Abstract
Continued smoking after a cancer diagnosis contributes to several negative health outcomes. Although many cancer patients attempt to quit smoking, high smoking relapse rates have been observed. This highlights the need for a targeted, evidence-based smoking-relapse prevention intervention. The design, method, and baseline characteristics of a randomized controlled trial assessing the efficacy of a self-help smoking-relapse prevention intervention are presented. Cancer patients who had recently quit smoking were randomized to one of two conditions. The Usual Care (UC) group received the institution’s standard of care. The smoking relapse-prevention intervention (SRP) group received standard of care, plus 8 relapse-prevention booklets mailed over a 3 month period, and a targeted educational DVD developed specifically for cancer patients. Four hundred and fourteen participants were enrolled and completed a baseline survey. Primary outcomes will be self-reported smoking status at 6 and 12-months after baseline. Biochemical verification of smoking status was completed for a subsample. If found to be efficacious, this low-cost intervention could be easily disseminated with significant potential for reducing the risk of negative cancer outcomes associated with continued smoking.
Keywords: smoking, relapse-prevention, randomized controlled trial, cancer patients
1. Introduction
The potential negative implications of continued smoking in cancer patients are well established. Persistent smoking after a cancer diagnosis places the individual at increased risk for a secondary malignancy, poor cancer treatment outcomes, and decreased quality of life. 1–3 In fact, the 2014 Surgeon General’s report concluded that sufficient evidence exists to demonstrate a causal link between continued smoking and poor cancer outcomes for patients and survivors. 4
Fortunately, cancer patients are highly motivated to quit smoking. 5 In a previous study, 84% of patients reported making at least one quit attempt and 69%, reported making multiple quit attempts.6 The majority of quit attempts occur at the time of diagnosis. 5,7 Although many cancer patients attempt to quit, studies have reported smoking relapse rates among cancer patients ranging from 13% to 60%, 8–13 with abstinence rates in half of these studies validated biochemically. Importantly, even low relapse rates are cause for concern due to the considerable negative health impact and quality of life consequences. Therefore, interventions are needed to prevent smoking relapse among cancer patients who have already achieved initial smoking cessation.
Brandon and colleagues 14,15 previously tested a self-help smoking relapse- prevention intervention for the general population (titled Forever Free®) and found that it was efficacious and cost-effective. This study extends the self-help relapse-prevention approach to a cancer patient population. Indeed, several smoking relapse risk factors, such as nicotine dependence, negative affect, and low self-efficacy, have been observed in both general and oncology populations. 16–20 Previous research has suggested that there is also a range of unique factors associated with relapse among this population that must be considered: pain and fatigue, delayed relapse rates, cancer-specific risks of continued smoking, and cancer-relevant benefits of quitting smoking. 5,21–23
Therefore, we developed an educational DVD, titled Surviving SmokeFree®, to target the unique needs of cancer patients. The Forever Free® booklets and Surviving SmokeFree® DVD address both the common and cancer-specific relapse risk factors, respectively. This multimodal intervention also represents a potentially cost-effective and highly disseminable smoking relapse-prevention intervention for cancer patients.
This paper describes the design, methods, and data analysis plan for a randomized controlled trial (RCT). Baseline characteristics of the sample are also presented to highlight the feasibility of recruiting patients who have diverse cancer sites and stages. The primary aim of the RCT is to test the efficacy of a multimodal empirically-based, targeted smoking relapse prevention intervention for cancer patients. Secondary aims will explore the influence of potential moderators on intervention outcomes and examine the degree to which the intervention impacts outcomes via theoretically-derived mediating variables.
2. Methods
2.1 Participants
Participants included recently diagnosed cancer patients receiving the first round of treatment at Moffitt Cancer Center, a NCI-designated Comprehensive Cancer Center in Tampa, Florida. At the time of enrollment, eligible patients were at least 18 years of age, able to speak and read English, had smoked at least 10 cigarettes per day (CPD) for at least one year prior to their cancer diagnosis, and had quit smoking within the past 90 days. A quit was defined as self-reported smoking abstinence for at least 24 hours. Previous research suggests that the risk of smoking relapse is highest among those who have recently quit, 24 thus our range was from 24 hours to 90 days quit, after which the risk of smoking relapse is reduced. Patients diagnosed with distant metastases (malignancies that have spread from the original tumor to distant organs or lymph nodes), were excluded to avoid added patient burden. Patients were recruited by trained research staff from inpatient units and various outpatient clinics. The study was approved by the University of South Florida’s institutional review board.
