Abstract
The purpose of this study was to deposit a thin layer of TiO2 on a Co-Cr substrate, serving as a deactivation film protecting the metallic fitting surface. The crystalline structure and surface morphology of the film were characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM). A scratch tester was used to examine the adhesion strength between the TiO2 film and the Co-Cr substrate. The water contact angles and antifungal efficacy against C. albicans of the TiO2-deposited Co-Cr samples were investigated and further compared with those of uncoated Co-Cr substrates. The results indicated that a pure anatase microstructure and dense and smooth surface texture as well as strong binding to the underlying metallic surface were obtained. The originally hydrophobic Co-Cr alloy surface turned hydrophilic after TiO2 film coating. Most importantly, the TiO2-coated surface showed a superior antifungal capability under UV-irradiation compared to those without TiO2 coating. This work contains meaningful results for the development of a new metallic framework coating with improved hydrophilicity and antifungal properties.
1. Introduction
The demand for prosthetic treatment is of increasing tendency as a result of the aging issue globally [1, 2]. Among all available techniques, removable partial denture (RPD) is regarded as a simple and economic option to repair the missing teeth [3]. Therefore, RPDs have been the commonly used treatment for edentulism to date. Co-Cr alloys have a wide range of applications as a material for metallic framework owing to their good mechanical properties as well as superior biocompatibility. However, it has been reported that yeasts and bacteria are prone to colonize on the fitting surface of the dentures forming biofilms [4, 5]. These biofilms consequently cause damage to the oral mucosa below, commonly associated with denture stomatitis (DS). DS affects up to two-thirds or even more of denture wearers, the majority of whom are asymptomatic and unaware of the problem [6–8].
The etiology of DS is multifactorial, among which the candida primarily C. albicans infection and poor denture hygiene have been widely accepted as critical risk factors [9–11]. Yoshijima et al. [12] have reported an association of hydrophobic surface with the colonization of C. albicans. It is therefore believed that a proper deactivation of the metallic fitting surface may alleviate the problem associated with biofilms. This is attributed to the increase of hydrophilicity and the antimicrobial properties relying on surface coating techniques. Recently, titanium dioxide (TiO2) coating has drawn great attentions due to its photocatalytic, antimicrobial, and self-cleaning properties [13–17]. In addition, TiO2 films have shown enhanced biocompatibilities and good corrosion resistance of the substrate [18, 19]. In the published studies, TiO2 thin film has been applied to denture base acrylic resin, resulting in increased hydrophilic properties and decreased attachment of food bolus accumulation, as well as an inhibitory effect on adhesion of microorganisms [20, 21]. However, to the best of our knowledge, the study of TiO2 film on Co-Cr alloy frameworks is still not available, since TiO2 films growing on a Co-Cr alloy surface with good bonding strength are challenging, restricting their applications so far.
A large magnitude of techniques, including sol-gel processes, chemical vapor deposition, electrophoretic deposition, plasma immersion ion implantation, plasma spray, and atomic layer deposition (ALD), have been made available for depositing TiO2 films on a variety of surfaces [22–25]. Among the aforementioned techniques, ALD has been widely applied due to its figure of merits including high-quality and large-area flat coating, perfect structure, and process controllability. Although significant efforts have been devoted to depositing TiO2 films on various substrates, such as silicon and metallic and polymer materials [23, 26, 27], there is little information in the literature with regard to the application of ALD in dentistry. Therefore, this study was innovatively designed to deposit a thin layer of TiO2 on a Co-Cr substrate, with the help of ALD. Concerning potential dental applications, our hypothesis was that the hydrophilic and antimicrobial capacity of TiO2 films might have the potential to inhibit the growth of C. albicans adhered to the metallic frameworks and thus lower the risk of associated denture stomatitis. In particular, an optimized ALD process was adopted via a home-made ALD system. The crystalline structure, microstructure, and adhesion strength of deposited TiO2 film were examined. Moreover, the water contact angles and antifungal properties of the TiO2-deposited Co-Cr samples were investigated and further compared with those of uncoated Co-Cr substrates.
