Abstract
The effects of ionizing radiation to human health are of great concern in the field of space exploration and for patients considering radiotherapy. However, to date, the effect of high-dose radiation on metabolism in the liver has not been clearly defined. In this study, 1H nuclear magnetic resonance (NMR)-based metabolomics combined with multivariate data analysis was applied to study the changes of metabolism in the liver of C57BL/6 mouse after whole-body gamma (3.0 and 7.8 Gy) or proton (3.0 Gy) irradiation. Principal component analysis (PCA) and orthogonal projection to latent structures analysis (OPLS) were used for classification and identification of potential biomarkers associated with exposure to gamma and proton radiation. The results show that the radiation exposed groups can be well separated from the control group. Where the same dose was received, the proton exposed group was nevertheless well separated from the gamma-exposed group, indicating that different radiation sources induce different alterations in the metabolic profile. Common among all high-dose gamma and proton exposed groups were the statistically decreased concentrations of choline, O-phosphocholine and trimethylamine N-oxide, while the concentrations of glutamine, glutathione, malate, creatinine, phosphate, betaine and 4-hydroxyphenylacetate were statistically and significantly elevated. Since these altered metabolites are associated with multiple biological pathways, the results suggest that radiation induces abnormality in multiple biological pathways. In particular, metabolites such as 4-hydroxyphenylacetate, betaine, glutamine, choline and trimethylamine N-oxide may be prediagnostic biomarkers candidates for ionizing exposure of the liver.
INTRODUCTION
The effects of ionizing radiation to human health are of great concern in the field of space exploration as well as to patients who are receiving radiotherapy. High-dose ionizing radiation carries enough energy to induce obvious physical symptoms, appearing as acute radiation syndrome, which include nausea, vomiting and fatigue, among others. These symptoms are believed to be the consequences of gene expression dysfunction after exposure (1). The radiation source, dosage and time after exposure are all contributing factors to the symptoms of acute radiation syndrome (2). Clinically, these symptoms are associated with a dramatic decrease in white blood cell counts (3). However, the underlying biological pathways, in particular the metabolic pathways, which are altered by high-dose radiation associated with different internal organs have not yet been clearly elucidated.
Metabolites are small molecules produced by various cellular regulatory processes with molecular weight less than 1,000 Da. Metabolites are the end products or intermediates in metabolic pathways that are directly regulated by enzymes, while enzymes are linked to genes. Thus, changes of metabolic profiles can provide information about the potentially affected biological pathways (4). Metabolomics is a method capable of detecting metabolic responses of a living system during and after exposure to external stimuli (5). Liquid-state nuclear magnetic resonance (NMR) is a nondestructive and unbiased analytical approach capable of detecting and quantifying metabolites in the biological tissues extracts. Since almost all metabolites contain hydrogen, which has natural abundance of 99.985% and possesses high-gyromagnetic ratio that results in high sensitivity, 1H NMR spectroscopy has become one of the most frequently used techniques in metabolomics (6).
Pattern recognition methods are an integral part of metabolomics (5, 7). The metabolomics data are analyzed with the aid of multivariate data analysis methods, such as principal component analysis (PCA) (8) and orthogonal projection to latent structure (OPLS) (9). Principal component analysis is an unsupervised pattern recognition method for class separation and for identifying the outliers, where the metadata are simplified and the most information is retained by reconstituting a new coordinate system formed with the orthogonal latent variables (10, 11). As a supervised statistical method, the orthogonal projection to latent structures analysis (OPLS) (12) has received increasing attention, owing to its superior capability of classification, interpretation and prediction (13). Based on the OPLS model, a powerful visualization tool, S-plot, has been proposed for interpreting the multivariate statistical model, enabling direct identification and extraction of the statistically significant discriminatory metabolites and potential biomarkers (14). The positive loadings indicate upregulated metabolites and negative loadings indicate downregulated metabolites.
The liver is an important organ that plays a critical role in metabolism with multiple functions in the human body, including regulation of glycogen storage, decomposition of red blood cells, synthesis of plasma protein and detoxification of toxins (15). In this study, 1H NMR-based metabolomics were used for metabolic profiling of hydrophilic tissue extracts from the excised livers of both control mice and whole-body irradiated mice (3.0 or 7.8 Gy gamma; or 3.0 Gy proton) at days 4 and 11 postirradiation. The spectral deconvolution technique was used to identify and quantify all metabolites from NMR spectra. Multivariate analyses (PCA and OPLS) were performed for pattern recognition and identification of metabolites in which concentrations were statistically significantly changed as a result of radiation exposure. Based on these findings, the metabolic pathways and networks that are influenced by radiation are discussed.
MATERIALS AND METHODS
Animal and Sample Preparation
A total of 27 female C57BL/6 seven-week-old mice were purchased from Jackson Laboratory (Bar Harbor, ME). Before the beginning of the one week acclimation time period at the animal facility of Pacific Northwest National Laboratory (PNNL) or Brookhaven National Laboratory (BNL), the animals were randomly divided into six groups and then were exposed to whole-body gamma or proton irradiation. The gamma and proton radiation energy at the position of the mice was measured beforehand based on the National Institute of Standards and Technology (NIST; Gaithersburg, MD). The individually housed animals were fed a standard diet and maintained in a controlled environment facility with an ambient temperature of 22–25°C and 45% relative humidity on a 12:12 h light:dark schedule.
Mice were exposed to whole-body gamma irradiation at the High Exposure Facility of PNNL, using a high-activity source (1,250 keV 60Co) with linear energy transfer (LET) associated with these fields in the range of 0.2–2 keV/μm. The mice were isolated in the corner of their polymer cages and placed a minimum of 100 cm from the collimated 6.000 Ci (222 TBq) 60Co source, and then exposed to the proposed doses, respectively. After irradiation, the isolation barrier was removed and mice were transferred to the PNNL Animal Facility. All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at PNNL. The absorbed dose rate at a depth of approximately 600 mg/cm2 was 0.83 Gy/min relative to tissue for both gamma and proton irradiation.
Mice received whole-body proton irradiation at the NASA Space Radiation Laboratory/Brookhaven National Laboratory (NSRL/BNL) Heavy Ion Facility. The irradiation protocol (16) previously used at NSRL/BNL was closely followed for performing the high-LET proton irradiations. Mice were placed in aerated polystyrene boxes immediately before the entrance region of the container was exposed to protons at the BNL Alternating Gradient Synchrotron (AGS) (1,055 MeV/nucleon at the target, with track-averaged LET = 148.2 keV/μm at target). All procedures for the control mice were identical to those of exposed animals, except that they were not irradiated. All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) at BNL.
The reasons for selecting these radiation doses (3.0 and 7.8 Gy) are explained below. Metabolomics have been successfully utilized for assessing potential biomarkers in urine (17), plasma (18) and serum (19) of mice exposed to 3.0 Gy of gamma radiation; and interesting results have been achieved. The lethal dose for mice is approximately 7.8 Gy (20), and in the case of an atomic bomb attack the survivors may be exposed to radiation that meets or exceeds the lethal dose. It has been reported that 24 h after high-dose (3.0 and 8.0 Gy) gamma irradiation, metabolites in mouse urine underwent a change (17). However, 3 days after lethal-dose gamma irradiation, metabolite levels in urine samples of nonhuman primates had some outliers compared to controls, indicating significant biological variations (21). Furthermore, mouse tissues were able to recover at day 11 after 2.0 Gy gamma irradiation (22). The goal of this study was to investigate changes in metabolites of liver tissue at time points close to days 3 and 11 postirradiation; ultimately, days 4 and 11 postirradiation were selected Six groups of animals were exposed to gamma radiation (n = 27; Supplementary Table S1; http://dx.doi.org/10.1667/RR14602.1.S1) [0 Gy (n = 4), 3.0 Gy (n = 10; 2 groups) and 7.8 Gy (n = 4)] or proton radiation [0 Gy (n = 4) or 3.0 Gy (n = 5)], respectively. At day 4 postirradiation, some of the mice exposed to gamma radiation [0 Gy (control, n = 4), 3.0 Gy (n = 5) and 7.8 Gy (n = 4)] were sacrificed with 70/30 CO2/O2, and the liver from each mouse was immediately removed, snap-frozen in liquid nitrogen, then weighed and stored at −80°C freezer until subsequent NMR analysis. At day 11 postirradiation, the remaining mice exposed to gamma radiation [3.0 Gy (n = 5)], and all mice exposed to proton radiation [0 Gy (control, n = 4), 3.0 Gy (n = 5)] were sacrificed with 70/30 CO2/O2; liver samples were collected using the same protocol as described above. Only small sample sizes were necessary for this study, since the high-dose radiation used here dramatically impacts metabolic changes (23). The changes induced by high-dose radiation are much more substantial than the biological variations; thus, a sample size of 4–5 animals was sufficient. In fact, the results of this study fully justify the use of such sample size; our finding that the exposed groups were well separated from the control group, is statistically based on 1H NMR metabolic profiling results. It is also worth noting that small sample sizes (n = 3–5) have been previously used in mouse models to study neurodevelopmental disorders by metabolomics, where reliable biomarkers related to the neurodevelopmental disorders have been successfully obtained (24, 25).