2.2. Study Design
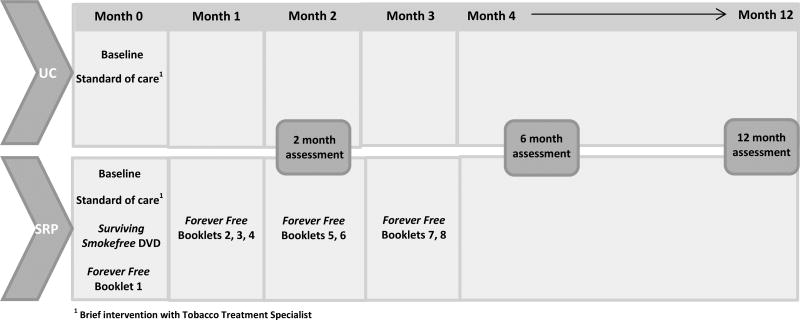
The initial phase of the study was dedicated to the development of the targeted supplementary educational DVD, titled Surviving SmokeFree®. Development of the DVD was guided by our formative work, 23 as well as relevant research and theory on smoking relapse-prevention. Our intervention was informed by Witkiewitz and Marlatt’s (2004) 25 reconceptualized cognitive-behavioral model of relapse, which posits that relapse risk is a dynamic interaction of distal and proximal risks. This framework includes the interaction of background factors (family history, dependence), physiological states (e.g., withdrawal, pain, fatigue), affective states, cognitive processes (e.g. motivation) and coping skills in influencing relapse. The randomized trial consisted of two arms, Usual Care (UC) and Smoking Relapse Prevention (SRP). The UC group received standard of care (i.e. a brief clinical smoking intervention provided by the institution’s Tobacco Treatment Specialist). Participants randomized to the SRP group received standard of care, viewed the Surviving SmokeFree® DVD, and received the first booklet of the Forever Free® series, along with a copy of Surviving SmokeFree® DVD. The remaining 7 booklets were mailed over a three month period. The primary outcome is smoking relapse as measured by self-reported 7-day point prevalence. We hypothesized that participants in the SRP group will demonstrate lower rates of smoking relapse at 6 and 12 months. Figure 1 summarizes the intervention and assessment distribution time points.
Figure 1.
Intervention and assessment distribution schedule
2.3. Intervention Conditions
2.3.1 Usual Care (UC)
Participants randomized to the UC group received the institution’s standard of care, which included a one-time routine assessment of smoking behavior and a brief clinical intervention (5–15 minutes) with the tobacco treatment specialist. Specifically, patients received brief counseling based on the 5 A’s Clinical Practice Guidelines; Ask about tobacco use, Advise to quit, Assess willingness to make a quit attempt, Assist in quit attempt, Arrange for follow-up.17 Depending on patient interest, patients were also offered information about local and state smoking resources (e.g., Quitline), educational brochures, and assistance with obtaining pharmacotherapy (i.e., varenicline, bupropion, or nicotine replacement therapy).
2.3.2 Smoking Relapse-Prevention Intervention (SRP)
Study participants randomized to the SRP group received standard care as described above, including the brief clinical intervention from the tobacco treatment specialist, as well as our newly developed smoking-relapse prevention intervention comprising the series of 8 Forever Free® booklets and the Surviving SmokeFree® DVD. Intervention components are described below.
Forever Free® Booklets
The first booklet in the series, received at study enrollment, presented a summary of the basic relapse-prevention principles and techniques. This booklet included topics such as nicotine dependence, situations that place the person at high risk for relapse, coping with urges to smoke, making lifestyle changes, and ways to handle an initial “slip”.14 The seven remaining booklets, mailed during the 3 subsequent months post-recruitment, provided more in-depth information on specific topics related to maintaining abstinence in the long term; “Smoking Urges”; Smoking and Weight”; What If You Have a Cigarette?”; “Your Health”; “Smoking, Stress, and Mood”; “Lifestyle Balance”; and “Life Without Cigarettes.” Given the demonstrated efficacy of the booklet series for reducing relapse in two previous clinical trials, 14,15 we did not modify the content of this validated intervention. The intervention distribution schedule is based on empirical support for the importance of continued contact and the delayed period of relapse risk observed in cancer patients, while balancing the ease in which the intervention can be disseminated. Booklets were 7×10 inches in size, ranged from 9 to 21 pages, with a mean of 15 pages, and were written at the fifth to sixth grade reading level.