2. Materials and Methods
2.1. Preparation of TiO2 Film
The experiments were carried out in a hot-wall ALD system (home-made system, School of Chemistry and Chemical Engineering, Nanjing University, China). The Co-Cr alloy (Bego, Bremen, Germany) was used as substrate material, with the alloy composition given in Table 1. Small disc samples (diameter = 15 mm; thickness = 2.5 mm) were casted from the Co-Cr alloy. Prior to deposition, the substrate specimens were mechanically grounded by water-proof SiC abrasive papers with various sizes of up to 2000 grit and then polished with 30–50 nm alumina powder. The alloy discs were further ultrasonically cleaned in diluted sodium hydroxide solution, acetone, ethanol, and deionized water sequentially to remove residual surface contamination completely and finally dried N2.
Table 1.
Chemical composition of the Co-Cr alloy.
| Element | Co | Cr | Mo | Ni | Others |
|---|---|---|---|---|---|
| (wt.%) | 62.3% | 29.3% | 6.2% | 1.0% | 1.2% |
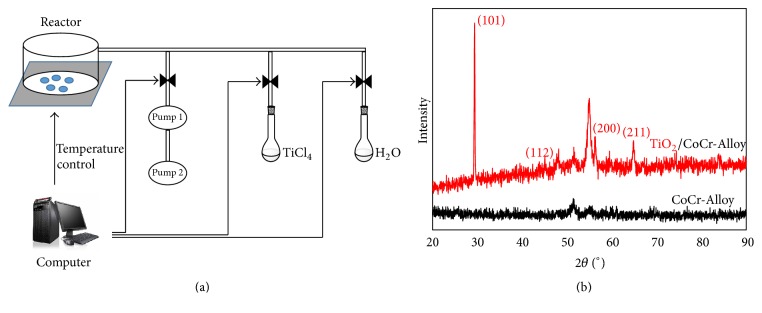
TiCl4 and water, held in separate external reservoirs at room temperature, were alternatively introduced into the reactor chamber. The sketch is shown in Figure 1(a). One ALD deposition cycle consists of 3.0 s TiCl4 pulse time, 250 s pump-down, 2.0 s water pulse time, and 300 s pump-down, respectively. Herein, a longer pump-down period was employed instead of using high purity N2/Ar gas as purging gas to remove any residual reactants and by-products. The deposition temperature was held at 300°C constantly and 1000 ALD cycles were deposited for each sample.
Figure 1.
(a) Sketch of ALD technique (Pump 1: turbomolecular pump. Pump 2: sliding-vane rotary vacuum pump) and (b) XRD pattern of the surface of the treated sample.
2.2. Film Characterization Measurements
The crystalline structure of films was identified by X-ray diffraction (XRD) (D8, Bruker AXS GmbH, Karlsruhe, Germany). CoKα1 radiation (λ = 0.178897 nm) was over a range from 20° to 90° (2θ). The surface morphology of the samples was examined by scanning electron microscopy (SEM) (S-3400N II, Hitachi, Tokyo, Japan). The cross-sectional microstructure and thickness of deposited films were determined by field emission scanning electron microscopy (Quanta FEG 250, FEI, Hillsboro, America).
A scratch tester was used to examine the adhesion strength between the TiO2 film and the Co-Cr substrate (UMT Multi-Specimen Test System, CETR, CA, USA). The coated surface was scratched using a conical Rockwell C tip with diameter of 5 μm. Scratch (3 mm in length) was made with the applied loads ranged from 0.15 to 5.0 Kg at a linear speed of 0.01 mm/s, where the acoustic emission (AE) signal intensity (due to interface exfoliating) was monitored at the same time. The critical load was identified with the continuous increase in the AE signal during scratching. After testing, the scratch on the surface of TiO2 film was evaluated by optical microscope (Gx41, Olympus, Tokyo, Japan) at 16x magnification.
The static contact angles of coated and uncoated samples were measured using the sessile drop method at room temperature (OCA30 video contact angle system, Dataphysics, Filderstadt, Germany). A 2.0 μL droplet of deionized water was dropped on the testing surface using a computer-controlled microsyringe. The contact angle was then determined from the magnified image collected with a camera in the system. Six samples from each group were selected randomly and three replicate measurements were performed at different positions. The averaged value was presented as the contact angle for each sample. The data is presented as the mean ± standard deviation. A t test was applied for statistical analysis, with P < 0.05 being considered significant.
