Abstract
Background
Evidence-based clinical practice guidelines (CPGs) are defined as “statements that are scientifically reviewed about evidence and systematically developed to assist in the doctors' and patients' decision making in certain clinical situations.” This recommendation aims to promote good clinical practice for the provision of safe and effective practices of conscious sedation in dentistry.
Methods
The development of this clinical practice guideline was conducted by performing a systematic search of the literature for evidence-based CPGs. Existing guidelines, relevant systematic reviews, policy documents, legislation, or other recommendations were reviewed and appraised. To supplement this information, key questions were formulated by the Guideline Development Group and used as the basis for designing systematic literature search strategies to identify literature that may address these questions. Guideline documents were evaluated through a review of domestic and international databases for the development of a renewing of existing conscious sedation guidelines for dentistry. Clinical practice guidelines were critically appraised for their methodologies using Appraisal of guidelines for research and evaluation (AGREE) II.
Results
A total of 12 existing CPGs were included and 13 recommendations were made in a range of general, adult, and pediatric areas.
Conclusion
The clinical practice guidelines for conscious sedation will be reviewed in 5 years' time for further updates to reflect significant changes in the field.
Keywords: Clinical practice, Conscious sedation, Dentistry, Evidence-based medicine, Guidelines
INTRODUCTION
In 1990, the Institute of Medicine (IOM) defined clinical practice guidelines that were “systematically developed to assist in the doctors' and patients' decision making in a particular situation” [1]. In 2011, evidence based medicine has since revised this definition to the following: “statements that are the assessment of their benefits and harms other treatment methods and the basis of the system through literature review including recommendations to optimize patient care” [2]. The definition in Korea was agreed upon via mutual consensus by a panel of experts based upon the modified Delphi method, to the following: “statements that are scientifically reviewed about evidence and systematically developed to assist in the doctors' and patients' decision making in certain clinical situations” [3]. With the prevalence of dental implantation in the field of dentistry, the need for behavioral control in pediatric patients and patients with special health care needs is increasing. Additionally, dental sedation has also been receiving particular attention. As dental sedation becomes more widely used in dental clinics, its benefits, such as the management of anxiety and pain, have also been noted. However, concurrent reports of adverse effects and the incidence of severe complications related to dental sedation have also increased. Therefore, the dental sedation clinical practice guidelines necessitated review, and a 5-year follow-up to the publication of the original guidelines was performed. The Korean Dental Society of Anesthesiology proposed a revision in 2014, and consequently, the “Dental Sedation Clinical Practice 2015” was an additional revised document of the original published by researchers under the recommendations of the academy of dental sedation at the Korean Academy of Dental Sciences. The American Dental Association defines evidence-based dentistry as “an approach to oral healthcare that requires the judicious integration of systematic assessments of clinically relevant scientific evidence, relating to the patient's oral and medical condition and history, with the dentist's clinical expertise and the patient's treatment needs and preferences” [4]. An evidence-based approach was developed to supplement the absence of explicit standards and methodologies within the former method. This new approach was developed along with an advancement of evidence-based medical methods [5]. This involved the introduction of bibliographic search software that allowed for a systematic examination of the literature and the minimization of errors in the evidence selection process. Moreover, the increase in meta-analyses performed and the creation of influential data sources, such as the Cochrane database, has recently facilitated evidence stratification. Consequently, recommendations were formulated after more convincing processes were made apparent. At present, an evidence-based development method is advantageous and more commonly employed because of its objective, evaluative, and deductive recommendations [6,7,8]. However, from the user's perspective, the question of ‘how the guideline has been developed’ persists. In addition, compared to earlier stages of guideline development, the more recent modifications of the guidelines could be considered confusing. Therefore, to validate the reliability of the guidelines, the Committee of Clinical Practice Guideline reviews the current progress in the development of clinical guidelines.