Surviving SmokeFree® DVD
Although the Forever Free® booklets cover important smoking relapse-prevention topics relevant to the majority of the general population, cancer patients experience unique challenges that also need to be considered. 5,26,27 Therefore, the Surviving SmokeFree® DVD addressed these challenges, such as specific cancer-related risks and benefits of smoking cessation. Key content areas included: coping with cancer-related negative affect, cancer-specific smoking risks (immediate and long-term), cancer-related benefits of quitting, cancer- related pain and fatigue, enhancing self-efficacy, pharmacotherapy options, and social support. This content was drawn from prior formative findings and relevant research theory about smoking relapse-prevention. 5 Interviews with health care providers and cessation experts were used to deliver health related messages. The video also included patients role-modeling behavioral coping strategies for dealing with urges to smoke to enhance the effectiveness of the message.28 Patient testimonials were included to relay the benefits of staying smoke free during cancer treatment, the potential for improved outcomes, and strategies that help maintain abstinence. Importantly, the DVD was developed via a multi-step iterative process using a learner verification approach to ensure the DVD was suitable for the intended audience. During this process, we evaluated if patients found the DVD appealing, gauged comprehension of the DVD’s key messages, and assessed if the patient testimonials were relatable and acceptable. Additional details describing the DVD development process are described elsewhere. 29 Participants view the Surviving SmokeFree® DVD at the time of enrollment and are provided a copy to take home.
2.4. Procedures
Participants (N = 414) were recruited between June 2012 and March 2015. Potential participants were identified via medical chart reviews, or via an electronic capture and trigger system that identified patients who were current smokers or had quit smoking in the past 90 days. Only cancer patients who reported quitting smoking within the previous 90 days were eligible for this study. The participants were then prescreened and, if deemed medically eligible, were seen by the Tobacco Treatment Specialist and provided standard of care. Patients were then approached by trained research staff and screened for study eligibility. If the patient was eligible and wished to participate, written informed consent was obtained and the timing of assessments was described. The baseline assessment was then administered.
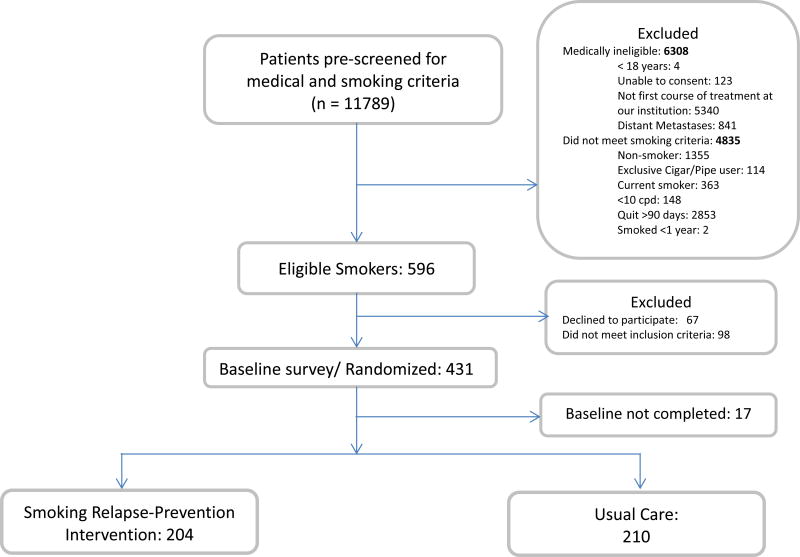
Participants were subsequently randomized into one of the two arms, UC or SRP, using balance-permuted block, stratified randomization with a block size of 10. Stratification variables included gender, length of smoking abstinence (i.e., less than 7 days or ≥ 7 days), and cancer site (i.e., Thoracic, Head & Neck, Other). A total of 596 cancer patients were approached and assessed for eligibility, 431 met inclusion criteria and were randomized, with 414 providing data for analyses. Figure 2 displays the study recruitment flowchart. After completing the baseline assessment, participants randomized to the SRP group viewed the 14-minute Surviving SmokeFree® DVD. Participants were then provided with the first booklet in the Forever Free® series (Booklet1; An Overview) and were told when the remaining Forever Free booklets would be mailed to their home. They were also provided a copy of the Surviving SmokeFree® DVD to keep. Breath CO samples were collected at baseline for all patients medically capable of performing the test. Follow-up assessments were scheduled to be completed at 2, 6, and 12 months post baseline. Participants received $25 for completing the baseline assessment, $25 for each completed follow-up, a $25 bonus payment for completing all of the assessments, and $20 for each biochemical sample provided.