2.3. Antifungal Property Tests
C. albicans strain (ATCC 10231) was used as the pathogen for the microbiological tests. Cultures of microorganisms were grown in Martin Broth, modified (liquid) at 37°C with 150 rpm shaking for 12 h. 10-fold serial dilutions of the incubation fluid were applied to reach the concentration of 107 colony-forming units per milliliter (CFU/ml). The tested samples were sterilized under ultraviolet light (245 nm) for 2 h, prior to the investigations. A total of 100 μL C. albicans diluted suspension was added onto the TiO2-coated and uncoated surface, respectively. After illumination under an 8 W UV lamp (365 nm) for 1 h, the droplets were washed from the sample surface using 0.9 ml saline solution repeatedly. Afterwards 100 μL of each serially diluted washing suspension was dispersed on the modified Martin Agar Medium and incubated for 24 h at 37°C. The number of colonies on the mediums was quantified via the direct counting method after incubation.
3. Results
3.1. Structural Characterization of TiO2 Film
Figure 1(b) displayed a typical XRD spectrum of the TiO2-deposited Co-Cr sample. Besides the typical peaks corresponding to elements of Co-Cr substrate, the dominating (101), (112), (200), and (211) crystalline peaks corresponded to the anatase TiO2 phase. This suggested a highly crystalline TiO2 film formed on the Co-Cr alloy. Anatase TiO2 was the dominating crystalline form of the surface layer under current experimental conditions.
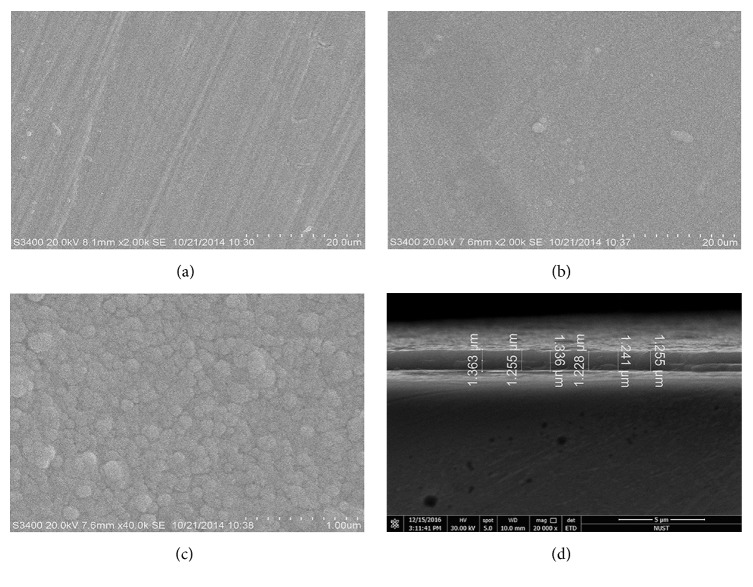
The microstructures of the sample with and without TiO2 film were shown in SEM images (Figures 2(a)–2(c)). The surface of the TiO2 film was relatively dense, uniform, and smooth without noticeable pinholes or cracks (Figure 2(b)). It was noted that the TiO2 film has entirely covered the polishing scratches on the Co-Cr surface (Figure 2(a)). In addition, a large number of rounded grains with the size of about 100 nm were observed in a high-magnification SEM image of TiO2 film (Figure 2(c)). It could be seen from the cross-sectional scanning of the TiO2-coated sample that the TiO2 layer was continuous, and its thickness was estimated to be 1.2–1.4 μm (Figure 2(d)).
Figure 2.
SEM images of (a) Co-Cr substrate in low magnification. (b) TiO2 film in low magnification. (c) TiO2 film in high magnification. (d) Cross-sectional view of TiO2 film on Co-Cr substrate.