Polar metabolites were extracted from liver tissues using a modified Folch method, according to published protocol (26). Briefly, after randomization of the samples, every pre-weighed intact frozen liver tissue was homogenized by a Tissue-Tearor (BioSpec Products Inc., Bartlesville, OK) in an ice-cold glass vial after adding 4 ml MeOH and 0.85 ml deionized H2O per g of liver tissue, followed by vortexing the mixture, adding 2 ml chloroform per g of tissue, then vortexing again. This process took 6 min and was performed exactly the same for each sample. For the next step, 2 ml each of chloroform and deionized H2O per g of tissue were added to the mixture, which was then vortexed again, followed by transferring of the different layers to glass vials separately with syringes, after the mixture (in ice bath) was centrifuged. Finally, the solvents of hydrophilic metabolites were removed using a lyophilizer and then stored in a −80°C freezer before NMR spectral measurements were performed.
NMR Spectroscopy
All NMR spectra were acquired at 293 K on a Varian 800 MHz NMR spectrometer (operating at 799.42 MHz for 1H) equipped with a Z-axis-gradient 5 mm HCN probe. The hydrophilic metabolites were reconstituted in 600 μl of D2O containing 0.05 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) for chemical shift reference and internal concentration standard and 0.2% sodium azide (w/v) as bacteriostatic agent to prevent biodegradation. Approximately 550 μl of the prepared sample was loaded into a standard 5 mm NMR tube (Wilmad-LabGlass, Buena, NJ). One-dimensional 1H NMR spectra were acquired from each sample using the standard Varian PRESAT pulse sequence with a single excitation and 1.5 s low-power pre-saturation at the water peak position to suppress the residual water signal. A total of 6 k transients were accumulated to ensure a high quality 1H spectrum was obtained with sufficient signal-to-noise ratio for metabolites with concentration as low as approximately 0.5 μM in the NMR tube. For metabolite signal assignment and confirmation purposes, two-dimensional (2D) NMR spectra, including 1H–1H correlation spectroscopy (COSY) and 1H J-resolved spectroscopy (JRES), were acquired at 293 K for selected samples.
NMR Data Processing and Multivariate Data Analysis
All free induction decays (FIDs) were multiplied by an exponential function with a Lorentz line broadening factor of 0.5 Hz and zero-filled to 128 k data points prior to Fourier transformation. All NMR spectra were manually corrected for phase and baseline distortions using the NMR Processor module (NMR suite 8.1, Professional; Chenomx, Edmonton, Canada). Chemical shift was referenced to the peak of methyl proton of DSS at 0 ppm.
The multivariate data can be generated by spectral binning and spectral deconvolution. Although spectral binning is very efficient for large-scale object matrix and can be easily automated (27), it is difficult to generate high-quality multivariate data due to the influences of residual water peak, rolling baseline and peak shift. Another disadvantage of spectral binning data is that a significant number of variables cannot be assigned to specific metabolites due to overlapping spectral peaks (28). In contrast, the technique of spectral deconvolution is capable of identifying and assigning the chemical identities of all metabolites with concentrations higher than the detection limits, including cases of severely overlapping peaks (29). Thus, spectral deconvolution can be used to quantitatively analyze the complex metabolic profiles (30). For these reasons, spectral deconvolution was performed to generate the multivariate data sets, using the NMR Profiler module (NMR suite 8.1, Professional; Chenomx), which contains a database of more than 330 common metabolites associated with mammals and bacteria. The concentration of each metabolite in the NMR tube was calculated by the well-established method in Chenomx with DSS as internal concentration standard. The absolute concentration of each metabolite was obtained by normalizing the corresponding metabolite concentration obtained from 1H NMR measurements to unit weight (mg) of liver tissue before extraction (31).
The normalized metadata sets were imported into SIMCA version 13.0.3 (Umetrics, Malmo, Sweden), followed by PCA and OPLS. Considering every variable has equal importance to the statistical results, prior to PCA the data were mean centered and unit-variance scaled (32). Both PCA and OPLS models were constructed using the nonlinear iterative partial least squares (NIPALS) algorithm (33) and the number of components was determined by the standard sevenfold cross-validation method (32). The quality of multivariate analysis models was evaluated with R2 values, indicating the explained variations, and Q2 values, representing the model predictabilities, respectively. Finally, the OPLS model significance was further assessed for the robustness with the CV-ANOVA approach (34) to ensure the statistical significance of the intergroup differentiations, with significance at P < 0.05.
For interpreting the multivariate classification models from OPLS, the S-plot was used, enabling direct inspection and extraction of statistically significant and potentially biochemically important metabolites. The cutoff value 0.811 or 0.878 (i.e., the critical values for Pearson correlation coefficient) was chosen for the current study based on the number of samples in each group (n = 4 or 5) and the discrimination significance of P < 0.05, which plays a critical role in extracting and identifying the statistically significant biomarkers (35).
RESULTS AND DISCUSSION
NMR Spectra of Liver Tissue Extracts
Figure 1 shows a representative 1H NMR spectrum obtained from the liver hydrophilic metabolites extracts from a mouse exposed to 3.0 Gy dose of gamma radiation, where peaks are assigned to specific metabolites with good confidence (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR14602.1.S1) based on spectral deconvolution using Chenomx and previously cited literature (28, 36), as well as validated by 2D 1H–1H correlation spectroscopy (COSY) and 1H J-resolved spectroscopy (JRES). Procedures for spectral deconvolution and the quality of fitting are shown in Supplementary Fig. S1. A total of 72 metabolites were identified, including a variety of amino acids, carbohydrates, glycolysis products, TCA cycle intermediates, choline metabolites, ethanolamine metabolites and organic bases. The detailed peak assignments are listed in Table 1 and the average concentrations are listed in Supplementary Table S2.
FIG. 1.
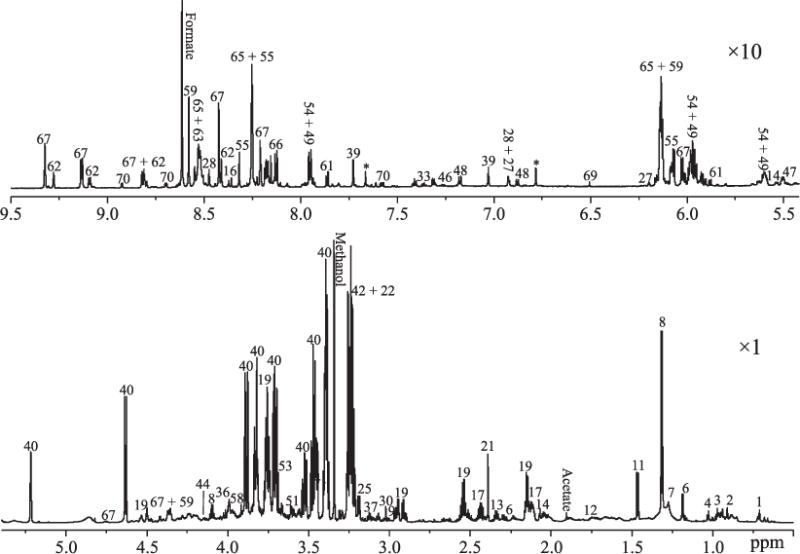
A typical 800 MHz liquid state 1H NMR metabolite spectrum of the hydrophilic extracts of liver excised from a 3.0 Gy gamma-exposed mouse. In the plot, the spectral region between 5.5 and 9.5 ppm is vertically expanded by 10 times while the 0.5–5.5 ppm spectral region is vertically expanded by one time. *Unassigned peak. A total of 72 metabolites have been identified and metabolite numbers are listed in Table 1.
TABLE 1.
