Abstract
Adhesion of cells to an extracellular matrix is characterized by several discrete morphological and functional stages beginning with cell-substrate attachment, followed by cell spreading, migration, and immobilization. We find that although arachidonic acid release is rate-limiting in the overall process of adhesion, its oxidation by lipoxygenase and cyclooxygenases regulates, respectively, the cell spreading and cell migration stages. During the adhesion of NIH-3T3 cells to fibronectin, two functionally and kinetically distinct phases of arachidonic acid release take place. An initial transient arachidonate release occurs during cell attachment to fibronectin, and is sufficient to signal the cell spreading stage after its oxidation by 5-lipoxygenase to leukotrienes. A later sustained arachidonate release occurs during and after spreading, and signals the subsequent migration stage through its oxidation to prostaglandins by newly synthesized cyclooxygenase-2. In signaling migration, constitutively expressed cyclooxygenase-1 appears to contribute ∼25% of prostaglandins synthesized compared with the inducible cyclooxygenase-2. Both the second sustained arachidonate release, and cyclooxygenase-2 protein induction and synthesis, appear to be regulated by the mitogen-activated protein kinase extracellular signal-regulated kinase (ERK)1/2. The initial cell attachment-induced transient arachidonic acid release that signals spreading through lipoxygenase oxidation is not sensitive to ERK1/2 inhibition by PD98059, whereas PD98059 produces both a reduction in the larger second arachidonate release and a blockade of induced cyclooxygenase-2 protein expression with concomitant reduction of prostaglandin synthesis. The second arachidonate release, and cyclooxygenase-2 expression and activity, both appear to be required for cell migration but not for the preceding stages of attachment and spreading. These data suggest a bifurcation in the arachidonic acid adhesion-signaling pathway, wherein lipoxygenase oxidation generates leukotriene metabolites regulating the spreading stage of cell adhesion, whereas ERK 1/2-induced cyclooxygenase synthesis results in oxidation of a later release, generating prostaglandin metabolites regulating the later migration stage.
INTRODUCTION
Cell adhesion to the extracellular matrix (ECM) is a sequential process of discrete temporal stages. Detached cells, e.g., leukocytes, metastasized cancer cells, or suspension-cultured cells, initially attach to an ECM protein substrate by means of plasma membrane receptors. Attached cells then undergo spreading, which results in flattening and the formation of focal adhesions. Subsequently, some cell types undergo migration on the ECM, whereas others remain stationary. Each of these stages of adhesion, i.e., attachment, spreading, migration, and immobilization, involves changes in morphology and cytoskeletal structure that are regulated by incompletely defined protein kinase and lipid second messenger pathways (reviewed in Huttenlocher et al., 1995; Heidemann and Buxbaum, 1998; Schwartz and Baron, 1999).
Our previous work with HeLa cells characterized a lipid and kinase-mediated signaling pathway regulating the attachment and spreading stages of cell adhesion on collagen. That work centered on the phospholipase A2 (PLA2)-mediated release of arachidonic acid (AA) from membrane phospholipids; stimulated by integrin receptor clustering that occurs during cell attachment to ECM. Oxidation of AA by a lipoxygenase (LOX), but not by cyclooxygenases (COXs), was required for subsequent activation of protein kinase C to enable cell spreading (Chun and Jacobson, 1992, 1993; Auer and Jacobson, 1995). PLA2-mediated AA release was also shown to be a requirement for β1-integrin-mediated NIH-3T3 cell spreading on a fibronectin (FN) matrix (Whitfield and Jacobson, 1999).
The 85-kDa cytosolic PLA2 (cPLA2) is a major source of intracellular AA release in signal transduction, because it exhibits specificity for phospholipids with AA in the sn2 position, whereas calcium-independent PLA2 (iPLA2) and the secretory PLA2 (sPLA2) do not show the same preference (reviewed in Murakami et al., 1997; Balsinde et al., 1999). AA participates in multiple signaling pathways by providing substrate to three types of oxidative enzymes, LOXs, COXs, and epoxygenase (EOX), all of which participate in diverse signal transduction pathways (reviewed in Piomelli, 1993; Seeds and Bass, 1999).
Epoxygenase metabolites are not known to be involved in cell adhesion although there are reports suggesting that they act in other signaling pathways (Madamanchi et al., 1998; Chen et al., 1999). Conversely, the LOX and COX pathways are widely involved in signaling a variety of cellular functions. The major lipoxygenases are 5-, 12-, and 15-LOX. The 12- and 15-LOXs form hydroxyeicosatetraenoic acids (HETEs), and 5-LOX catalyzes the formation of 5-HETE and leukotrienes (LTs). HETEs and LTs are known to be involved in some aspects of adhesion (Damtew and Spagnuolo, 1997; Trikha and Honn, 1997; Rice et al., 1998; Honda et al., 1999) as well as inflammation (Lee et al., 1997; Natarajan et al., 1997; Conrad, 1999). There are two COX isoforms. COX-1 is constitutively expressed in many tissues, generating basal levels of prostaglandins for “housekeeping” functions. COX-2 is the product of an inducible immediate-early gene, and its synthesis is rapidly up-regulated by a variety of mitogens, generating prostaglandins involved in inflammation. Overexpression of either COX-1 or COX-2 has been reported to induce malignant transformation in some cells (DuBois et al., 1996; Oshima et al., 1997; Sheng et al., 1997; Tsujii et al., 1997; Sheng et al., 1998; Tsujii et al., 1998).
Interest has also focused recently on the role of the mitogen-activated protein kinases, particularly the p42-p44 extracellular signal-regulated kinases 1 and 2 (ERK1/2), in regulating various aspects of the arachidonate adhesion-signaling pathway. ERK1/2 are known to be rapidly activated by phosphorylation immediately after cell contact with the ECM (Chen et al., 1994; Takahashi and Berk, 1996; Heuertz et al., 1997; Wei et al., 1998; Redlitz et al., 1999). It was previously suggested that their activity might be a requirement for cPLA2 activation and subsequent cell spreading (Clark and Hynes, 1996; Cybulsky and McTavish, 1997). However, it has been shown also that inhibition of ERK activation does not limit spreading in several types of cells (Reszka et al., 1997; Crawford and Jacobson, 1998; Miranti et al., 1999). Other work also indicated that ERK1/2 may be overexpressed in some highly motile transformed cell lines, and its activity is in fact required for migration in several primary and tissue culture cell lines (Chikahisa et al., 1997; Graf et al., 1997; Klemke et al., 1997; Hinton et al., 1998; Lundberg et al., 1998; Ogura and Kitamura, 1998; Xi et al., 1999). These studies taken together suggest that ERK1/2 might be involved in regulating the migration stage of cell adhesion, but not the preceding spreading stage.
This presents an interesting question: How might ERK1/2 activated during the spreading stage regulate later migration without influencing spreading? ERK1/2 is known to mediate the up-regulation of the inducible COX-2 in several mitogen-response assay systems, particularly in signaling pathways stimulated by interleukins and growth factors (Niiro et al., 1998; Sheng et al., 1998; Subbaramaiah et al., 1998; Zakar et al., 1998; Adderley and Fitzgerald, 1999; Barry et al., 1999). A relationship between ERK1/2 and COX-2 synthesis has not yet been suggested or examined with respect to cell-extracellular matrix substrate adhesion, however. Independent of those studies, COX-2 activity and production of prostaglandins was shown to be involved in cell migration in several other experimental systems, but also without mention of any relationship to ERK1/2 (Tsujii and DuBois, 1995; Kreuzer et al., 1996; Daniel et al., 1999). These various studies suggest that AA, LOX, COX, and ERK1/2 are all involved in cell adhesion, but how these second messengers interact is not yet clearly defined.
The HeLa cell-collagen spreading pathway previously characterized showed that oxidation of AA by a lipoxygenase, but not either of the cyclooxygenases or epoxygenase, was required for spreading. Additionally, ERK1/2 was rapidly phosphorylated during adhesion of HeLa cells, although cell spreading was not affected by inhibition of its activation (Crawford and Jacobson, 1998). It was similarly shown by others that inhibition of ERK1/2 activation by a dominant negative Ras in NIH-3T3 cells did not affect cell spreading, although it did significantly reduce PLA2-mediated AA release, calling into question whether AA release was a requirement for spreading in these cells (Clark and Hynes, 1996). We showed that AA release was required for NIH-3T3 cell spreading (Whitfield and Jacobson, 1999), and our later data indicated this spreading was not sensitive to ERK1/2 inhibition. It remained to be explained why the decrease in ERK1/2-mediated PLA2 activity seen by others did not affect spreading in NIH-3T3 cells, although AA release was required for spreading. We proposed to test whether a kinetic or mass effect of AA release regulated by ERK1/2 is involved in signaling cell spreading or a later stage of adhesion, and what relationship this might have to a potential induction of COX-2 expression.
Interestingly, in HeLa cells LOX metabolism of AA signaled spreading; however, AA release continued even after spreading was complete (Auer and Jacobson, 1995). A similarly sustained AA release after spreading in initial assays was noted in NIH-3T3 cells as well. It therefore seemed possible that a modulation in AA oxidation could potentially regulate the transition from cell spreading to migration, based on a shift from early LOX-dominated oxidation of AA to a later oxidation by COX. An ERK1/2-mediated induction of COX-2, to make use of such a sustained AA release as an oxidation substrate to generate metabolites required for migration, would demonstrate one clear function for the adhesion-stimulated activation of ERK1/2 in the absence of any role for it in spreading. We therefore hypothesized that the induction of and increased oxidative activity by COX-2 during adhesion provides a mechanism that shifts AA metabolism from the LOX-dominated oxidation necessary for the cell spreading stage of cell adhesion to a COX-dominated oxidation essential for the migration stage.
Herein, we show that during NIH-3T3 cell spreading and migration on fibronectin, a kinetically and functionally distinct biphasic AA release occurs. A small transient ERK1/2-independent release is detectable within the first 5 min of cell plating, and is sufficient to signal 80–90% of control levels of spreading by means of oxidation to leukotrienes by 5-lipoxygenase. A later appearing and sustained AA release is partially sensitive to ERK1/2 inhibition and is not required for spreading, but does signal migration by means of oxidation to prostaglandins, predominantly by cyclooxygenase-2. Finally, we show that blockade of ERK1/2 activation prevents both the induction of COX-2 synthesis and downstream cell migration. Taken together, these data support the idea of a kinetic and functional divergence in the AA adhesion pathway, where metabolites generated from LOX oxidation of transiently released AA support initial spreading in an ERK-independent manner, whereas later-appearing COX-2 metabolites derived from a second sustained AA release influence postspreading migration. ERK1/2 appears to have a dual role in regulating the transition from spreading to migration, by both inducing the expression of COX-2, and by enhancing the later sustained AA release to provide additional substrate for the COX-mediated production of prostaglandins required for signaling migration.
MATERIALS AND METHODS
Reagents
NIH-3T3 cells were from American Type Culture Collection (Rockville, MD). Cells were maintained as subconfluent monolayers in DMEM supplemented with 10% (wt/vol) calf serum (Atlanta Biologicals, Atlanta, GA), 100 μg/ml dihydrostreptomycin, and 60 U/ml penicillin (Sigma, St. Louis, MO), in a humidified 37°C incubator with 5% CO2. For most assays, cells were serum-starved for 24 h in serum-free DMEM with 0.5% fatty-acid-free bovine serum albumin (BSA). Tissue culture plates and flasks were from VWR (West Chester, PA). Tritium-labeled AA (specific activity = 200–230 Ci/mmol) was from American Radiolabeled Chemicals (St. Louis, MO). Fatty-acid-free AA was from Cayman Chemical (Ann Arbor, MI). Mepacrine (quinacrine), a PLA2 inhibitor; PD98059, which specifically prevents phosphorylation of ERK1/2/by inhibition of mitogen-activated protein kinase kinase (MEK)1; AACOCF3, a selective inhibitor of both cPLA2 and iPLA2; HELSS, a specific inhibitor of iPLA2 only, and resveratrol, a COX-1 specific inhibitor were all from CalBiochem (San Diego, CA). SC236, a specific inhibitor of COX-2, was a gift from Searle (Skokie, IL). AA-861, a specific 5-LOX inhibitor, and baicalein, a 12-LOX inhibitor, were from BIOMOL (Plymouth Meeting, PA). PD146176, a 15-LOX specific inhibitor, was a gift from Parke-Davis (Morris Plains, NJ). FN and BSA were from Sigma.
Cell Spreading and Inhibition Assays
Cells were detached and prepared as previously described with some minor modifications (Whitfield and Jacobson, 1999). Briefly, serum-starved cells were detached with 0.01% trypsin-EDTA in Hanks' balanced salt solution (HBSS), washed with 0.01% trypsin inhibitor in HBSS, and resuspended in fresh serum-free DMEM-BSA with or without indicated inhibitors and/or metabolites, at concentrations indicated in figure legends. Cell density was adjusted to 1–2 × 105 cells/ml at parity between control and treated cells. Cells treated with most inhibitors or other agents were incubated for 15–30 min, depending on inhibitor, at room temperature on a reciprocal shaker at low speed, and then plated on either 35- or 60-mm2 tissue-culture polystyrene plates coated with either FN for spreading, or BSA as a negative control, because NIH-3T3 cells do not spread on BSA. With assays with the use of PD98059 to prevent activation of ERK1/2, cells were serum-starved for 24 h, incubated in serum-free culture with the inhibitor for 2 h before detachment, and then resuspended in the same concentration of inhibitor. FN coating on polystyrene plates was 20 μg/plate in HBSS overnight at 4°C, rinsed with phosphate-buffered saline (PBS), and then blocked with 1% BSA in PBS for 30 min, rinsed with PBS, and briefly air-dried. BSA plates were 5% BSA in PBS, otherwise prepared as for FN coating. Plated cells in coated dishes were placed in a 37°C water bath, and spreading was evaluated at indicated intervals by phase-contrast microscopy. An individual cell was counted as “spread” when diameter was minimally twice that of the nucleus. The percentage of spread cells was evaluated as (number of spread cells ÷ total of number cells in field) × 100 = percentage of spreading. Data from three to seven assays were analyzed for statistical significance by analysis of variance (ANOVA), and are shown graphically as mean ± SE.