3.2. Adhesion Strength
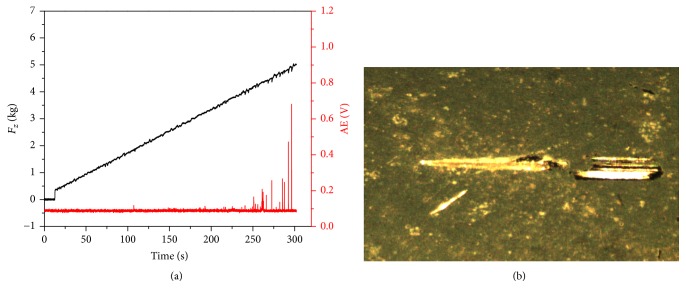
The adhesion strength between the coating layer and substrate is listed as one of the most important factors determining the lifetime and performance of the coated component. The association curve between AE signal and the load on C tip showed that the TiO2 coating starts to peel off when the load reached approximately 4.0 Kg with continuously peaks (Figure 3(a)). The scratch morphology image (Figure 3(b)) revealed neither observed neighboring cracks nor coating detachment, besides the scratch corresponding to the applied force. All aforementioned observations suggested a good adhesion between the TiO2 film and Co-Cr substrate.
Figure 3.
Scratch test (a) applied load and AE signal intensity. (b) Optical image of the scratch test specimen.
3.3. Water Contact Angle
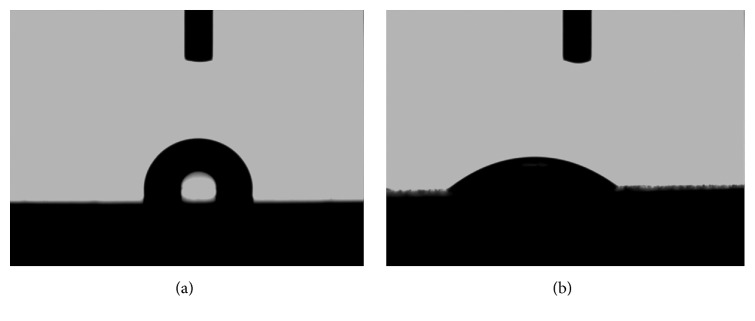
The optical images of water drops sprayed onto the tested samples demonstrated that the average surface contact angle of deionized water was 103.0° ± 1.2° on the Co-Cr alloy and 37.3° ± 3.8° on the TiO2 -coated samples, respectively (Figure 4), indicating that the water contact angle of Co-Cr alloy showed a significant decrease (P < 0.05) after the TiO2 film deposition.
Figure 4.
Optical images of a water droplet in contact with Co-Cr (a) and TiO2 (b) surface.
3.4. TiO2-Coated Film Exhibited a Powerful Antifungal Property
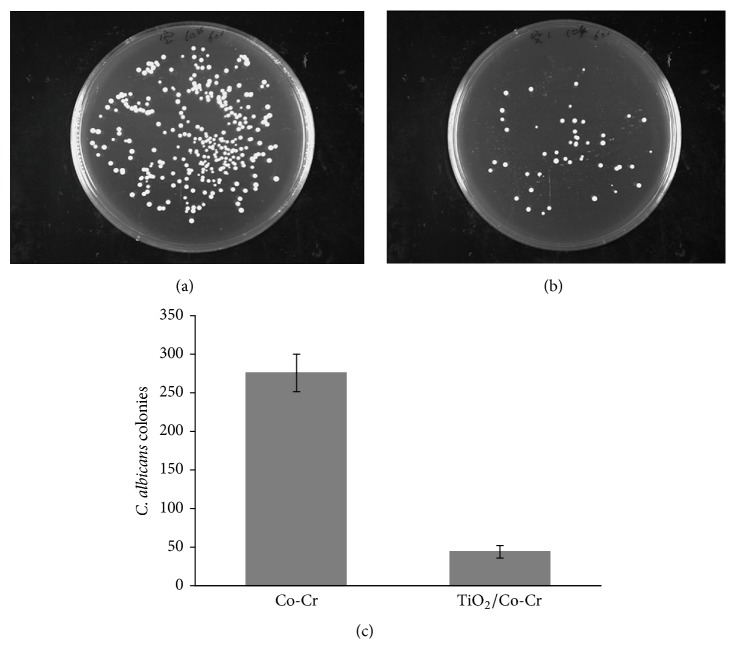
In order to clarify the relative number of survival C. albicans on tested surface after 1 h UV illumination, the diluted suspensions washed from the uncoated and TiO2-coated sample surface were incubated, respectively. Figures 5(a) and 5(b) showed photo images of colonies on the media after incubation for 24 h. The number of survival C. albicans on TiO2-coated sample was much lesser than that on uncoated one. As shown in Figure 5(c), the quantified number of colonies from TiO2-coated group was significantly less than uncoated group (P < 0.05). These results demonstrated that the TiO2 film presented a powerful antifungal effect under UV illumination.