Hydrophilic Metabolites Peaks Assignments and Average Concentrations in Liver Tissues
| Concentration (μM/mg), mean ± SD
|
||||
|---|---|---|---|---|
| Key | Metabolites | δ 1H (ppm) and multiplicity | Control | 7.8 Gya |
| 1 | Cholate | 0.72(s), 0.94(d), 1.65(m) | 0.57 ± 0.08 | 0.31 ± 0.03 |
| 2 | Isoleucine | 0.93(t), 1.0(d), 3.66(d) | 0.52 ± 0.06 | 0.49 ± 0.03 |
| 3 | Leucine | 0.94(t), 0.99(d), 1.27(m), 1.46(m), 1.95(m), 3.64(d) | 1.06 ± 0.19 | 1.01 ± 0.08 |
| 4 | Valine | 0.97(d), 1.02(d), 2.27(m), 3.6(d) | 0.93 ± 0.25 | 0.86 ± 0.09 |
| 5 | Isobutyrate | 1.05(d), 2.38(m) | 0.10 ± 0.02 | 0.14 ± 0.01 |
| 6 | 3-Hydroxybutyrate | 1.2(d), 2.3(dd), 2.39(dd), 4.14(dd) | 3.37 ± 0.31 | 0.78 ± 0.09 |
| 7 | Threonine | 1.33(d), 3.58(d), 4.26(m) | 2.91 ± 0.23 | 3.03 ± 0.21 |
| 8 | Lactate | 1.33(d), 4.11(q) | 11.79 ± 1.39 | 16.07 ± 2.69 |
| 9 | Lysine | 1.43(m), 1.51(m), 1.72(m), 1.89(m), 1.91(m), 3.03(t), 3.75(t) | 0.91 ± 0.25 | 1.00 ± 0.10 |
| 10 | Saccharopine | 1.45(m), 1.75(m), 1.88(m), 2.1(m), 2.4(dd), 3.06(m), 3.61(t), 3.75(t) | 1.54 ± 0.41 | 1.34 ± 0.10 |
| 11 | Alanine | 1.48(d), 3.78(q) | 6.21 ± 1.01 | 9.88 ± 1.16 |
| 12 | Arginine | 1.65(m), 1.74(m), 1.9(m), 1.92(m), 3.23(t), 3.77(t) | 2.59 ± 0.35 | 2.93 ± 0.33 |
| 13 | Glutamate | 2.05(m), 2.14(m), 2.34(m), 2.37(m), 3.76(dd) | 3.10 ± 0.08 | 3.59 ± 0.99 |
| 14 | UDP-N-acetylglucosamine | 2.06(s), 3.54(t), 3.84(m), 4.0(m), 4.3(t), 5.51(dd), 5.97(dd), 7.94(d), 8.28(d) | 0.32 ± 0.05 | 0.21 ± 0.03 |
| 15 | 1,3-Diaminopropane | 2.08(m), 3.12(m) | 0.42 ± 0.01 | 0.45 ± 0.06 |
| 16 | S-adenosylhomocysteine | 2.1(m), 2.67(t), 3.0(dd), 3.1(dd), 4.33(dd), 4.42(t), 6.06(d), 8.26(s), 8.36(s) | 0.18 ± 0.02 | 0.18 ± 0.02 |
| 17 | Glutamine | 2.12(m), 2.43(m), 3.77(t) | 2.25 ± 0.11 | 5.96 ± 1.92 |
| 18 | O-acetylcarnitine | 2.13(s), 2.5(dd), 2.63(dd), 3.18(s), 3.61(d), 5.6(m) | 0.10 ± 0.02 | 0.15 ± 0.03 |
| 19 | Glutathione | 2.16(m), 2.18(m), 2.52(m), 2.57(m), 2.95(dd), 2.98(dd), 3.74(d), 3.77(m), 4.58(m) | 6.28 ± 0.86 | 12.72 ± 1.66 |
| 20 | Malate | 2.36(dd), 2.67(dd), 4.3(m) | 0.62 ± 0.17 | 1.50 ± 0.30 |
| 21 | Succinate | 2.39(s) | 2.54 ± 0.30 | 2.85 ± 0.46 |
| 22 | Carnitine | 2.42(m), 3.21(s), 3.4(m), 4.56(m) | 1.56 ± 0.21 | 1.17 ± 0.13 |
| 23 | 2-Oxobutyrate | 2.44(t), 2.99(t) | 0.19 ± 0.03 | 0.14 ± 0.02 |
| 24 | Citrate | 2.52(d), 2.65(d) | 0.06 ± 0.01 | 0.16 ± 0.02 |
| 25 | β-Alanine | 2.53(t), 3.20(t) | 2.55 ± 0.17 | 2.17 ± 0.42 |
| 26 | Aspartate | 2.65(dd), 2.8(dd), 3.89(dd) | 0.80 ± 0.18 | 0.84 ± 0.28 |
| 27 | NADPH | 2.73(dd), 2.85(dd), 4.04(m), 4.2(m), 5.98(d), 6.19(d), 6.92(s), 8.22(s), 8.47(s) | 0.14 ± 0.02 | 0.11 ± 0.04 |
| 28 | NADH | 2.74(dd), 4.04(dd), 4.2(m), 5.98(d), 6.2(d), 6.92(s), 8.22(s), 8.47(s) | 0.13 ± 0.03 | 0.08 ± 0.02 |
| 29 | Trimethylamine | 2.88(s) | 0.10 ± 0.02 | 0.13 ± 0.02 |
| 30 | Creatine phosphate | 3.01(s), 3.94(s) | 0.43 ± 0.10 | 0.33 ± 0.10 |
| 31 | Creatine | 3.04(s), 3.91(s) | 0.14 ± 0.02 | 0.16 ± 0.01 |
| 32 | Creatinine | 3.04(s), 3.98(s) | 0.16 ± 0.03 | 0.28 ± 0.06 |
| 33 | Phenylalanine | 3.11(dd), 3.27(dd), 3.99(dd), 7.33(m), 7.38(m), 7.43(m) | 0.18 ± 0.02 | 0.20 ± 0.01 |
| 34 | Histidine | 3.14(dd), 3.24(dd), 3.98(dd), 7.06(s), 7.79(s) | 0.03 ± 0.01 | 0.07 ± 0.00 |
| 35 | Ethanolamine | 3.14(m), 3.82(m) | 0.83 ± 0.17 | 1.17 ± 0.22 |
| 36 | O-phosphoethanolamine | 3.2(t), 3.98(dd) | 1.30 ± 0.11 | 2.61 ± 0.20 |
| 37 | Choline | 3.21(s), 3.52(m), 4.07(m) | 0.56 ± 0.07 | 0.15 ± 0.04 |
| 38 | O-phosphocholine | 3.21(s), 3.56(m), 4.19(m) | 0.89 ± 0.24 | 0.38 ± 0.06 |
| 39 | τ-Methylhistidine | 3.22(dd), 3.3(dd), 3.73(s), 3.95(dd), 6.8(s), 8(s) | 0.43 ± 0.04 | 0.50 ± 0.07 |
| 40 | Glucose | 3.23(m), 3.4(m), 3.5(m), 3.53(dd), 3.7(dd), 3.72(dd), 3.78(m), 3.83(m), 3.84(m), 3.94(dd), 4.65(d), 5.23(d) | 47.55 ± 7.13 | 58.91 ± 2.52 |
| 41 | Trimethylamine N-oxide | 3.23(s) | 1.95 ± 0.20 | 0.62 ± 0.15 |
| 42 | sn-Glycero-3-phosphocholine | 3.23(s), 3.6(dd), 3.67(m), 3.68(dd), 3.86(m), 3.92(m), 3.95(m), 4.32(m) | 0.95 ± 0.12 | 0.53 ± 0.02 |
| 43 | Glucose-6-phosphate | 3.25(t), 3.5(m), 3.57(m), 3.72(t), 3.96(m), 5.21(d) | 2.60 ± 0.21 | 5.27 ± 0.53 |
| 44 | Betaine | 3.27(s), 3.91(s) | 0.74 ± 0.12 | 3.16 ± 0.43 |
| 45 | Myo-inositol | 3.27(t), 3.52(dd), 3.61(t), 4.04(m) | 0.82 ± 0.14 | 0.75 ± 0.16 |
| 46 | Tryptophan | 3.3(dd), 3.48(dd), 4.05(dd), 7.2(dd), 7.29(dd), 7.32(s), 7.54(d), 7.74(d) | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 47 | Maltose | 3.4(t), 3.61(m), 3.72(m), 5.22(m), 5.4(dd) | 0.52 ± 0.21 | 0.92 ± 0.21 |
| 48 | 4-Hydroxyphenylacetate | 3.43(s), 6.87(d), 7.17(d) | 0.10 ± 0.01 | 0.18 ± 0.01 |
| 49 | UDP-glucose | 3.51(dd), 3.58(m), 3.78(dd), 4.13(d), 4.17(m), 4.23(m), 4.27(m), 4.36(m), 5.61(dd), 5.97(d), 5.99(d), 7.97(d) | 0.25 ± 0.06 | 0.29 ± 0.06 |
| 50 | UDP-glucuronate | 3.51(dd), 3.58(m), 3.78(dd), 4.13(d), 4.17(m), 4.23(m), 4.27(m), 4.36(m), 5.61(dd), 5.97(d), 5.99(d), 7.97(d) | 0.19 ± 0.02 | 0.21 ± 0.05 |
| 51 | Glycerol | 3.56(dd), 3.65(dd), 3.78(m) | 1.99 ± 0.30 | 2.18 ± 0.19 |
| 52 | Mannose | 3.57(t), 5.17(s) | 0.22 ± 0.07 | 0.31 ± 0.05 |
| 53 | Mannitol | 3.7(dd), 3.8(d), 3.9(dd) | 1.48 ± 0.18 | 1.96 ± 0.33 |
| 54 | UDP-galactose | 3.72(dd), 3.75(dd), 3.81(m), 3.91(dd), 4(d), 4.16(m), 4.19(m), 4.24(m), 4.28(m), 4.37(m), 5.63(dd), 5.98(m), 7.95(d) | 0.61 ± 0.14 | 0.51 ± 0.05 |
| 55 | Adenosine | 3.83(dd), 3.91(dd), 4.29(m), 4.42(dd), 4.79(dd), 6.05(d), 8.24(s), 8.34(s) | 0.55 ± 0.06 | 0.32 ± 0.08 |
| 56 | Inosine | 3.84(dd), 3.92(dd), 4.27(m), 4.44(m), 4.76(t), 6.11(d), 8.23(s), 8.35(s) | 0.11 ± 0.01 | 0.10 ± 0.03 |
| 57 | Serine | 3.84(dd), 3.95(dd), 3.99(dd) | 1.53 ± 0.45 | 1.94 ± 0.35 |
| 58 | Glycolate | 3.92(s) | 0.73 ± 0.15 | 0.59 ± 0.13 |
| 59 | AMP | 4.01(m), 4.36(m), 4.49(m), 4.78(dd), 6.11(d), 8.24(s), 8.62(s) | 0.88 ± 0.29 | 0.30 ± 0.08 |
| 60 | IMP | 4.1(m), 4.36(m), 6.14(d), 8.2(s), 8.55(s) | 0.40 ± 0.07 | 0.37 ± 0.08 |
| 61 | Uridine | 4.12(m), 4.22(t), 4.33(t), 5.89(dd), 7.85(d) | 0.32 ± 0.11 | 0.41 ± 0.10 |
| 62 | NADP+ | 4.2(m), 4.3(m), 6.0(d), 6.08(d), 8.13(s), 8.18(t), 8.41(s), 8.8(d), 9.09(d), 9.27(s) | 0.25 ± 0.08 | 0.26 ± 0.03 |
| 63 | ADP | 4.2(m), 4.37(m), 4.57(dd), 4.74(dd), 6.13(dd), 8.27(s), 8.58(s) | 0.56 ± 0.09 | 0.48 ± 0.03 |
| 64 | Nicotinic acid adenine dinucleotide | 4.2(m), 4.38(m), 6.03(dd), 8.07(t), 8.15(s), 8.42(s), 8.76(d), 9.0(d), 9.13(s) | 0.03 ± 0.01 | 0.02 ± 0.01 |