MATERIALS AND METHODS
The 2015 REVISED dental sedation clinical practice guidelines, or Guidance for the development of clinical practice guidelines Ver 1.0 [9], were developed using the Appraisal of guidelines for research and evaluation (AGREE) II framework for a Korean context (Table 1). A review panel of experts from the Committee of Clinical Practice Guideline (CPG) of the Korean Academy of Medical Sciences (KAMS) reviewed the literature and revised the existing clinical guidelines. These revised guidelines reflect the reality of dental sedation more accurately than the previous version published in 2010 [10], and were based on evidence obtained from clinically useful questions (PICOH) (Table 2).
Table 1. The stages of Guideline development.
| Guidelines Development in Planning Phase | 1. Guideline development relevant components of the Committee. |
| 2. Development planning and consensus on the design phase. | |
| Guidelines Development Preparation step | 1. Final question and a clinical question about health. |
| 2. Search of practice guidelines | |
| 3. Evaluation of care guidelines | |
| 4. Selection of the quality of guidelines to be used on the adaptation | |
| Development phase I (The stage of developing recommendations) | 1. Preparation of draft recommendations. |
| 2. Feedback inquiry and Results should be reflected in recommendations. | |
| 3. Recommendation for adaption agreement. | |
| Development phase II (The stage of writing phase) The creation of a draft practice guidelines. | 1. The summary of recommendations |
| 2. The descriptions of the development process and methods. | |
| 3. The descriptions of evidence or background. | |
| 4. Presentation of evidence | |
| 5. Presentation of the Summary and any exhibits hereto. | |
| Review and final stage instructions. | 1. Internal and external reviews |
| 2. Take guidelines committee. | |
| 3. Confirmation of the final practice guidelines | |
| Validation and dissemination phase. | 1. Authentication and publication of the Academy of dental science |
| 2. Consideration of the obstacles associated with supply and use. | |
| 3. Monitor supply and execution of guidelines is developed. |
Table 2. A search example with Medical Subject Headings (MeSH) terms for the PICOH questions.
| 5. Child_midazolam+N2O_Pubmed search 26th Jun 2015 | ||
|---|---|---|
| Search | Query | Results |
| #1 | Midazolam[Mesh] | 7,406 |
| #2 | Midazolam[TIAB] OR Dormicum[TIAB] OR Versed[TIAB] OR "Ro 21-3981"[TIAB] OR "Ro 21 3981"[TIAB] | 11,080 |
| #3 | #1 OR #2 | 12,283 |
| #4 | Nitrous Oxide[Mesh] | 12,248 |
| #5 | Nitrous Oxide[TIAB] OR N2O[TIAB] OR Laughing Gas[TIAB] OR Nitrogen Protoxide[TIAB] | 16,736 |
| #6 | #4 OR #5 | 21,182 |
| #7 | Dentistry[Mesh] OR Dental Health Services[Mesh]OR Dental Clinics[Mesh] OR Dental Offices[Mesh] OR Anesthesia, Dental[Mesh] | 347,358 |