Figure 5.
Photo images of incubated C. albicans colonies washed from surface of uncoated (a) and TiO2 -coated sample (b). (c) The numbers of C. albicans colonies.
4. Discussion
The majority of ALD reactions rely on two gaseous precursors introduced into the chamber in a sequential, self-limiting manner. An inert gas, such as Ar and nitrogen, is infused between the precursor pulses to remove extra reactants and by-products. For example, Cheng et al. obtained TiO2 films using TiCl4 and H2O, employing Ar as the purging gas, on a magnitude of substrate materials [28]. In the current study, the application of ALD has been successfully extended to coat TiO2 films on a Co-Cr alloy. Moreover, the method optimization includes longer pump-down instead of purging gas. This modification greatly simplified the experimental procedure by obviating additional purging gases. On the other hand, the experimental period was slightly extended. Further optimization in terms of pumping capacity and tubing design will help to improve the working efficiency of current setup.
It is well-known that the phase composition and photocatalytic properties of the ALD TiO2 films are dependent on many parameters such as the chamber pressure, the deposition temperature, and the substrate material. On the basis of XRD pattern, it was concluded that the film grown under the current experimental conditions at 300°C was pure anatase structure. Earlier studies have also shown that the anatase structure with lower conduction bandedge exhibits a stronger photocatalytic and bactericidal ability [22, 28–30].
The surface smoothness is considered as an important factor influencing the adherence of oral pathogenic microorganisms [31–33]. Surface scratches and cracks can enhance the attachment of microorganisms and the growth of biofilms [8, 34]. Furthermore, wettability is also an important property of biomaterials because hydrophilic surfaces are more resistant to microbial adhesion than hydrophobic surfaces [12, 35, 36]. Usually, surface with a contact angle more than 70° is identified as hydrophobic, while a hydrophilic surface has a contact angle below 70° [37]. Results from the water contact angle measurements suggested that the Co-Cr alloy was hydrophobic and then became hydrophilic when coated with the TiO2 film. Yoshijima et al. [12] have reported that the decreasing surface hydrophobicity can diminish the ability of C. albicans to attach and colonize the denture surface. Our results revealed that coating TiO2 film can cover the polished scratches and increase the surface hydrophilicity of Co-Cr alloy at the same time. This may prevent the microbial attachment to certain extent and thus resist the development of biofilms leading to denture stomatitis.
More importantly, some studies have identified TiO2 as a surface coating to enhance the antibacterial capability of substrate materials [13, 26, 27]. This observation is most likely due to its photo-induced superhydrophilicity and photocatalytic property, though the exact bactericidal mechanisms of TiO2 under UV-irradiation are still under debate [22]. In general, the cell wall and membrane damage by reactive oxygen species generated from the photocatalytic activity of TiO2 is the mostly accepted killing action [38]. Furthermore, not only bacteria but also viruses, fungi, and other microorganisms can also be killed by UV-irradiated TiO2 [39]. Evaluation of microbial survivability is one of in vitro techniques to assess antimicrobial properties [22]. Our study complemented the previous studies by evaluating growth inhibition of C. albicans on TiO2-coated and uncoated samples. As can be observed from the antifungal experiment, the TiO2 coating exhibited a great inhibitory effect against C. albicans, with a statistically significant reduction in the number of colony-forming units (CFU) compared to the control group (P < 0.05). Thus, it is fair to assume that the powerful oxidative ability of TiO2 can induce the apoptosis and necrosis in C. albicans cells, although the multilayer composition of C. albicans cell wall is more complicated and resistant to antimicrobial activity than that of bacteria [40]. To better understand the underlying mechanism and mimic the real scenario, it is beneficial to perform a time-resolved antifungal experiment lasting a longer period of days or even weeks. Moreover, the illuminating UV conditions in terms of strength and duration can be further studied to match more practical values.