| 65 | ATP | 4.2(m), 4.4(m), 4.57(dd), 4.74(dd), 6.15(d), 8.23(s), 8.38(s) | 0.49 ± 0.08 | 0.82 ± 0.14 |
| 66 | GTP | 4.21(m), 4.32(m), 5.92(d), 8.12(d) | 0.54 ± 0.08 | 0.43 ± 0.05 |
| 67 | NAD+ | 4.21(m), 4.37(m), 4.5(m), 6.05(dd), 8.15(s), 8.19(t), 8.41(s), 8.8(d), 9.13(d), 9.3(s) | 0.87 ± 0.13 | 0.92 ± 0.18 |
| 68 | Allantoin | 5.4(s), 6.0(s) | 0.22 ± 0.02 | 0.50 ± 0.10 |
| 69 | Fumarate | 6.52(s) | 0.06 ± 0.01 | 0.07 ± 0.01 |
| 70 | Niacinamide | 7.6(dd), 8.26(m), 8.71(m), 8.94(d) | 0.20 ± 0.05 | 0.30 ± 0.06 |
| 71 | Oxypurinol | 8.19(s) | 0.45 ± 0.02 | 0.43 ± 0.19 |
| 72 | Hypoxanthine | 8.19(s), 8.21(s) | 0.29 ± 0.04 | 0.21 ± 0.05 |
Notes. UDP-glucuronate = uridine diphosphate glucoronate; UDP-galactose = uridine diphosphate galactose; AMP = adenosine triphosphate; ADP = adenosine diphosphate; GTP = guanosine-5′-triphosphate. Multiplicity indicated by: singlet = (s) +; doublet = (d); triplet = (t); quartet = (q); multiplet = (m); and double of doublet = (dd).
Day 4 after 7.8 Gy gamma irradiation.
Statistical Results Based on Spectral Deconvolution
Spectral deconvolution was performed to obtain the concentration of each metabolite in the NMR spectrum and then normalized to unit tissue weight to obtain the estimated absolute metabolite concentration in liver tissue. The absolute concentrations of metabolites then constitute the X-matrix (each row represents a sample and each column represents a metabolite). For example, the average concentrations in tissues and the associated standard deviation for the control group and the 7.8 Gy exposed group are summarized in Table 1.
The absolute concentration data were then used for multivariate data analysis. The PCA score plot indicates that the six groups (2 control groups and 4 exposed groups) were clearly separate from each other without any outliers (Fig. 2). Therefore, all the 27 samples were kept for further OPLS modeling. To maximize the correlation between the X- and Y-matrix (the class information) as well as the variation in X-matrix, OPLS was performed to evaluate variable importance and identify significant variables responsible for separating treatment groups. The statistical parameters, R2 and Q2, show good quality of the generated OPLS models, and P values from CV-ANOVA further confirm the validity of these models (Table 2).
FIG. 2.
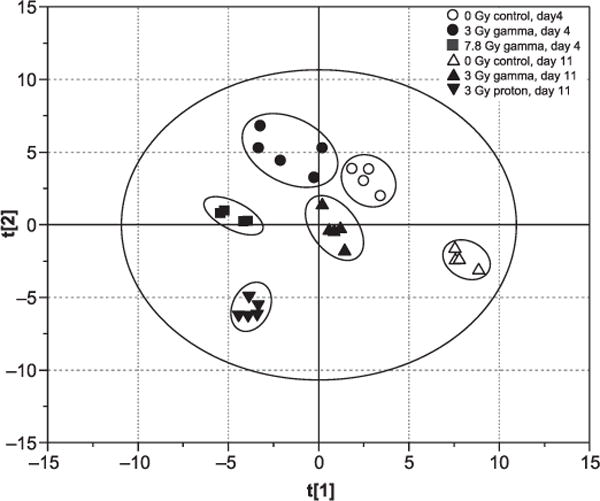
PCA score plot of liver tissue extracts from all control and exposed groups based on 72 metabolites. The PCA score plot shows that the six groups are clearly separate from each other without any outliers, with model statistical parameters of R2 = 0.724 and Q2 = 0.474, respectively.
TABLE 2.
The Multivariate Statistical Parameters R2 and Q2 Based on PCS and OPLS
| Model | Days | Sample | R2X | Q2 | P |
|---|---|---|---|---|---|
| PCA | — | All | 0.724 | 0.474 | – |
| OPLS | 4 | 3.0 Gy gamma vs. control | 0.705 | 0.960 | 0.1148 |
| 3.0 Gy gamma vs. control | 0.569 | 0.934 | 0.0124a | ||
| 7.8 Gy gamma vs. control | 0.754 | 0.988 | 0.0299 | ||
| 11 | 3.0 Gy gamma vs. control | 0.654 | 0.970 | 0.0026 | |
| 3.0 Gy proton vs. control | 0.657 | 0.984 | 0.0008 |
With 7 metabolites removed before OPLS modeling, the two groups are now statistically significantly separated.
The statistical results of all metabolites obtained from S-plot are summarized in Supplementary Tables S3 and S4 (http://dx.doi.org/10.1667/RR14602.1.S1). As shown in Supplementary Tables S3 and S4, the statistically significant metabolites that are responsible for separating the exposed groups from the control groups depend on radiation dose, time after exposure and radiation source. For example, the P value is 0.11 (>0.05) at day 4 after 3.0 Gy gamma irradiation among the control and exposed groups, indicating these two groups have no significant statistical difference, using all the metabolites observed. In contrast, the P value is 0.03 (<0.05) at day 4 after 7.8 Gy gamma irradiation among the control and exposed groups, indicating that the two groups are statistically significantly separate. This result further indicates that the effect of a higher dose appears earlier or sooner after exposure. The difference between the two treatment groups is due to the dose of gamma radiation (Table 3). As shown in Table 3, compared to the control group, there are some differences at day 4 postirradiation in the 3.0 and 7.8 Gy gamma-exposed groups; at these doses, radiation induced upregulation of 7 and 12 metabolites, respectively, and downregulation of 5 and 6 metabolites, respectively. Nine metabolites/biomarkers were identified as common to both exposure groups. As the gamma-radiation dose was increased from 3.0 to 7.8 Gy, some of these biomarkers changed significantly. Relative to the 3.0 Gy exposed group, the further upregulated metabolites in the 7.8 Gy exposed group are alanine, histidine, ATP and allantoin, while the further downregulated metabolites are sn-Glycero-3-phosphocholine, 2-oxobutyrate, 3-hydroxybutyrate and carnitine (Table 3). Thus, the higher dose of gamma radiation (7.8 Gy) has more potent effects in liver metabolism disturbance, and these 8 metabolites showed a more remarkable dose-response effect. It was also found that β-alanine, NADPH and oxypurinol concentrations were significantly changed in the 3.0 Gy exposed group, while insignificant in the 7.8 Gy exposed group. Although the P value (P = 0.11) at day 4 postirradiation in the 3.0 Gy exposed and control groups is insignificant, using all 72 metabolites observed, the P value at day 11 postirradiation in the 3.0 Gy exposed and control groups is 0.0026 (<0.05), which means that the two groups are statistically significantly separated. The differences in liver tissue extracts at days 4 and 11 postirradiation in the 3.0 Gy gamma-exposed groups reveals an apparent latent period for the sequelae of gamma radiation, when mouse liver dysfunction, in terms of all the NMR observable metabolic changes, is not observable (Table 4). Some metabolites were insignificant at day 4 postirradiation but became significant at day 11 postirradiation, while others, such as NADPH, trimethylamine, O-phosphoethanolamine and oxypurinol, were recovered at day 11 after 3.0 Gy gamma exposure.