Figure 2.
Participant flow diagram.
2.5. Measures
2.5.1 Baseline Assessment
Socio-demographic characteristics and smoking characteristics and history were assessed using standardized survey items including the Fagerström Test for Nicotine Dependence (FTND). 30 Medical characteristics, including cancer site and stage, were obtained via a medical record review. A modified version of the situation specific abstinence self-efficacy scale, 31 was included to evaluate cessation self-efficacy. Pain and fatigue severity and interference were evaluated utilizing the Brief Pain Inventory (BPI), 32 and the Brief Fatigue Inventory (BFI), 33 respectively. The Partner Interaction Questionnaire (PIQ) was administered to assess negative and positive partner behaviors related to smoking. 34The use of cognitive and behavioral coping strategies was assessed using an adapted version of the Coping with Temptation Inventory. 35 For the secondary aims, partner support, sex, cancer site (smoking-related vs. non-smoking related), and level of nicotine dependence will be evaluated as potential moderators of cessation outcomes.17,20,36 Self-efficacy 31 and coping strategies will be tested as possible mediators.
2.5.2 Follow-Up Assessments
Follow-up assessments were completed at 2, 6, and 12 months post-baseline assessment (Figure 1). Follow-ups assessments were conducted primarily by phone. Special accommodations were made for in-person or mailed assessments to accommodate medical circumstances or when patients could not be reached. At each follow-up, tobacco use and any use of smoking cessation aids since the previous contact was assessed. Participants randomized to the intervention group also completed an adapted version of the eight-item Client Satisfaction Questionnaire 37 to assess their use and evaluation of the intervention materials. Use and satisfaction of the Surviving SmokeFree® DVD and Forever Free® intervention elements were assessed separately. To assess use of the Forever Free® booklets, participants were asked whether they read the booklets and whether they read them more than once. To measure DVD usage, participants were asked the number of times they watched the DVD, as well as whether they had a DVD player or computer at home to view the DVD.
The primary outcome variable is self-reported smoking status at 6 and 12-months using 7-day point-prevalence criteria (i.e., individuals who report that they smoked a cigarette in the 7 days preceding will be classified as relapsed). The time-line follow-back procedure 38 was used to allow for calculation of continuous abstinence since the previous follow-up point.
Breath carbon monoxide (CO) samples were collected for a subsample of participants who reported abstinence at 6 and 12-months and were receiving their follow-up care at the institution or lived within 50 miles (N = 162 samples collected at 6-month follow up, which represents 91.5% of the sample who reported abstinence and lived within 50 miles; N = 133 samples collected at 12 month follow-up, which represents 93.6% of the sample who reported abstinence and lived within 50 miles). The breath sample was collected via a portable CO monitor (Vitalograph Inc., Lenexa, KS) utilizing 8 parts per million as the cutoff for abstinence verification. The breath CO data collected will be used to estimate the level of misreporting of smoking status in the sample and to adjust estimated abstinence rates accordingly.
2.6. Data Analyses Plan for RCT Outcomes
The demographic, cancer-related, smoking, mediating, and moderating variables described above will be summarized using descriptive statistics. Intervention conditions will be compared on baseline characteristics using the appropriate statistical tests (e.g., t-test or Chi-square). Variables with group differences at p < 0.1 will be included as covariates in subsequent analyses of the intervention. To evaluate attrition bias, all variables will be compared between participants who complete the study and those who are lost to follow-up at 2-, 6- and 12- months.
To test the efficacy of the proposed SRP intervention for cancer patients, percentages of smoking relapse (7-day point prevalence and continuous abstinence) at 2-, 6- and 12- month follow ups, and their 95% confidence intervals, will be calculated using the exact binomial method. The difference of relapse between the intervention groups will be evaluated using Fisher’s exact test. The pair-wise agreements and associations among 2-, 6- and 12-month relapse will be measured using the Kappa coefficient and McNemar’s test. Data will be analyzed and reported using both the intent-to-treat approach, in which missing participants will be coded as smoking, and the responders-only approach.
