Abstract
Wnt-4 is expressed in developing neural and renal tissue and is required for renal tubulogenesis in mouse and Xenopus. The function of Wnt-4 in neural differentiation is unknown so far. Here we demonstrate that Wnt-4 is required for eye development in Xenopus laevis. This effect of Wnt-4 depends on the activation of a β-catenin-independent, noncanonical Wnt signaling pathway. Furthermore, we report the identification of EAF2, a component of the ELL-mediated RNA polymerase II elongation factor complex, as a target gene of Wnt-4 signaling. EAF2 is specifically expressed in the eye and EAF2 expression was dependent on Wnt-4 function. Loss of EAF2 function results in loss of eyes and loss of Wnt-4 function could be rescued by EAF2. In neuralized animal caps, EAF2 has properties characteristic for an RNA polymerase II elongation factor regulating the expression of the eye-specific transcription factor Rx. These data add a new layer of complexity to our understanding of eye development and give further evidence for the importance of noncanonical Wnt pathways in organ development.
Keywords: EAF2, eye development, RNA polymerase II elongation, Wnt-4
Introduction
Specification of cells during development becomes evident by differential gene expression and is regulated by extracellular growth factors. Wnt proteins form a family of secreted glycoproteins which can activate different intracellular signaling pathways. The canonical Wnt/β-catenin pathway involves stabilization of cytoplasmic β-catenin, which can act as a transcriptional coactivator (Logan and Nusse, 2004). Noncanonical Wnt pathways are per definition independent of β-catenin signaling (Kühl et al, 2000a; Veeman et al, 2003). These pathways involve activation of calcium-sensitive enzymes like protein kinase C and calcium calmodulin-dependent kinase II or activation of Jun-N-terminal kinase (JNK) through members of the rho family of small GTPases. This latter pathway is also known as the planar cell polarity pathway, which is involved in Drosophila epithelial cell polarity as well as Drosophila eye development (Mlodzik, 1999). In vertebrates, noncanonical Wnt signaling is activated by Wnt-5A, Wnt-11, and Wnt-4 (Du et al, 1995) and regulates dorso-ventral patterning of the embryo, cell migration, and heart development (Veeman et al, 2003). In Xenopus, mouse, and chicken embryos, Wnt-4 is expressed in distinct expression domains in neural tissues as well as the developing excretory system (McGrew et al, 1992; Parr et al, 1993; Hollyday et al, 1995; Saulnier et al, 2002). Loss-of-function studies have shown that Wnt-4 is required for kidney organogenesis in mouse and Xenopus. Wnt-4 has also been linked to axonal pathfinding in rat embryos (Lyuksyutova et al, 2003). The role for Wnt-4 in regulating gene expression during neural differentiation, however, is poorly defined so far.
The eye derives from the anterior neural tissue, the forebrain. The early eye field is characterized by expression of several marker genes including the homeobox transcription factor Rx and the paired box transcription factor Pax-6. During neurulation this initially continuous eye field is divided, resulting in two lateral expression domains of Rx and Pax-6 by a midline-derived inhibitory signal. Pax-6, Rx, and other transcription factors expressed in the developing eye are thought to constitute a positive autoregulatory feedback loop (Zuber et al, 2003). However, upstream signaling pathways and growth factors that contribute to this feedback loop are not known. In a recent report, the Wnt receptor Xenopus Frizzled-3 (Fz-3) has been implicated in eye development, suggesting that Wnt signaling might play a role during early eye development (Rasmussen et al, 2001). The Wnt ligand activating Fz-3 during eye development, however, is unknown so far.
As the neural functions of Wnt-4 have been poorly investigated to date, we aimed to (i) uncover the potential roles for Wnt-4 in neural patterning and (ii) to identify the downstream factors by which Wnt-4 can mediate its effects. Here, we show that Wnt-4 and its downstream factor EAF2 are required for eye development in Xenopus laevis. Furthermore, our data implicate noncanonical Wnt signaling in early eye development.
Results
Wnt-4 is required for eye development in Xenopus
During Xenopus embryogenesis, Wnt-4 expression starts at the onset of neurogenesis at stage 12.5–13 with two characteristic spots in the anterior brain region that persist until later stages (McGrew et al, 1992; Figure 1A–C). Whole-mount in situ analyses using marker genes specific for the early eye field (Pax-6, Rx) or the midbrain marker Pax-2 indicate that Wnt-4 is expressed immediately adjacent to the early eye field (Figure 1D–F) and marks the forebrain/midbrain boundary. Additionally, Wnt-4 is expressed in the pronephros (Figure 1C) as well as in the floor plate. To uncover a potential role of Wnt-4 during neural development, we persued two independent strategies: We first performed neural specific loss-of-function analyses for Wnt-4 and, second, we aimed to identify genes that are transcriptionally upregulated by Wnt-4 in neuralized animal caps of Xenopus embryos.
Figure 1.
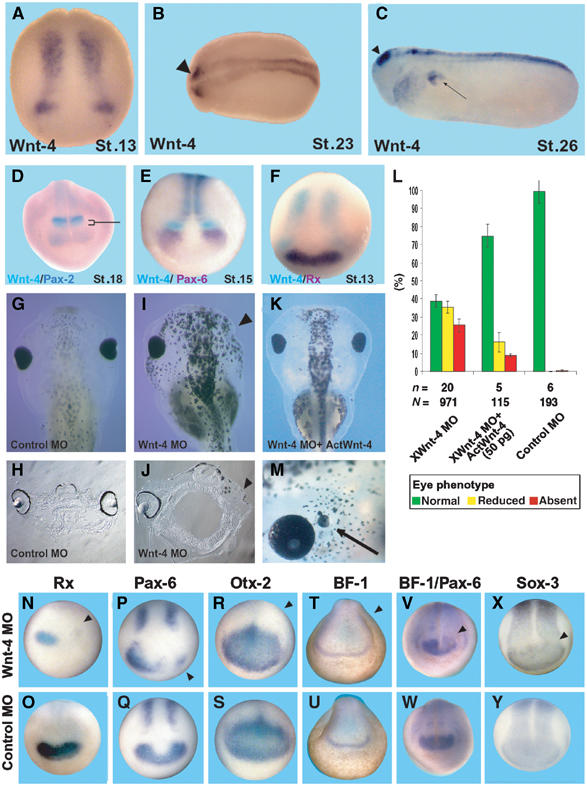
Inhibition of Wnt-4 function by an antisense MO leads to loss of eye structures. (A–C) Wnt-4 is expresssed in neural tissue as monitored by whole-mount in situ hybridization of X. laevis embryos of different stages as indicated. Expression of Wnt-4 in neural tissue starts at stage 13. Two characteristic spots in the anterior neural plate persist until later stages (arrowhead). Arrow in (C): pronephros expression. (D–F) Mapping of the anterior-most neural expression domain of Wnt-4. (D) Double in situ hybridization with the midbrain marker Pax-2, (E) the early eye field marker genes Pax-6 or (F) Rx, indicate that Wnt-4 is expressed at the forebrain–midbrain boundary immediately caudally to the eye field. The bracket in (D) indicates the expression of Wnt-4 in the forebrain region that does not overlap with Pax-2. (G, I) Injection of a Wnt-4 MO, but not a control MO, interferes with eye development as judged by a loss of RPE. (H, J) Sections of those embryos indicate the complete loss of eyes. (K) Co-injection of Wnt-4 MO and an activin–Wnt-4 fusion is able to revert the phenotype in a significant manner. (L) Statistical evaluation of experiments. n=number of independent experiments, N=number of embryos scored. Error bars indicate standard error means. (M) Overexpression of Wnt-4 leads to development of ectopic eye structures in rare cases (Arrow). (N–Y) Wnt-4 MO or control MO unilaterally injected embryos were analyzed by whole-mount in situ hybridization against Rx (N, O), Pax-6 (P, Q), Otx-2 (R, S), BF-1 (T, U), BF-1/Pax-6 simultaneously (V, W) or Sox-3 (X, Y). Loss of Wnt-4 function specifically leads to a loss of Rx and Pax-6 in the early eye field, but not of Otx-2, BF-1, or Sox-3. Arrowheads in (I, J, N, P, R, T, V, X) indicate the injected side of the embryo.
