that the same may be true for CxFV and our previous finding of an association between CxFV and WNV may be environmentally mediated.
To date, relatively few studies have examined CxFV and WNV coinfection in Culex species mosquitoes or mosquito cell culture, and results have been inconsistent. Kent et al. (2010), found no difference in WNV replication kinetics in Aedes albopictus C6/36 cells or in C. quinquefasciatus sequentially infected with a strain of CxFV, but did identify an increase in WNV transmission in a strain of C. quinquefasciatus when WNV was coinoculated with CxFV. However, CxFV is vertically transmitted and likely precedes WNV infection (Saiyasombat et al. 2011). Conversely, in a study of WNV transmission in a laboratory population of C. pipiens naturally infected with CxFV, WNV dissemination was delayed in coinfected individuals, but no significant difference in transmission was observed (Bolling et al. 2012). In that study, however, the mosquito colonies were from different geographic regions and may have differed in vector competence (Bennett et al. 2002, Vaidyanathan and Scott 2007). In this study, we report a total of 20 CxFV and WNV coinfected Culex species mosquitoes collected from 2005 to 2012 from our field site. These results show that WNV/CxFV coinfection is common in nature, although they do not indicate whether the two viruses infect the same cells or modify infectivity in the mosquito.
Viruses may affect mosquitoes in ways that influence transmission. For example, CxFV is efficiently transovarially transmitted in C. pipiens, whereas WNV is not (Baqar et al. 1993, Dohm et al. 2002b, Goddard et al. 2003, Saiyasombat et al. 2011). The effects of primary CxFV infection on the transovarial transmission of WNV are currently unknown. Similarly, no studies to our knowledge have investigated the potential influence of CxFV infection on mosquito feeding behavior. Infection of Ae. aegypti with another flavivirus, dengue virus, has been shown to influence feeding behavior, potentially reflecting infection of the mosquito nervous system (Platt et al. 1997). We recently found that CxFV infection alters the flight activity of C. pipiens collected from our study site in suburban Chicago by reducing the overall activity (Newman et al. 2016). How this reduction in flight activity influences coinfections with WNV and the results we report in this study is unknown. The potential behavioral effects of CxFV on Culex mosquitoes and the indirect effects on WNV transmission have yet to be examined. Our results suggest that CxFV and WNV respond to similar environmental drivers in nature, but they do not preclude the possibility of virus–virus interaction within mosquitoes.
Acknowledgments
We thank the village of Oak Lawn, IL, for providing laboratory facilities and logistical support. We also thank private landowners in the villages of Oak Lawn and Alsip, IL, and the Archdiocese of Chicago for allowing us to conduct our research. We thank our 2011/2012 field crew members: Diane Gohde, Tim Thompson, Marija Gorinshteyn, Allison Gardner, Shawn Janairo, Carl Hutter, Mike Glester, Patrick Kelly, Charlie Hartley, Amanda Dolinski, Axel Adams, and Nichar Gregory, as well as Rebecca Richman, for assistance with planning, mosquito collections, sample processing, and analytical advice. USDA is an equal opportunity provider and employer. This research was supported by the National Science Foundation/National Institutes of Health Ecology of Infectious Diseases Program under award no. 0840403. C. Newman was provided support through the National Institutes of Health T32 Parasite and Vector Biology Training Program through the Department of Pathobiological Sciences at the University of Wisconsin—Madison.
Author Disclosure Statement
No competing financial interests exist.