The intervention outcomes include binary 6- and 12-month relapse (self-reported 7-day point prevalence). The following prospective mediators of relapse will be assessed: participants’ use and evaluation of intervention materials, quitting self- efficacy, and use of cognitive and behavioral strategies for coping with urges to smoke. Mediation will be evaluated using Hayes’ (2013) regression-based approach and structural equation modeling. To evaluate 6-month relapse, the 2-month scores of the mediating variables will be applied. Similarly, to evaluate 12-month relapse, the 6-month scores will be applied.
Logistic regression will be applied for evaluating prospective moderators: cancer site (smoking-related vs. non-smoking related), cancer treatment, level of nicotine dependence, and level of social support. Interaction terms will be evaluated in the presence of their main effects adjusting for potential confounds.
2.7. Sample Size Calculation
The sample size calculation focused on Aim 1: To test the efficacy of a multimodal empirically-based, targeted smoking relapse-prevention intervention for cancer patients. The primary hypothesis predicts that cancer patients who received the SRP intervention would demonstrate lower rates of smoking relapse at the final 12- month follow-up assessment. Based on previous research, 13–15 we estimated that the 12-month smoking relapse rate for the UC group and SRP group would be 40% and 25%, respectively. A sample size of 165 per group (total 330) achieves 80% power to detect the 15% relapse difference between the conditions using the two-sided Fisher’s exact test. A significance level of 0.05 will be used. Assuming 20% attrition, accrual of 414 participants was required.
3. Baseline Results
Descriptive statistics for demographic, smoking, and cancer-related variables are presented in Table 1. Of the 414 patients who completed a baseline assessment, half were female, the majority were Caucasian, and 7% self-identified as Hispanic or Latino. The mean age was 55.0 (SD = 10.74), and 41.0% reported an annual household income below $30,000. Two-thirds of the sample reported having financial concerns regarding their cancer diagnosis and treatment. With respect to previous smoking history, participants smoked an average of 21.0 (SD = 9.5) cigarettes per day and were moderately nicotine dependent as suggested by an average Fagerström score of 5.1 (2.2). The most prevalent cancer diagnoses in our sample were: Thoracic (21.3%), followed by Head and Neck (18.4%), and Gastrointestinal (12.3%). Nearly half of the sample had early stage disease (i.e., Stage I and Stage II). No significant differences between conditions were found for any of the variables presented in Table 1.
Table 1.
Patient Demographics, Disease, and Tobacco-Use Characteristics at Study Enrollment
| SRP | UC | Total | |
|---|---|---|---|
| Characteristics | n = 204 | n = 210 | N = 414 |
| M (SD) or (N, %) | M (SD) or (N, %) | M (SD) or (N, %) | |
| Gender (Female) | 102 (50.0%) | 106 (51.0%) | 208 (50.2%) |
| Age | 55.0 (11.0) | 55.0 (10.5) | 55.0 (10.7) |
| Race | |||
| White | 193 (95.0%) | 189 (90.0%) | 382 (92.3%) |
| Black/African American | 3 (1.0%) | 10 (5.0%) | 13 (3.1%) |
| Other | 4 (2.0%) | 7 (3.0%) | 11 (2.7%) |
| Hispanic ethnicity | 13 (6.0%) | 16 (8.0%) | 29 (7.0%) |
| Marital Status: Married or living with a partner | 113 (55.0%) | 111 (53.0%) | 224 (54.0%) |
| Education: High school graduate or below | 94 (46.0%) | 96 (46.0%) | 190 (46.0%) |
| Income: Below $30,000/year | 84 (41.0%) | 86 (41.0%) | 170 (41.0%) |
| Employed | 117 (57.0%) | 112 (53.3%) | 229 (55.3%) |
| Financial concerns regarding cancer Dx/Tx: Yes | 122 (59.8%) | 128 (60.9%) | 250 (60.3%) |
| Current financial situation | |||
| Difficult/Very Difficult | 58 (28.4%) | 58 (27.6%) | 116 (28.0%) |
| Cancer Site | |||
| Thoracic | 43 (21.1%) | 45 (21.4%) | 88 (21.3%) |
| Head &Neck | 38 (18.6%) | 38 (18.1%) | 76 (18.4%) |
| Gastrointestinal | 25 (12.3%) | 26 (12.4%) | 51 (12.3%) |
| Genitourinary | 26 (12.8%) | 17 (8.1%) | 43 (10.4%) |
| Breast | 22 (10.8%) | 26 (12.4%) | 47 (11.6%) |
| Gynecological | 20 (9.8%) | 16 (7.6%) | 36 (8.7%) |
| Hematological | 16 (7.8%) | 25 (11.9%) | 41 (9.9%) |
| Cutaneous | 10 (4.9%) | 10 (4.8%) | 20 (4.8%) |
| Other | 4 (2.0%) | 7 (3.3%) | 11 (2.7%) |
| Disease stage (TNM) | |||
| Unstaged | 20 (9.8%) | 38 (18.1%) | 58 (14.0%) |
| Stage 0 | 7 (3.4%) | 2 (1.0%) | 9 (2.2%) |
| Early (I–II) | 111 (54.4%) | 90 (42.9%) | 201 (48.6%) |
| Late (III–IV) | 66 (32.4%) | 80 (38.1%) | 146 (35.3%) |