In order to analyze the function of Wnt-4 in neural tissues, we used a characterized Wnt-4 antisense morpholino oligo (Wnt-4 MO) that has previously been shown to interfere with translation of the endogenous Wnt-4 protein (Saulnier et al, 2002) and has been used to study the function of Wnt-4 during pronephros development. The Wnt-4 MO or a control MO was injected unilaterally into one dorsal-animal blastomere at the eight-cell stage to target the presumptive anterior neural region. In all, 61% of Wnt-4 MO- but not control MO-injected embryos showed strongly reduced (35%) or absent eyes (26%) at stage 42 of development (Figure 1G, I, and L), as judged by the size of the retinal pigment epithelium (RPE). This result was confirmed by histological sections (Figure 1H and J). Whereas normally developed eyes have a multilayered structure, this is missing in Wnt-4 MO-treated embryos on the injected side. As a complete loss of RPE is a simple and reliable way to monitor developmental eye defects, we used this phenotype to analyze the outcome of further experiments described below. To rescue the Wnt-4 MO-induced phenotype, we used an Activin-Wnt-4 fusion construct which consists of the activin-derived propeptide and the mature Wnt-4 protein. The Wnt-4 MO does not target RNA coding for this fusion protein and after translation cleavage of the propeptide releases a mature and functional Wnt-4 protein (Saulnier et al, 2002). Coinjecting 50 pg of this RNA with Wnt-4 MO significantly reduced the number of embryos with eye defects on the injected side (Figure 1K and L). In addition, we overexpressed Wnt-4 RNA in dorsal-animal blastomeres of eight-cell stage embryos to analyze Wnt-4 gain-of-function embryos. Most of these embryos displayed defects in gastrulation movements as described earlier by others (Du et al, 1995). However, in rare cases (1–2%) embryos were obtained with ectopic eye structures (Figure 1M) similar to those injected with the Wnt receptor Fz-3 (Rasmussen et al, 2001) or Pax-6 (Chow et al, 1999).
We next characterized the loss-of-function defects in eye development at the molecular level. Unilateral injection of Wnt-4 MO into dorsal-animal blastomeres resulted in a loss of Rx and Pax-6 expression at stages 13/14 on the injected side (Figure 1N–Q). This effect on gene expression was specific for Pax-6 and Rx expression as the fore- and midbrain marker gene Otx-2 or the forebrain marker BF-1 was not affected (Figure 1R–U). Note that Otx-2 is not considered to be an eye marker gene at this stage of development (see Supplementary Figure 1). Pax-6 expression in the spinal cord (Figure 1P and Q) and the expression of the pan-neural marker Sox-3 were not affected (Figure 1X and Y). These data indicate that Wnt-4 is specifically required for eye-specific marker gene expression. At later stages, both the olfactory and the otic placode were established normally (data not shown). In addition, double in situ staining for Pax-6 and BF-1 revealed that a knockdown of Wnt-4 function interfered solely with Pax-6 expression (Figure 1V and W). As a consequence, the possibility that the loss of eyes is due to defects in gastrulation movements can be excluded. Our findings thus clearly establish a specific requirement for Wnt-4 in early eye development of Xenopus.
Wnt-4 exerts its function through a noncanonical Wnt pathway
We next addressed the question of which intracellular signaling pathway is triggered by Wnt-4 during eye development. Wnt-4 has recently been suggested to activate β-catenin-independent, noncanonical Wnt signaling pathways, which can be activated by a deletion mutant of dishevelled (dsh), dshΔDIX (Du et al, 1995; Boutros et al, 1998; Veeman et al, 2003). Coinjection of dshΔDIX RNA and Wnt-4 MO resulted in a significant reversal of the Wnt-4 MO phenotype (Figure 2A, C, and E). Injection of GFP RNA had no effect. In contrast, overexpression of β-catenin RNA can mimic signaling via the canonical Wnt/β-catenin pathway. Neural specific overexpression of β-catenin RNA was unable to rescue the Wnt-4 MO phenotype (Figure 2A, B, and E). As an early overexpression of β-catenin might interfere with aspects of early axial and neural patterning (Kiecker and Niehrs, 2001), we also overexpressed β-catenin later in development by means of plasmid injections. CMV-driven β-catenin overexpression after onset of zygotic transcription (Stage 8) did not rescue the Wnt-4 MO phenotype (Figure 2E). We therefore conclude that Wnt-4 signals through β-catenin-independent signaling pathways.
Figure 2.
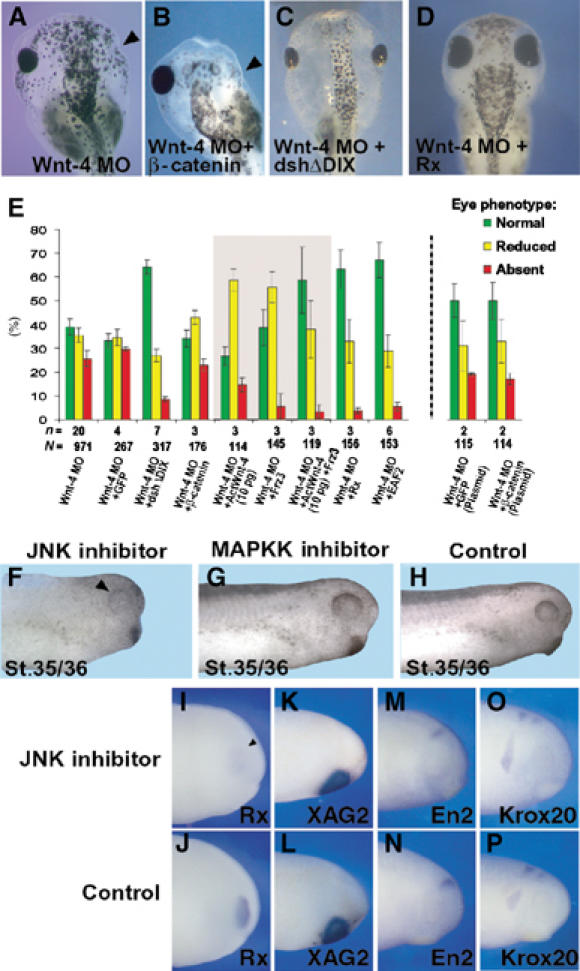
The effect of Wnt-4 is mediated through a β-catenin-independent noncanonical Wnt pathway. (A–C) Injection of dshΔDIX (C), but not β-catenin RNA (B), rescues the eye phenotype of Wnt-4 MO (A)-injected embryos. (D) Injection of Rx RNA rescues the eye phenotype of Wnt-4 MO. Arrowheads indicate missing eyes. (E) To quantify the rescue experiments, embryos were characterized according to the phenotype observed as normal, reduced eye or absent eye. Mean values of n experiments are given. N=number of embryos scored. Error bars indicate standard error means. For the β-catenin DNA rescue experiments, an independent set of Wnt-4 MO embryos is given for comparison. (F–H) Treatment of embryos with the JNK inhibitor SP600125 from stage 11 to 22 results in strongly reduced RPE (arrowhead) at stages 35/36. (G, H) Treatment with the MAPKK inhibitor PD98059 does not affect eye development. (I–P) Marker gene analyses of JNK inhibitor-treated embryos by in situ hybridization (stage 24) using different marker genes as indicated.