| #8 | Dental Clinic*[TIAB] OR Dental Office*[TIAB] OR Dental[TIAB] OR dentistr*[TIAB] OR oral sedation*[TIAB] OR oral sedativ*[TIAB] OR oral conscious sedation*[TIAB] OR Dental Anesthes*[TIAB] OR jsubsetd[text] | 618,556 |
| #9 | #7 OR #8 | 647,830 |
| #10 | #9 AND ("infant"[MeSH] OR "child"[MeSH] OR "adolescent"[MeSH] OR Children*[TIAB] OR chlid[TIAB] OR childs[TIAB] OR Preschool[TIAB] OR Adolescent*[TIAB] OR Adolescenc*[TIAB] OR Teen[TIAB] OR Teenager*[TIAB] OR Youth*[TIAB]) | 121,257 |
| #11 | Dental Care for Children[Mesh] | 3,076 |
| #12 | #10 OR #11 | 121,766 |
| #13 | #3 AND #6 AND #12 | 84 |
| #14 | #13 AND (Meta-Analysis[ptyp] OR "Meta-Analysis as Topic"[Mesh] OR Meta-Analys*[TIAB] OR Randomized Controlled Trial[ptyp] OR "Randomized Controlled Trials as Topic"[Mesh] OR Randomized[TIAB] OR Randomised[TIAB] OR radom[TIAB] OR randomly[TIAB] OR RCT[TIAB] OR ((Double* OR single* OR treb* OR tripl*) AND (Blind* OR mask*)) OR systematic[sb] OR "Consensus Development Conference" [PT] OR "Consensus Development Conference, NIH" [PT] OR "Guideline" [PT] OR "Practice Guideline" [PT] OR Guideline* OR Practice guideline* OR Clinical Practice guideline* OR Recommend* OR Consensus*) | 43 |
| Searching formulation (RCT/SR/Meta/Practice guideline Article type) | (Midazolam[Mesh] OR Midazolam[TIAB] OR Dormicum[TIAB] OR Versed[TIAB] OR "Ro 21-3981"[TIAB] OR "Ro 21 3981"[TIAB]) AND (Nitrous Oxide[Mesh] OR Nitrous Oxide[TIAB] OR N2O[TIAB] OR Laughing Gas[TIAB] OR Nitrogen Protoxide[TIAB]) AND (((Dentistry[Mesh] OR Dental Health Services[Mesh]OR Dental Clinics[Mesh] OR Dental Offices[Mesh] OR Anesthesia, Dental[Mesh] OR Dental Clinic*[TIAB] OR Dental Office*[TIAB] OR Dental[TIAB] OR dentistr*[TIAB] OR oral sedation*[TIAB] OR oral sedativ*[TIAB] OR oral conscious sedation*[TIAB] OR Dental Anesthes*[TIAB] OR jsubsetd[text]) AND ("infant"[MeSH] OR "child"[MeSH] OR "adolescent"[MeSH] OR Children*[TIAB] OR chlid[TIAB] OR childs[TIAB] OR Preschool[TIAB] OR Adolescent*[TIAB] OR Adolescenc*[TIAB] OR Teen[TIAB] OR Teenager*[TIAB] OR Youth*[TIAB])) OR Dental Care for Children[Mesh]) AND (Meta-Analysis[ptyp] OR "Meta-Analysis as Topic"[Mesh] OR Meta-Analys*[TIAB] OR Randomized Controlled Trial[ptyp] OR "Randomized Controlled Trials as Topic"[Mesh] OR Randomized[TIAB] OR Randomised[TIAB] OR radom[TIAB] OR randomly[TIAB] OR RCT[TIAB] OR ((Double* OR single* OR treb* OR tripl*) AND (Blind* OR mask*)) OR systematic[sb] OR "Consensus Development Conference" [PT] OR "Consensus Development Conference, NIH" [PT] OR "Guideline" [PT] OR "Practice Guideline" [PT] OR Guideline* OR Practice guideline* OR Clinical Practice guideline* OR Recommend* OR Consensus*) | 43 |
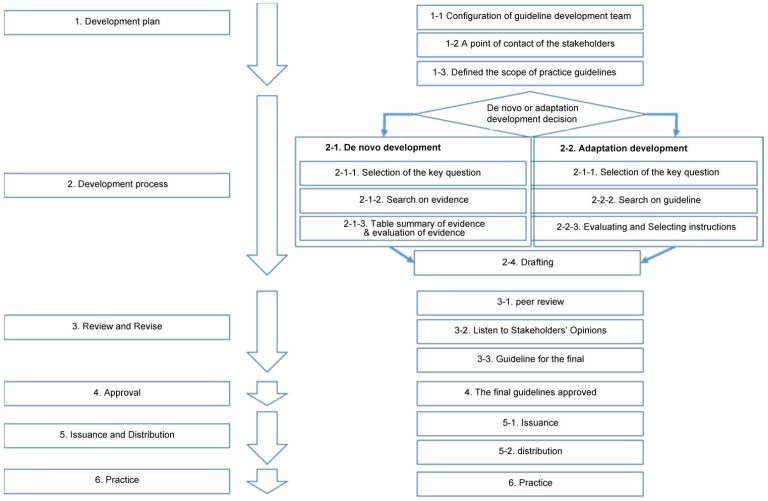
A hybrid method that combined the novel and adaptive development approaches was used for the development of the guidelines (Fig. 1).