TABLE 3.
The Significantly Changed Metabolites in Liver Tissue Extract at Day 4 after 3.0 and 7.8 Gy Gamma Irradiation
| Key | Metabolites | 4D3GyG | 4D7GyG | 4D37G* |
|---|---|---|---|---|
| 1 | Cholate | —a | ↓b | — |
| 6 | 3-Hydroxybutyrate | ↓ | ↓ | ↑ |
| 11 | Alanine | — | ↑ | ↑ |
| 17 | Glutamine | ↑ | ↑ | — |
| 19 | Glutathione | ↑ | ↑ | — |
| 20 | Malate | — | ↑ | — |
| 22 | Carnitine | — | — | ↓ |
| 23 | 2-Oxobutyrate | — | — | ↓ |
| 24 | Citrate | ↑ | ↑ | — |
| 25 | β-Alanine | ↓ | — | — |
| 27 | NADPH | ↓ | — | — |
| 32 | Creatinine | — | ↑ | — |
| 34 | Histidine | — | ↑ | ↑ |
| 36 | O-phosphoethanolamine | ↑ | ↑ | — |
| 37 | Choline | ↓ | ↓ | — |
| 38 | O-phosphocholine | — | ↓ | — |
| 41 | Trimethylamine N-oxide | ↓ | ↓ | — |
| 42 | sn-Glycero-3-phosphocholine | — | ↓ | ↓ |
| 43 | Glucose-6-phosphate | — | ↑ | — |
| 44 | Betaine | ↑ | ↑ | — |
| 48 | 4-Hydroxyphenylacetate | ↑ | ↑ | — |
| 65 | ATP | — | — | ↑ |
| 68 | Allantoin | — | ↑ | ↑ |
| 71 | Oxypurinol | ↑ | — | — |
Notes. It is apparent that a higher dose of gamma radiation (7.8 Gy) has more potent effects in liver metabolism. Also, a metabolite is listed if it had statistical significance in at least one treatment group. 4D3GyG = day 4 postirradiation in 3.0 Gy gamma-exposed and control groups; 4D7GyG = day 4 postirradiation in 7.8 Gy gamma-exposed and control groups; 4D37G = day 4 postirradiation in 3.0 Gy compared to 7.8 Gy gamma-exposed groups.
0 = 3.0 Gy gamma radiation, 1 = 7.8 Gy gamma radiation when performing OPLS analysis; positive and negative coefficients indicate up and downregulated metabolites, respectively.
No difference between control and exposed groups.
The concentrations of metabolites increase or decrease between the two groups.
TABLE 4.
3.0 Gy Gamma Irradiation at Days 4 and 11 Induced Changes in Liver Tissue Extracts
| Key | Metabolites | 4D3GyG | 11D3GyG |
|---|---|---|---|
| 6 | 3-Hydroxybutyrate | ↓ | —a |
| 11 | Alanine | — | ↑b |
| 14 | UDP-N-acetylglucosamine | ↓ | ↓ |
| 17 | Glutamine | ↑ | ↑ |
| 18 | O-acetylcarnitine | — | ↑ |
| 19 | Glutathione | ↑ | ↑ |
| 20 | Malate | — | ↑ |
| 21 | Succinate | — | ↑ |
| 24 | Citrate | ↑ | ↑ |
| 25 | β-Alanine | ↓ | ↓ |
| 26 | Aspartate | — | ↑ |
| 27 | NADPH | ↓ | — |
| 28 | NADH | — | ↑ |
| 29 | Trimethylamine | ↑ | — |
| 31 | Creatine | — | ↑ |
| 32 | Creatinine | — | ↑ |
| 34 | Histidine | — | ↓ |
| 35 | Ethanolamine | — | ↑ |
| 36 | O-Phosphoethanolamine | ↑ | — |
| 37 | Choline | ↓ | ↓ |
| 38 | O-Phosphocholine | — | ↓ |
| 40 | Glucose | — | ↑ |
| 41 | Trimethylamine N-oxide | ↓ | ↓ |
| 43 | Glucose-6-phosphate | — | ↑ |
| 44 | Betaine | ↑ | ↑ |
| 47 | Maltose | — | ↑ |
| 48 | 4-Hydroxyphenylacetate | ↑ | ↑ |
| 52 | Mannose | — | ↑ |
| 55 | Adenosine | — | ↑ |
| 57 | Serine | — | ↑ |
| 58 | Glycolate | — | ↑ |
| 61 | Uridine | — | ↑ |
| 63 | ADP | — | ↑ |
| 65 | ATP | — | ↑ |
| 71 | Oxypurinol | ↑ | — |
Notes. A metabolite is listed if it had statistical significance in at least one treatment group. D3GyG = day 4 postirradiation in 3.0 Gy gamma-exposed and control groups; 11D3GyG = day 11 postirradiation in 3.0 Gy gamma-exposed and control groups.
No difference between control and exposed group.
Concentrations of metabolites increase or decrease between the two groups.
Based on the PCA score plot, a clear separation was achieved during the 11 days postirradiation for both 3.0 Gy gamma- and proton-exposed groups (Fig. 2). A set of discriminatory metabolites for separating the two treated groups were identified with the corresponding correlation coefficients obtained from S-Plot analysis, and results were tabulated (Table 5). A total of 25 discriminatory metabolites were found, and six of these (glutathione, choline, O-phosphocholine, glucose-6-phosphate, betaine and 4-hydroxyphenylacetate) were the same as those discriminatory metabolites that separate the exposed groups (both gamma and proton) and the control group at day 4 postirradiation. These results indicate that using different radiation sources, metabolic changes vary after exposure. Despite these source-dependent differences, both gamma and proton radiation change the metabolic profiles in mice livers. More discriminatory metabolites are apparent with proton radiation (e.g., isobutyrate, 3-hydroxybutyrate, lactate, 1,3-diaminopropane, NADPH, trimethylamine and sn-Glycero-3-phosphocholine), which are not present in gamma-exposed group. This finding indicates that at the same dose, proton radiation is more potent than gamma radiation. The same conclusion can be drawn from the fold changes, as is shown in Fig. 3.
TABLE 5.
Liver Metabolites Responsible for Discrimination at Day 11 after 3.0 Gy Whole-Body Gamma- and Proton Irradiation and The Corresponding Correlation Coefficients Obtained from S-plot
| Key | Metabolites | 11D3GyG | 11D3GyP | 11DGP* |
|---|---|---|---|---|
| 1 | Cholate | — | —a | ↓b |
| 5 | Isobutyrate | — | ↓ | ↓ |
| 6 | 3-Hydroxybutyrate | — | ↓ | ↓ |
| 8 | Lactate | — | ↑ | ↑ |
| 11 | Alanine | ↑ | ↑ | ↑ |
| 15 | 1,3-Diaminopropane | — | ↓ | ↓ |
| 17 | Glutamine | ↑ | ↑ | — |
| 18 | O-acetylcarnitine | ↑ | ↑ | — |
| 19 | Glutathione | ↑ | ↑ | ↑ |
| 20 | Malate | ↑ | ↑ | — |
| 21 | Succinate | ↑ | ↑ | ↑ |
| 23 | 2-Oxobutyrate | — | — | ↓ |
| 24 | Citrate | ↑ | — | ↓ |
| 25 | β-Alanine | ↓ | ↓ | — |
| 26 | Aspartate | ↑ | ↑ | — |
| 27 | NADPH | — | ↑ | ↑ |
| 28 | NADH | ↑ | — | ↓ |
| 29 | Trimethylamine | — | ↓ | ↓ |
| 31 | Creatine | ↑ | ↑ | ↓ |
| 32 | Creatinine | ↑ | ↑ | — |
| 34 | Histidine | ↓ | — | — |
| 35 | Ethanolamine | ↑ | — | ↓ |
| 37 | Choline | ↓ | ↓ | ↓ |
| 38 | O-Phosphocholine | ↓ | ↓ | ↑ |
| 40 | Glucose | ↑ | ↑ | — |
| 41 | Trimethylamine N-oxide | ↓ | ↓ | — |
| 42 | sn-Glycero-3-phosphocholine | — | ↓ | ↓ |
| 43 | Glucose-6-phosphate | ↑ | ↑ | ↑ |
| 44 | Betaine | ↑ | ↑ | ↑ |
| 47 | Maltose | ↑ | ↑ | ↑ |
| 48 | 4-Hydroxyphenylacetate | ↑ | ↑ | ↑ |
| 52 | Mannose | ↑ | ↑ | — |
| 55 | Adenosine | ↑ | ↑ | — |
| 57 | Serine | ↑ | — | — |
| 58 | Glycolate | ↑ | — | — |
| 59 | AMP | — | — | ↓ |
| 61 | Uridine | ↑ | ↑ | — |
| 63 | ADP | ↑ | — | — |
| 68 | Allantoin | — | — | ↑ |
| 70 | Niacinamide | — | — | ↑ |
Notes. At day 11 days postirradiation, it is clear that 3.0 Gy gamma and proton irradiation induce different metabolic changes in mouse liver. Gamma irradiation induced upregulation of 24 metabolites and downregulation of 6 metabolites, while proton irradiation induced upregulation of 19 metabolites and downregulation of 10 metabolites. In both 3.0 Gy gamma and proton exposed groups, 22 biomarkers were commonly identified at day 11 postirradiation. A metabolite is listed if it had statistical significance in at least one treatment group. 11D3GyG = day 11 postirradiation in 3.0 Gy gamma-exposed and control groups; 11D3GyP = day 11 postirradiation in 3.0 Gy proton exposed and control groups; 11DGP = day 11 postirradiation in 3.0 Gy gamma and proton exposed groups.