Recently, Wnt-4 has been shown to activate JNK (Cai et al, 2002) as does dshΔDIX (Boutros et al, 1998). In support of a role for JNK activity in Wnt-4 induced noncanonical signaling, treatment of Xenopus embryos with the JNK inhibitor SP600125, but not an inhibitor against MAPKK, during stages of early eye development (St.11–22) resulted in a small eye phenotype at stage 30 (Figure 2F–H). A molecular analysis of this phenotype revealed a downregulation of Rx expression at stage 24, whereas XAG2, a cement gland marker, En-2, a marker for the midbrain–hindbrain border, as well as Krox-20, a hindbrain marker, remained unchanged (Figure 2I–P). Thus, these data indicate that Wnt-4 regulates eye development in Xenopus through a noncanonical Wnt pathway and implicate JNK to be involved in this pathway.
As Fz-3 has been implicated in eye development and in noncanonical Wnt signaling (Rasmussen et al, 2001), we tested whether Fz-3 RNA is able to rescue the Wnt-4 MO phenotype. Indeed, a low amount of Fz-3 RNA (10 pg) reduced the number of eyeless embryos but did not reduce the overall occurrence of an eye phenotype. Interestingly, injection of a low dose of Act-Wnt-4 RNA (10 pg) had also only a modest rescuing activity. However, coinjection of Fz-3 and low dose of Act-Wnt-4 RNA (10 pg) synergistically rescued the Wnt-4 MO phenotype (Figure 2E). Taken together, these data implicate Fz-3 in Wnt-4-mediated signaling.
Finally, we tested the ability of Rx as a potential downstream gene to rescue the Wnt-4 MO phenotype. Therefore, we injected Rx RNA together with Wnt-4 MO and monitored eye development. Interestingly, injection of Rx RNA was able to significantly revert the Wnt-4 MO phenotype in Xenopus (Figure 2A, D, and E).
Identification of Wnt-4 downstream genes
We performed a PCR-mediated subtractive cDNA screen (Figure 3) using the animal cap assay to elucidate in an alternative approach the role of Wnt-4 in neural patterning and to identify potential Wnt-4 downstream genes in neural tissue. Animal caps were neuralized by injecting noggin RNA into two-cell stage Xenopus embryos. FGF was subsequently added to neuralized animal cap cultures, resulting in caps expressing anterior as well as posterior neural marker genes. These noggin/FGF-treated caps define the default state for the screen and we asked which genes are upregulated by overexpressing Wnt-4 by RNA coinjection into two-cell stage embryos. A subtractive cDNA library was constructed from treated animal caps when controls reached stage 22. This procedure results in a cDNA library consisting of cDNA clones likely to be enriched in the noggin/FGF/Wnt-4 pool versus the noggin/FGF pool. To eliminate false-positive clones, inserts of 600 clones were PCR amplified, spotted onto nylon membranes and subsequently hybridized with radioactively labeled cDNA probes derived from both RNA pools. Using this experimental approach, we identified 44 genes that were more highly expressed after treatment with Wnt-4. To narrow down the number of genes of potential interest, these clones were sequenced and their spatial expression patterns were determined by in situ hybridization techniques using Xenopus embryos at different developmental stages. A list of genes upregulated by Wnt-4 treatment in FGF/noggin-treated animal caps is given in Supplementary Table 1.
Figure 3.
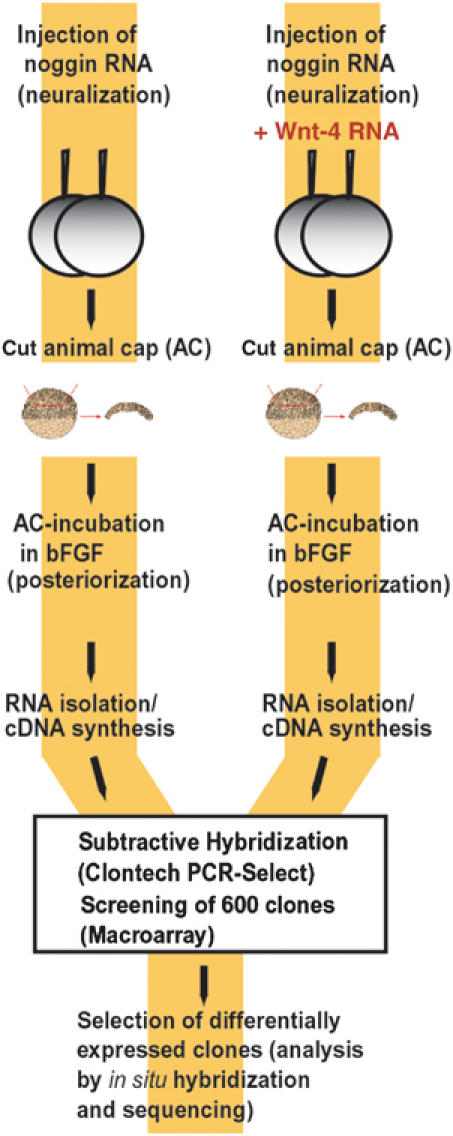
Identification of Wnt-4 target genes by subtractive cDNA cloning. Schematic drawing of the subtractive cDNA screen. Animal caps were treated either with noggin and FGF (left branch) or with noggin, FGF, and Wnt-4 (right branch) as indicated. RNA was isolated from these caps and enriched for those RNA species to be upregulated by Wnt-4 following the Clontech protocol. Of those clones obtained, 600 were spotted as a macroarray and rehybridized with radioactively labeled cDNA pools of both samples. Verified clones were sequenced and the spatio-temporal expression was determined by whole-mount in situ hybridization. See Supplementary Table for a list of identified genes.
EAF2 is a Wnt-4 downstream factor
One of the potential downstream genes identified in the screen is the Xenopus homolog of EAF2 (Figure 4A), which is an ELL-associated factor and part of the ELL-mediated RNA polymerase II elongation factor complex (Li et al, 2003; Simone et al, 2003). In Xenopus, EAF2 is expressed maternally in a ubiquitous manner (data not shown). Tissue-specific, zygotic expression of EAF2 starts at stage 18.5 in the developing eye and the posterior neural plate (Figure 4B). Additional specific expression domains include the pronephric tubule anlage and, at lower levels, the somites and the epiphysis (Figure 4C and H). The lens does not express EAF2 (Figure 4D). Parasagittal sections indicate that the expression of EAF2 in the eye is immediately adjacent to the expression of Wnt-4 in the posterior diencephalon at stage 18.5 (data not shown). In both cases, eye and pronephros, Wnt-4 expression timely precedes that of EAF2 (see Figure 4E–H for pronephros expression; compare Figures 4B and 1A for anterior neural expression).
Figure 4.