| Smoking-related variables | |||
| Cigarettes per day | 21.6 (9.4) | 20.3 (9.6) | 21.0 (9.5) |
| Number of years smoking | 35.0 (12.6) | 34.3 (11.5) | 34.6 (12.1) |
| Fagerström Test for Nicotine Dependence | 5.3 (2.1) | 5.0 (2.2) | 5.1 (2.2) |
| Quitting self-efficacy | 37.9 (7.6) | 38.4 (7.5) | 38.2 (7.6) |
4. Discussion
There is growing recognition that cancer patients should be provided with smoking cessation assistance. 39,40 To our knowledge, this is the first study specifically designed to test the efficacy of a targeted smoking relapse-prevention intervention for cancer patients. This study has several strengths. First, our focus on relapse-prevention is novel. Prior studies have focused primarily on the development and testing of smoking cessation interventions for current smokers, as opposed to helping those who have recently quit to maintain their abstinence. 20,41 Second, the majority of previous trials have focused solely on patients diagnosed with a thoracic or head and neck malignancy, whereas our trial includes a diverse sample of cancer sites. The inclusion of recent quitters with tobacco-related as well as non-tobacco related cancers will allow us to examine the impact of cancer site on outcomes and is essential to extend the reach of clinically-relevant interventions.
Our study represents one of the largest smoking trials conducted with cancer patients. The identification and recruitment of eligible patients required a recruitment period of 33 months to achieve our target sample size, with several key lessons learned in the process. Paramount to successfully accruing our sample was our evolving screening and recruitment methodology. We worked closely with our institution’s clinical systems and the information shared services departments to modify current data sources that capture smoking status to develop a more accurate and efficient electronic trigger method for identifying potential participants. Given the vast volume of data contained within the medical record system, investigators are encouraged to examine how existing patient data can be leveraged for recruitment purposes. Recruitment was also facilitated by research staff training. In collaboration with social work, staff were trained on patient communication and managing the complexities of a busy clinical setting (e.g., how to communicate with the medical team). These efforts likely contributed to our low patient refusal rates. Taken together, the implementation of these steps resulted in a faster pace of recruitment as the study progressed.
There are some limitations of the present study that should be acknowledged. First, the generalizability of our findings to a racially and ethnically-diverse population will be limited due to the low racial/ethnic diversity of our sample. For example, cancer patients treated at community hospitals may differ with respect to race, ethnicity, and socioeconomic status. 42 It is important to note however that social-economic status variability is present among participants. Two-thirds of our sample reported having financial concerns regarding their diagnosis. Another limitation of our study is that, because the Forever Free® booklets and Surviving SmokeFree® DVD were provided together, it will be difficult to ascertain the relative contribution of each intervention component. Importantly, both intervention components are low-cost and easily portable; thus, knowledge gained about the relative efficacy of each component is not paramount. The Surviving Smokefree® video was offered only via the DVD, so a potential limitation is that participants who did not have access to a DVD player may not have been able to re-view the video after seeing it upon enrollment. We will be able to measure whether this was indeed a limitation because we assess DVD usage at follow-up.
Additionally, given logistical and resource considerations, we will only have the collection of biochemical samples from a subsample of patients (i.e., those receiving their follow-up care at the institution or those who reside within 50 miles). However, these data will be helpful to further evaluate the value of the inclusion of biochemical verification in future smoking research with cancer patients. Previous studies have reported the misreporting of smoking status to range from 2.5 – 33% of patients. 43,44 Finally, research aspects of an intervention trial, including participant compensation and data collection, may represent a limitation to generalizability to non-research settings.