Arachidonic Acid Release Assays
Cells were cultured for 18–24 h in serum-free DMEM-BSA with 2.0 μCi/ml [3H]AA equivalent to 4.0 μM AA. Labeled cells were detached with trypsin-EDTA, washed with trypsin inhibitor in HBSS, and resuspended in fresh DMEM-BSA with indicated inhibitors, at concentrations indicated in figure legends, as described above. After incubation a final wash was followed by resuspension in fresh DMEM-BSA with or without inhibitors, and then 5 ml each of control and treated cells was plated separately on 60-mm2 FN- or BSA-coated dishes and incubated in a water bath at 37°C. Immediately before plating a 200-μl sample was removed, centrifuged at 13,000 rpm for 1 min to pellet loose cells, and 3× 30-μl samples of supernatant analyzed for preplating counts. At indicated intervals, a similar volume of medium was removed, centrifuged, and processed for analysis of counts. An equivalent amount of fresh medium was added back to the plates to maintain the original plated volume. AA release was evaluated as appearance of label in the medium supernatant; under these conditions AA is the predominant labeled product released into medium for ∼60 min (Auer and Jacobson, 1995; Lloret and Moreno, 1996; Muthalif et al., 1998). Sample cpm was averaged per time point and presented as a percentage of the counts in the preplating medium at the beginning of the experiment. Data from three to seven assays were analyzed for statistical significance by ANOVA and shown as means with SE (SigmaStat software; Jandel Scientific, San Rafael, CA).
SDS-PAGE and Immunoblotting
Cells prepared as described above for spreading assays were plated on 60-mm2 FN-coated plates in a 37°C water bath. At indicated times postplating, medium was aspirated and spread cells were lysed at 4°C with buffer containing 50 mM Tris-HCl, pH 7.2, 150 mM NaCl, 1.5 mM EDTA, 1.5 mM EGTA, 1% Triton X-100, 0.1% deoxycholate, 0.1% SDS, and protease and phosphatase inhibitor cocktails optimized for mammalian cells (P2850 and P5766; Sigma). Lysed cells and buffer were collected with a cell scraper, sonicated for 2–5 s, and centrifuged at 13,000 rpm for 10 min at 4°C. Supernatant was aspirated and boiled for 5 min with loading buffer (188 mM Tris-HCl, pH 6.8, 6% SDS, 30% glycerol, 0.1% β-mercaptoethanol, and bromophenol blue dye) after removing aliquots for Bradford protein determination (Bio-Rad, Cambridge, MA). A 10% SDS-polyacrylamide gel was loaded with 50 μg of protein/well and electrophoresed at 100 V, 4°C according to the Laemmli method. Protein was then transferred to nitrocellulose. Blots were probed with 1:1000 anti-pan ERK antibody against total ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA); 1:500 anti-phosphoERK antibody against activated ERK1/2 (PerkinElmer Life Science Products, Boston MA); 1:500 anti-COX-1 antibody (Santa Cruz Biotechnology), or 1:500 anti-COX-2 antibody (Dr. Dan Dixon, University of Utah, Salt Lake City, UT). Blots were washed with Tris-buffered saline-Tween, incubated with an appropriate secondary antibody conjugated with horseradish peroxidase (Sigma), washed, and exposed to Kodak BioMax x-ray film after Bio-Rad enhanced chemiluminescence development.
Prostaglandin and Leukotriene Enzymeimmunoassays
Quantitation of total prostaglandin E2 (PGE2) produced by spreading cells was by use of a Cayman Chemical STAT-Prostaglandin E2 immunoassay kit 514131, according to the manufacturer's recommended protocol. Briefly, cells were prepared ± inhibitors at concentrations indicated in figure legends and plated on FN- or BSA-coated plates as described above; both cells and medium were collected at 4°C with kit-provided lysis buffer at indicated times, maintained at −70°C until all samples were collected, and subjected to enzymeimmunoassay for quantitation of PGE2 as an indicator of cyclooxygenase activity. Cayman Chemical immunoassay kits for analyzing cysteinyl leukotrienes LTC4/LTD4/LTE4 (520501) or for leukotriene B4 (LTB4) only (520503) were used to quantitate total cell leukotriene (LT) levels, according to the manufacturer's directions. Briefly, samples of spreading cells and medium ± indicated inhibitors were collected as described above; lipids were extracted with methanol and purified by chromatography with the use of AmPrep C18 columns, dried under nitrogen, and resuspended in kit assay buffer. Data from triplicate assays for each LT type were analyzed for statistical significance by ANOVA.
Migration Assay
Migration was evaluated by use of modified Boyden chambers in 24-well polystyrene plates (Costar, Cambridge, MA). Both sides of the Boyden insert filter were coated with FN as described above for plates, and then inserts were equilibrated in serum-free DMEM-BSA containing the indicated inhibitor at concentrations indicated in figure legends. Cells were prepared and incubated as described above with or without indicated inhibitors ± PGE2, and then 200 μl of suspended cells adjusted to 1 × 10 4 cells/ml was pipetted into an insert and permitted to attach and spread on the inner filter surface for 1 h. Insert inner filters were briefly examined by microscope to ascertain equal cell spreading in all treatment inserts. Inserts with spread cells on the inner filter were then moved to wells containing fresh DMEM-BSA with or without inhibitors, ± PGE2, to permit random migration across the filter. For assays with later additions of inhibitors or prostaglandin, cells were pipetted into the filters in medium without additions to permit attachment and early spreading to occur first, and then at 30 min postplating medium in both upper and outer chambers was exchanged for that containing the indicated inhibitors, ± PGE2. At indicated times, cells on filters were fixed in 4% paraformaldehyde and stained with 1% Coomassie blue dye in 40:10:40 (vol/vol) methanol/glacial acetic acid/double distilled H2O. Cells remaining on the inside filter surface were gently removed with a cotton swab to permit visualization and counting of cells on the outer filter surface. Migration was evaluated by phase-contrast microscopy as the number of cells migrated to the outer filter surface, normalized to percentage of control cell migration at 3–4 h postplating, depending on the assay.
Measurement of Spread Cell Surface Area
Cells plated on an FN substrate, with or without inhibitors or other additions as described above, were photographed at 60 min postplating under a phase-contrast microscope with the use of a Nikon camera and Kodak T-Max 400 film, at the same magnification for all treatments. Scanned negatives were digitized at identical resolutions, and three random fields were selected for each treatment containing minimally 200 cells/field. Mean cell surface area was determined with the use of SigmaScanPro image analysis software (Jandel Scientific), and normalized as percentage of control spread cell surface area.
Data Analysis
Data from replicate assays were grouped and analyzed by ANOVA with the use of SigmaStat statistical software (Jandel Scientific). The number of included experiments and the statistical significance of the data presented are indicated in RESULTS, or in the figure legends.
RESULTS
Cell Spreading Kinetics and Morphology
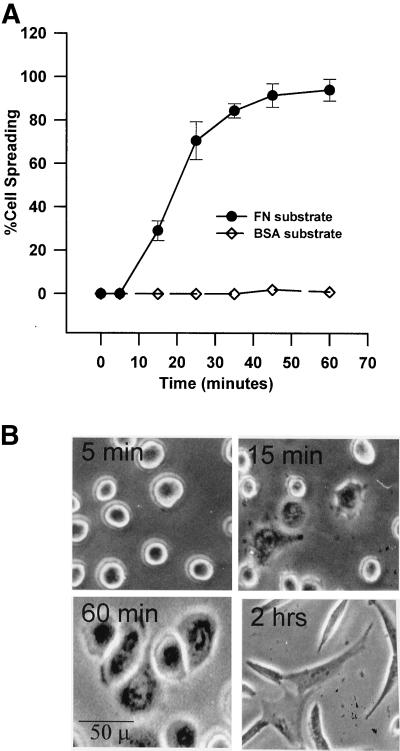
The percentage of NIH-3T3 cells that spread versus time was determined with the use of a fibronectin substrate, an ECM protein for which the cells have integrin receptors, and compared with a nonspecific substrate, BSA, which is not permissive to spreading (Figure 1A). Under the conditions used herein, cells attached to FN- coated culture dishes within 5 min of plating but remain rounded, although they exhibit distinct refractive changes indicative of initiation of early spreading (Figure 1B, 5 min). Partial spreading of 20–25% of the plated cells is observed at ∼15 min (15 min); full spreading of 90–95% of the cell population is seen at ∼60 min (60 min) and is characterized by a flattened “fried-egg” appearance. NIH-3T3 cells assume a typically fibroblast pyramidal shape that is associated with onset of migration ∼2 h after plating. Cells plated on the nonspecific BSA substrate remain rounded and do not spread over the time of the assay. The BSA-plated cells remained viable and spread if collected and replated on FN.
Figure 1.
Kinetics and morphology of NIH-3T3 cell spreading on fibronectin. (A) Percentage of cells spread versus time. Detached cells were suspended in serum-free medium and plated on plastic dishes coated with 20 μg of FN or 50 μg of BSA. Percentage of spreading was determined as described in MATERIALS AND METHODS. Points with error bars represent means with SEs (n = 4). (B) Morphology of cell spreading over time on FN. Under the conditions used herein, cells attached to fibronectin but remain rounded within 5 min of plating (5 min). Partial spreading is observed commencing at ∼15 min (15 min); full spreading of 90–95% of the cell population is seen at ∼60 min (60 min). The typically fibroblast pyramidal shape seen 2 h postplating appears to be the migratory phenotype in these cells (2 h).
Regulation of Cell Spreading
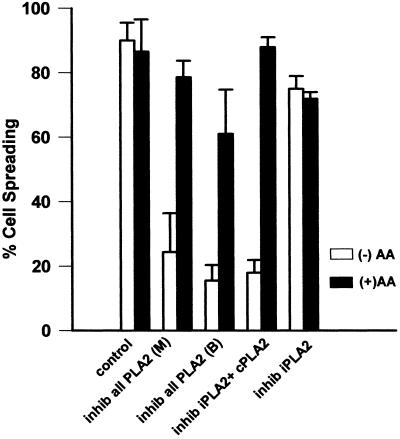
We had previously shown with the use of a nonselective inhibitor of the various forms of PLA2 that AA release was essential for both HeLa cell spreading on a collagen substrate (Chun and Jacobson, 1992) and NIH-3T3 cell spreading on a fibronectin substrate (Whitfield and Jacobson, 1999). Herein, we confirm this and demonstrate that it is most likely the cytosolic form of PLA2 that appears to be responsible for most of the AA released by the cells during adhesion (Figure 2). In addition to the nonselective inhibitors of all PLA2 forms, mepacrine [inhib all PLA2 (M)] and 4-bromophenacyl bromide (BPB) [inhib all PLA2 (B)], AACOCF3, a more selective inhibitor of both the calcium-dependent cytosolic cPLA2 and the calcium-independent iPLA2 but not the secretory PLA2, also inhibited cell spreading (inhib iPLA + cPLA2). Additionally, a specific inhibitor of iPLA2, HELSS (inhib iPLA2), was also used to subtractively differentiate between cPLA2 and iPLA2 by comparison with AACOCF3. No difference in spreading response was seen between the general PLA2 inhibitors and AACOCF3, as all produced dose-dependent inhibition of spreading. The iPLA2-specific inhibitor HELSS caused a 10–15% reduction in spreading at any concentration that was of questionable significance (p ≤ 0.05). Figure 2 shows the results with the use of a single higher concentration of each respective inhibitor (white bars) that in the case of cPLA2 inhibition by means of mepacrine, BPB, or AACOCF3, achieved significant inhibition of cell spreading (p ≤ 0.01) and was reversible by addition of the immediate downstream metabolite AA.
Figure 2.
(A) 3T3 cell spreading is sensitive to inhibition of a phospholipase A2. Detached cells were suspended in serum-free medium with or without the indicated inhibitor for 15 min and then plated on a FN substrate. Percentage of spreading was evaluated at 60 min. Bars indicate treatment with 30 μM mepacrine [inhib all PLA2 (M)] or 3 μM BPB [inhib all PLA2 (B)], general inhibitors of all PLA2 isoforms; or with AACOCF3 (25 μM), an inhibitor of both iPLA2 and cPLA2 (inhib cPLA2 + iPLA2), or with the iPLA2-specific inhibitor HELSS (10 μM) (inhib iPLA2). All are shown either with (black bars) or without (white bars) 15 μM arachidonic acid (n = 3; means with SEs shown). Mepacrine, BPB, and AACOCF3 all produce dose-dependent spreading inhibition; a single higher concentration of each is shown herein that is reversible with exogenous AA. HELSS treatment produced a small 10–15% reduction at any concentration but the effect was not reversible by addition of AA.
To distinguish between inhibition of spreading that is directly related to the blockade of PLA2 as opposed to nonspecific cytotoxic effects of the inhibitor, the reversal of spreading inhibition by the addition of exogenous AA was evaluated (Figure 2, black bars). BPB proved to be more cytotoxic than either mepacrine or AACOF3, because reversal by exogenous AA of spreading inhibition was incomplete at concentrations of BPB necessary to block spreading to the same degree as that seen with cells treated with the other inhibitors. Both mepacrine and AACOCF3 were essentially the same with regard to spreading inhibition and its full reversal by exogenous AA (p ≤ 0.05). The small amount of spreading inhibition seen with HELSS was not reversible by exogenous AA, indicating a probable slight toxicity of the inhibitor. The observation that all general inhibitors of all PLA2s inhibited spreading (mepacrine and BPB), the more selective inhibitor of both iPLA2 and cPLA2 also inhibited spreading (AACOCF3), but the specific inhibitor of iPLA2 did not (HELSS), supports the idea that cPLA2 is the predominant isoform of PLA2 signaling cell spreading in fibroblasts, as was previously shown with HeLa cells (Crawford and Jacobson, 1998). The above-mentioned results are also consistent with previous work demonstrating that the amount of AA produced by cells during adhesion is rate-limiting with respect to the rate and extent of cell spreading (Chun and Jacobson, 1992; Crawford and Jacobson, 1998).