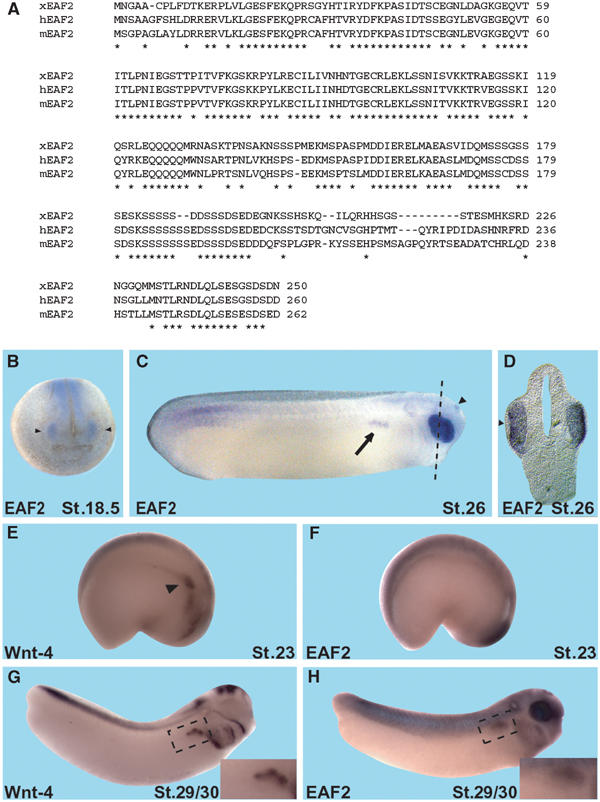
Sequence and expression of Xenopus EAF2. (A) Xenopus EAF2 (xEAF2, GenBank Acc. # AJ579576) codes for a 250 amino-acid protein with homologs in humans (hEAF2, GenBank Acc. # NP_060926) and mouse (mEAF2, GenBank Acc. # AAK59701). The amino-acid identity between xEAF2 and hEAF2 and mEAF2 was calculated as 64 and 63%, respectively. Asterisks indicate conserved amino acids. (B) During Xenopus embryogenesis, EAF2 is expressed in the developing eye (arrowheads) as early as stage 18.5. (C) At stage 26, EAF2 expression is also detected in the pronephric tubule anlage (arrow) and faintly in the epiphysis (arrowhead). Dotted line indicates level of section shown in (D). (D) Transverse sections indicate that EAF2 is not expressed in the lens (arrowhead). (E–H) Wnt-4 and EAF2 are co-expressed in the pronephric anlage and in developing pronephric tubules. Wnt-4 (E, G) and EAF2 (F, H) transcripts were detected by whole-mount in situ hybridization. Embryos are shown in lateral views with anterior to the right and dorsal to the top. Insets show enlargements of the pronephric region. Wnt-4 expression is present in the pronephric anlage (E, arrowhead) of a stage 23 embryo, whereas EAF2 expression is not yet detected at this stage (F). At stage 29/30, both Wnt-4 (G) and EAF2 (H) are expressed in the pronephric tubule anlage.
If EAF2 is indeed regulated by Wnt-4 in vivo, a loss of Wnt-4 function should result in a reduced or absent expression of EAF2. In Wnt-4 MO-injected embryos, EAF2 gene expression is significantly downregulated, as determined by semiquantitative RT–PCR analyses (Figure 5A). Targeted injections of Wnt-4 MO but not a control MO into single blastomeres, which contribute primarily to anterior neural tissue or the pronephros, indicate that expression of EAF2 in both organs depends on Wnt-4 (Figure 5B–E). In addition, inhibiting JNK function by SP600125 treatment from stage 11 to 22 resulted in downregulation of EAF2 expression (Figure 5F–H). We subsequently addressed the question whether EAF2 might be a direct target gene of noncanonical Wnt signaling. Due to the lack of purified Wnt-4, we made use of characterized inducible Frizzled receptors encompassing extracellular and transmembrane domains of the β2 adrenergic receptor (β2AR) and intracellular loops of Frizzled receptors (Figure 5I). These receptors can be stimulated or inhibited by β-adrenergic agonist (isoproterenol) or antagonists (propranolol), respectively. The β2AR/Rfz-1 chimeric receptor couples to the Wnt/β-catenin pathway, whereas the β2AR/Rfz-2 chimeric receptor couples to noncanonical Wnt signaling (Liu et al, 1999, 2001; Kühl et al, 2000b). These receptors were stably transfected into human embryonic kidney cells (HEK293), challenged with isoproterenol or propranolol, and the expression of human EAF2 was monitored by RT–PCR. Interestingly, hEAF2 was induced by the activated β2AR/Rfz-2, but not by the β2AR/Rfz-1 chimeric receptor (Figure 5J). Upregulation of EAF2 transcription was observed as early as 30 min after stimulation of the β2AR/Rfz-2 receptor (Figure 5K). This activation was resistent to treatment with cycloheximide, an inhibitor of eukaryotic translation. This observation is consistent with the idea that EAF2 upregulation might be a direct response in the choosen cell line upon cell stimulation. In summary, we have identified EAF2 as a gene regulated by Wnt-4 activity in explant cultures and during embryonic organogenesis.
Figure 5.
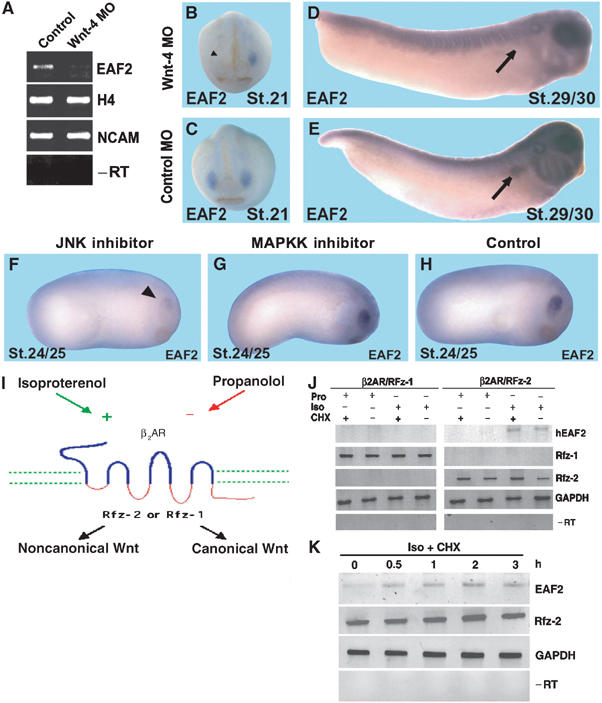
EAF2 is a Wnt-4 downstream factor. (A) RT–PCR analysis demonstrate that EAF2 gene expression is downregulated in Wnt-4 loss-of-function embryos, confirming the results obtained in the Wnt-4 gain-of-function screen. (B–E) Targeted injection of the Wnt-4 MO (B, D), but not a control MO (C, E), into blastomeres that will give rise to anterior neural tissue (B, C) or the pronephric kidneys (D, E), respectively, indicate that expression of EAF2 in these tissues depends on Wnt-4 gene function at stage 21 or stage 29/30, respectively. Arrowhead in (B) indicates missing expression of EAF2 in the eye anlage after Wnt-4 MO injection. Arrows in (D) and (E) highlight the pronephric anlage. (F–H) Treatment of embryos with the JNK inhibitor, but not the MAPKK inhibitor, from stage 11 to 22 leads to a downregulation of EAF2 expression in the eye at stage 24/25. (I) Schematic drawing of inducible Frizzled receptors and their signaling specificity as previously reported. (J) HEK293 cells were stably transfected with inducible β2AR/Rfz-1 or β2AR/Rfz-2 receptors, as indicated and verified by RT–PCR for Rfz-1 or Rfz-2 intrarcellular loops. Activation of β2AR/Rfz-2, but not β2AR/Rfz-1, by the β2-adrenergic agonist isoproterenol (Iso), but not the antagonist propranolol (Pro), leads to activation of EAF2 gene expression, as monitored by RT–PCR within 4 h of treatment. Activation is resistant to treatment with cycloheximide (CHX). GAPDH served as loading control. −RT: negative control. (K) Time course of EAF2 activation after stimulation as indicated.