References
- Baqar S, Hayes CG, Murphy JR, Watts DM. Vertical transmission of West Nile virus by Culex and Aedes species mosquitoes. Am J Trop Med Hyg 1993; 48:757–762 [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1–8. 2015. Available at http://CRAN.R-project.org/package=lme4
- Beasley DWC, Barrett ADT, Tesh RB. Resurgence of West Nile neurologic disease in the United States in 2012: What happened? What needs to be done? Antiviral Res 2013; 99:1–5 [DOI] [PubMed] [Google Scholar]
- Bennett KE, Olson KE, de Lourdes Muñoz M, Fernandez-Salas I, et al. Variation in vector competence for dengue 2 virus among 24 collections of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg 2002; 67:85–92 [DOI] [PubMed] [Google Scholar]
- Bertolotti L, Kitron UD, Walker ED, Ruiz MO, et al. Fine-scale genetic variation and evolution of West Nile virus in a transmission “hot spot” in suburban Chicago, USA. Virology 2008; 374:381–389 [DOI] [PubMed] [Google Scholar]
- Biggerstaff B. 2009. Pooled infection rate, version 4.0. Division of Vector-borne Infectious Disease, National Center for Infectious Diseases, Centers for Disease Control and Prevention. Available at www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html
- Bolling BG, Eisen L, Moore CG, Blair CD. Insect-specific flaviviruses from Culex mosquitoes in Colorado, with evidence of vertical transmission. Am J Trop Med Hyg 2011; 85:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling BG, Olea-Popelka FJ, Eisen L, Moore CG, et al. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on the vector competence for West Nile virus. Virology 2012; 427:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF, Hamer GL, Walker ED, Brown WM, et al. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance. Ecosphere 2011;2:art70 [Google Scholar]
- Cook S, Moureau G, Harbach RE, Mukwaya L, et al. Isolation of a novel species of flavivirus and a new strain of Culex flavivirus (Flaviviridae) from a natural mosquito population in Uganda. J Gen Virol 2009; 90:2669–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohm DJ, O'Guinn ML, Turell MJ. Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol 2002a; 39:221–225 [DOI] [PubMed] [Google Scholar]
- Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera:Culicidae). J Med Entomol 2002b; 39:640–644 [DOI] [PubMed] [Google Scholar]
- Ewing DA, Cobbold CA, Purse BV, Nunn MA, et al. Modelling the effect of temperature on the seasonal population dynamics of temperate mosquitoes. J Theor Biol 2016; 400:65–79 [DOI] [PubMed] [Google Scholar]
- Gardner AM, Hamer GL, Hines AM, Newman CM, et al. Weather variability affects abundance of larval Culex (Diptera: Culicidae) in storm water catch basins in suburban Chicago. J Med Entomol 2012; 49:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerry PR, Holub RE. Seasonal abundance and control of Culex spp. in catch basins in Illinois. J Am Mosq Control Assoc 1989; 5:537–540 [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vertical transmission of West Nile virus by three California Culex (Diptera: Culicidae) species. J Med Entomol 2003; 40:743–746 [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Brawn JD, Loss SR, et al. Culex pipiens (Diptera: Culicidae): a bridge vector of West Nile virus to humans. J Med Entomol 2008a; 45:125–128 [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kitron UD, Goldberg TL, Brawn JD, et al. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am J Trop Med Hyg 2009; 80:268–278 [PubMed] [Google Scholar]
- Hamer GL, Walker ED, Brawn JD, Loss SR, et al. Rapid amplification of West Nile virus: The role of hatch-year birds. Vector Borne Zoonotic Dis 2008b; 8:57–67 [DOI] [PubMed] [Google Scholar]
- Hayes EB, Gubler DJ. West Nile virus: Epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 2006; 57:181–194 [DOI] [PubMed] [Google Scholar]
- Hayes EB, Komar N, Nasci RS, Montgomery S, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis 2005; 11:1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Isawa H, Tsuda Y, Yano K, et al. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology 2007; 359:405–414 [DOI] [PubMed] [Google Scholar]