Roles of Arachidonic Acid Oxidative Enzymes and ERK1/2 in Cell Spreading
Arachidonic acid produced by PLA2 during cell attachment can be oxidized by three classes of enzymes that generate downstream second messengers potentially involved in cell spreading. One class is the lipoxygenases: 5-LOX, 12-LOX, and 15-LOX, which are distinguished by the position of the carbon atom that is oxidized. Another class is the cyclooxygenases, either a constitutively expressed COX-1 or an inducible COX-2. Lastly, EOX also oxidizes AA.
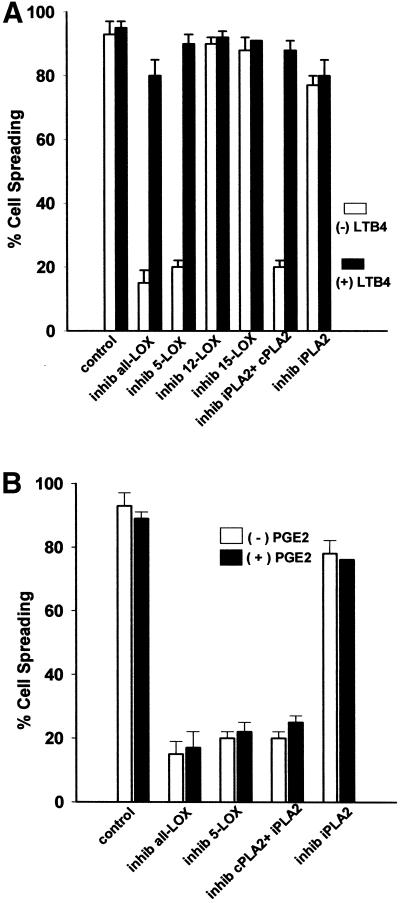
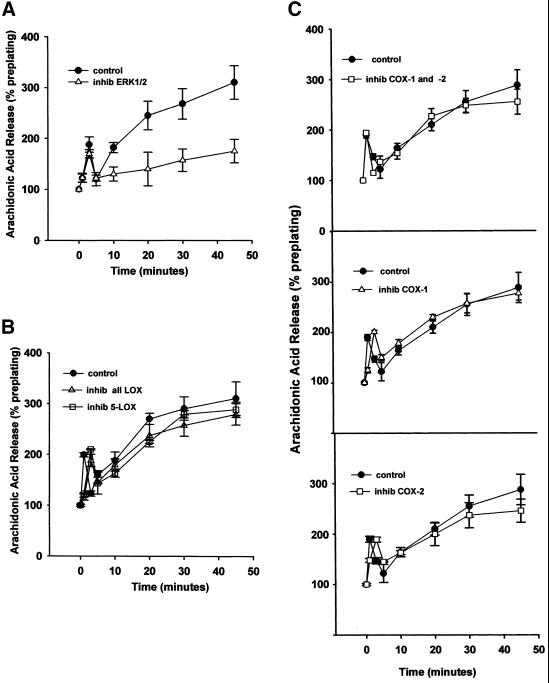
The role of lipoxygenases in cell spreading was evaluated with both nonselective and specific LOX inhibitors (Figure 3A). Nordihydroguaretic acid (NDGA), a nonselective inhibitor of all lipoxygenases, inhibited cell spreading in a dose-dependent manner (inhib all LOX), as did the specific 5-LOX inhibitor AA861 (inhib 5-LOX) (p ≤ 0.01); whereas the specific 12-LOX and 15-LOX inhibitors baicalein (inhib 12-LOX) and PD14617 (inhib 15-LOX), respectively, did not (Figure 3A, white bars). In addition, the inhibition of cell spreading by both the pan-LOX inhibitor NDGA and the 5-LOX inhibitor was reversed to control levels of spreading by the addition of exogenous LTB4 (black bars). Although the results are consistent with 5-LOX being responsible for cell spreading, it is not known from these data whether the 12-LOX and 15-LOX enzymes are not involved, or merely absent from the cell. Furthermore, although the inhibitors of the LOXs are very specific except for the pan-LOX inhibitor NDGA, we do not have an independent measure at this point that the 12-LOX and 15-LOX inhibitors were selective in the fibroblasts in the absence of readily available activity assays for these enzymes. Reduction in spreading was dose-dependent with NDGA (inhib all LOX) and AA861 (inhib 5-LOX) (p ≤ 0.01); representative higher concentrations are shown herein. Exogenous LTB4 also reversed the spreading inhibition due to the PLA2 inhibitor AACOCF3 (inhib iPLA2 + cPLA2), indicating that the leukotriene spreading signal is downstream of PLA2 and AA release. The black bars in Figure 3B show that the addition of 50 nM PGE2, a major cyclooxygenase product, does not reverse the spreading inhibition caused by LOX or PLA2 blockade.
Figure 3.
(A) Leukotriene B4 reverses spreading blockade from inhibitors of 5-lipoxygenase or cytosolic phospholipase A2. General inhibition of lipoxygenases with 30 μM NDGA (inhib all LOX), and specific inhibition of 5-LOX with 25 μM AA861 (inhib 5-LOX) reduced NIH-3T3 cell spreading, whereas inhibition of either 12-LOX with up to 100 μM baicalein (inhib 12-LOX), or of 15-LOX with up to 25 μM PD146176 (inhib 15-LOX) had no effect. The decrease from NDGA or AA861 was dose-dependent; representative higher concentrations are shown herein. The spreading blockade due to inhibition of any LOX by NDGA or 5-LOX by AA861, or of cPLA2 and iPLA2 by AACOCF3 (inhib iPLA2 + cPLA2) was reversible by addition of the 5-LOX oxidative product 100 nM LTB4 (black bars). Detached cells were incubated with indicated treatment and then plated on FN-coated dishes for 60 min at 37°C (n = 3; means with SEs). (B) Exogenous prostaglandin E2 does not reverse spreading blockade due to phospholipase A2 or lipoxygenase inhibitors. Cells were treated with inhibitors and allowed to spread as in A, except with the cyclooxygenase oxidative product PGE2, at 50 nM (n = 2).
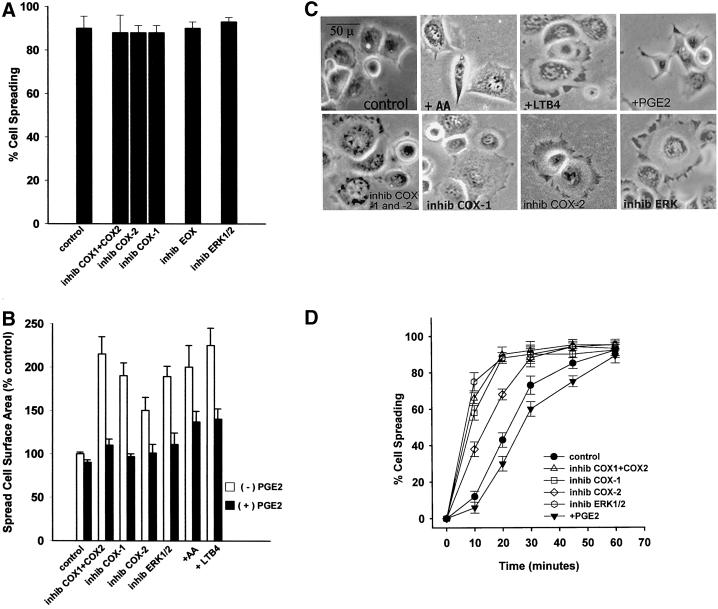
To examine the potential role of the other oxidative enzymes in cell spreading, a nonselective inhibitor of both cyclooxygenases, indomethacin (inhib COX1 + COX2), was used, as were SC236 to specifically inhibit COX-2 (inhib COX-2) and resveratrol to specifically inhibit COX-1 (inhib COX-1) (Figure 4A). Additionally, metyrapone was used to inhibit epoxygenase, the remaining branch enzyme of AA metabolism (inhib EOX). Multiple concentrations of each inhibitor were tested, and representative higher concentrations are shown herein. An inhibitor of the mitogen-activated protein kinase ERK1/2, PD98059, was also used and is shown herein at 50 μM (inhib ERK1/2), because we were evaluating a potential relationship between ERK and the COXs in adhesion. No COX or ERK inhibitor produced a reduction in cell spreading, (p ≤ 0.01) (Figure 4A). Furthermore, although the percentage of the cells that were spread at 60 min was not affected by the COX or ERK inhibitors, all enhanced the extent to which individual cells spread as measured by mean surface area (Figure 4B, white bars), with the greatest increases in surface area from the pan-COX inhibitor indomethacin and the ERK inhibitor PD98059. Exogenous AA (+AA) and LTB4 (+LTB4) were also tested for effects on mean spread cell surface area and were found to produce similar effects in increasing surface area of spread cells at 60 min. Increased spread cell surface area due to inhibition of COXs or ERK, or due to addition of AA or LTB4, were all reversed to near control mean surface area by addition of 50 nM PGE2, a major COX oxidative product (Figure 4B, black bars). Addition of 50 nM PGE2 alone reduced surface area ∼10% compared with control cells, not a statistically significant change (p ≤ 0.05), although the overall shape of the PGE2-treated cells was less rounded than control cells and appeared to be more akin to the later 2-h migratory cells seen in Figure 1B. Photographs of representative cells in Figure 4C show the distinctive spreading morphology stimulated by addition of AA, LTB4, or PGE2; or by the inhibition of ERK or COXs. Photo panels are labeled as in Figure 4A.
Figure 4.
(A) Cyclooxygenase, epoxygenase, and ERK activity are not required for cell spreading. Cells were detached, suspended in serum-free medium, and incubated with varying concentrations of COX, EOX, or ERK1/2/inhibitors before plating on FN-coated plates at 37°C. Single representative higher concentrations are shown herein: 50 μM indomethacin against both cyclooxygenases (inhib COX1 + COX2), 25 μM resveratrol against COX-1 (inhib COX-1), 25 μM SC236 against COX-2 (inhib COX-2), 50 μM metyrapone against epoxygenase (inhib EOX), or 50 μM PD98059 against ERK1/2 (inhib ERK1/2). Percentage of cell spreading was evaluated at 60 min (bars indicate means and SE; n = 3). (B) Mean spread cell surface area at 60 min is increased by exogenous arachidonic acid or leukotriene B4, and by cyclooxygenase-1/2 or ERK1/2 inhibitors. Cells treated as in A were photographed at the same magnification and mean cell surface area measured by SigmaScanPro surface area analysis program. Bars are labeled as in A and indicate means and SE normalized to percentage of control surface area. Exogenous PGE2 (50 nM) reverses the spreading cell surface area enhancement seen with addition of AA, LTB4, indomethacin, resveratrol, SC236, or PD98059 at concentrations shown in A. Addition of 50 nM PGE2 alone reduced surface area at 60 min by ∼10% compared with control untreated cells (n = 200 cells/treatment/field × three fields). (C) Morphology of spreading cells with arachidonic acid or leukotriene B4, and with lipoxygenase, cyclooxygenase or ERK inhibitors. Representative cell spreading at 60 min shown with or without 50 μM PD98059 to block ERK1/2 (inhib ERK), 50 μM indomethacin to block both COX-1 and COX-2 (inhib COX-1 and -2), 25 μM SC236 to block COX-2 (inhib COX-2), or 25 μM resveratrol to inhibit COX-1 (inhib COX-1) at concentrations indicated in A. The enhancement of spread cell surface area induced by these inhibitors resembles those of cells treated with 15 μM AA alone (+AA), or with 50 nM LTB4 (+LTB4), an oxidative product of 5-LOX, whereas addition of 50 nM PGE2 (+PGE2) slightly reduces surface area and alters overall shape. (D) Cell spreading kinetic rate is also increased by cyclooxygenase inhibitors. Cells treated with the same inhibitors at the concentrations as in A were measured for the rate of cell spreading over time as described in Figure 1A. COX inhibitors increased cell spreading rate over the first 30 min of spreading in a dose-dependent manner; representative higher concentrations are shown herein (means and SEs; n = 4).
Figure 4D shows that in addition to increasing mean spread cell surface area at 60 min, COX or ERK inhibition significantly increases the kinetic rate of cell spreading during the first 30 min of adhesion (p ≤ 0.05), although by 60 min the spreading percentage is equal between control and inhibited cells.
These data suggest that AA mass is rate-limiting for cell spreading, and that its oxidation by lipoxygenases signals spreading, whereas cyclooxygenase activity produces a slowing effect on spreading by competing with LOX for available AA. It is reasonable to attribute the faster spreading in the presence of COX inhibitors to increased LOX activity due to more AA being available to LOX enzymes. Additionally, the reversal of spreading inhibition by exogenous LTB4 applied to blockade caused by cPLA2, pan-LOX, and 5-LOX inhibitors suggests that of all the potential AA oxidative enzymes only 5-LOX participates significantly in signaling cell spreading, and its effect is downstream of PLA2-mediated AA release.