0 = gamma radiation, 1 = proton radiation when performing OPLS analysis; positive and negative coefficients indicate up and downregulated metabolites, respectively.
No difference between the two groups.
The concentrations of metabolites increase or decrease between the two groups.
FIG. 3.
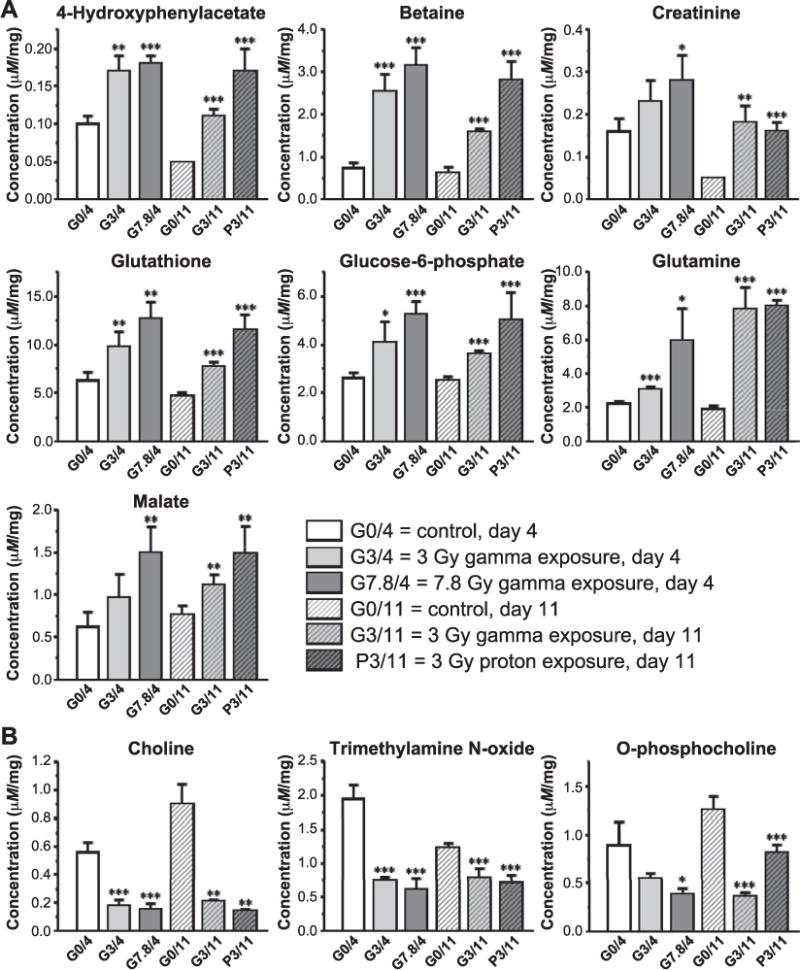
The absolute concentrations of statistically significantly changed metabolites captured by OPLS models derived from the control and exposed groups include seven upregulated metabolite markers (panel A) and three downregulated metabolite markers (panel B). Statistical significance for each was calculated using Welch’s t test between control (sham irradiated) and treated groups. *P < 0.05, **P < 0.01 and ***P < 0.001. The absolute concentration of each metabolite was obtained by normalizing the corresponding metabolite concentration obtained from 1H NMR measurements to the unit weight (mg) of liver tissue before extraction.
Based on all the valid OPLS models (P < 0.05), the statistically significant metabolites in the liver extracts that contributed to the separation of the treatment groups from the control groups were extracted from S-plots and tabulated (Table 6). As shown in Table 6 and Supplementary Table S2 (http://dx.doi.org/10.1667/RR14602.1.S1), compared to the control group, the upregulated metabolites in the exposed groups were glutamine, glutathione, malate, creatinine, phosphate, betaine and 4-hydroxyphenylacetate, and the downregulated metabolites were choline, O-phosphocholine and trimethylamine N-oxide. Figure 3 shows the bar graph highlighting the changes of the estimated absolute concentrations of these statistically significant metabolites, including seven upregulated metabolite markers and three downregulated metabolite markers. Statistical significance among the control and exposed groups was calculated with Welch’s t test. (Supplementary Table S5) All of these metabolites can be considered as latent and potential biomarkers of exposure to either gamma or proton radiation in liver tissue. Although the exposed and control groups were not statistically separated at day 4 after 3.0 Gy gamma irradiation (based on all 72 metabolites identified from the NMR spectra), when the noise data were discarded by removing seven variables with loading absolute value <0.027 in PCA loading plot (Supplementary Fig. S2) before the second multivariate data analysis (i.e. PCA and OPLS), improved separations were obtained. The PCA score plot (Supplementary Fig. S3) shows that six groups are clearly separated from each other and without any outliers, and the statistical parameters (Supplementary Table S6) indicate that excellent quality models are achieved using only 65 (seven metabolites removed) metabolites. OPLS modeling now shows that these two groups are statistically significantly separated with P = 0.0124 (Table 2). Based on the analysis using either all of the 72 metabolites or 65 metabolites, at the relatively early period (4 days) after 3.0 Gy gamma irradiation, the correlation coefficients from the S-plot showed statistically significant changes in several metabolites as prediagnostic biomarkers in mouse liver. The upregulated metabolites were glutamine, betaine and 4-hydroxyphenylacetate, and the downregulated metabolites were choline and trimethylamine N-oxide.
TABLE 6.
Radiation-Induced Metabolic Changes in Liver Tissue Extracts
| Key | Metabolites | Correlation coefficient |
|---|---|---|
| 17 | Glutamine | ↑* |
| 19 | Glutathione | ↑ |
| 20 | Malate | ↑ |
| 32 | Creatinine | ↑ |
| 37 | Choline | ↓ |
| 38 | O-phosphocholine | ↓ |
| 41 | Trimethylamine N-oxide | ↓ |
| 43 | Glucose-6-phosphate | ↑ |
| 44 | Betaine | ↑ |
| 48 | 4-Hydroxyphenylacetate | ↑ |
Concentrations of metabolites increase or decrease between the two groups.
Molecule Pathway Discussion
The major results from the combined 1H NMR metabolic profiling and multivariate date analysis of liver tissue extracts are summarized in this article. The four exposed groups were well separated from the control group based on metabolites obtained from the hydrophilic extracts. A suit of discriminatory metabolites that play a critical role in separating the exposed groups have been identified. We found that the concentrations of glutamine, glutathione, malate, creatinine, phosphate, betaine and 4-hydroxyphenylacetate were statistically significantly increased, while the concentrations of choline, O-phosphocholine and trimethylamine N-oxide were significantly decreased according to all of the valid OPLS models across all high-dose exposed groups.