EAF2 is required for eye development in X. laevis
Recently, it has been demonstrated that RNA polymerase II elongation factors like ELL, Foggy/Spt5, or Pandora/Spt6 play important roles during development (Guo et al, 2000; Eissenberg et al, 2002; Keegan et al, 2002). We therefore asked whether EAF2 is required for eye development. We first tested whether an EAF2 MO inhibits translation of EAF2 RNA (5′UTR+EAF2) in an in vitro transcription and translation assay (Figure 6A). The EAF2 MO clearly blocked translation of EAF2 RNA, whereas a control MO had no effect. An EAF2 control RNA construct lacking the 5′-UTR (Δ5′UTR+EAF2) was not targeted by the EAF2 MO and was therefore used for rescue experiments. We next injected the EAF2 MO into those blastomeres that will give rise to the eye region. Unilateral injection of EAF2 MO results in 50% of embryos with reduced or absent eyes on the injected side. Loss of complete eye structures was observed in 24% of the embryos (Figure 6B–D) as judged by loss of RPE. The loss-of-eye phenotype could be rescued by coinjection of an EAF2 construct (Δ5′UTR+EAF2) that is not a target for the EAF2 MO, whereas injection of RNA coding for GFP had no effect (Figure 6D). Molecular analysis indicated that EAF2 loss-of-function embryos display a loss of Rx, Pax-6, and Otx-2 expression in the eye region consistent with the morphological phenotype (Figure 6E–J). As EAF2 expression in the eye starts at stage 18.5, we also analyzed expression of marker genes at earlier stages of development (St.13/14). As expected, we did not observe an effect on marker gene expression at this stage, implicating that EAF2 is involved in maintenance of eye-specific marker gene expression. Interestingly, overexpression of EAF2 does not result in enlarged or ectopic eye structures (data not shown), implicating a permissive rather than an instructive function of EAF2 during eye development. Taken together, our data clearly indicate that EAF2 function is required for eye development and highlight an essential function of EAF2 during embryonic development. As EAF2 expression in the eye depends on Wnt-4 function and both, Wnt-4 MO and EAF2 MO injections result in the same phenotype, we next analyzed whether EAF2 RNA injection is sufficient to rescue the Wnt4 MO phenotype. Coinjection experiments clearly indicate that EAF2 can revert the effect elicited by Wnt-4 MO (Figure 2E), indicating that both might act through a common downstream target.
Figure 6.
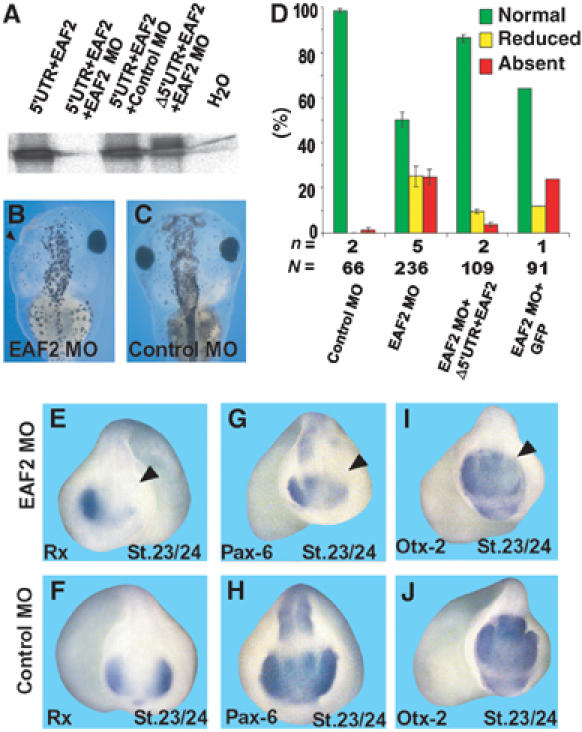
EAF2 is required for eye development. (A) The EAF2 MO used inhibits translation of EAF2 RNA (5′UTR+EAF2), whereas the control MO does not. An EAF2 construct lacking the 5′UTR (Δ5′UTR+EAF2) is not targeted by the MO. Data of transcription and translation assays, resulting in production of 35S-labeled proteins, are shown. (B, C) Unilateral injection of EAF2 MO, but not control MO, into dorsal-animal blastomeres of eight-cell stage embryos results in loss of eyes, as indicated by a loss of RPE (arrow head). (D) Statistical evaluation of phenotypes observed. n=number of independent experiments, N=number of embryos scored. Error bars indicate standard error means. (E–J) Marker gene analysis for Rx, Pax-6 or Otx-2 expression, as indicated by whole-mount in situ hybridization of unilaterally EAF2 MO-injected embryos (E, G, I) or control MO-injected embryos (F, H, J). All the three marker genes are downregulated in the developing eye. The arrowheads in panels (E, G, I) indicate the injected sides of the embryos.
EAF2 functions as an RNA polymerase II elongation factor
We next explored whether EAF2 indeed functions as a component of an RNA polymerase II elongation complex. To address this point, we adopted an established RNA polymerase II elongation assay to the Xenopus animal cap system (Guo et al, 2000). We focused these experiments on the regulation of Rx transcription as Rx was able to rescue Wnt-4 MO-injected embryos and Rx expression depends on Wnt-4, EAF2, and JNK. Due to RNA polymerase II pausing and template escape during transcription, a population of RNA transcripts is generated from the Rx gene locus that differs in RNA length in noggin-neuralized animal caps. This can be shown by RT–PCR using primers specific for different regions of the generated RNAs (Figure 7A), that is, the signal for a primer pair proximal to the transcription start site is stronger than the signal for more distal primer pairs although all of them show comparable signals on a DNA template. Overexpression of EAF2 in noggin-neuralized caps led to an increase in signal strength for the distal primer, Rx(d) (Figure 7B). The signal for the proximal primer pair Rx(p) remained unchanged, indicating that EAF2 indeed increases the number of Rx full-length transcripts and thus likely functions as an RNA polymerase II elongation factor. This effect on Rx transcription was not observed when GFP RNA was overexpressed instead of EAF2 RNA. We also did not observe an effect of EAF2 on Pax-6 transcription (Figure 7B). This effect of EAF2 on Rx expression thus might explain why EAF2 is able to rescue the Wnt-4 MO phenotpype.
Figure 7.
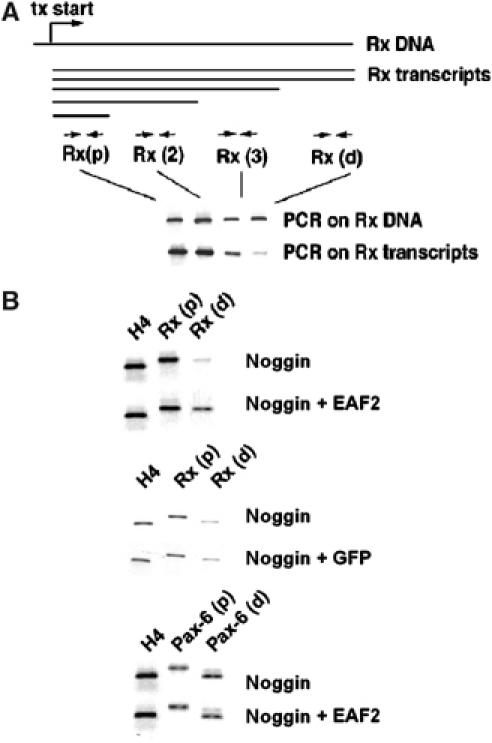
EAF2 has properties of an RNA polymerase II elongation factor. (A) During transcription, RNA transcripts of different lengths are generated due to pausing and template escape, as indicated in the schematic drawing. Relative occurrence of Rx transcripts of different lengths can be monitored by RT–PCR in noggin-neuralized animal caps using different RT–PCR primer sets (Rx (p), Rx (2), Rx(3), Rx(d)). Tx: transcription start site. (B) Overexpression of EAF2, but not GFP RNA, in noggin-neuralized animal caps leads to an increase in full-length transcripts as monitored by the distal Rx primer, Rx(d). H4 served as loading control. Transcription of Pax-6 was not affected by EAF2 overexpression.
Discussion
Detailed studies of transcription factors that are expressed at early stages of eye specification and morphogenesis of the eye have given us great insight into the molecular steps required for eye development (Chow and Lang, 2001). However, the molecular nature of growth factors involved in early eye development has remained largely unknown so far. Here, we report that Wnt-4 is required for early steps of eye development in X. laevis. This finding is based on loss-of-function experiments with subsequent analyses of marker gene expression. Furthermore, our gain-of-function studies led to the identification of a novel Wnt-4 downstream factor, EAF2, which is specifically expressed in the developing eye and which is required for eye development. In this context, Wnt-4 acts through a noncanonical Wnt pathway that is independent of β-catenin but involves activation of JNK.