Arachidonic Acid Release
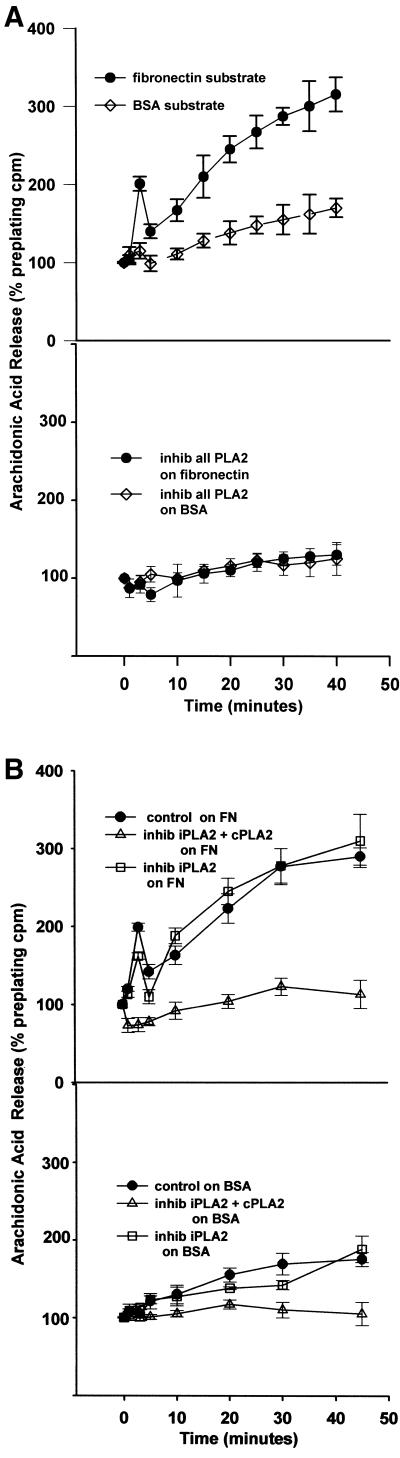
To further understand the role of AA in cell adhesion we determined the kinetics of AA released from [3H]AA prelabeled cells adhering to a fibronectin substrate. A kinetically biphasic release of [3H]AA was seen when NIH-3T3 cells attached and spread on FN (Figure 5A). The first phase was a burst or transient release of AA that occurred within the first 5 min of cell plating (closed circles, top), and coincided with the suspended plated cells settling and contacting the FN substrate. This was followed by a second phase, which was a sustained increase in AA release occurring over the next hour. As a control, cells were also plated on a BSA substrate where they do not spread. On a BSA substrate the cells produced only the later sustained release of [3H]AA, albeit ∼50% less than that seen with cells on FN (Figure 5A, top, open diamonds). All phases of [3H]AA release, whether the cells were on FN or BSA, were inhibited by the addition of the general PLA2 inhibitor mepacrine (bottom), indicating the AA was produced by a PLA2.
Figure 5.
(A) NIH-3T3 cell spreading on a fibronectin substrate produces a biphasic release of arachidonic acid from phospholipase A2 activity. (Top) Cells were incubated in 4 μM [3H]arachidonic acid for 18 h, detached, suspended in serum-free medium, and plated on either a FN (black circles) or a BSA (open diamonds) substrate. Cells spread fully on FN but do not attach or spread on BSA. Samples of medium were removed at the indicated intervals, and AA evaluated as appearance of label in the medium. Data are normalized as a percentage of [3H]AA counts in the medium before plating (means with SE; n = 4). (Bottom) [3H]AA release from cells treated with 30 μM mepacrine, an inhibitor of all PLA2s, before plating on either substrate. (B) Treatment of cells with 25 μM AACOCF3 (open diamonds), an inhibitor of both iPLA2 and cPLA2, also reduces AA release from cells plated on a FN or BSA substrate, whereas treatment with 10 μM HELSS (open squares), a specific inhibitor of iPLA2 only, does not block AA release (n = 3).
To further test the identity of PLA2 involved, the same assay was repeated with AACOCF3, an inhibitor of iPLA2 and cPLA2, and the iPLA2-specific inhibitor HELSS (Figure 5B). Whereas HELSS (open squares) did not affect AA release at any phase or on either FN or BSA, AACOCF3 (open triangles) blocked both stages of AA release on FN, and suppressed overall release on BSA as well. This comparative use of the PLA2 inhibitors supports that cPLA2 is the predominant PLA2 involved in signaling cell spreading by mediating AA release.
The observation that the initial transient release of AA only occurred on an FN substrate where cells spread, and not on the nonspreading BSA substrate, suggested that the initial AA release alone without respect to the second sustained release was sufficient to signal cell spreading. This is consistent with the initial transient release of [3H]AA experienced by cells on the FN substrate being due to the ligation and clustering of integrin receptors, because it has been previously shown that clustering FN receptors stimulates AA release (Auer and Jacobson, 1995; Szabo et al., 1995; Haimovich et al., 1999; Zhu et al., 1999; Whitfield and Jacobson, 1999). Additional assays were conducted to test this possibility (Figure 6A). The general PLA2 inhibitor mepacrine added at 5 min after cell plating on a FN-coated dish (open squares) significantly reduced the second AA release, but not the first (p ≤ 0.05) (Figure 6A, bottom), yet the cells were still spread to ∼80% of control cell spreading (Figure 6A, top, inhib all PLA2, 5 min postplating). However, mepacrine added to cells before plating reduced both phases of AA release (open triangles, bottom) and blocked 80–90% of cell spreading (top, preplating). This demonstrates that the initial transient AA release is sufficient to signal most cell spreading. In Figure 6B, the same assay is shown with the use of AACOCF3 to inhibit both cPLA2 and iPLA2, added to cells either preplating (open triangles) or 5 min postplating (open squares) after the first transient AA release. The results with AACOCF3 are very similar to those described above with mepacrine, with respect to both AA release and cell spreading, in that preincubation of cells with the iPLA2 + cPLA2 inhibitor reduced cell spreading and both phases of AA release, whereas addition after 5 min permitted the initial transient release and also spreading equal to control (p ≤ 0.05). Figure 6C repeats this experiment with the use of the iPLA2-specific inhibitor HELSS. Addition of HELSS at either preplating or at 5 min postplating caused a 10% reduction in the later phase of AA release that was not significant (p ≤ 0.05) (top), with a slight 10–15% reduction in spreading (bottom). These data also support a predominant role for cPLA2 and not iPLA2 in signaling NIH-3T3 cell spreading.
Figure 6.
(A) Initial transient arachidonic acid release signals most cell spreading. The general PLA2 inhibitor mepacrine (30 μM), added to plated cells at 5 min postplating after the early transient AA release seen in Figure 5A had already occurred, suppressed the second AA release but not the first (bottom; open squares) and still permitted most cell spreading (top; inhib all PLA2 5 min post-plating). Treatment with the same inhibitor before plating suppresses both phases of AA release (bottom; open triangles), and cell spreading (top; inhib all PLA2 pre-plating). (B, top) As with mepacrine in A, 25 μM AACOCF3 (inhib iPLA2 + cPLA2), added to cells after the initial transient AA release at 5 min, blocks the second AA release but not the first, and still permits most cell spreading (bottom) (means and SEs; n = 3). (C) HELSS (10 μM) (inhib iPLA2) added to cells either at 5 min after the initial AA release, or incubated before plating, does not significantly block either phase of AA release (top), or cell spreading (bottom).
If as indicated above, the initial transient AA release that mediates cell spreading is in response to FN receptor clustering, what activates the second sustained release of AA? Based upon previous work showing that ERK1/2 activation during spreading in some systems regulated PLA2 phosphorylation and AA release, we evaluated AA release from cells in the presence of an inhibitor of ERK1/2 activation. Cells treated with PD98059, a specific inhibitor of MEK activation of ERK1/2, also displayed a biphasic AA release (Figure 7A). Although the initial transient AA release was unaffected, the second sustained release was significantly diminished, ∼50% compared with control between 10 and 60 min (p ≤ 0.05). This suggests that the transient initial AA release signaling spreading appears to be ERK-independent with respect to PLA2 activation. However, the later phase of release is clearly PD98059-sensitive; indicating that ERK1/2 is modulating PLA2 activity after the first release, to increase the second release by ∼50% after the spreading signal has been completed.
Figure 7.
(A) Inhibition of ERK1/2 phosphorylation with PD98059 partially suppresses the second, but not the initial transient arachidonic acid release, during cell spreading. Cells were treated with 50 μM PD98059 to inhibit activation of ERK1/2 as described in MATERIALS AND METHODS. Control untreated (closed circles) and ERK-inhibited (open triangles) cells respond similarly until 10–15 min postplating, at the beginning of the sustained second phase of AA release; ERK inhibition results in ∼50% reduction in the second AA release without blocking the first transient AA release (means and SEs; n = 3.), while not inhibiting cell spreading (Figure 4A). (B) 5-Lipoxygenase inhibition does not affect AA release. Cells treated with either the general LOX inhibitor NDGA at 30 μM (inhib all LOX) or the 5-LOX inhibitor AA861 (25 μM) (inhib 5-LOX) do not experience reduced AA release in either phase (n = 2). (C) Cyclooxygenase inhibition does not affect AA release. Cells treated with 50 μM indomethacin to inhibit COX-1 and -2 (inhib COX-1 and -2), 25 μM resveratrol to inhibit COX-1 (inhib COX-1), or 25 μM SC236 to inhibit COX-2 ([inhib COX-2] were tested for AA release during spreading as in A. (n = 3).
Figure 7B shows that addition of the pan-LOX inhibitor NDGA (inhib all LOX) or the 5-LOX specific inhibitor AA861 (inhib 5-LOX) does not significantly affect AA release in treated cells versus control cells. Figure 7C additionally demonstrates that inhibition of both COX-1 and COX-2 by indomethacin (top), inhibition of COX-1 by resveratrol (middle), or of COX-2 by SC236 (bottom) also do not significantly affect either phase of AA release (p ≤ 0.05). These data suggest that regulation of cPLA2-mediated AA release during adhesion is partially modulated by ERK1/2, but is not subject to feedback regulation by products of AA oxidation from either LOX or COX pathways.
Cell Migration
Although the results of the above-mentioned experiments indicate that COX oxidation of AA is not essential for the spreading stage of cell-substrate adhesion, it was possible that activity of one or both COX isoforms could be necessary for the cell migration stage. To test a potential role for cyclooxygenases and ERK1/2 in the migration stage, NIH-3T3 cells were serum-starved for 24 h to down-regulate COX-2 expression, treated with various COX or ERK inhibitors, and then plated on FN-coated 0.8-μm filters in modified Boyden chambers. The number of cells that migrated across the filter in the next 4 h was then determined as indicated in MATERIALS AND METHODS. Figure 8A shows that indomethacin to inhibit both COXs, SC236 to inhibit COX-2, or PD98059 to inhibit ERK1/2 all resulted in significant and dose-dependent reductions in migration (white bars) that were reversed by addition of exogenous PGE2 (black bars). Resveratrol, a COX-1 specific inhibitor, also reduced migration, but to a significantly lesser extent than COX-2 inhibition, in that even at higher concentrations of the COX-1 inhibitor migration was not reduced to less than ∼70% of control. This was also reversible by exogenous PGE2. The ability of PGE2 to overcome the blockade of ERK indicates that COX activity is downstream of ERK in the adhesion-signaling pathway. Furthermore, added PGE2 alone enhanced migration almost twofold over that of control, suggesting that the amount of prostaglandins endogenously produced is rate-limiting with regard to the cell migration stage of cell-substrate adhesion. The significantly different levels of migration reduction due to COX-1 or COX-2 inhibition, respectively, also suggests that COX-2 contributes more of the total prostaglandin mass signaling migration than does COX-1.
Figure 8.
Cell migration requires ERK1/2 and cyclooxygenase activity. (A) NIH-3T3 cell migration was evaluated with Boyden chamber assays as described in MATERIALS AND METHODS. Cells were treated with 50 μM PD98059 to inhibit ERK1/2 (inhib ERK1/2), 50 μM indomethacin to inhibit both cyclooxygenases (inhib COX1 + COX2), 25 μM SC236 to inhibit cyclooxygenase-2 (inhib COX-2), or 25 μM resveratrol to inhibit cyclooxygenase-1 (inhib COX-1). White bars indicate inhibitor alone, black bars indicate addition of the cyclooxygenase oxidation product PGE2 (50 nM) with inhibitor. Dose-dependent reduction in migration was seen with PD98059, indomethacin, and SC236, the figure shows a typical higher concentration of each reversible by exogenous PGE2. Resveratrol inhibition of COX-1 was dose-dependent for a limited range, but did not reduce migration to <70% of control at concentrations >25 μM (means and SEs; n = 3) (B) Inhibitors of COX-2 or PLA2 added after 30 min of cell spreading block most cell migration. Inhibitors were added to cells after 30 min of cell plating on FN after the initial AA release had taken place, to test the requirement for AA generated in the second sustained release as a substrate for cyclooxygenase activity. Addition of 25 μM AACOCF3 (inhib iPLA2 + cPLA2), 50 μM indomethacin (inhib COX1 + COX2), or 25 μM SC236 (inhib COX-2), after 30 min of cell spreading, reduces migration in a dose-dependent manner; a single representative inhibitory concentration is shown herein that is reversible by PGE2 addition. The COX-1 inhibitor resveratrol at 25 μM (inhib COX-1) producesonly ∼25% reduction in migration when added after 30 min of spreading. Inhibition of ERK1/2 with 50 μM PD98059 at 30 min postspreading also reduced migration only to ≥70% of control, compared with the migration of ≤15% of control seen in A at the same concentration added preplating. Post 30 min spreading addition of 10 μM HELSS (inhib iPLA2) produced a 10–15% reduction in migration that was not dose-dependent. Addition of 30 μM NDGA (inhib all LOX) or 25 μM AA861 (inhib 5-LOX) at 30 min postplating reduced migration also by only 15–20%.