5. Conclusion
In conclusion, TiO2 thin coatings have been successfully deposited on Co-Cr substrate via an optimized ALD process. The figure of merits included pure anatase structure, dense and smooth surface, strong bonding, and full coverage to the substrate surface. More importantly, the TiO2 coatings possessed a high antifungal activity that eliminates most of the C. albicans after the UV-irradiation. Therefore, there is a great potential of TiO2 coating on future RPD items against possible denture stomatitis.
Acknowledgments
This work was supported by Translational Medicine Center of Nanjing.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Lijuan Huang and Shuanglin Jing contributed equally to this work.
References
- 1.Douglass C. W., Gammon M. D., Atwood D. A. Need and effective demand for prosthodontic treatment. The Journal of Prosthetic Dentistry. 1988;59(1):94–104. doi: 10.1016/0022-3913(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 2.Douglass C. W., Shih A., Ostry L. Will there be a need for complete dentures in the United States in 2020? Journal of Prosthetic Dentistry. 2002;87(1):5–8. doi: 10.1067/mpr.2002.121203. [DOI] [PubMed] [Google Scholar]
- 3.Lima J. M. C., Anami L. C., Araujo R. M., Pavanelli C. A. Removable partial dentures: Use of rapid prototyping. Journal of Prosthodontics. 2014;23(7):588–591. doi: 10.1111/jopr.12154. [DOI] [PubMed] [Google Scholar]
- 4.Pereira-Cenci T., Del Bel Cury A. A., Crielaard W., Ten Cate J. M. Development of Candida-associated denture stomatitis: New insights. Journal of Applied Oral Science. 2008;16(2):86–94. doi: 10.1590/S1678-77572008000200002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor R., Maryan C., Verran J. Retention of oral microorganisms on cobalt-chromium alloy and dental acrylic resin with different surface finishes. The Journal of Prosthetic Dentistry. 1998;80(5):592–597. doi: 10.1016/S0022-3913(98)70037-X. [DOI] [PubMed] [Google Scholar]
- 6.Reichart P. A. Oral mucosal lesions in a representative cross-sectional study of aging Germans. Community Dentistry and Oral Epidemiology. 2000;28(5):390–398. doi: 10.1034/j.1600-0528.2000.028005390.x. [DOI] [PubMed] [Google Scholar]
- 7.Budtz-Jörgensen E. Clinical aspects of Candida infection in denture wearers. The Journal of the American Dental Association. 1978;96(3):474–479. doi: 10.14219/jada.archive.1978.0088. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G., Tomsett K., Wickes B. L., López-Ribot J. L., Redding S. W. Denture stomatitis: a role for Candida biofilms. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2004;98(1):53–59. doi: 10.1016/j.tripleo.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Rocha Gusmão J. M., Ferreira Dos Santos S. S., Piero Neisser M., Cardoso Jorge A. O., Ivan Faria M. Correlation between factors associated with the removable partial dentures use and Candida spp. in saliva. Gerodontology. 2011;28(4):283–288. doi: 10.1111/j.1741-2358.2010.00390.x. [DOI] [PubMed] [Google Scholar]
- 10.Gendreau L., Loewy Z. G. Epidemiology and etiology of denture stomatitis. Journal of Prosthodontics. 2011;20(4):251–260. doi: 10.1111/j.1532-849X.2011.00698.x. [DOI] [PubMed] [Google Scholar]
- 11.Aoun G., Cassia A. Evaluation of denture-related factors predisposing to denture stomatitis in a lebanese population. Materia Socio Medica. 2016;28(5):392–396. doi: 10.5455/msm.2016.28.392-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshijima Y., Murakami K., Kayama S., et al. Effect of substrate surface hydrophobicity on the adherence of yeast and hyphal Candida: original article. Mycoses. 2010;53(3):221–226. doi: 10.1111/j.1439-0507.2009.01694.x. [DOI] [PubMed] [Google Scholar]