XWnt-4 is required for Xenopus eye development
Several transcription factors are known to pattern the anterior neural plate of vertebrate embryos and function in eye development (Zuber et al, 2003). These transcription factors regulate each other's expression and form a network controlling specification of eye fate. Expression of these genes occurs after neural induction, but growth factors that initiate their expression or contribute to maintenance of expression are presently unknown. A similar network of transcription factors is involved in specification of eye fate in Drosophila, where signaling by EGFR, Notch, Hedgehog, and Wingless has been implicated in eye specification (Royet and Finkelstein, 1997). We have shown here that Wnt-4, a vertebrate member of the Wnt/wingless family of secreted glycoproteins, is required for eye development in a vertebrate embryo, X. laevis.
Based on several observations, our experiments suggest that Wnt-4 is required for maintenance rather than initial induction of eye-specific marker genes. First, expression of Pax-6 or Rx in the anterior neural plate starts during gastrulation at stage 11–12 and is strongly upregulated at stage 12.5 (Zuber et al, 2003). However, neural Wnt-4 expression is not detectable until stage 12.5–13. Thus, the initial expression of eye marker genes precedes the expression of Wnt-4. Second, overexpression of noggin in animal caps was sufficient to induce Pax-6 and Rx in the absence of Wnt-4 expression, further supporting the notion that Wnt-4 is not required for Pax-6 or Rx induction (data not shown). However, we observed a loss of Pax-6 and Rx expression in the prospective eye field as early as stage 13 in Wnt-4 MO-injected embryos, clearly indicating that Wnt-4 function is required at this stage and thus for maintenance of marker gene expression. In rescue experiments, we were able to show that Rx is able to rescue the Wnt-4 MO phenotype, whereas initial experiments suggest that Pax-6 is unable to revert the Wnt-4 MO phenotype. Indeed, previous publications placed Rx upstream of Pax-6 in eye development (Mathers et al, 1997; Zhang et al, 2000; Zuber et al, 2003). Rx−/− mice do not develop an optic vesicle and lack expression of Pax-6 and Six-3, whereas Pax-6−/− mice develop an optic vesicle, which is, however, abnormally small and malformed (small eye phenotype). Our data implicate Rx as a target for Wnt-4 action, which will require analyses of the Xenopus Rx promoter. Experiments aimed at analyzing the Xenopus Rx promotor are currently underway.
The effect of Wnt-4 on eye development might be mediated by binding to the Wnt receptor Fz-3. Fz-3 is expressed in the diencephalon and loss-of-function experiments reveal the requirement of Fz-3 for eye development and eye-specific marker gene expression (Rasmussen et al, 2001). Just recently, Fz-3 has been shown to be functionally coupled to Wnt-4 during axonal pathfinding in the rat neural tube (Lyuksyutova et al, 2003). In addition, it has been suggested that Fz-3 is able to activate a noncanonical Wnt pathway (Kühl et al, 2000a, 2000b; Rasmussen et al, 2001). This is consistent with our observation described above that Wnt-4 signals through noncanonical Wnt pathways during eye development and that low doses of Wnt-4 and Fz-3 synergistically rescue the Wnt-4 MO phenotype.
Strikingly, Wnt-4 knockout mice were reported to lack any obvious eye phenotype (Stark et al, 1994). It needs to be noted though that other Wnt members are coexpressed with Wnt-4 in the posterior diencephalon in mice (Parr et al, 1993), but not Xenopus (Wolda and Moon, 1992), including Wnt-7B. This Wnt member was reported not to fall into the class of Wnt proteins that act through β-catenin (Naylor et al, 2000). Accordingly, Wnt-7B was mentioned to activate a noncanonical Wnt signaling pathway (Rosso et al, 2005). Therefore, Wnt-7B may compensate for the loss of Wnt-4 in Wnt-4−/− mice.
Wnt-4 signals through a noncanonical Wnt pathway
Wnt-4 has recently been shown to act through activation of JNK (Cai et al, 2002) and thus through the Wnt/JNK pathway. Here we provide further evidence for the existence of a Wnt-4-triggered Wnt/JNK signaling pathway as a deletion mutant of dsh, dshΔDIX, that has been shown to activate noncanonical Wnt, and JNK signaling is sufficient to rescue the Wnt-4 MO phenotype. In addition, treatment of embryos with the JNK inhibitor SP600125 during stages of early eye development resulted in eye defects. Thus, a decrease in JNK signaling activity can phenocopy a Wnt-4 loss-of-function situation and activation of JNK signaling can revert the Wnt-4 MO phenotype. Taken together, these findings strongly indicate that Wnt-4 signals through JNK during eye development. This observation is further supported by the finding that JNK1−/−, JNK2+/− mutant mice also display an eye phenotype with reduced Pax-6 expression although during later stages of eye development (Weston et al, 2003). In parallel, we were able to exclude a role for Wnt/β-catenin signaling during these early stages of eye development, as coinjection of Wnt-4 MO and β-catenin RNA or DNA did not rescue the eye-specific phenotype. Further supporting our conclusion, Wnt-4 did not posteriorize neuralized animal caps, whereas Wnt-3A and β-catenin did (data not shown). This, however, does not exclude the possibility that Wnt/β-catenin signaling might act at later stages of eye development, that is, lens development, as recently suggested (Stump et al, 2003).
Noncanonical Wnt signaling has been shown to regulate cell migration processes, that is, during gastrulation or migration of neuronal precursor cells (Veeman et al, 2003). These processes involve the transmembrane proteins strabismus or prickle to be part of a noncanonical Wnt signaling pathway resembling the Drosophila planar cell polarity pathway. However, when we injected a strabismus MO (Darken et al, 2002) into animal neural blastomeres, we never observed the loss of eye phenotype which is characteristic for the Wnt-4 MO (data not shown). In addition, our marker gene analyses also indicate that Wnt-4 MO injection into dorsal-animal blastomeres does not interfere with cell movements. In summary, we conclude that the effect of neural Wnt-4 MO injections is on differentiation rather than cell migration.
Wnt-4 as a long-range signaling molecule
Wnt-4 is expressed at the forebrain/midbrain boundary and its expression does not overlap with the primary eye field, raising the question how Wnt-4 mediates its effect on eye development. As Wnts are lipid modified and as they strongly interact with proteoglycans (Logan and Nusse, 2004), it is widely assumed that they act as short-range signaling molecules. However, recent findings also argue for a long-range signaling activity of Wnts. Recently, it has been shown that Wnt-3A can induce β-catenin signaling with properties consistent as a long-range signaling molecule (Kiecker and Niehrs, 2001) during anterior–posterior patterning of the neural tube. During inner ear development, Wnt-7A is also able to signal over a long distance (Dabdoub et al, 2003). Patterning of the somites occurs by Wnt proteins derived from the adjacent dorsal neural tube as well as the ectoderm at least in part through noncanonical Wnt signaling (Chen et al, 2004). Wnt-4 has also been implicated as a long-range signaling molecule during anterior–posterior guidance of commissural axons in rat embryos (Lyuksyutova et al, 2003). Consistent with this hypothesis, the Drosophila homolog wingless also can signal over a long distance (Zecca et al, 1996; Neumann and Cohen, 1997). Our findings that EAF2 can be regulated by Wnt-4 signaling and that the expression domains of both genes in neural tissue are just adjacent to each other support the idea of Wnt-4 to signal as a long-range molecule.
A potential function of RNA polymerase II elongation for eye development
Using a subtractive cloning strategy, we identified EAF2 as a potential downstream factor in neural Wnt-4 signaling, which is known to interact in mammals with the RNA polymerase II elongation factor ELL. Within eukaryotes, several elongation factors exist that regulate the activity of RNA polymerase II. These elongation factors are regulated by interacting proteins and show some specificity with respect to the genes they regulate (Shilatifard et al, 2003). Interfering with RNA polymerase II elongation by specifically targeting individual components of elongation complexes thus might result in specific phenotypes during development. In Xenopus, EAF2 is highly enriched in the developing eye with lower expression in the pronephros, the epiphysis, and the somites. This expression pattern is conserved between species as the mouse EAF2 homolog is expressed in the eye, the developing kidney, and the somites as well (Li et al, 2003). Whether this reflects a conserved function of EAF 2 is currently unknown as knockout mice for EAF2 are not available. On a functional level, our data demonstrate that EAF2 is essential for normal eye development as EAF2 regulates Rx expression.