A second series of experiments was designed to determine whether both the LOX and COX oxidation pathways are essential for cell migration after spreading is complete, and whether the second sustained AA release was providing a substrate for oxidative conversion to metabolites essential for signaling cell migration. Cells were first permitted to attach and spread on FN-coated Boyden chamber filters for 30 min, during which time the initial transient AA release and early spreading are completed, and then inhibitors of PLA2, LOX, or COXs were added to the medium in both the upper and lower chambers for the duration of the migration assay (Figure 8B, white bars). It was ascertained that addition of these inhibitors did not cause detachment from the filter or death of the spread cells. Dose-dependent inhibition of migration by the cPLA2 and iPLA2 inhibitor AACOCF3, but not by the iPLA2 inhibitor HELSS, added 30 min after plating indicates that the second release of AA is cPLA2-mediated and is essential for cell migration. A single higher concentration of each is shown herein. This result is in contrast to AACOCF3 inhibition of cell spreading, where it was shown that the initial transient release of AA was required for spreading (Figure 6B). NDGA, the general LOX inhibitor (inhib all LOX), and the 5-LOX inhibitor AA861 (inhib 5-LOX) at concentrations sufficient to inhibit spreading if applied before plating the cells, caused a small reduction to 80–85% of control migration, suggesting that the LOX signaling effect is largely complete by 30 min postplating. Indomethacin (inhib COX-1 + COX-2) blockade of both COXs, or SC236 inhibition of COX-2 (inhib COX-2) produced a significantly larger reduction in migration to 15% of control, similar to that seen when inhibitors were added before plating in Figure 6A (p ≤ 0.05). Resveratrol inhibition of COX-1 (inhib COX-1) added at 30 min postplating reduced migration only to ∼75% of control, very close to that seen when added preplating in Figure 6A. Addition of PD98059 to inhibit ERK1/2 after 30 min postplating reduced migration to ∼50% of control, significantly less inhibitory than the reduction to 15% of control migration seen when it was added preplating. This suggests that the signals contributed by ERK to migration are also predominantly complete before 30 min, but are nonetheless still needed to optimize migration after 30 min. Exogenous PGE2 (black bars) reversed any inhibition of migration due to any inhibitor, even when added to the iPLA2 inhibitor that did not significantly reduce migration, albeit to a lesser extent than that seen with PGE2 alone. These results, as well as those in Figures 3, 4, and 6, suggest that although the LOX oxidative branch of AA is required for the cell spreading stage of adhesion, it optimizes but is not absolutely required for the migration stage during the 3- to 4-h postplating that migration was measured. In addition, these results indicate that the leukotrienes signaling spreading probably come from AA produced during the first 30 min of cell adhesion, when the initial transient release of AA takes place. On the other hand, the COX oxidative branch of AA that is required for signaling migration appears to use the cPLA2-generated AA produced during the later sustained release to generate prostaglandins that signal migration. Although both COX-1 and COX-2 appear to use the sustained release of AA, COX-2 seems to be predominantly required to signal optimal migration. This idea was further tested by analyzing the respective COX activities during spreading and migration as described in the after section.
LOX and COX Activity Kinetics During Spreading
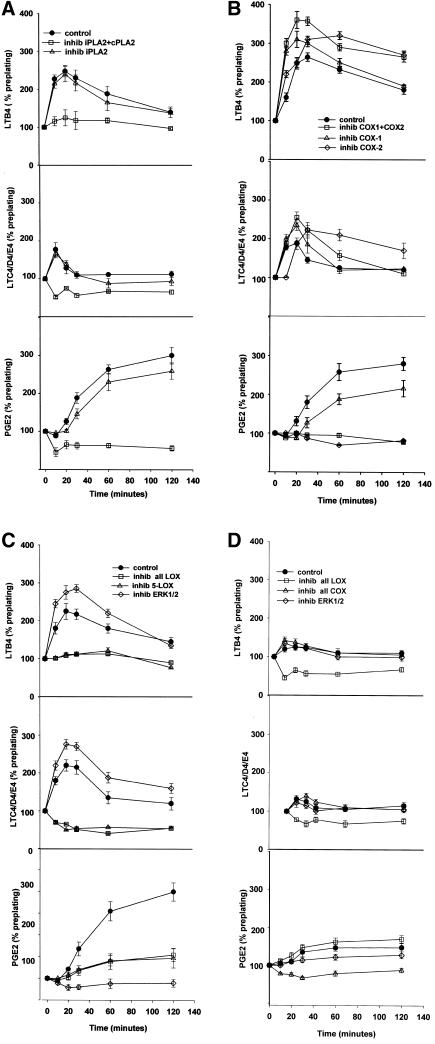
To further explore the possibility that there is a shift from the LOX to the COX oxidative branches of AA oxidation during cell-substrate adhesion, and whether ERK1/2 is involved in regulating the shift, we measured the production of prostaglandins and leukotrienes during spreading and early migration with the use of enzymeimmunoassays (Figure 9, A–D). Prostaglandin production was evaluated by measuring PGE2 levels. Leukotriene production was measured separately for both LTB4 and the cysteinyl leukotrienes LTC4/D4/E4. For all immunoassays, total cell production was evaluated by collecting cells plus the surrounding medium during the indicated time postplating. The amounts of LTs and PGE2 shown are normalized as a percentage of preplating levels. In Figure 9A, cells were treated with AACOCF3 to inhibit both iPLA2 and cPLA2 (open squares), or HELSS to inhibit iPLA2 alone (open triangles). Untreated control cells (closed circles) plated on FN show a transient increase in both LTB4 of more than twofold (top), and in the cysteinyl LTs (middle) of ∼1.8-fold, between 10 and 30 min postplating, followed by a slow decrease to near preplating levels by 120 min. Conversely, PGE2 synthesis lags during the first 30 min of cell spreading, followed by a threefold increase between 30 and 120 min (bottom).
Figure 9.
Lipoxygenase and cyclooxygenase activity during cell adhesion to FN is modulated by the competing oxidative branch enzymes, and by ERK1/2. (A) Arachidonic acid produced by cPLA2, but not iPLA2, is the substrate for both LOX and COX oxidative activities. Cells were detached, incubated in serum-freemedium with either 25 μM AACOCF3 (inhib iPLA2 + cPLA2) or 10 μM HELSS (inhib iPLA2), and plated on FN-coated plates as described in MATERIALS AND METHODS. Cells plus medium were collected at indicated time points and assayed by enzymeimmunoassay for the 5-LOX oxidative products LTB4 (top) and cysteinyl leukotrienes LTC4/D4/E4 (middle), and for the cyclooxygenase product PGE2 (bottom). (B) Inhibition of cyclooxygenases increases 5-lipoxygenase activity. Cells were treated with or without 50 μM indomethacin (inhib COX1 + COX2), 25 μM resveratrol (inhib COX-1), or 25 μM SC236 (inhib COX-2), plated on FN-coated plates, and collected over time as in A. (C) Inhibition of 5-LOX blocks leukotriene synthesis but does not increase prostaglandin levels; inhibition of ERK1/2 blocks PGE2 synthesis and also increases leukotriene levels. Cells were treated with 50 μM PD98059 (inhib ERK1/2), 30 μM NDGA (inhib all LOX), or 25 μM AA861 (inhib 5-LOX) and spread on FN-coated plates before collection and immunoassay as described in MATERIALS AND METHODS. (D) Leukotriene and prostaglandin E2 synthesis is generally reduced in cells plated on a BSA substrate. Cells treated with 30 μM NDGA (inhib all LOX), 50 μM indomethacin (inhib all COX), or 50 μM PD98059 to prevent ERK1/2 activation (inhib ERK1/2), were plated on a BSA substrate where no spreading occurs, to compare with prostanoid synthesis on a FN substrate as seen in A–C (means and SEs; n = 3).
No significant difference was seen between control (closed circles) and HELSS-treated cells with respect to any LT or to PGE2 synthesis (p ≤ 0.05). AACOCF3 addition reduced production of LTB4 (top), cysteinyl LTs (middle), and PGE2 (bottom), indicating that cPLA2 was producing the AA substrate for both 5-LOX and the cyclooxygenases. In Figure 9B, cells were treated with 50 μM indomethacin (open squares), 25 μM resveratrol (open triangles), or 25 μM SC236 (open diamonds), and plated on FN-coated plates as described in Figure 9A. Indomethacin prevented most PGE2 synthesis (bottom), and conversely significantly increased both cysteinyl LT and LTB4 synthesis (middle and top) by ∼60% over control (closed circles) at all times from initial plating to 120 min postplating (p ≤ 0.05). Inhibition of COX-1 by resveratrol also increased LTB4 (top) and cysteinyl LT production (middle) early in the first 30 min of spreading, but the effect diminished after 30 min postplating. COX-1 inhibition also caused a lesser reduction in PGE2 synthesis than did indomethacin, with PGE2 levels rising to ∼60% of control level between 60–120 min (bottom).
Inhibition of COX-2 caused a reduction in PGE2 synthesis equal to that of indomethacin after 30 min postplating (open diamonds, bottom). COX-2 inhibition also produced an increase in all LTs, but to a lesser degree than did indomethacin or resveratrol, and the increase in LT synthesis was delayed compared with the other COX inhibitors (top and middle, open diamonds). These data suggest that because indomethacin has an inhibitory effect on both COXs, and the COX-2 effect is more delayed, that COX-1 acts earlier than COX-2 in competing with 5-LOX for AA as an oxidative substrate.
In Figure 9C, cells were treated with 30 μM NDGA to inhibit all LOXs (open squares), or 25 μM AA861 (open triangles). Addition of either LOX inhibitor reduced LT synthesis to less than preplating levels (top and middle), but failed to stimulate greater PGE2 production more than control (bottom) (p ≤ 0.05). Whether this was due to a general failure in the downstream signaling pathway when spreading is blocked, or some other regulatory effect, is uncertain from this data.
Additionally, the role of ERK1/2 in PGE2 and leukotriene production was evaluated by inhibiting ERK1/2 activation with the MEK inhibitor PD98059 (Figure 9C, open diamonds). The production of both cysteinyl leukotrienes and LTB4 by NIH-3T3 cells was found to transiently increase ∼60% over control during the first 30 min of cell attachment (top and middle). This was followed by a slow decrease to background levels, and was accompanied by a concomitant suppression of PGE2 synthesis (bottom).
In Figure 9D, untreated control cells and cells treated with the pan-LOX inhibitor NDGA, the pan-COX inhibitor indomethacin, or the ERK1/2 inhibitor PD98059 were plated on a BSA substrate where no spreading occurs, to compare prostanoid synthesis versus that indicated above in the FN-mediated spreading. With untreated cells on BSA (closed circles), a small rise is seen early in LTB4 and cysteinyl LT synthesis (top and middle), accompanied by a rise in PGE2 accumulation after 30 min (bottom), a small increase by comparison with cells on the FN substrate. Inhibition of LOXs reduces synthesis of all LTs (top and middle), but does not cause an increase in PG synthesis. The prostanoid levels seen in cells plated and not spreading on BSA appear to indicate a small basal synthesis in the absence of adhesion-stimulated signaling.
It should be emphasized that the increased synthesis of leukotrienes in COX-inhibited, ERK-inhibited, and uninhibited cells spreading on FN took place within the first 30 min of cell attachment and early spreading. During this same 15-min period, there was a lag in the synthesis of PGE2 in control cells (bottom panels) for the first 30 min postplating, which was followed by a threefold increase over the next 2 h. This supports the idea that the initial transient release of AA observed during the first 5–10 min of cell attachment (Figures 5 and 6) is used for leukotriene synthesis that signals cell spreading. Conversely, ERK inhibition resulted in a reduction of PGE2 production to a low but not null level, whereas LOX inhibition did not have such a reductive effect. This suggests that normally ERK may have a direct positive regulatory effect on COX activity. The failure of ERK to affect LT or PGE2 synthesis in cells plated on BSA suggests that ERK may fail to be activated on BSA. This idea is further tested in the after section.
Overall, these data support the idea that in the early stages of cell spreading, 5-LOX competes with one or both of the COXs for available AA as an oxidative substrate. The suppression of PGE2 synthesis by inhibiting ERK suggests that ERK may regulate one or both COXs, either directly or by influencing induction of synthesis. Therefore, we also examined both COX-1 and COX-2 protein levels during the process of spreading and migration as described in the next section.
ERK1/2 Induction of COX-2 Synthesis
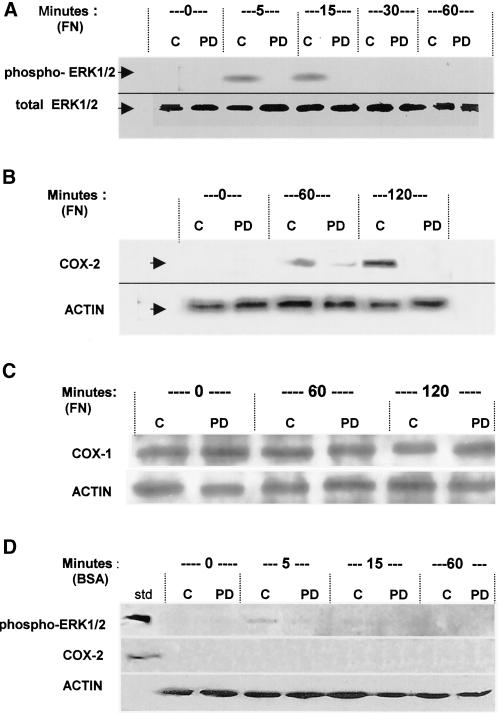
From the results described above indicating that both COXs and ERK1/2 were involved in the cell migration stage of adhesion, we tested whether this was due to an induction of COX-2 synthesis and whether the induction was modulated by ERK1/2. Cells were allowed to attach, spread, and migrate with or without the ERK inhibitor PD98059 on a FN substrate, and at various time points, they were lysed and prepared as described in MATERIALS AND METHODS for SDS-PAGE and Western blotting (Figure 10). Blots were probed for pan-ERK1/2 (total ERK 1/2) expression, or activated phosphorylated ERK (phospho-ERK) levels (Figure 10A), and for both COX-1 (COX-1; Figure 10C) and COX-2 (COX-2; Figure 10B) protein levels with the use of the appropriate antibodies as described in MATERIALS AND METHODS. ERK1/2 was seen to be rapidly phosphorylated within 5 min of plating and then dephosphorylated by 30 min postplating in control cells (C), whereas the amount of total ERK did not change over time (Figure 10A). This ERK1/2 activation by phosphorylation is a well-known effect of integrin-mediated cell interaction with ECM (Miyamoto et al., 1996; Cybulsky and McTavish, 1997; Langholz et al., 1997; Bourdoulous et al., 1998; Crawford and Jacobson, 1998). Addition of 50 μM PD98059 prevented phosphorylation as shown by the PD lanes. The results herein show that the experimental concentration of PD98059 used in subsequent experiments does prevent ERK phosphorylation and activation, which is rapid and transient in untreated cells.