Fig. 1. Hybrid method for guideline development.
The term used in clinical practice guidelines is based on the fifth edition of the medical glossary of terms published by the Korean Medical Association. The development of these clinical practice guidelines involved a systematic search of the literature based on the availability of evidence-based CPGs. Existing guidelines, relevant systematic reviews, policy documents, legislation, or other recommendations, were reviewed and appraised. The Cochrane Library was searched for systematic reviews and literature from Medline and Embase were used to address key questions.
To supplement this information, key questions were proposed by the Guidance Development Group and used as the basis for designing systematic literature search strategies to identify further literature that may address these questions. Literature on the development of existing guidelines were acquired through domestic (Korean)/international channels and a manual search was performed to further contribute towards the development of dental sedation guidelines. In addition, the following internet sites were reviewed: the Korean Medical Guideline Information Center (KOMGI), Korean Guideline Clearinghouse, Guidelines International Network (GIN) International Guideline Library, Agency for Healthcare Research and Quality (AHRQ) National Guideline Clearinghouse, National Institute for Health and Care Excellence (NICE), Scottish Intercollegiate Guideline Network (SIGN), National Health and Medical Research Council (NHMRC), New Zealand Guidelines Group, Canadian Collaboration on Clinical Practice Guidelines in Dentistry, National Guidelines Clearinghouse, FDI World Dental Federation, National Electronic Library for Health Guideline Finder, and Medline.
The AGREE II framework, which is an appraisal tool, was used to evaluate and critically appraise the CPGs. The title and abstract of each reference was screened for relevance independently by two researchers who were not members of the Guidance Development Group. Disagreements about the inclusion of specific individual references requiring further consideration were resolved by discussion, and if necessary, the opinion of a third researcher was sought. For the inclusion of CPGs, original reports published in English and Korean that described the intervention strategies in sufficient detail were included. A second requirement was that the CPGs needed to be based on a systematic review of the relevant literature. Additionally, only the most recently updated version of the CPGs were assessed. CPGs were excluded if they were not evidence-based, or if they included guidelines intended only for patients, or if they were translations of foreign guidelines.
A total of 60 guidelines were assessed, and 18 were selected for in-depth analyses using the IOM trustworthy standards; the evaluation items of AGREE II were used for reference (Table 3).
Table 3. Korean version of the Appraisal of guidelines for research and evaluation II (AGREE II).
| Contents | score | |||||||
|---|---|---|---|---|---|---|---|---|
| Scope and intent | Guidelines specifically the overall purpose of technology. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Described as a specific clinical question was covered in the instructions. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| application guidelines, specifically about the technology to patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| The involvement of stakeholders | All relevant group of experts included in guideline development group. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Including the patient's views and preferences | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| User instructions are clearly defined. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| The rigidity of the development | Target user, developed guidelines for the prior investigation.The rigidity of the development | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Search for a systematic way | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Select the basis of criteria are specified | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| It described a method of reaching recommendations | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| To reach health benefits, taking into account the risk of adverse events and recommendations | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Evidence to support the recommendations and are explicitly connected. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Received external review by an expert before the published guidelines. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| There are guidelines for the renewal process. Clarity and expression | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Clarity and expression | Specific and unambiguous recommendations. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| A variety of ways that can be selected in clinical situations is clearly presented. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| The main recommendations can be found easily. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Application | There are guidelines for applying the instruments | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| May be to apply the recommendations that may occur in thinking about the potential barriers. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Taking into account costs that can occur when to apply the recommendations. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Guidelines present the implementation of major criteria that can be monitored and evaluated. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Editorial indipendence | Independent from the a group sponsored by the contents of the guidelines. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Guidelines Development members and record the problems of conflict of interest. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Total score | ||||||||
Twelve guidelines were considered for further adaptation (Table 4). Nonetheless, some of the guidelines that were included, targeted children as their intended audience, and therefore, may reduce the scope of this review. Therefore, the details of the key questions were considered as a novel development.