If found efficacious, the next step will be to ascertain the most efficient and cost-effective way to disseminate this newly developed smoking relapse-prevention intervention for cancer patients. The potential integration of this self-help intervention into standard care may also be explored. In addition, to further extend the reach of this intervention, it would be valuable to have versions in other languages, with Spanish as the highest priority. Alternative modalities could be considered as well, in order to expand the reach, such as mobile or online versions. Careful consideration of the target population will need to be considered, of course, as older cancer patients may prefer a more traditional modality, 45 such as booklets and DVD.
In conclusion, our current study represents the first of its kind to test a smoking relapse-prevention intervention for cancer patients. We have enrolled a large sample of cancer patients with a variety of cancer types. If the intervention is effective, this study has the potential to reduce smoking relapse, and thereby improve cancer patient outcomes.
Acknowledgments
This work was supported by grant R01 CA154596 from the National Cancer Institute.
References Cited
- 1.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garces YI, Schroeder DR, Nirelli LM, et al. Tobacco use outcomes among patients with head and neck carcinoma treated for nicotine dependence: a matched-pair analysis. Cancer. 2004;101(1):116–124. doi: 10.1002/cncr.20350. [DOI] [PubMed] [Google Scholar]
- 3.Westmaas JL, Alcaraz KI, Berg CJ, Stein KD. Prevalence and correlates of smoking and cessation-related behavior among survivors of ten cancers: findings from a nationwide survey nine years after diagnosis. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1783–1792. doi: 10.1158/1055-9965.EPI-14-0046. [DOI] [PubMed] [Google Scholar]
- 4.General USS. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. 2014 http://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf.
- 5.Simmons VN, Litvin EB, Jacobsen PB, et al. Predictors of smoking relapse in patients with thoracic cancer or head and neck cancer. Cancer. 2013;119(7):1420–1427. doi: 10.1002/cncr.27880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995;75(2):569–576. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Park ER, Japuntich SJ, Rigotti NA, et al. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012;118(12):3153–3164. doi: 10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley ME, Sarna L, Kotlerman J, et al. Smoking cessation is challenging even for patients recovering from lung cancer surgery with curative intent. Lung Cancer. 2009;66(2):218–225. doi: 10.1016/j.lungcan.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison GDM. Smoking habits of long-term survivors of surgery for lung cancer. Thorax. 1982;37:331–333. doi: 10.1136/thx.37.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110(5):1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 11.Gritz ER, Carr CR, Rapkin DA, Chang C, Beumer J, Ward PH. A smoking cessation intervention for head and neck cancer patients: trial design, patient accrual, and characteristics. Cancer Epidemiol Biomarkers Prev. 1991;1(1):67–73. [PubMed] [Google Scholar]
- 12.Walker MS, Larsen RJ, Zona DM, Govindan R, Fisher EB. Smoking urges and relapse among lung cancer patients: findings from a preliminary retrospective study. Prev Med. 2004;39(3):449–457. doi: 10.1016/j.ypmed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 14.Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consult Clin Psychol. 2000;68(1):103–113. doi: 10.1037//0022-006x.68.1.103. [DOI] [PubMed] [Google Scholar]
- 15.Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, Cantor AB. Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: dismantling the effects of amount of content versus contact. J Consult Clin Psychol. 2004;72(5):797–808. doi: 10.1037/0022-006X.72.5.797. [DOI] [PubMed] [Google Scholar]
- 16.Cinciripini PM, McClure JB. Smoking cessation: recent developments in behavioral and pharmacologic interventions. Oncology (Williston Park) 1998;12(2):249–256. 259. discussion 260, 265, 242. [PubMed] [Google Scholar]
- 17.Fiore MJC, Baker T, Bailey W, Benowitz N, Curry S. In: Treating tobacco use and dependence: 2008 update. US Department of Health and Human Services. Public Health Services, editor. Rockville (MD): 2008. [Google Scholar]
- 18.Gritz ER, Carr CR, Rapkin D, et al. Predictors of long-term smoking cessation in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1993;2(3):261–270. [PubMed] [Google Scholar]
- 19.Schnoll RA, Rothman RL, Newman H, et al. Characteristics of cancer patients entering a smoking cessation program and correlates of quit motivation: implications for the development of tobacco control programs for cancer patients. Psychooncology. 2004;13(5):346–358. doi: 10.1002/pon.756. [DOI] [PubMed] [Google Scholar]