Figure 10.
Inhibition of ERK1/2 activation blocks the induction and synthesis of COX-2 protein during NIH-3T3 cell adhesion. Cells with or without 50 μM PD98059 to inhibit activation of ERK1/2 were allowed to attach and spread on FN (A–C), or plated on BSA where no spreading occurs (D), for the indicated times in minutes. Cells were lysed and subjected to SDS-PAGE and immunoblotting as described in MATERIALS AND METHODS. In all blots, control untreated cell lanes are indicated with C, and cells treated with PD98059 are indicated with PD. (A) ERK1/2 expression and activation by phosphorylation during cell adhesion to FN. Total ERK1/2 as a loading indicator is shown in the bottom row, detected with anti-pan-ERK antibody (total ERK1/2). Activated ERK1/2 is shown in the top row, detected with anti-phosphoERK1/2 antibody (phospho-ERK1/2). (B) Inducible COX-2 expression during cell adhesion to FN. COX-2 protein was detected with an anti-COX-2 antibody (COX-2), and actin was probed (ACTIN) as a loading control. (C) COX-1 expression during cell adhesion to FN. COX-1 protein expression was probed with anti-COX-1 antibody (COX-1), with actin probed as a loading control (ACTIN). (D) ERK1/2 and COX-2 in nonspreading cells plated on BSA. The top blot strip was probed with anti-phosphoERK; a phospho-ERK protein standard is the upper left lane (std). The middle strip was probed for COX-2, also with a standard in the left lane. The bottom strip shows actin in the same cells. The blots shown are typical representatives of three experiments.
COX-2 protein was not detectable in the serum-starved cells before plating, but protein synthesis was significantly increased in response to cell-ECM adhesion by 60 min postplating in control cells, and continued to increase in expression well into the times these cells were beginning to migrate at 120 min (Figure 10B). Inhibition of ERK activation by PD98059 prevented the induced synthesis of COX-2 protein, as seen in the +PD lanes, compared with uninhibited control cells in the C lanes. A trace of COX-2 is apparent in the ERK-inhibited cells at 60 min, but is entirely absent by 2 h, whereas in control cells expression increases between 60 and 120 min. COX-1 protein levels were not affected by ERK1/2 inhibition, consistent with it being constitutively present in NIH-3T3 cells (Figure 10C). The above-mentioned data also support that the prolonged reduction in PGE2 synthesis associated with inhibition of ERK1/2 as seen in Figure 9C is due largely to a failure to induce COX-2 expression and synthesis.
Cells plated on a BSA substrate where no spreading occurs, with or with the ERK inhibitor, are shown in Figure 10D. A trace of activated ERK is seen in control cells on BSA (C) at 5 min postplating, but is absent at all other times. Addition of PD98059 prevented any phosphorylation (PD) at any time. This supports that ERK activation is largely dependent on cell-ECM attachment, as has been widely reported. Furthermore, COX-2 also failed to appear in cells on the BSA substrate (Figure 10D). This also suggests that the ERK signaling required for COX-2 up-regulation is adhesion-dependent.
DISCUSSION
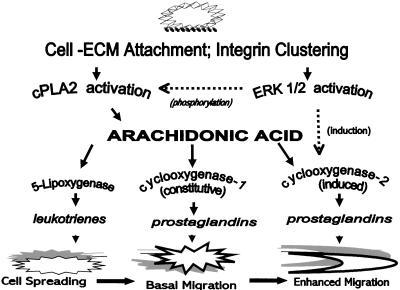
Our results indicate that the various stages of NIH-3T3 fibroblast adhesion, i.e., cell attachment, spreading, and migration, are independently regulated by different arachidonic acid metabolites. In brief, adhesion of the cells to FN in vitro begins with the integrin receptor-mediated attachment stage, during which cPLA2 is activated to release AA in a transient burst. This is followed by the spreading stage, which is signaled by leukotrienes synthesized through LOX oxidation of the rapidly released AA. Last, the migration stage is set in motion by prostaglandins formed by COX oxidation of AA released during the late spreading stage. In addition to showing that the cell spreading and migration stages are sequentially regulated and modulated by the divergence of LOX and COX branches of AA oxidation, we show that migration is also influenced at the level of gene transcription by the induction of COX-2 synthesis. The above-mentioned conclusions are schematically presented in Figure 11, and the discussion of the results that led to them is given below.
Figure 11.
Model of a bifurcated arachidonic acid pathway regulating a transition from cell spreading to cell migration. NIH-3T3 cell spreading on fibronectin is by means of α5β1-integrin receptors in the plasma membrane initially binding extracellular matrix fibronectin. The signaling pathway begins with integrin receptor clustering during cell-ECM attachment, which stimulates rapid cPLA2 activation and an immediate transient AA release oxidized by 5-lipoxygenase, generating leukotrienes to signal cell spreading. ERK1/2 is also rapidly activated during the cell attachment phase, and modulates cPLA2 activity to enhance a later sustained AA release, as well as inducing the immediate expression of COX-2 protein. Up-regulation of COX-2 increases total prostaglandin production over that contributed by constitutive COX-1, to stimulate a postspreading transition to migration.
Although the spreading stage of NIH-3T3 cells on fibronectin, as with that of HeLa cells on a collagen substrate, uses an AA signaling pathway summarized by PLA2 → AA → LOX → LT (Figures 1–3; Chun and Jacobson, 1992, 1993; Auer and Jacobson, 1995; Chun et al., 1996, 1997; Crawford and Jacobson, 1998; Whitfield and Jacobson, 1999), less is known of whether other oxidative enzymes of AA are involved. Roles for both the LOX and COX branches of AA metabolism have been implicated in growth factor-induced cytoskeletal changes such as stress fiber breakdown (Peppelenbosch et al., 1992) but how this impacts cell adhesion is not known. Observations that prompted us to look more carefully into the LOX and COX branches of AA metabolism in cell adhesion were that any treatment of HeLa cells or 3T3 fibroblasts that increased the flow of AA through the LOX branch also increased the extent to which cells spread, whereas when the flow of AA through the COX branch was stimulated, the extent to which the cells spread was decreased and migration was increased (Figures 4 and 5; Chun et al., 1997). In other words, there appears to be an opposing effect on the utilization of the available AA that is known to be released in rate-determining amounts with regard to LOX and COX activities during cell substrate adhesion. Furthermore, reports that COX appears to be associated with cell motility (Kreuzer et al., 1996; Minami et al., 1996; Tsujii et al., 1997; Daugschies and Ruttkowski, 1998; Horie-Sakata et al., 1998; Tsujii et al., 1998; Daniel et al., 1999) also support the hypothesis that LOX and COX differentially regulate the cell spreading and migration stages of adhesion.
The approach used to investigate the dual role of AA metabolism in regulating, respectively, the spreading and migration stage of NIH-3T3 fibroblast adhesion was primarily pharmacological knockout and rescue of enzyme activity. For such an approach to yield clear conclusions four criteria should be met: 1) production of PLA2, LOX, or COX metabolites must take place before or at least concomitantly with the function being evaluated; 2) inhibitors used to block these enzymatic reactions must be shown to actually function when given to the cells; 3) the effect of the inhibitors in blocking function must be reversible when the product of the inhibited reaction is exogenously provided to the cells; and 4) the inhibitors do not block function simply because they are cytotoxic. It should be emphasized that in all cases reported herein, cells were synchronized with regard to cell adhesion to enable a study of the regulation of the sequential stages of adhesion. NIH-3T3 fibroblasts grown in monolayers were synchronized by serum-starvation to down-regulate expression of COX-2, trypsinizing the cells, and then plating them as a suspension onto FN-coated culture dishes. These cells were harvested from preconfluent monolayers, because confluent monolayers yielded inconsistent results.
With the above-mentioned approach, the pivotal role of AA in adhesion of 3T3 fibroblasts was shown by demonstrating that inhibitors of cPLA2, but not sPLA2 or iPLA2, blocked the production of AA during cell attachment, with concomitant blockage of cell spreading and migration (Figures 2, 5, and 6). Next, it was found that the blockade of cell spreading and migration by the PLA2 inhibitors was reversed by the addition of exogenous AA to the cells, further confirming the role of AA in adhesion and indicating that the inhibitors were not cytotoxic. It was also shown that the AA formed by the action of PLA2 during adhesion that was used to signal cell spreading was released during the attachment stage, before the onset of cell spreading and migration (Figures 5 and 6).
The same pharmacological approach was used to determine how LOX and COX function in NIH-3T3 fibroblast spreading and migration. LOX metabolism of AA was found to regulate cell spreading by showing that 1) leukotrienes were produced by the cells before and during cell spreading; 2) leukotriene production by the cells, and cell spreading, were blocked by LOX inhibitors (Figure 9C); and 3) blockade of cell spreading from LOX inhibition was overcome by exogenous LTB4 (Figure 3A). It was also shown that COX metabolism was not only not required for cell spreading (Figure 4A), but also its suppression enhanced both the rate of cell spreading (Figure 4D) and the surface area of spread cells (Figure 4B). Finally, inhibition of COX blocked both migration (Figure 8A) and the production of prostaglandins by the cells and caused an increase in LOX activity (Figure 9B), whereas addition of exogenous PGE2 to COX-inhibited cells reversed the effect of the inhibitor on migration (Figure 8B). Prostaglandin E2 production by the cells was delayed during attachment and spreading, but began well before the cells started to migrate (Figure 9A). Taken together, the above-mentioned evidence indicates that the cell spreading stage of NIH-3T3 fibroblast adhesion to FN is dependent upon the metabolism of AA to leukotrienes by LOX, and that migration is dependent upon the oxidation of AA by COX to prostaglandins, as depicted by the model in Figure 11.
When the kinetics of AA production was measured with the cells synchronously attaching to FN, a biphasic release was found. An initial transient release occurring concomitantly with the cell attachment stage was followed by a second sustained release that occurred simultaneously with the spreading stage (Figure 5A). Further testing with LOX and COX inhibitors given to adhering cells either before or after the first transient AA release had taken place indicated that this initial release was required for cell spreading, whereas the second sustained release was not required for spreading but was essential for later cell migration (Figure 6, A and B). Additional support for the sequential nature of the adhesion stages of the NIH-3T3 cells to FN comes from experiments on the kinetics of leukotriene and prostaglandin synthesis. The LOX-mediated release of leukotrienes required for cell spreading began during the preceding attachment stage and then diminished after spreading, whereas COX-mediated release of prostaglandins essential for cell migration began later during the spreading stage, but before the migration stage (Figure 9). In addition to confirming the sequential nature of cell adhesion, these data also suggest that the initial transient release of AA provides substrate primarily for leukotriene synthesis, and the later sustained release of AA is primarily used for prostaglandin synthesis.
Although LOX metabolism of AA is essential for the cell spreading stage to take place, it appears that the later migration stage largely proceeds with minimal LOX activity. Not only does LOX activity diminish after 60 min of spreading (Figure 9B) but also 80% of control migration can still occur when LOX is inhibited after cell spreading is complete (Figure 8B). Interestingly, blockade of LOX activity by preincubation of cells with a LOX inhibitor before plating on FN did not result in a proportionately larger increase in prostaglandin production along the lines of the elevated LT levels from inhibition of COX (Figure 9C), as might have been expected. This may be because cell spreading was blocked by the LOX inhibitors, and the spreading block disrupted further downstream steps in the adhesion pathway, i.e., COX-2 synthesis and activity. Because COX-2 expression was not up-regulated in nonspreading cells plated on BSA, and there was also no difference in PGE2 levels between LOX-inhibited and control cells plated on the BSA substrate (Figure 9D), it is possible that the same situation occurred in cells prevented from spreading on FN by preincubation in the LOX inhibitor, although this idea was not tested herein. COX-2 up-regulation and activity appear to be responsible for ∼70% of the PGE2 rise after 60 min of cell spreading on FN (Figure 9B), so a failure in COX-2 induction and activity would leave only the constitutively expressed COX-1 to produce lesser amounts of PGE2 during later stages of adhesion. This would result in a situation comparable to that of the cells plated on BSA, wherein similarly lesser amounts of PGE2 were produced by COX-1 only (Figure 9D). In support of this is the observation that the amount of PGE2 produced by nonspreading LOX-inhibited cells on FN, although reduced substantially from control cells, is equivalent to the PGE2 production of the nonspreading cells plated on BSA where COX-2 expression was not induced. Because AA release from cells was not affected by preplating incubation in LOX inhibitors (Figure 7B), a lack of AA substrate for COX-1 or COX-2 is not responsible for the reduction in PGE2 seen with LOX-inhibited cells on FN. It should be emphasized that cells initially prevented from spreading by a LOX inhibitor are also not able to migrate because of the sequential nature of the adhesion process. It should not be concluded from the reduction in PGE2 production after preincubation in LOX inhibitors that LOX activity is also required during migration. LOX inhibitors added to cells after they had already fully spread had little effect on migration (Figure 8B), supporting the idea that LOX signaling of spreading is largely completed by the time migration ensues. Future work planned will further examine these remaining issues of regulation and expression between 5-LOX and COX-2.