How is the function of EAF2 coupled to the function of Wnt-4 during eye development? Whereas we observed a downregulation of eye-specific marker genes after knockdown of Wnt-4 function as early as stage 13, eye-specific expression of EAF2 starts not earlier than stage 18. Nevertheless, EAF2 can rescue the phenotype observed after Wnt-4 MO treatment, suggesting an identical molecular target. Indeed, we were able to demonstrate that Rx can rescue the Wnt-4 MO phenotype, whereas Rx itself is regulated by EAF2. This suggests that Wnt-4 is required for early aspects of eye development in the absence of EAF2 expression. Later, Wnt-4 induces EAF2, which itself is involved in maintaining the expression of eye-specific marker genes in a stable feedback loop.
We also analysed a potential function of EAF2 during pronephros development. Knockdown of pronephric EAF2 gene function resulted in abnormal pronephric development affecting, however, not only the EAF2-expressing tubules but also the pronephric duct (data not shown). Pronephric defects occurred with high frequency in conjuction with abnormal somitogenesis, as revealed by marker gene analysis. As the paraxial mesoderm, which gives rise to the somites, is an important source of signals inducing pronephric development, it is likely that the large-scale nature of the pronephric defects is a consequence of disrupting an earlier, EAF2-dependent step essential for paraxial mesoderm differentiation. This precludes at present a detailed analysis of EAF2 function in pronephric development.
In summary, our data provide a first example for a growth factor regulating the expression of an RNA polymerase II elongation factor component. Thereby we also add a new layer of complexity on the regulation of gene expression by extracellular factors. Having established a link between noncanonical Wnt-4 signaling and EAF2 expression and function, our data also provide a new molecular mechanism as to how biological effects of noncanonical Wnt signaling might be mediated.
Materials and methods
Embryos
Xenopus embryos were obtained using standard procedures and staged according to Nieuwkoop and Faber (1967).
RNA transcription, microinjections, and explant cultures
Capped RNAs were transcribed in vitro using the SP6 or T7 Message Machine Kit (Ambion). For the animal cap assay, embryos were injected at the two-cell stage and caps were dissected at stage 8. The explants were cultured in LCMR (43 mM NaCl, 0.85 mM KCl, 0.37 mM CaCl2, 0.19 mM MgCl2, 5 mM HEPES, pH 7.2) including 0.5% BSA and penicillin/streptomycin (Invitrogen, final concentrations: penicillin 20 U/ml, streptomycin 20 μg/ml) until uninjected control embryos reached stage 22. RNA amounts injected were: noggin 400 pg; XWnt-4 1000 pg. For FGF treatment, bFGF (Promega) was added at a concentration of 200 ng/ml. The JNK inhibitor SP600125 (Alexis) was used at a concentration of 12.5 μM, consistent with the inhibitory function of this compound against JNK in different cell-based assays (Bennett et al, 2001). Optimal concentration and timing of inhibitor treatment were evaluated experimentally (see Supplementary Figure 2). The MAPKK inhibitor PD98059 (Calbiochem) was used at 2 μM.
RNA isolation and RT–PCR analyses
Total RNA was isolated from explanted tissues using Purescript RNA Isolation Kit (Biozym) and subsequently analyzed by RT–PCR. First-strand cDNA was synthesized according to the Invitrogen protocol using Superscript II reverse transcriptase. The PCR reaction mixtures were prepared using the Master Amp PCR Core Kit (Epicentre Technologies) and PCR was performed under standard conditions at 55°C annealing temperature. Primer sequences are given in Supplementary data.
Whole-mount in situ hybridization and histology
For the analyses of marker gene expression by whole-mount in situ hybridization, embryos were injected unilaterally into one dorsal-animal blastomere at the eight-cell stage and cultured until indicated stages. Embryos were fixed in MEMFA (0.1 M MOPS (pH 7.4), 2 mM EGTA, 1 mM MgSO4, and 4% formaldehyde) for 1.5–2 h at room temperature, washed in 1 × MEM solution and dehydrated in EtOH. Whole-mount in situ hybridization was performed according to a standard protocol. For histology, embryos were refixed in MEMFA for 1–2 h and embedded in gelatine/BSA. Vibratome sections were cut at 30 μm thickness and coverglass mounted with 90% glycerol, 10% 0.1 M Tris–Cl (pH 7.4).
Wnt-4 and EAF2 antisense MO
The Wnt-4 MO was used as published (Saulnier et al, 2002). A standard MO with the base composition 5′-CCTCTTACCTCAGTTACAATTTATA-3′ or a Wnt-4 4-base mismatch MO (Saulnier et al, 2002) was used as a control. The EAF2 MO used was a 25-mer MO with the base composition 5′-AGCTGCTCCATTCATCCTGCCGGCC-3′. The functionality of the EAF2 MO was tested using the transcription and translation assay TNT-Kit (Promega). The 5′UTR+EAF2 construct consists of the whole open reading frame and an additional 10 bp of the 5′UTR. These 10 bp are missing in the Δ5′UTR+EAF2 construct. Oligos were resuspended in sterile water and injected unilaterally into a single blastomere at the eight-cell stage in doses of 5 ng per embryo. One dorsal-animal blastomere was selected to target anterior neural tissue, while a ventral vegetal blastomere was selected to target the developing pronephric kidney. For rescue experiments, 5 ng of Wnt-4 MO was injected together with different RNAs or DNA as indicated. Amounts of RNA were: dshΔDIX: 50 pg, β-catenin RNA: 150 pg, β-catenin DNA: 100 pg, EAF2: 1 ng, Activin-Wnt-4: 10 or 50 pg, Fz-3: 10 pg, Rx: 200 pg, Pax-6: 200 pg. The β-catenin RNA induced secondary axes when overexpressed on the ventral side of four-cell stage embryos, indicating the functionality of the RNA used.
Subtractive cDNA screen
In order to identify Wnt-4 downstream genes activated in neuralized animal caps, we followed the protocols of the following Clontech kits: SMART PCR cDNA synthesis kit, PCR-Select cDNA subtraction kit and PCR-Select differential screening kit. The original identified EAF2 clone contained 950 bp of the 3′UTR and we completed the EAF2 sequence by a RACE procedure (GeneRacer Kit, Invitrogen). The 5′ RACE primer used was: 5′-GAT CTG CCA GGC ACT GAG GGA ATT T-3′ using an annealing temperature of 65°C, resulting in a 1800 bp fragment with the 5′-UTR and the open reading frame. The full sequence of EAF2 was deposited under the accession number AJ579576. A related EST clone was found in the database with the accession number CA988261, which likely represents an allelic variation.
RNA polymerase II elongation assay
For the RNA polymerase II elongation assay, animal caps were neuralized by injection of 400 pg of noggin RNA at two-cell stage. Caps were cut at stage 8 and cultured until stage 20 in 1 × MBSH (10 mM HEPES (pH 7.5), 88 mM NaCl, 2.5 mM KCl, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 2.4 mM NaHCO3). The length of RNA transcripts was monitored by RT–PCR. Primer sequences are given in Supplementary data.