- 13.Yu J. C., Ho W., Lin J., Yip H., Wong P. K. Photocatalytic activity, antibacterial effect, and photoinduced hydrophilicity of TiO2 films coated on a stainless steel substrate. Environmental Science and Technology. 2003;37(10):2296–2301. doi: 10.1021/es0259483. [DOI] [PubMed] [Google Scholar]
- 14.Ma C., Nagai A., Yamazaki Y., et al. Electrically polarized micro-arc oxidized TiO 2 coatings with enhanced surface hydrophilicity. Acta Biomaterialia. 2012;8(2):860–865. doi: 10.1016/j.actbio.2011.09.021. [DOI] [PubMed] [Google Scholar]
- 15.Hossain M. F., Biswas S., Takahashi T., Kubota Y., Fujishima A. Influence of direct current power on the photocatalytic activity of facing target sputtered TiO2 thin films. Thin Solid Films. 2008;517(3):1091–1095. doi: 10.1016/j.tsf.2008.06.020. [DOI] [Google Scholar]
- 16.Pleskova S. N., Golubeva I. S., Verevkin Y. K. Bactericidal activity of titanium dioxide ultraviolet-induced films. Materials Science and Engineering C. 2016;59:807–817. doi: 10.1016/j.msec.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Cao S., Wang Y., Cao L., et al. Preparation and antimicrobial assay of ceramic brackets coated with TiO2 thin films. Korean Journal of Orthodontics. 2016;46(3):146–154. doi: 10.4041/kjod.2016.46.3.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishna D. S. R., Sun Y., Chen Z. Magnetron sputtered TiO2 films on a stainless steel substrate: selective rutile phase formation and its tribological and anti-corrosion performance. Thin Solid Films. 2011;519(15):4860–4864. doi: 10.1016/j.tsf.2011.01.042. [DOI] [Google Scholar]
- 19.Shan C. X., Hou X., Choy K.-L. Corrosion resistance of TiO2 films grown on stainless steel by atomic layer deposition. Surface and Coatings Technology. 2008;202(11):2399–2402. doi: 10.1016/j.surfcoat.2007.08.066. [DOI] [Google Scholar]
- 20.Kado D., Sakurai K., Sugiyama T., Ueda T. Evaluation of Cleanability of a Titanium. Prosthodontic Research Practice. 2005;4(1):69–76. doi: 10.2186/prp.4.69. [DOI] [Google Scholar]
- 21.Arai T., Ueda T., Sugiyama T., Sakurai K. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. Journal of Oral Rehabilitation. 2009;36(12):902–908. doi: 10.1111/j.1365-2842.2009.02012.x. [DOI] [PubMed] [Google Scholar]
- 22.Visai L., de Nardo L., Punta C., et al. Titanium oxide antibacterial surfaces in biomedical devices. International Journal of Artificial Organs. 2011;34(9):929–946. doi: 10.5301/ijao.5000050. [DOI] [PubMed] [Google Scholar]
- 23.Cheng H.-E., Chen C.-C. Morphological and photoelectrochemical properties of ALD TiO2 films. Journal of the Electrochemical Society. 2008;155(9):D604–D607. doi: 10.1149/1.2952659. [DOI] [Google Scholar]
- 24.Zhang P., Wang T., Gong J. Passivation of surface states by ALD-grown TiO2 overlayers on Ta3N5 anodes for photoelectrochemical water oxidation. Chemical Communications. 2016;52(57):8806–8809. doi: 10.1039/c6cc03411j. [DOI] [PubMed] [Google Scholar]
- 25.Ren W., Zhou W., Zhang H., Cheng C. ALD TiO2-coated flower-like MoS2 nanosheets on carbon cloth as sodium ion battery anode with enhanced cycling stability and rate capability. ACS Applied Materials & Interfaces. 2017;9(1):487–495. doi: 10.1021/acsami.6b13179. [DOI] [PubMed] [Google Scholar]
- 26.Haenle M., Fritsche A., Zietz C., et al. An extended spectrum bactericidal titanium dioxide (TiO2) coating for metallic implants: In vitro effectiveness against MRSA and mechanical properties. Journal of Materials Science: Materials in Medicine. 2011;22(2):381–387. doi: 10.1007/s10856-010-4204-4. [DOI] [PubMed] [Google Scholar]