The observation that there was a lower level of PGE2 produced during cell attachment compared with that produced during later cell spreading suggested that the inducible form of COX might be activated during cell adhesion to FN. Both COX-1 and COX-2 have been associated with migration, both in normal and transformed cells (Kreuzer et al., 1996; Daugschies and Ruttkowski, 1998; Horie-Sakata et al., 1998; Daniel et al., 1999). COX-1 is constitutively expressed in many cells and produces basal low levels of prostaglandins at a steady rate. We showed herein that COX-1 protein levels are unchanged in these cells over the time course of adhesion (Figure 10C). Therefore, it can be expected to be contributing a fairly stable level of PGs to the total cell level at any given time, and its expression and activity are not diminished by serum-starvation as is the case with COX-2.
It is reasonable to propose that COX-2 expression is induced during cell adhesion because ERK1/2 is activated during adhesion, and induction of COX-2 expression by growth-factor mitogens is mediated by ERK1/2 (Miyamoto et al., 1996; Cybulsky and McTavish, 1997; Hwang et al., 1997; Karim et al., 1997; Langholz et al., 1997; Bourdoulous et al., 1998; Miller et al., 1998; Sheng et al., 1998; Adderley and Fitzgerald, 1999). Our measurements indicate that COX-2 protein appeared in control cells at ∼45–60 min postplating, after spreading was essentially complete, whereas blockade of ERK1/2 prevented induction and synthesis of COX-2 (Figure 10B). ERK1/2 inhibition of COX-2 protein synthesis also reduced prostaglandin production to very low levels (Figure 9C). Basal PGE2 production in the presence of the ERK inhibitor therefore reflects the continuing presence of COX-1, which contributes lesser amounts of prostaglandins to cell maintenance. This is highlighted in Figure 9B wherein inhibition of COX-1 without inhibiting COX-2 still permits ∼70% of control cell PGE2 accumulation after 60 min. COX-1 protein expression was not sensitive to ERK1/2 inhibition (Figure 10C), which is consistent with its expression being constitutive. ERK1/2 inhibition also resulted in an increase in leukotriene production during the first 30 min of spreading (Figure 9C). Whether this is due to a negative ERK1/2 regulation of COX-1 or 5-LOX remains uncertain from these data, since the 30-min time zone of increased LT production precedes significant COX-2 synthesis. This remains to be evaluated in the future.
The eventual effects of the lipid metabolite pathway on cell morphology, spreading, and migration appear to be at the level of actin cytoskeleton polymerization. In previous work, LOX has been found to facilitate cell spreading by activation of protein kinase Cε (PKCε), resulting in the induction of actin polymerization (Chun and Jacobson, 1993; Timar et al., 1993; Chun et al., 1997). In the work presented herein, the marginal inhibition of migration (<20%) when LOX activity was inhibited after the cells were allowed to spread could reflect the need for turnover between monomeric and polymeric actin during the process of migration. Work in progress indicates that although LOX activity leads to PKC activation and actin polymerization producing spreading-associated stress fibers, COX activity leads to protein kinase A activation, stress fiber reduction, and actin filament bundling that appear to be essential for the cell migration stage (Glenn and Jacobson, personal communication).
In addition to the up-regulation of COX-2, ERK1/2 activation appears to be involved in directly modulating the later phase of AA release. It has been shown that ERK, as well as PKC and protein kinase A, can phosphorylate cPLA2 and thereby enhance its activity from 1.5- to 3-fold (Lin et al., 1993; Nemenoff et al., 1993). Although the inhibition of ERK1/2 did not impair the initial transient AA release, it did reduce the second sustained AA release by ∼50% (Figure 7A), while having no negative effect on cell spreading (Figure 4B). This suggests that ERK1/2 may increase cPLA2 activity by phosphorylation to enhance the second later release, after the initial transient AA release is complete. Unfortunately, we were unable to demonstrate phosphorylation of cPLA2 by a shift in its electrophoretic mobility, as done by others with fibroblasts (Clark and Hynes, 1996) as well as by us in with the use of HeLa cells (Crawford and Jacobson, 1998). However, the increased AA availability by cPLA2 activation in the second sustained release that begins ∼20 min postplating correlates well with both the advent of COX-2 synthesis and the increase in PGE2 production, both of which are dependent on ERK activation and required for optimal cell migration.
Interestingly, the reduction in the second sustained AA release seen herein with ERK1/2 inhibition also clears up another puzzle: why other work showed that ERK1/2 inhibition by use of a Ras dominant negative cell line did not affect NIH-3T3 cell spreading, but did reduce PLA2 activity, calling into question whether these cells needed PLA2-mediated AA to signal spreading (Clark and Hynes, 1996). The discrepancy can be explained by noting that the aforementioned work measured total AA release over ∼45 min, but omitted the very early postplating time points where the transient release of AA takes place as shown herein. Our work supports their observation of an overall reduction in AA release with ERK inhibition, as seen herein with respect to the second later AA release, but also adds detail in showing a previously undescribed novel early transient AA release that is ERK-independent and sufficiently signals spreading.
The evidence presented herein strongly supports that a bifurcation of AA oxidation into LOX and COX pathways is a critical aspect of cell spreading and subsequent cell migration. Furthermore, the degree of spreading and migration is rate-limited by the mass of leukotriene and prostaglandin produced during adhesion. This is supported by the observations that addition of exogenous LTB4 to NIH-3T3 fibroblasts increased the rate and extent of cell spreading (Figures 3A and 4C), while addition of exogenous PGE2 increased the rate of migration (Figure 8). These data support that spreading and migration rates are limited by the mass of AA oxidized, respectively, by LOX and COX, and are an extension of previous work showing that the overall rate of adhesion is limited by the mass of AA produced by the cells (Chun and Jacobson, 1992). In essence, the regulation of spreading and migration by AA conforms to the law of mass action as elucidated by Waage and Guldberg in 1879 (Janin, 1996). The ratio of LOX and COX products of arachidonic acid oxidation or their respective enzyme activities would determine whether cells tend to spread and stay spread, or subsequently migrate. It should be emphasized that the observed effects of leukotrienes or prostaglandins on spreading or migration are far downstream of LOX versus COX substrate competition position in the overall adhesion pathway. It is seen in several of the experiments herein that addition of either exogenous LTB4 or PGE2 in the absence of enzyme inhibitors had direct effects on cell morphology and/or surface area that bypassed the upstream competition between COX and LOX for AA substrate. Exogenous LTB4 increased spread cell surface area even in the absence of COX inhibitors, and exogenous PGE2 reversed the increased spread cell surface area from inhibition of either COX or ERK1/2 even without LOX inhibitors (Figure 4B). Although addition of PGE2 alone to control cells did not reduce surface area more than ∼15–20%, this may simply reflect the inability of the cells to compress beyond a minimal point, and the morphology of the cells with added PGE2 was altered (Figure 4C). As described above, our ongoing work indicates that these effects on morphology and behavior from leukotrienes or prostaglandins are attributable to changes in actin polymerization. These particular experiments with the use of exogenous metabolites, but not enzyme inhibitors, did not shift the balance of activity between LOX and COX by perturbing competition for AA substrate, but addressed effects of their respective metabolite products further downstream by changing the overall mass ratio of LTB4 to PGE2. In the absence of enzyme inhibitors or exogenous enzyme products however, the mass effect model presumably would be the central regulatory scheme. This presents an interesting paradox such that the many effects attributed to COX inhibition and reduced prostaglandin production might be in reality due to an increase in formation of LOX metabolites such as leukotrienes.
Last, it is reasonable to speculate that the bifurcation of AA between LOX and COX activities regulates in vivo adhesive functions in a manner similar to what we have observed with the NIH-3T3 fibroblasts in vitro. A reasonable example is wound healing in the cornea, where healing requires two forms of corneal endothelial cell movement; migration of individual cells as well as spreading of groups of cells into the wounded area (Joyce et al., 1995). Consistent with our results on the ratio of LOX and COX metabolites being important in the regulation of spreading and migration is the observation in that work that the addition of PGE2 to corneal wounds increased healing by cell migration, but suppressed healing by cell spreading. It has also been noted that COX is overexpressed in some types of cancers (DuBois et al., 1996; Tsujii et al., 1997; Dore et al., 1998; Tsujii et al., 1998), which is better understood with respect to the data presented herein by inferring that malignantly transformed cells exposed to a constitutively elevated level of prostaglandins would tend to more easily undergo metastatic tumor cell migration. In conclusion, it appears that regulation of cell spreading and migration in adhesion in vitro, and possibly adhesion or wound repair in vivo, are regulated in part by mass action of metabolites, i.e., the concentrations of AA, LTs, and PGs produced, respectively, by PLA2, LOX, and COX.
ACKNOWLEDGMENTS
This research was partially supported by the National Institutes of Health Grant GM-29127, and partially by the University of Massachusetts at Amherst Chemistry-Biology Interface Program National Institutes of Health Training Grant T32-GM08515. We thank Dr. Lou Roberts, Dr. John Crawford, Adam Kellogg, Honor Glenn, Dr. Mira Menon, and Angelo Green for their assistance and advice.
Abbreviations used:
- AA
arachidonic acid
- BPB
4-bromophenacyl bromide
- BSA
bovine serum albumin
- COX
cyclooxygenase
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FN
fibronectin
- HBSS
Hanks' balanced salt solution
- LOX
lipoxygenase
- NDGA
nordihydroguairetic acid
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- PLA2
phospholipase A2
- LT
leukotriene
REFERENCES
- Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- Auer KL, Jacobson BS. Beta-1 integrins signal lipid second messengers required during cell adhesion. Mol Biol Cell. 1995;6:1305–1313. doi: 10.1091/mbc.6.10.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsinde J, Balboa MA, Insel PA, Dennis EA. Regulation and inhibition of phospholipase A2. Annu Rev Pharmacol Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- Barry OP, Kazanietz MG, Pratico D, Fitzgerald GA. Arachidonic acid in platelet microparticles up-regulates cyclooxygenase-2-dependent prostaglandin formation via a protein kinase C/mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1999;274:7545–7556. doi: 10.1074/jbc.274.11.7545. [DOI] [PubMed] [Google Scholar]
- Bourdoulous S, Orend G, Mackenna DA, Pasqualini R. Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J Cell Biol. 1998;143:267–276. doi: 10.1083/jcb.143.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Kinch MS, Lin TH, Burridge K, Juliano RL. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- Chen JK, Wang DW, Faulk JR, Capdevila J, Harris RC. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J Biol Chem. 1999;274:4764–4769. doi: 10.1074/jbc.274.8.4764. [DOI] [PubMed] [Google Scholar]
- Chikahisa L, Matsuo K-I, Yamado Y. Modulation of tumor cell motility by plasmin. Invasion Metastasis. 1997;17:323–333. [PubMed] [Google Scholar]
- Chun J, Auer KA, Jacobson BS. Arachidonate initiated protein kinase C. activation regulates HeLa cell spreading on a gelatin substrate by inducing F-actin formation and exocytotic upregulation of beta 1 integrin. J Cell Physiol. 1997;173:361–370. doi: 10.1002/(SICI)1097-4652(199712)173:3<361::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Chun J-S, Ha M-J, Jacobson BS. Differential translocation of protein kinase CSymbol“ § 12 during HeLa cell adhesion to a gelatin substratum. J Biol Chem. 1996;271:13008–13012. doi: 10.1074/jbc.271.22.13008. [DOI] [PubMed] [Google Scholar]
- Chun JS, Jacobson BS. Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol Biol Cell. 1992;3:481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun JS, Jacobson BS. Requirement for diacylglycerol and protein kinase C in HeLa cell-substratum adhesion and their feedback amplification of arachidonic acid production for optimum cell spreading. Mol Biol Cell. 1993;4:271–281. doi: 10.1091/mbc.4.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Hynes RO. Ras activation is necessary for integrin-mediated activation of extracellular-signal-regulated kinase 2 and cytosolic phospholipase A2 but not for cytoskeletal organization. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- Conrad DJ. The arachidonate 12/15 lipoxygenases. A review of tissue expression and biologic function. Clin Rev Allergy Immunol. 1999;17:71–89. doi: 10.1007/BF02737598. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Jacobson BS. Extracellular calcium regulates HeLa cell morphology during adhesion to gelatin: role of translocation and phosphorylation of cytosolic phospholipase A2. Mol Biol Cell. 1998;9:3429–3443. doi: 10.1091/mbc.9.12.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky AV, McTavish AJ. Extracellular matrix is required for MAP kinase activation and proliferation of rat glomerular epithelial cells. Biochem Biophys Res Commun. 1997;231:160–166. doi: 10.1006/bbrc.1997.6064. [DOI] [PubMed] [Google Scholar]