Table 4. List of Adaption development guidelines.
| 1 | 2015 | Standards for Conscious Sedation in the Provision of Dental Care 2015. provided by The Dental Faculties of the Royal Colleges of Surgeons and the Royal College of Anaesthetists https://www.rcseng.ac.uk/fds/Documents/dental-sedation-report-2015-web-v2.pdf |
| 2 | 2014 | Guidelines on Sedation and/or Analgesia for Diagnostic and Interventional Medical, Dental or Surgical Procedures provided byAustralian and New Zealand College of Anaesthetists (ANZCA) |
| 3 | 2013 | Guidance for CommissioningNHS England Dental Conscious SedationServicesprovided by Society for Advancement of Anaesthesia in Dentistry (SAAD) |
| 4 | 2012 | Guidelines for the Use of Sedation and GeneralAnesthesia by Dentistsprovided by American dental association (ADA) |
| 5 | 2012 | Conscious Sedation in Dentistry. provided by Scottish Dental Clinical Effectiveness Programme (SDCEP) 2nd edn. Dundee |
| 6 | 2011 | Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures provided byAmerican Association of Pediatric Dentistry (AAPD) |
| 7 | 2011 | Conscious sedationprovided byBritish Dental Association (BDA) |
| 8 | 2010 | Sedation in children and young people. Sedation for diagnostic and therapeutic procedures in children and young people provided by National Institute for Health and Care Excellence (NICE) |
| 9 | 2010 | Guidelines for the safe use of procedural sedation and analgesia for diagnostic and therapeutic procedures in adults: provided by South African Society of Anaesthesiologists |
| 10 | 2010 | Guidelines for the safe use of procedural sedation and analgesia for diagnostic and therapeutic procedures in children:provided by South African Society of Anaesthesiologists |
| 11 | 2003 | Guidelines on sedation in paediatric dentistryprovided byEuropean Academy of Paediatric Dentistry (EAPD) |
| 12 | 2002 | Practice Guidelines for Sedation and Analgesia provided byAmerican Society of Anesthesiologists (ASA) |
RESULTS
In a survey of guidelines from developed nations, a total of 60 CPGs were included. From this figure, a total of 12 CPGs were evaluated in a detailed review (Table 4). Levels of evidence of literature (or guidelines), used as the scientific evidence from which the recommendations were derived, were classified into four different groups based on their level of evidence by the Clinical Guideline Development Group (Table 5). Modified GRADE (Grading of Recommendations Assessments, Development, and Evaluation) was used as the standard for grading recommendations. Recommendation grades were classified based on the level of evidence, benefit and risk, utilization at clinical sites, and other factors (Table 6). A detailed review of the CPGs revealed 13 main recommendations for general, adult, and pediatric areas (Table 7).
Table 5. Classification of level of evidence.
| Level of Evidence | Definition |
|---|---|
| A | Evidence from which the recommendation was derived is clear. |
| One or more randomized controlled trials (RCT), meta-analyses, or systematic review (SR). | |
| B | Evidence from which the recommendation was derived is credible. |
| One or more well-conducted case control studies or non-randomized controlled trials, such as cohort studies. | |
| C | Evidence from which the recommendation was derived exists, but is not credible. |
| Low quality evidence such as observational studies and case reports. | |
| D | Evidence of recommendation is based on clinical experience and expert opinions. |
Table 6. Grading of recommendations.