- Kent RJ, Crabtree MB, Miller BR. Transmission of West Nile virus by Culex quinquefasciatus Say infected with Culex flavivirus Izabal. PLoS Negl Trop Dis 2010; 4:e671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent Crockett R, Burkhalter K, Mead D, Kelly R, et al. Culex flavivirus and West Nile virus in Culex quinquefasciatus populations in the Southeastern United States. J Med Entomol 2012; 49:165–174 [DOI] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP, et al. Host heterogeneity dominates West Nile virus transmission. Proc Biol Sci 2006; 273:2327–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, et al. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis 2005; 11:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog 2008; 4:e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Guzman H, Bueno R, Jr., Dennett JA, et al. Characterization of Culex flavivirus (Flaviviridae) strains isolated from mosquitoes in the United States and Trinidad. Virology 2009; 386:154–159 [DOI] [PubMed] [Google Scholar]
- Koenraadt CJ, Harrington LC. Flushing effect of rain on container-inhabiting mosquitoes Aedes aegypti and Culex pipiens (Diptera: Culicidae). J Med Entomol 2008; 45:28–35 [DOI] [PubMed] [Google Scholar]
- Loetti V, Schweigmann N, Burroni N. Developmental rates, larval survivorship, and wing length of Culex pipiens (Diptera: Culicidae) at constant temperatures. J Nat Hist 2011; 45:2203–2213 [Google Scholar]
- Mazerolle MJ. 2016. AICcmodavg: Model selection and multimodel inference based on (Q)AICc. R package version 2.1–0. Available at http://CRAN.R-project.org/package=AICcmodavg
- Morales-Betoulle ME, Monzón Pineda ML, Sosa SM, Panella N, et al. Culex flavivirus isolates from mosquitoes in Guatemala. J Med Entomol 2008; 45:1187–1190 [DOI] [PubMed] [Google Scholar]
- Moureau G, Cook S, Lemey P, Nougairede A, et al. New insights into flavivirus evolution, taxonomy and biogeographic history extended by analysis of canonical and alternative coding sequences. PLoS One 2015; 10:e0117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CM, Anderson TK, Goldberg TL. Decreased flight activity in Culex pipiens (Diptera:Culicidae) naturally infected with Culex flavivirus. J Med Entomol 2016; 53:233–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CM, Cerutti F, Anderson TK, Hamer GL, et al. Culex flavivirus and West Nile virus mosquito coinfection and positive ecological association in Chicago, United States. Vector Borne Zoonotic Dis 2011; 11:1099–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOAA National Climate Data Center. State of the climate: National overview for annual 2012. 2012. Available at www.ncdc.noaa.gov/sotc/national/2012/13
- NOAA National Weather Service Forecast Office. Chicago, IL: Annual Climate Report; 2015. Available at http://w2.weather.gov/climate/index.php?wfo=lot [Google Scholar]
- Platt KB, Linthicum KJ, Myint KS, Innis BL, et al. Impact of dengue virus infection on feeding behavior of Aedes aegypti. Am J Trop Med Hyg 1997; 57:119–125 [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2014. Available at www.R-project.org [Google Scholar]
- Reddy MR, Lepore TJ, Pollack RJ, Kiszewski AE, et al. Early evening questing and oviposition activity by the Culex (Diptera: Culicidae) vectors of West Nile virus in northeastern North America. J Med Entomol 2007; 44:211–214 [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol 2006; 43:309–317 [DOI] [PubMed] [Google Scholar]
- Reisen WK, Thiemann T, Barker CM, Lu H, et al. Effects of warm winter temperature on the abundance and gonotrophic activity of Culex (Diptera: Culicidae) in California. J Med Entomol 2010; 47:230–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Chaves LF, Hamer GL, Sun T, et al. Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors 2010; 3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiyasombat R, Bolling BG, Brault AC, Bartholomay LC, et al. Evidence of efficient transovarial transmission of Culex flavivirus by Culex pipiens (Diptera: Culicidae). J Med Entomol 2011; 48:1031–1038 [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O Guinn ML, et al. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol 2005; 42:57–62 [DOI] [PubMed] [Google Scholar]
- US Geological Survey (USGS). Disease Maps Home: West Nile virus, Historical Data. US Department of the Interior 2011, 2012. 2015. Available at http://diseasemaps.usgs.gov/wnv_us_human.html
- Vaidyanathan R, Scott TW. Geographic variation in vector competence for West Nile virus in the Culex pipiens (Diptera: Culicidae) complex in California. Vector Borne Zoonotic Dis 2007; 7:193–198 [DOI] [PubMed] [Google Scholar]
- Village of Alsip. West Nile Virus Information. Village of Alsip, Illinois. 2015. Available at http://villageofalsip.org/wp/west-nile-virus/
- Wagenmakers E, Farrell S. AIC model selection using Akaike weights. Psychon Bull Rev 2004; 11:192–196 [DOI] [PubMed] [Google Scholar]
- Wang J, Ogden NH, Zhu H. The impact of weather conditions of Culex pipiens and Culex restuans (Diptera: Culicidae) abundance: A case study in Peel region. J Med Entomol 2011; 48:468–475 [DOI] [PubMed] [Google Scholar]