- Damtew B, Spagnuolo PJ. Tumor cell-endothelial cell interactions: evidence for roles for lipoxygenase products of arachidonic acid in metastasis. Prostaglandins Leukot Essent Fatty Acids. 1997;56:295–300. doi: 10.1016/s0952-3278(97)90573-1. [DOI] [PubMed] [Google Scholar]
- Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis. Cancer Res. 1999;59:4574–4577. [PubMed] [Google Scholar]
- Daugschies A, Ruttkowski B. Modulation of migration of Esophagostomum dentatum larvae by inhibitors and products of eicosanoid metabolism. Int J Parasitol. 1998;28:355–362. doi: 10.1016/s0020-7519(97)00153-7. [DOI] [PubMed] [Google Scholar]
- Dore M, Cote L, Mitchell A, Sirois J. Expression of prostaglandin G/H synthase type 1, but not type 2, in human ovarian adenocarcinomas. J Histochem Cytochem. 1998;46:77–84. doi: 10.1177/002215549804600110. [DOI] [PubMed] [Google Scholar]
- DuBois RN, Radhika A, Reddy BS, Entingh AJ. Increased cyclooxygenase-2 levels in carcinogen-induced rat colonic tumors. Gastroenterology. 1996;110:1259–1262. doi: 10.1053/gast.1996.v110.pm8613017. [DOI] [PubMed] [Google Scholar]
- Graf K, Xi XP, Yang D, Fleck E, Hsueh WA, Law RE. Mitogen-activated protein kinase activation is involved in platelet-derived growth factor-directed migration by vascular smooth muscle cells. Hypertension. 1997;29:334–339. doi: 10.1161/01.hyp.29.1.334. [DOI] [PubMed] [Google Scholar]
- Haimovich B, Ji P, Ginales E, Kramer R, Greco R. Phospholipase A2 enzymes regulate alpha IIb beta3-mediated, but not Fc gammaRII receptor-mediated, pp125FAK phosphorylation in platelets. Thromb Haemost. 1999;81:618–624. [PubMed] [Google Scholar]
- Heidemann SR, Buxbaum RE. Cell crawling: first the motor, now the transmission. J Cell Biol. 1998;141:1–4. doi: 10.1083/jcb.141.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuertz RM, Hamann KJ, Hershenson MB, Jeff AK. Adhesion of bovine airway smooth muscle cells activates extracellular signal-regulated kinases. Am J Respir Cell Mol Biol. 1997;17:456–461. doi: 10.1165/ajrcmb.17.4.2702. [DOI] [PubMed] [Google Scholar]
- Hinton DR, He S, Graf K, Yang D, Hseuh WA, Ryan SJ, Law RE. Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp Cell Res. 1998;239:11–15. doi: 10.1006/excr.1997.3873. [DOI] [PubMed] [Google Scholar]
- Honda HM, Leitinger N, Frankel M, Goldhaber JI, Natarajon R, Nedler JL, Weiss JN, Berliner JA. Induction of monocyte binding to endothelial cells by MM-LDL: role of lipoxygenase metabolites. Arterioscler Thromb Vasc Biol. 1999;19:680–686. doi: 10.1161/01.atv.19.3.680. [DOI] [PubMed] [Google Scholar]
- Horie-Sakata K, Shimada T, Hiraishi H, Terano A. Role of cyclooxygenase 2 in hepatocyte growth factor-mediated gastric epithelial restitution. J Clin Gastroenterol. 1998;27,suppl 1:S40–S46. doi: 10.1097/00004836-199800001-00008. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- Hwang D, Jang BC, Yu G, Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide. Mediation through both the mitogen-activated protein kinase and NF-κβ signaling pathways in macrophages. Biochem Pharmacol. 1997;54:87–96. doi: 10.1016/s0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Janin J. For Guldberg and Waage: with love and cratic entropy. Proteins. 1996;24:i–ii. doi: 10.1002/prot.340240402. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Joyce SJ, Powell SM, Meklir B. EGF and PGE2: effects on corneal endothelial cell migration and monolayer spreading during wound repair in vitro. Curr. Eye Res. 1995;14:601–609. doi: 10.3109/02713689508998408. [DOI] [PubMed] [Google Scholar]
- Karim S, Berrou E, Levy Toldano S, Brychaert M, MacLouf J. Regulatory role of prostaglandin E2 in induction of cyclooxygenase-2 by a thromboxane A2 analogue ( U46619) and basic fibroblast growth factor in porcine aortic smooth-muscle cells. Biochem J. 1997;326:593–599. doi: 10.1042/bj3260593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke RL, Cai S, Giannini AL, Gallegher PJ, deLanerolle P, Cheresh DA. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:581–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer J, Denger S, Jahn L, Bader J, Ritter K, von Hodenberg E, Kubler W. LDL stimulates chemotaxis of human monocytes through a cyclooxygenase-dependent pathway. Arterioscler Thromb Vasc Biol. 1996;16:1481–1487. doi: 10.1161/01.atv.16.12.1481. [DOI] [PubMed] [Google Scholar]
- Langholz O, Roeckel D, Petersohn D, Broermann E, Eckes B, Krieg T. Cell-matrix interactions induce tyrosine phosphorylation of MAP kinases ERK1 and ERK2 and PLCγ-1 in two-dimensional and three-dimensional cultures of human fibroblasts. Exp Cell Res. 1997;235:22–27. doi: 10.1006/excr.1997.3640. [DOI] [PubMed] [Google Scholar]
- Lee S, Felts KA, Perry GC, Armacost LM, Cobb RR. Inhibition of 5-lipoxygenase blocks IL-1 beta-induced vascular adhesion molecule-1 gene expression in human endothelial cells. J Immunol. 1997;158:3401–3407. [PubMed] [Google Scholar]
- Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cP.L.A2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Lloret S, Moreno JJ. Role of kinases and G-proteins on arachidonate release induced by zymosan in mouse peritoneal macrophages. Int J Biochem Cell Biol. 1996;28:465–472. doi: 10.1016/1357-2725(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Lundberg MS, Curto KA, Bilato C, Montecone RE, Crow MT. Regulation of vascular smooth muscle migration by mitogen-activated protein kinase and calcium/calmodulin-dependent protein kinase II signaling pathways. J Mol Cell Cardiol. 1998;30:2377–2389. doi: 10.1006/jmcc.1998.0795. [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Bukoski RD, Ruge MS, Rao GN. Arachidonic acid activates Jun N-terminal kinase in vascular smooth muscle cells. Oncogene. 1998;16:417–422. doi: 10.1038/sj.onc.1201551. [DOI] [PubMed] [Google Scholar]
- Miller C, Zhang M, He Y, Zhao J, Peiletier JP, diBattista JA, Martel-Pelletier J. Transcriptional induction of cyclooxygenase-2 gene by okadaic acid inhibition of phosphatase activity in human chondrocytes: co-stimulation of AP-1 and CRE nuclear binding proteins. J Cell Biochem. 1998;69:392–413. [PubMed] [Google Scholar]
- Minami T, Zushi S, Shinomura Y, Matsuzawa Y. Phospholipase A2 stimulation of rat intestinal epithelial cell (IEC-6) migration. Am J Physiol. 1996;271:G664–G668. doi: 10.1152/ajpgi.1996.271.4.G664. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Ohno S, Brugge JS. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J Biol Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Nakatani Y, Atsumi G, Inoue K, Kudo I. Regulatory functions of phospholipase A2. Crit Rev Immunol. 1997;17:225–283. doi: 10.1615/critrevimmunol.v17.i3-4.10. [DOI] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Uddin MR, Harper JL, Malik KU. Signal transduction mechanisms involved in angiotensin-(1-7)-stimulated arachidonic acid release and prostanoid synthesis in rabbit aortic smooth muscle cells. J Pharmacol Exp Ther. 1998;284:388–398. [PubMed] [Google Scholar]
- Natarajan R, Rosdahl J, Gonzales N, Bai W. Regulation of 12-lipoxygenase by cytokines in vascular smooth muscle cells. Hypertension. 1997;30:873–879. doi: 10.1161/01.hyp.30.4.873. [DOI] [PubMed] [Google Scholar]
- Nemenoff RA, Winitz S, Gian NX, Van Patten V, Johnson GL, Hessler LE. Phosphorylation and activation of a high molecular weight form of phospholipase A2 by p42 microtubule-associated protein 2 kinase and protein kinase C. J Biol Chem. 1993;268:1960–1964. [PubMed] [Google Scholar]
- Niiro H, Otsuka T, Ogami E, Yamaoka K, Nagano S, Akahashi M, Nakashima H, Arinobu Y, Izuhara K, Niho Y. MAP kinase pathways as a route for regulatory mechanisms of IL-10 and IL-4 which inhibit COX-2 expression in human monocytes. Biochem Biophys Res Commun. 1998;250:200–205. doi: 10.1006/bbrc.1998.9287. [DOI] [PubMed] [Google Scholar]
- Ogura M, Kitamura M. Oxidant stress incites spreading of macrophages via extracellular signal-regulated kinases and p38 mitogen-activated protein kinase. J Immunol. 1998;161:3569–3574. [PubMed] [Google Scholar]
- Oshima H, Oshima M, Kobayashi M, Tsuksumi M, Taketo MM. Morphological and molecular processes of polyp formation in Apc (delta716) knockout mice. Cancer Res. 1997;57:1644–1649. [PubMed] [Google Scholar]
- Peppelenbosch MP, Tertoolen LG, den Hertog J, deLoat S. Epidermal growth factor activates calcium channels by phospholipase A2/5-lipoxygenase-mediated leukotriene C4 production. Cell. 1992;69:295–303. doi: 10.1016/0092-8674(92)90410-e. [DOI] [PubMed] [Google Scholar]
- Piomelli D. Arachidonic acid in cell signaling. Curr Opin Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- Redlitz A, Daum G, Sage EH. Angiostatin diminishes activation of the mitogen-activated protein kinases ERK-1 and ERK-2 in human dermal microvascular endothelial cells. J Vasc Res. 1999;36:28–34. doi: 10.1159/000025623. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Bulinski JC, Krebs EB, Fischer EH. Mitogen-activated protein kinase/extracellular signal-regulated kinase 2 regulates cytoskeletal organization and chemotaxis via catalytic and microtubule-specific interactions. Mol Biol Cell. 1997;8:1219–1232. doi: 10.1091/mbc.8.7.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RL, Tang DG, Haddad M, Honn KV, Taylor JD. 12(S)-Hydroxyeicosatetraenoic acid increases the actin microfilament content in B16a melanoma cells: a protein kinase-dependent process. Int J Cancer. 1998;77:271–278. doi: 10.1002/(sici)1097-0215(19980717)77:2<271::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Baron V. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]
- Seeds MC, Bass DA. Regulation and metabolism of arachidonic acid. Clin Rev Allergy Immunol. 1999;17:5–26. doi: 10.1007/BF02737594. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beauchamp RD, DuBois RN. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest. 1997;99:2254–2259. doi: 10.1172/JCI119400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng H, Williams CS, Liang P, DuBois RN, Beauchamp RD. Induction of cyclooxygenase-2 by activated Ha-ras oncogene in Rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J Biol Chem. 1998;273:22120–22127. doi: 10.1074/jbc.273.34.22120. [DOI] [PubMed] [Google Scholar]
- Subbaramaiah K, Chung WJ, Dannenberg AJ. Ceramide regulates the transcription of cyclooxygenase-2. Evidence for involvement of the extracellular-signal-regulated kinase/c-Jun N-terminal kinase and p38 mitogen-activated protein kinase pathways. J Biol Chem. 1998;273:32943–32949. doi: 10.1074/jbc.273.49.32943. [DOI] [PubMed] [Google Scholar]
- Szabo MC, Teague TK, McIntyre BW. Regulation of lymphocyte pseudopodia formation by triggering the integrin alpha 4/beta 1. J Immunol. 1995;154:2112–2124. [PubMed] [Google Scholar]
- Takahashi M, Berk BC. Mitogen-activated protein kinase (ERK 1/2) activation by shear stress and adhesion in endothelial cells. Essential role for a herbimycin-sensitive kinase. J Clin Invest. 1996;98:2623–2631. doi: 10.1172/JCI119083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timar J, Tang D, Bazaz R, Haddad MM, Kimler VA, Taylor JD, Honn KV. PKC mediates 12(S)-HETE-induced cytoskeletal rearrangement in B16a melanoma cells. Cell Motil Cytoskeleton. 1993;26:49–65. doi: 10.1002/cm.970260106. [DOI] [PubMed] [Google Scholar]
- Trikha M, Honn KV. Role of 12-lipoxygenase and protein kinase C in modulating the activation state of the integrin alpha IIb beta 3 on human tumor cells. Adv Exp Med Biol. 1997;407:55–60. doi: 10.1007/978-1-4899-1813-0_8. [DOI] [PubMed] [Google Scholar]
- Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase-2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujii M, Kawano S, Isuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- Wei J, Shaw LM, Mercurio AM. Regulation of mitogen-activated protein kinase activation by the cytoplasmic domain of the alpha6 integrin subunit. J Biol Chem. 1998;273:5903–5907. doi: 10.1074/jbc.273.10.5903. [DOI] [PubMed] [Google Scholar]
- Whitfield RA, Jacobson BS. The β1-integrin cytosolic domain optimizes phospholipase A2-mediated arachidonic acid release required for NIH-3T3 cell spreading. Biochem Biophys Res Commun. 1999;258:306–312. doi: 10.1006/bbrc.1999.0642. [DOI] [PubMed] [Google Scholar]
- Xi XP, Graf K, Goetze S, Fleck E, Hsueh WA, Law RE. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:73–82. doi: 10.1161/01.atv.19.1.73. [DOI] [PubMed] [Google Scholar]
- Zakar T, Mijovic JE, Eyster KM, Bhardwaj D, Olson DM. Regulation of prostaglandin H2 synthase-2 expression in primary human amnion cells by tyrosine kinase dependent mechanisms. Biochim Biophys Acta. 1998;1391:37–51. doi: 10.1016/s0005-2760(97)00195-1. [DOI] [PubMed] [Google Scholar]
- Zhu AJ, Haase I, Watt FM. Signaling via beta1 integrins and mitogen-activated protein kinase determines human epidermal stem cell fate in vitro. Proc Natl Acad Sci USA. 1999;96:6728–6733. doi: 10.1073/pnas.96.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]