| Recommendation Grade | Definition | Transcription |
|---|---|---|
| Class I | The recommendation’s level of evidence (A) and benefits are clear; utilization at clinical sites is high. | (Is recommended) |
| Class Iia | The recommendation’s level of evidence (B) and benefits are credible; utilization at clinical sites is high or moderate. | (Should be considered) |
| Class Iib | The recommendation’s level of evidence (C or D) and benefits are not credible, but utilization at clinical sites is high or moderate. | (May be considered) |
| Class III | The recommendation’s level of evidence (C or D) and benefits are not credible with the risk of harmful results; utilization at clinical sites is low. | (Is not recommended) |
Table 7. The main recommendations following a detailed review of the 13 clinical practice guidelines.
| Recommendation contents | Recommendation grade | Levels of evidence |
|---|---|---|
| General | ||
| 1. Target patients for conscious sedation | ||
| Conscious sedation is recommended for dental patients who satisfy the following conditions: ASA of 1 or 2 and no serious behavioral problems, upper airway infections, difficulty in airway maintenance, serious gastro-esophageal reflux, or a history of allergy to the drugs that will be administered. | I | D |
| 2. Fasting before sedation | ||
| The patient is advised to fast before undergoing sedation according to the following guidelines: clean water – at least 2 hours before sedation; breast milk – at least 4 hours; milk or light meal – at least 6 hours. | IIa | A |
| 3. Materials for conscious sedation | ||
| Preparation of the following materials is recommended for conscious sedation: oxygen supplier – at least 2 oxygen tanks, pressure regulator, oxygen flow meter; oxygen administration device – facial mask, nasal cannula; patient monitoring device – ECG, blood pressure gauge, respiration monitoring device (stethoscope or capnogram), pulse oximetry, thermostat, glucose meter; properly functioning aspirator that can be used at any time; rescue medication – atropine, epinephrine, flumazenil; airway management devices for emergency – oropharyngeal airway, nasopharyngeal airway, Combitube, laryngeal mask, i-gel, bag valve mask; automated external defibrillator; and device for intravenous access if possible. | I | D |
| 4. Patient monitoring | ||
| The patients are monitored by the following parameters during sedation: level of sedation – sedation scales (OAA/S, etc.); respiration – respiratory sound, respiratory exercise, end-tidal CO2 tension; oxygenation –pulse oximetry, skin and mucous membrane color, and time-based regular monitoring – heart rate, blood pressure, capillary refill time. | I | A |
| 5. Conscious sedation records | ||
| The following details are recorded every 5 minutes during sedation: sedation scales and vital signs (heart rate, respiratory rate, blood pressure, pulse oximetry). | I | D |
| The following details should be recorded with regards to the drug that is being administered: drug name, dose, route, time, reason, patient's response after the administration; method of supplying oxygen. | I | D |
| 6. Discharge criteria | ||
| The following details are checked according to discharge criteria (e.g., the Aldrete Score sheet) after confirming that there are no abnormal symptoms associated with dental treatment: recovery to the conscious state prior to sedation; level of oxygen saturation in the atmosphere > 92%; normal movement; ability to take a deep breath and cough; less than 20 mmHg difference in systolic pressure before and after sedation. | I | C |
| A patient is considered for discharge if the following conditions are met after sedation: vital signs within a normal range; patient has controllable pain, nausea, and vomiting; the precautions after sedation has been explained to the patient; the patient is accompanied by a responsible guardian; the patient has not experienced complications associated with dental treatment. | IIa | D |
| 7. Nitrous oxide ventilation facilities | ||
| The following facility maintenance is recommended for nitrous oxide sedation: installation of effective ventilation facilities; regular measurement of nitrous oxide concentration; regular ventilation facility inspection. | I | B |
| Adults | ||
| 1. Midazolam conscious sedation | ||
| The recommended maximum dose of midazolam for adult dental patients is as follows: oral: 0.25–0.5 mg/kg, maximum 7.5 mg; intramuscular 0.075 mg/kg; intranasal: 0.2 mg/kg 1 bolus per administration; injection: 2 mg; continuous infusion: 0.1 mg/kg/h (maximum 10 mg/h). | I | A |
| 2. Triazolam conscious sedation | ||
| The recommended maximum dose of triazolam for adult dental patients is as follows: oral or sublingual: 0.125–0.5 mg. | IIa | B |