- 20.Schnoll RA, Rothman RL, Wielt DB, et al. A randomized pilot study of cognitive-behavioral therapy versus basic health education for smoking cessation among cancer patients. Ann Behav Med. 2005;30(1):1–11. doi: 10.1207/s15324796abm3001_1. [DOI] [PubMed] [Google Scholar]
- 21.Gritz ER, Vidrine DJ, Fingeret MC. Smoking cessation a critical component of medical management in chronic disease populations. Am J Prev Med. 2007;33(6 Suppl):S414–422. doi: 10.1016/j.amepre.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Gritz ER, Demark-Wahnefried W. Health behaviors influence cancer survival. J Clin Oncol. 2009;27:1930–1932. doi: 10.1200/JCO.2008.21.3769. [DOI] [PubMed] [Google Scholar]
- 23.Simmons VN, Litvin EB, Patel RD, et al. Patient-provider communication and perspectives on smoking cessation and relapse in the oncology setting. Patient Educ Couns. 2009;77(3):398–403. doi: 10.1016/j.pec.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 25.Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol. 2004;59(4):224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- 26.Ditre JW, Gonzalez BD, Simmons VN, Faul LA, Brandon TH, Jacobsen PB. Associations between pain and current smoking status among cancer patients. Pain. 2011;152(1):60–65. doi: 10.1016/j.pain.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egestad H, Emaus N. Changes in health related quality of life in women and men undergoing radiation treatment for head and neck cancer and the impact of smoking status in the radiation treatment period. Eur J Oncol Nurs. 2014;18(4):339–346. doi: 10.1016/j.ejon.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Meade CD. Producing videotapes for cancer education: methods and examples. Oncol Nurs Forum. 1996;23(5):837–846. [PubMed] [Google Scholar]
- 29.Meltzer LR, Meade CD, Diaz DB, et al. Development of a targeted smoking relapse-prevention intervention for cancer patients. 2016 doi: 10.1007/s13187-016-1089-z. (Manuscript submitted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: an integrative model. Addict Behav. 1990;15(3):271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 32.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in Pain Research and Therapy; Issues in Pain Measurement. Vol. 12. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 33.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Cohen SLE. Partner behaviors that support quitting smoking. J Consult Clin Psychol. 1990;58:304–309. doi: 10.1037//0022-006x.58.3.304. [DOI] [PubMed] [Google Scholar]
- 35.Shiffman S. Behavioral assessment. In: Marlatt DMDGA, editor. Assessment of addictive behavior. New York: Guilford Press; 1998. pp. 139–199. [Google Scholar]
- 36.Schnoll RA, James C, Malstrom M, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25(3):214–222. doi: 10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- 37.Attkisson CCG, T K. Client satisfaction questionnaire-8 and service satisfaction scale-30. In: Maruish ME, editor. The use of psychological testing for treatment planning and outcome assessment. Hillsdale, NJ: Erlbaum; 1994. pp. 402–420. [Google Scholar]
- 38.Sobell LCS, M B. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Allen RZLaJP., editor. Measuring alcohol consumption: Psychosocial and biochemical methods. Clifton, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 39.Shileds PG. New NCCN Guidelines: Smoking Cessation for Patients with Cancer. J Nat Comp Cancer Network. 2015;13(5S):643–645. doi: 10.6004/jnccn.2015.0191. [DOI] [PubMed] [Google Scholar]
- 40.Toll BA, Brandon TH, Gritz ER, Warren GW, Herbst RS ACCR Subcommittee on Tobacco and Cancer. Assessing tobacco use by cancer patients and facilitating cessation: an American Association for Cancer Research policy statement. Clin Cancer Res. 2013;19:1941–1948. doi: 10.1158/1078-0432.CCR-13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson Cox L, Patten CA, Ebbert JO, et al. Tobacco use outcomes among patients with lung cancer treated for nicotine dependence. J Clin Oncol. 2002;20(16):3461–3469. doi: 10.1200/JCO.2002.10.085. [DOI] [PubMed] [Google Scholar]
- 42.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20(8):2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 43.Ostroff JS, Burkhalter JE, Cinciripini PM, et al. Randomized trial of a presurgical scheduled reduced smoking intervention for patients newly diagnosed with cancer. Health Psychol. 2014;33(7):737–747. doi: 10.1037/a0033186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warren GW, Arnold SM, Valentino JP, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103(1):45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampson L, Papadakos J, Milne V, et al. Preferences for the Provision of Smoking Cessation Education Among Cancer Patients. J Cancer Educ. 2016 doi: 10.1007/s13187-016-1035-0. [DOI] [PubMed] [Google Scholar]