Cell culture
Human embryonic kidney cells (HEK293) were stably transfected with expression plasmids coding for β2AR/Rfz-1 or β2AR/Rfz-2. Correct expression of these constructs was verified by RT–PCR using primers specific for Rfz-1 or Rfz-2 intracellular loops. To activate or inactivate these receptors, cells were treated with the β2-adrenergic agonist isoproterenol (100 μM) or the β2-adrenergic antagonist propranolol (30 μM) and gene expression was analyzed by RT–PCR. Primers are given in Supplementary data. Cycloheximide was used at 500 ng/ml simultaneously to agonist/antagonist treatment. Functionality of the cycloheximide used was tested in a TNT assay.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Table 1
Supplementary Materials and Methods
Acknowledgments
We thank Drs RT Moon, C Niehrs, T Pieler, C Malbon, M Andreazzoli, and T Hollemann for providing cDNA clones. We also like to thank D Weber, J Brohl, P Dietmann, and M Läsche for technical support and A Nolte for sequencing. MK is supported by the Deutsche Forschungsgemeinschaft (SFB 497, Tp A6) and AWB by the Swiss National Science Foundation (No. 3100A0-101964).
References
- Bennett BL, Sasaki DT, Murra BW, O'Leary EC, Sakata ST, Xu W, Leistern JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW (2001) SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 98: 13681–13686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M (1998) Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell 94: 109–118 [DOI] [PubMed] [Google Scholar]
- Cai Y, Lechner MS, Nihalani D, Prindle MJ, Holzman LB, Dressler GR (2002) Phosphorylation of Pax2 by the c-Jun N-terminal kinase and enhanced Pax2-dependent transcription activation. J Biol Chem 277: 1217–1222 [DOI] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, Fan CM (2004) Protein kinase A signaling via CREB controls myogenesis induced by Wnt proteins. Nature, November 28, epub ahead of print [DOI] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A (1999) Pax6 induces ectopic eyes in a vertebrate. Development 126: 4213–4222 [DOI] [PubMed] [Google Scholar]
- Chow RL, Lang RA (2001) Early eye development in vertebrates. Annu Rev Cell Dev Biol 17: 255–296 [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW (2003) Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development 130: 2375–2384 [DOI] [PubMed] [Google Scholar]
- Darken RS, Scola AM, Rakeman AS, Das G, Mlodzik M, Wilson PA (2002) The planar cell polarity gene strabismus regulates convergent extension movements in Xenopus. EMBO J 21: 976–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du SJ, Purcell SM, Christian JL, McGrew LL, Moon RT (1995) Identification of distinct classes and functional domains of Wnts through expression of wild-type and chimeric proteins in Xenopus embryos. Mol Cell Biol 15: 2625–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg JC, Ma J, Gerber MA, Christensen A, Kennison JA, Shilatifardi A (2002) dELL is an essential RNA polymerase II elongation factor with a general role in development. Proc Natl Acad Sci USA 99: 9894–9899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Yamaguchi Y, Schilbach S, Wada T, Lee J, Goddard A, French D, Handa H, Rosenthal A (2000) A regulator of transcriptional elongation controls neuronal development. Nature 408: 366–369 [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP (1995) Wnt expression patterns in chick embryo nervous system. Mech Dev 52: 9–25 [DOI] [PubMed] [Google Scholar]
- Keegan BR, Feldman JL, Lee DH, Koos DS, Ho RK, Stainier DYR, Yelon D (2002) The elongation factors Pandora/Spt6 and Foggy/Spt5 promote transcription in the zebrafish embryo. Development 129: 1623–1632 [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C (2001) A morphogen gradient of Wnt/β-catenin signaling regulates anteroposterior neural patterning in Xenopus. Development 128: 4189–4201 [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Malbon CC, Moon RT (2000b) Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and frizzled homologs and promotes ventral cell fates in Xenopus. J Biol Chem 275: 12701–12711 [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT (2000a) The Wnt/Ca2+ pathway a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16: 279–283 [DOI] [PubMed] [Google Scholar]
- Li M, Wu X, Zhuang F, Jiang S, Jiang M, Liu YH (2003) Expression of murine ELL-associated factor 2 (EAF2) is developmentally regulated. Dev Dynam 228: 273–280 [DOI] [PubMed] [Google Scholar]
- Liu T, DeConstanzo AJ, Liu X, Wang H, Hallaghan S, Moon RT, Malbon CC (2001) G protein signaling from activated rat frizzled-1 to the beta-catenin-Lef-Tcf pathway. Science 292: 1718–1722 [DOI] [PubMed] [Google Scholar]
- Liu X, Liu T, Slusarski DC, Yang-Snyder DC, Malbon CC, Moon RT, Wang H (1999) Activation of a frizzled-2/beta-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc Natl Acad Sci USA 96: 14383–14388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y (2003) Anterior–posterior guidance of commissural axons by Wnt-frizzled signaling. Science 302: 1984–1988 [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M (1997) The Rx homeobox gene is essential for vertebrate eye development. Nature 387: 603–607 [DOI] [PubMed] [Google Scholar]
- McGrew LL, Otte AP, Moon RT (1992) Analysis of Xwnt-4 in embryos of Xenopus laevis: a Wnt family member expressed in the brain and floor plate. Development 115: 463–473 [DOI] [PubMed] [Google Scholar]
- Mlodzik M (1999) Planar polarity in the Drosophila eye: a multifaceted view of signaling specificity and cross-talk. EMBO J 18: 6873–6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor S, Smalley MJ, Robertson D, Gusteros BA, Edwards PAW, Dale TC (2000) Retroviral expression of Wnt-1 and Wnt-7b produces different effects in mouse mammary epithelium. J Cell Sci 113: 2129–2138 [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorso-ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J (1967) Normal Table of Xenopus laevis (Daudin). Amsterdam, The Netherlands: North-Holland Publishing Company [Google Scholar]
- Parr BA, Shea MJ, Vassileva G, McMahon AP (1993) Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119: 247–261 [DOI] [PubMed] [Google Scholar]
- Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML (2001) Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci USA 98: 3861–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Sussmann D, Wanshaw-Boris A, Salinas PC (2005) Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci 8: 34–42 [DOI] [PubMed] [Google Scholar]
- Royet J, Finkelstein R (1997) Establishing primordia in the Drosophila eye-antennal imaginal disc: the roles of decapentaplegic, wingless, and hedgehog. Development 124: 4793–4800 [DOI] [PubMed] [Google Scholar]
- Saulnier DME, Ghanbari H, Brändli AW (2002) Essential function of Wnt-4 for tubulogenesis in the Xenopus pronephric kidney. Dev Biol 248: 13–28 [DOI] [PubMed] [Google Scholar]
- Shilatifard A, Conaway RC, Conaway JW (2003) The RNA polymerase II elongation complex. Annu Rev Biochem 72: 693–715 [DOI] [PubMed] [Google Scholar]
- Simone F, Luo RT, Polak PE, Kaberlien JJ, Thirman MJ (2003) ELL-associated factor 2 (EAF2), a functional homolog of EAF1 with alternative ELL binding properties. Neoplasia 101: 2355–2362 [DOI] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683 [DOI] [PubMed] [Google Scholar]
- Stump RJW, Ang S, Chen Y, von Bahr T, Lovicu FJ, Pinson K, de Iongh RU, Yamaguchi TP, Sassoon DA, McAvoy JW (2003) A role for Wnt/β-catenin signaling in lens epithelial differentiation. Dev Biol 259: 48–61 [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT (2003) A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5: 367–377 [DOI] [PubMed] [Google Scholar]
- Weston CR, Wong A, Hall JP, Goad MEP, Flavell RA, Davis RJ (2003) JNK initiates a cytokine cascade that causes Pax2 expression and closure of the optic fissure. Genes Dev 17: 1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolda SL, Moon RT (1992) Cloning and developmental expression in Xenopus laevis of seven additional members of the Wnt family. Oncogene 7: 761–766 [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
- Zhang L, Mathers PH, Jamrich M (2000) Function of rx, but not pax6, is essential for the formation of retinal progenitor cells in mice. Genesis 28: 135–142 [PubMed] [Google Scholar]
- Zuber ME, Gestri G, Viczian AS, Barsacchi G, Harris WA (2003) Specification of the vertebrate eye by a network of eye field transcription factors. Development 130: 5155–5167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Table 1
Supplementary Materials and Methods