| 3. Nitrous oxide conscious sedation | ||
| The following protocol is recommended for pediatric dental patients: Mix nitrous oxide with 3–5 L of oxygen every minute, and monitor the patient's response; slowly increase the amount of nitrous oxide by 10% every 1–2 minutes, up to 50% at maximum; upon the completion of nitrous oxide administration, ventilate with 100% oxygen for 3–5 minutes to prevent diffusion hypoxia. | I | B |
| Pediatric patients | ||
| 1. Midazolam conscious sedation | ||
| The recommended dose and the maximum dose of midazolam for conscious sedation in pediatric dental patients are as follows: Oral administration: 0.3–0.75 mg/kg, maximum 7.5 mg; intramuscular injection: 0.15–0.2 mg/kg; intranasal administration: 0.2 mg/kg. | I | A |
| If sedative effects of midazolam alone are insufficient, 50% N2O may be used in conjunction with midazolam. | Ia | C |
| 2. Chloral hydrate conscious sedation | ||
| The recommended and maximum doses of chloral hydrate for pediatric dental patients are as follows: oral administration: 25–50 mg/kg, maximum 1 g. | IIb | C |
| If sedative effects of chloral hydrate alone are insufficient, 50% N2O may be used in conjunction with chloral hydrate. | IIb | C |
| 3. Nitrous oxide conscious sedation | ||
| The following protocol is recommended for pediatric dental patients: mix nitrous oxide with 3–5 L of oxygen every minute, and monitor the patient's response; slowly increase the amount of nitrous oxide by 10% every 1–2 minutes, up to 50% at maximum; upon the completion of nitrous oxide administration, ventilate with 100% oxygen for 3–5 minutes to prevent diffusion hypoxia | I | B |
DISCUSSION
Developed nations have established policies for practice guidelines and disseminated and implemented high-quality guidelines on both a national and regional level. The approach towards developing practice guidelines have also changed, with a more systematic approach being adopted. These changes have helped validate many of the practice guidelines. Insofar, developed nations have implemented national policies regulating the development, expansion, and execution of practice guidelines. In a study evaluating the practice guidelines of different Europe countries, the UK, Finland, France, Germany, Italy, and Netherlands were reported to have implemented national policies supplementing clinical practice guidelines since the 1990s [11].
The recent scrutiny behind the development and application of clinical practice guidelines was attributable to the appraisal of scientific and objective evidence, considered the gold standard for evidence-based medicine, in clinical settings [12]. Clinical practice guidelines validate the doctor's decision-making processes in clinical situations, which are ultimately aimed at improving the quality of care.
The developers and users of clinical practice guidelines vary. Professional clinical associations, government and public institutions, and health-related companies use clinical practice guidelines, which can then be used as a framework for health care plans. However, recommendations to clinical practice guidelines cannot be trusted if the evidence from which they were developed is unreliable (lack of evidence, low-quality of evidence, etc.,) or if its development process was not transparent. This can create confusion and obfuscate the administrative aspects of clinical practice or health policies. In essence, they share many commonalities covered by the generic AGREE instruments (and the updated AGREE II [2010] framework), including the same domains [13], which evaluate the following aspects for quality development. First, explicit scope and purpose, the overall objective (s), clinical questions, and target population are explicated. Second, stakeholder involvement, the patient is involved in guideline development and all audiences have been clearly defined and involved in the pilottesting. Third, rigor of the development, recommendations are linked explicitly to supporting evidence and there is a discussion of the health benefits or risks; recommendations are reviewed externally before publication and the development groups are regularly updated. Fourth, clarity of presentation, recommendations are not ambiguous and do consider different possible options; key recommendations are easily identified; and a summary document and patient education materials are provided. Fifth, applicability, organizational changes and cost implications of applying recommendations and review criteria for monitoring guidelines use are explicated. Sixth, editorial independence, views or interests of the funding body have not influenced the final recommendations; members of the guideline group have declared possible conflicts of interest.