Gamma and proton radiation can induce metabolic disturbance in the highly radiosensitive organs and tissues, such as hematopoietic and gastrointestinal organs, in addition to liver (37) and affect gene expression (38). The genes in DNA encode protein molecules that are the workhorses of all cells. Almost all enzymes, including those metabolizing nutrients and synthesizing new cellular constituents, are proteins. The metabolites are the end products or intermediates of cellular regulatory processes, where almost all biochemical reactions are catalyzed by enzymes. The liver is an important organ that plays a critical role in metabolism with multiple functions, in which a variety of the metabolic pathways exist. 4-hydroxyphenylacetate is an oxidative deaminated metabolite of tyramine under high oxidase activity (39). A previously published study has shown a correlation between upregulated 4-hydroxyphenylacetate and oxidative stress (40). Betaine, a derivative of choline, is produced in the organism from choline and glycine, methyl groups that contribute to the conversion of homocysteine to methionine. As a methyl donor (41), betaine participates in the methionine cycle, primarily in the liver and kidney (42). Thus, betaine aids in liver function, detoxification and cellular functioning within the organism. Betaine protects the liver against hepatotoxins that damage the liver and enter the body through certain prescription medications (43). Creatinine is a breakdown product of creatine, a molecule that is produced by the liver and of major importance for energy production in muscle metabolism. Since the muscles in the body are relatively constant from day to day, the production of creatinine normally remains essentially unchanged on a daily basis. As a chemical waste molecule, creatinine is transported through the bloodstream to the kidneys and filtered out and disposed in the urine. Therefore, when the kidneys become damaged, the creatinine level in the liver will rise due to poor clearance of creatinine by the kidneys. An abnormally high level of creatinine is thus a sign of possible kidney malfunction or failure. (44) Glucose 6-phosphate is very common in cells, since the vast majority of glucose entering a cell will become phosphorylated during glycolysis metabolism. In the liver, a specific enzyme, i.e., glucose 6-phosphatase, which hydrolyzes glucose 6-phosphate, yields glucose and leads to an increase in blood glucose concentration. (45) The elevated glucose 6-phosphate is the result of energy metabolism disturbed by irradiation. The liver-relevant glutamine synthesis and its role in glutamine metabolism are involved more with regulation than production, since the liver absorbs large amounts of glutamine derived from the gut (46). Glutamine is metabolized through the citric acid cycle under persistent histanoxia, and glutamine contributes significantly to citrate carbons (47). A previously published study showed that exposure to radiation resulted in the concurrent conversion of glucose to lactate and the oxidation of glutamine via the TCA cycle (48). Glutathione is an important antioxidant in organisms capable of preventing damage to important cellular components caused by reactive oxygen species such as ionizing radiation (49). While all cells in the mouse body are capable of synthesizing glutathione, it has been shown that liver glutathione synthesis is essential (50). As an intermediate of citric acid cycle, malate plays an important role in organism, formed by the addition of an–OH group on the face of fumarate (51) or formed from pyruvate via anaplerotic reactions (52). Choline is important in nerve transmission and acetylcholine is capable of storing labile methyl groups. The principal metabolic destiny of choline is by irreversible oxidation to betaine in the liver (53). Choline is an essential nutrient and the liver is a central organ responsible for choline metabolism so its concentration reflects liver function (54). O-phosphocholine is an intermediate in the synthesis of phosphatidylcholine in a reaction that is catalyzed by choline kinase and converts choline into O-phosphocholine, which has potential benefits for liver repair (55). Trimethylamine N-oxide is a common metabolite in animals and a product of the oxidation of trimethylamine that is derived from choline (56). Trimethylamine N-oxide alters cholesterol metabolism in the liver, and in the presence of trimethylamine N-oxide, there is increased deposition of cholesterol and decreased removal of cholesterol from, peripheral cells such as those in the artery wall (57). Based on these results from our metabolomics study and the metabolic fates described in the Small Molecule Pathway Database (58), we propose that the mouse liver metabolic pathways (see Fig. 4) are effected by ionizing radiation exposure.
FIG. 4.
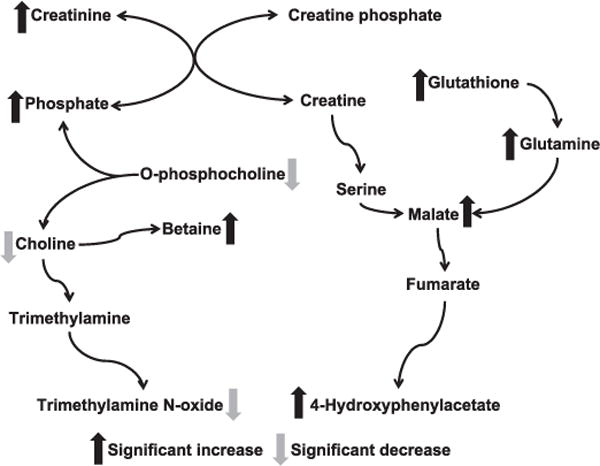
Proposed molecular pathway networks associated with the significantly altered metabolites after irradiation in mouse liver based on the findings from this work and the diverse metabolic fates depicted in the Small Molecule Pathway Database (SMPDB).
CONCLUSION
We have shown that the combined use of 1H NMR metabolomics on tissue extracts and multivariate data analysis (i.e., PCA and OPLS) are powerful tools for studying the effects of ionizing radiation in mouse liver. As many as 72 metabolites have been identified in the hydrophilic extracts of liver tissue. Both PCA and OPLS models showed that the treatment groups are well separated from the control groups, with better separation obtained by OPLS. The effects of 7.8 Gy gamma irradiation appeared early, at 4 days after whole-body irradiation using all of the 72 metabolites for statistical analysis. However, with the use of 72 metabolites, there was no statistically significant difference between the 3.0 Gy gamma-exposed and control groups at day 4 postirradiation. By filtering out seven metabolites with high variance, the 3.0 Gy gamma-exposed group at day 4 postirradiation was able to be statistically significantly separated from the control group. There were significant differences at day 11 postirradiation among the 3.0 Gy gamma and proton exposed groups, and a group of metabolites were identified to be responsible for the discrimination between the two groups. Both gamma and proton irradiation induce metabolic changes in mouse liver tissue, resulting in statistically significant downregulation of choline, O-phosphocholine and trimethylamine N-oxide, accompanied by elevation of glutamine, glutathione, malate, creatinine, phosphate, betaine and 4-hydroxyphenylacetate. These significantly changed metabolites are associated with multiple biological pathways and may be candidate biomarkers for ionizing radiation in liver. In particular, several metabolites may be considered as potential pre-diagnostic biomarkers in mouse liver based on the findings common to both gamma and proton radiation at various doses and times postirradiation, including 4-hydroxyphenylacetate, betaine, glutamine, choline and trimethylamine N-oxide.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences of the National Institute of Health (NIH; award no. R01ES022176). Collection of the gamma and proton irradiated samples was supported by the NASA Space Radiation Program (grant no. NNX07AU44G). All of the NMR experiments were performed in the Environmental Molecular Sciences Laboratory, a national scientific user facility sponsored by the Dept. of Energy (DOE) Office of Biological and Environmental Research, and located at Pacific Northwest National Laboratory (PNNL). PNNL is a multi-program national laboratory operated for the DOE by Battelle Memorial Institute under contract no. DE-AC06-76RLO 1830. We also thank Dr. Qibin Zhang, Mr. Donald N. Rommereim and Mr. Mark K. Murphy for their assistance with animal radiation exposure experiments and sample collection.
Footnotes
Editor’s note.
The online version of this article (DOI: 10.1667/RR14602.1) contains supplementary information that is available to all authorized users.
References
- 1.Bertho JM, Roy L, Souidi M, Benderitter M, Gueguen Y, Lataillade JJ, et al. New biological indicators to evaluate and monitor radiation-induced damage: An accident case report. Radiat Res. 2008;169:543–50. doi: 10.1667/RR1259.1. [DOI] [PubMed] [Google Scholar]
- 2.Sanzari JK, Wan XS, Krigsfeld GS, Wroe AJ, Gridley DS, Kennedy AR. The effects of gamma and proton radiation exposure on hematopoietic cell counts in the ferret model. Gravit Space Res. 2013;1:79–94. [PMC free article] [PubMed] [Google Scholar]
- 3.Maks CJ, Wan XS, Ware JH, Romero-Weaver AL, Sanzari JK, Wilson JM, et al. Analysis of white blood cell counts in mice after gamma- or proton-radiation exposure. Radiat Res. 2011;176:170–6. doi: 10.1667/RR2413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuelsson LM, Larsson DGJ. Contributions from metabolomics to fish research. Mol Biosyst. 2008;4:974–9. doi: 10.1039/b804196b. [DOI] [PubMed] [Google Scholar]
- 5.Nicholson JK, Lindon JC, Holmes E. ‘Metabonomics’: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–9. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson JK, Lindon JC. Systems biology - metabonomics. Nature. 2008;455:1054–6. doi: 10.1038/4551054a. [DOI] [PubMed] [Google Scholar]
- 7.Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nat Biotechnol. 2000;18:1157–61. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- 8.Xia JM, Wu XJ, Yuan YJ. Integration of wavelet transform with PCA and ANN for metabolomics data-mining. Metabolomics (Los Angel) 2007;3:531–7. [Google Scholar]
- 9.Wan QF, Wu GY, He QH, Tang HR, Wang YL. The toxicity of acute exposure to T-2 toxin evaluated by the metabonomics technique. Mol Biosyst. 2015;11:882–91. doi: 10.1039/c4mb00622d. [DOI] [PubMed] [Google Scholar]