- 27.Lin H., Xu Z., Wang X., et al. Photocatalytic and antibacterial properties of medical-grade PVC material coated with TiO2 film. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;87(2):425–431. doi: 10.1002/jbm.b.31120. [DOI] [PubMed] [Google Scholar]
- 28.Cheng H.-E., Hsu C.-M., Chen Y.-C. Substrate materials and deposition temperature dependent growth characteristics and photocatalytic properties of ALD TiO2 films. Journal of the Electrochemical Society. 2009;156(8):D275–D278. doi: 10.1149/1.3138723. [DOI] [Google Scholar]
- 29.Lilja M., Welch K., Åstrand M., Engqvist H., Strømme M. Effect of deposition parameters on the photocatalytic activity and bioactivity of TiO 2 thin films deposited by vacuum arc on Ti-6Al-4V substrates. Journal of Biomedical Materials Research - Part B Applied Biomaterials. 2012;100(4):1078–1085. doi: 10.1002/jbm.b.32674. [DOI] [PubMed] [Google Scholar]
- 30.Miao L., Tanemura S., Kondo Y., Iwata M., Toh S., Kaneko K. Microstructure and bactericidal ability of photocatalytic TiO2 thin films prepared by rf helicon magnetron sputtering. Applied Surface Science. 2004;238(1-4):125–131. doi: 10.1016/j.apsusc.2004.05.193. [DOI] [Google Scholar]
- 31.An Y. H., Friedman R. J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. Journal of Biomedical Materials Research. 1998;43(3):338–348. doi: 10.1002/(SICI)1097-4636(199823)43:3<338::AID-JBM16>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 32.Mori T., Togaya T., Jean-Louis M., Yabugami M. Titanium for removable dentures. I. Laboratory procedures. Journal of Oral Rehabilitation. 1997;24(5):338–341. doi: 10.1111/j.1365-2842.1997.tb00337.x. [DOI] [PubMed] [Google Scholar]
- 33.Bollen C. M., Lambrechts P., Quirynen M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: a review of the literature. Dental Materials. 1997;13(4):258–269. doi: 10.1016/S0109-5641(97)80038-3. [DOI] [PubMed] [Google Scholar]
- 34.von Fraunhofer J. A., Loewy Z. G. Factors involved in microbial colonization of oral prostheses. Gen Dent. 2009;57(2):136–143. [PubMed] [Google Scholar]
- 35.Fujimoto K., Tadokoro H., Ueda Y., Ikada Y. Polyurethane surface modification by graft polymerization of acrylamide for reduced protein adsorption and platelet adhesion. Biomaterials. 1993;14(6):442–448. doi: 10.1016/0142-9612(93)90147-T. [DOI] [PubMed] [Google Scholar]
- 36.Francois P., Vaudaux P., Nurdin N., Mathieu H. J., Descouts P., Lew D. P. Physical and biological effects of a surface coating procedure on polyurethane catheters. Biomaterials. 1996;17(7):667–678. doi: 10.1016/0142-9612(96)86736-6. [DOI] [PubMed] [Google Scholar]
- 37.Choi H. W., Dauskardt R. H., Lee S.-C., Lee K.-R., Oh K. H. Characteristic of silver doped DLC films on surface properties and protein adsorption. Diamond and Related Materials. 2008;17(3):252–257. doi: 10.1016/j.diamond.2007.12.034. [DOI] [Google Scholar]
- 38.Sunada K., Watanabe T., Hashimoto K. Studies on photokilling of bacteria on TiO2 thin film. Journal of Photochemistry and Photobiology A: Chemistry. 2003;156(1–3):227–233. doi: 10.1016/S1010-6030(02)00434-3. [DOI] [Google Scholar]
- 39.Foster H. A., Ditta I. B., Varghese S., Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Applied Microbiology and Biotechnology. 2011;90(6):1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Behzadnia A., Montazer M., Rashidi A., Rad M. M. Rapid sonosynthesis of N-doped Nano TiO2 on wool fabric at low temperature: Introducing self-cleaning, hydrophilicity, antibacterial/antifungal properties with low alkali solubility, yellowness and cytotoxicity. Photochemistry and Photobiology. 2014;90(6):1224–1233. doi: 10.1111/php.12324. [DOI] [PubMed] [Google Scholar]