As evidence-based approaches have become more prevalent in research, academic interest in clinical practice guidelines has increased, and guideline policies are becoming increasingly developed from an academic standpoint [14]. Other developed countries have implemented systematic frameworks for the development and dissemination of clinical practice guidelines at the national level [12], while in Korea, only clinical professional societies have undertaken efforts for the development and dissemination of such guidelines. Therefore, the government bodies must adopt a policy-based approach in cooperation with professional societies at the national level to effectively support the development and dissemination of trustworthy clinical practice guidelines.
CONCLUSION
Guidelines need to provide critical and objective information about the diagnoses, benefits, and limitations of available treatments. This would allow doctors to effectively use the guidelines to review the treatment options available to each patient. In addition, accredited dental anesthesiologists collaborating with allied health professions for dental care can provide stakeholders with clinical expertise and knowledge, which can then be applied to primary health care and outpatient clinics. Nonetheless, the implementation of clinical treatment guidelines for deep sedation are not comprehensive, and will require a review in 5 years' time to evaluate if the progress of significant changes.
References
- 1.Field MJ, Lohr KN, editors. Committee to advise the public health service on clinical practice guidelines, Institute of Medicine. Clinical practice guidelines: directions for a new program. Washington DC: National Academy Press; 1990. [Google Scholar]
- 2.Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, editors. Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Board on Health Care Services, Institute of Medicine. Clinical practice guidelines we can trust. Washington DC: National Academy Press; 2011. [PubMed] [Google Scholar]
- 3.Ji SM, Kim SY, Shin SS, Heo DS, Kim NS. Consensus on definition and quality standard of clinical practice guideline using RAND method. Korean J Health Policy Adm. 2010;20:1–16. [Google Scholar]
- 4.American Dental Association. Guidelines for the use of sedation and general anesthesia by dentists, 2007. 2012. [Google Scholar]
- 5.Evidence-Based Medicine Working Group. Evidence-based medicine. A new approach to teaching the practice of medicine. JAMA. 1992;268:2420. doi: 10.1001/jama.1992.03490170092032. [DOI] [PubMed] [Google Scholar]
- 6.Nair R, Aggarwal R, Khanna D. Seminars in arthritis and rheumatism. WB Saunders; 2011. Methods of formal consensus in classification/diagnostic criteria and guideline development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rycroft-Malone J. Formal consensus: the development of a national clinical guideline. Qual Health Care. 2001;10:238–244. doi: 10.1136/qhc.0100238... [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson N, Liu J, Lee MS. Clinical guidelines: the way for best practice. Eur J Integr Med. 2014;2:133–134. [Google Scholar]
- 9.Kim S, Jee S, Lee S, Lee Y, Park J, Nam M. Guidance for development of clinical practice guidelines. National Evidence-based Healthcare Collaborating Agency; 2011. pp. 20–77. [Google Scholar]
- 10.Korean Academy of Dental Sciences. 2010 Guidelines for the Use of Sedation by Dentists. Koonja publishing Inc; 2010. [2014 Feb 21]. Available from http://www.kadents.or.kr/modules/bbs/index.php?code=pds&mode=view&id=6&___M_ID=100. [Google Scholar]
- 11.Littlejohns P, Thomason M, Cluzeau F. Guideline development in Europe-An international comparison. Int J Technol Assess Health Care. 2000;16:1039–1049. doi: 10.1017/s0266462300103101. [DOI] [PubMed] [Google Scholar]
- 12.Turner T, Misso M, Harris C, Green S. Development of evidence-based clinical practice guidelines (CPGs): comparing approaches. Implement Sci. 2008;3:1. doi: 10.1186/1748-5908-3-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho HS, Shin IS, Oh MK, Lee YK, Kim JK, Jung YM, et al. A Strategy for Dissemination and Implementation of Clinical Practice Guidelines in Korea. National Evidence-based Healthcare Collaborating Agency; 2014. [Google Scholar]