- 10.Hotelling H. Analysis of a complex of statistical variables into principal components. J Educ Psychol. 1933;24:417–41. [Google Scholar]
- 11.Abdi H, Williams LJ. Principal component analysis. Wiley Interdiscip Rev Comput Stat. 2010;2:433–59. [Google Scholar]
- 12.Trygg J, Wold S. Orthogonal projections to latent structures (OPLS) J Chemometr. 2002;16:119–28. [Google Scholar]
- 13.Cloarec O, Dumas ME, Trygg J, Craig A, Barton RH, Lindon JC, et al. Evaluation of the orthogonal projection on latent structure model limitations caused by chemical shift variability and improved visualization of biomarker changes in H-1 NMR spectroscopic metabonomic studies. Anal Chem. 2005;77:517–26. doi: 10.1021/ac048803i. [DOI] [PubMed] [Google Scholar]
- 14.Wiklund S, Johansson E, Sjostrom L, Mellerowicz EJ, Edlund U, Shockcor JP, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80:115–22. doi: 10.1021/ac0713510. [DOI] [PubMed] [Google Scholar]
- 15.Holzhutter HG, Drasdo D, Preusser T, Lippert J, Henney AM. The virtual liver: a multidisciplinary, multilevel challenge for systems biology. Wires Syst Biol Med. 2012;4:221–35. doi: 10.1002/wsbm.1158. [DOI] [PubMed] [Google Scholar]
- 16.Pecaut MJ, Dutta-Roy R, Smith AL, Jones TA, Nelson GA, Gridley DS. Acute effects of iron-particle radiation on immunity. Part I: Population distributions. Radiat Res. 2006;165:68–77. doi: 10.1667/rr3493.1. [DOI] [PubMed] [Google Scholar]
- 17.Tyburski JB, Patterson AD, Krausz KW, Slavik J, Fornace AJ, Gonzalez FJ, et al. Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res. 2008;170:1–14. doi: 10.1667/RR1265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C, Yang J, Nie JH. Plasma phospholipid metabolic profiling and biomarkers of rats following radiation exposure based on liquid chromatography-mass spectrometry technique. Biomed Chromatogr. 2009;23:1079–85. doi: 10.1002/bmc.1226. [DOI] [PubMed] [Google Scholar]
- 19.Khan AR, Rana P, Devi MM, Chaturvedi S, Javed S, Tripathi RP, et al. Nuclear magnetic resonance spectroscopy-based metabonomic investigation of biochemical effects in serum of gamma-irradiated mice. Int J Radiat Biol. 2011;87:91–7. doi: 10.3109/09553002.2010.518211. [DOI] [PubMed] [Google Scholar]
- 20.Mortazavi SMJ, Mosleh-Shirazi MA, Tavassoli AR, Taheri M, Mehdizadeh AR, Namazi SAS, et al. Increased radioresistance to lethal doses of gamma rays in mice and rats after exposure to microwave radiation emitted by a GSM mobile phone simulator. Dose Response. 2013;11:281–92. doi: 10.2203/dose-response.12-010.Mortazavi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson CH, Patterson AD, Krausz KW, Kalinich JF, Tyburski JB, Kang DW, et al. Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res. 2012;178:328–40. doi: 10.1667/rr2950.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schreurs AS, Shirazi-Fard Y, Shahnazari M, Alwood JS, Truong TA, Tahimic CG, et al. Dried plum diet protects from bone loss caused by ionizing radiation. Sci Rep. 2016;6:21343. doi: 10.1038/srep21343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Hu JZ, Rommereim DN, Murphy MK, Phipps RP, Huso DL, et al. Application of high-resolution H-1 MAS NMR spectroscopy to the analysis of intact bones from mice exposed to gamma radiation. Radiat Res. 2009;172:607–16. doi: 10.1667/RR1715.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viola A, Saywell V, Villard L, Cozzone PJ, Lutz NW. Metabolic fingerprints of altered brain growth, osmoregulation and neurotransmission in a Rett syndrome model. PLoS One. 2007;2:e157. doi: 10.1371/journal.pone.0000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blankman JL, Long JZ, Trauger SA, Siuzdak G, Cravatt BF. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci U S A. 2013;110:1500–5. doi: 10.1073/pnas.1217121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beckonert O, Keun HC, Ebbels TMD, Bundy JG, Holmes E, Lindon JC, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc. 2007;2:2692–703. doi: 10.1038/nprot.2007.376. [DOI] [PubMed] [Google Scholar]
- 27.Craig A, Cloareo O, Holmes E, Nicholson JK, Lindon JC. Scaling and normalization effects in NMR spectroscopic metabonomic data sets. Anal Chem. 2006;78:2262–7. doi: 10.1021/ac0519312. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Hu M, Feng J, Liu M, Hu JZ. H-1 NMR metabolomics study of metastatic melanoma in C57BL6J mouse spleen. Metabolomics (Los Angel) 2014;10:1129–44. doi: 10.1007/s11306-014-0652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wishart DS. Quantitative metabolomics using NMR. TrAC trends in analytical chemistry. 2008;27:228–37. [Google Scholar]
- 30.Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM. Targeted profiling: Quantitative analysis of H-1 NMR metabolomics data. Anal Chem. 2006;78:4430–42. doi: 10.1021/ac060209g. [DOI] [PubMed] [Google Scholar]
- 31.Xiao X, Hu M, Liu ML, Hu JZ. 1H NMR metabolomics study of spleen from C57BL/6 mice exposed to gamma radiation. Metabolomics (Los Angel) 2016;6:1–11. doi: 10.4172/2153-0769.1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metabolomics. 2013;1:16. doi: 10.2174/2213235X11301010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wold S, Esbensen K, Geladi P. Proceedings of the Multivariate Statistical Workshop for Geologists and Geochemists. Principal component analysis. Chemometr Intell Lab Syst. 1987;2:37–52. [Google Scholar]
- 34.Eriksson L, Trygg J, Wold S. CV-ANOVA for significance testing of PLS and OPLS (R) models. J Chemom. 2008;22:594–600. [Google Scholar]
- 35.Weathington BL, Cunningham CJL, Pittenger DJ. Appendix B: Statistical tables Understanding business research. Hoboken: John Wiley & Sons, Inc; 2012. pp. 435–83. [Google Scholar]
- 36.Jiang LM, Huang J, Wang YL, Tang HR. Metabonomic analysis reveals the CCl4-induced systems alterations for multiple rat organs. J Proteome Res. 2012;11:3848–59. doi: 10.1021/pr3003529. [DOI] [PubMed] [Google Scholar]
- 37.Macia i Garau M, Lucas Calduch A, Lopez EC. Radiobiology of the acute radiation syndrome. Rep Pract Oncol Radiother. 2011;16:123–30. doi: 10.1016/j.rpor.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gridley DS, Mao XW, Cao JD, Bayeta EJM, Pecaut MJ. Protracted low-dose radiation priming and response of liver to acute gamma and proton radiation. Free Radic Res. 2013;47:811–20. doi: 10.3109/10715762.2013.826351. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein M, Anagnost B, Goldstei MN. Tyramine-H3: deaminated metabolites in neuroblastoma tumors and in continuous cell line of a neuroblastoma. Science. 1968;160:767–8. doi: 10.1126/science.160.3829.767. [DOI] [PubMed] [Google Scholar]
- 40.Liao W, Wei H, Wang XY, Qiu YP, Gou XJ, Zhang XL, et al. Metabonomic variations associated with aom-induced precancerous colorectal lesions and resveratrol treatment. J Proteome Res. 2012;11:3436–48. doi: 10.1021/pr300284h. [DOI] [PubMed] [Google Scholar]
- 41.Alirezaei M, Jelodar G, Ghayemi Z. Antioxidant defense of betaine against oxidative stress induced by ethanol in the rat testes. Int J Pept Res Ther. 2012;18:239–47. [Google Scholar]
- 42.Craig SAS. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–49. doi: 10.1093/ajcn/80.3.539. [DOI] [PubMed] [Google Scholar]
- 43.Schoental R. Liver disease and “natural” hepatotoxins. Bull World Health Organ. 1963;29:823–33. [PMC free article] [PubMed] [Google Scholar]
- 44.Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh A, Shieh JJ, Pan CJ, Sun MS, Chou JY. The catalytic center of glucose-6-phosphatase. HIS176 is the nucleophile forming the phosphohistidine-enzyme intermediate during catalysis. J Biol Chem. 2002;277:32837–42. doi: 10.1074/jbc.M201853200. [DOI] [PubMed] [Google Scholar]
- 46.Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr. 2003;133:2068S–72S. doi: 10.1093/jn/133.6.2068S. [DOI] [PubMed] [Google Scholar]
- 47.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Le A, Lane Andrew N, Hamaker M, Bose S, Gouw A, Barbi J, et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–21. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pompella A, Visvikis A, Paolicchi A, Tata VD, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y, Yang Y, Miller ML, Shen DX, Shertzer HG, Stringer KF, et al. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–28. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- 51.Edwards CB, Copes N, Brito AG, Canfield J, Bradshaw PC. Malate and fumarate extend lifespan in Caenorhabditis elegans. PLoS One. 2013;8:17. doi: 10.1371/journal.pone.0058345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owen OE, Kalhan SC, Hanson RW. The key role of anaplerosis and cataplerosis for citric acid cycle function. J Biol Chem. 2002;277:30409–12. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 53.Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem. 1999;274:7816–24. doi: 10.1074/jbc.274.12.7816. [DOI] [PubMed] [Google Scholar]
- 54.Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroen. 2012;28:159–65. doi: 10.1097/MOG.0b013e32834e7b4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Niebergall LJ, Jacobs RL, Chaba T, Vance DE. Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim Biophys Acta. 2011;1811:1177–85. doi: 10.1016/j.bbalip.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 56.Baker J, Chaykin S. The biosynthesis of trimethylamine-N-oxide. Biochim Biophys Acta. 1960;41:548–50. doi: 10.1016/0006-3002(60)90062-7. [DOI] [PubMed] [Google Scholar]
- 57.Koeth RA, Wang ZE, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jewison T, Su YL, Disfany FM, Liang YJ, Knox C, Maciejewski A, et al. SMPDB 2.0: big improvements to the Small Molecule Pathway Database. Nucleic Acids Res. 2014;42:D478–84. doi: 10.1093/nar/gkt1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


