Abstract
Background
Some studies have suggested antipsychotic-naive patients with nonaffective psychosis (NAP) have glucose intolerance.
Aims
To conduct a systematic review and meta-analysis of fasting glucose (FG), two hour values in the oral glucose tolerance test (2HG), fasting insulin concentration (INS), and insulin resistance (IR).
Method
We identified possibly relevant studies, then selected studies, following usual guidelines, with two authors reviewing the manuscripts. We required studies to include subjects with nonaffective psychosis and control subjects.
Results
There were 911 patients and 870 control subjects in the analysis of FG; their average ages were respectively 28.7 and 29.5 years. Significant differences were found for all four variables, with effect size estimates ranging from 0.21 to 0.58.
Conclusions
As a group, at the time of first clinical contact for psychosis, people with NAP have a slight increase in FG, which most of them maintain in the normal range despite a small increase in IR by secreting additional INS. When faced with a physiological challenge such as a glucose tolerance test or antipsychotics, they are no longer able to maintain a normal glucose concentration.
Keywords: schizophrenia, diabetes, antipsychotic-naïve, insulin, glucose tolerance
1. Introduction
Most research on nonaffective psychosis (NAP) understandably focuses on brain function and biology, but people with this group of disorders have a number of physiological and anatomical abnormalities in the periphery as well (Kirkpatrick et al., 2014). These include increased systemic inflammation (Miller et al., 2011, 2014), low birth weight (Abel et al., 2010; Cannon et al., 2002), a low body mass index prior to antipsychotic use (Wahlbeck et al., 2001), and an increased prevalence of minor physical anomalies from head to toe (Weinberg et al., 2007; Xu et al., 2011).
Family studies have found an increased risk of type 2 diabetes or abnormal glucose in the first degree relatives of people with NAP (Fernandez-Egea et al., 2008a, 2008b; Mukherjee et al., 1989; Van Welie et al., 2013), suggesting patients with psychosis may have an increased risk of diabetes that exists prior to exposure to antipsychotics, many of which increase the risk of diabetes (American Diabetes Association et al., 2004). Some studies of antipsychotic-naive patients with NAP have also found increased fasting glucose (Ryan et al., 2003) or abnormal glucose tolerance on a glucose tolerance test (Fernandez-Egea et al., 2009), but the results have been inconsistent. Confirmation that the increased risk of diabetes in this group is present independently of antipsychotics would imply that glucose monitoring should not be confined to those who gain weight with these medications, or even confined to patients taking antipsychotics.
We performed a systematic review and a meta-analysis to assess glucose intolerance in people with NAP prior to antipsychotic use. We assessed fasting glucose (FG), two hour values in the oral glucose tolerance test (2HG), fasting insulin (INS), and insulin resistance (IR).
2. Materials and Methods
2.1 Search strategy
We followed PRISMA guidelines but we began our project before registering, so our study was not registered. A preliminary search was done for systematic reviews to avoid publishing a duplicate review. Studies were identified by systematic searches from 1950 to 2016 in Medline, PsycINFO and Web of Science in independent searches by two authors (AG and LGB). Search terms included: schizophrenia, psychosis, naïve, untreated, antipsychotic naïve, and antipsychotic free, fasting glucose, diabetes, glucose tolerance test, insulin or insulin resistance. The titles and abstracts were screened for eligibility by two reviewers (AG and either LGB or BK). The search covered articles through Feburary, 2016. The search was supplemented by reference lists from relevant review articles and the articles included in the study. These articles were examined by two of these same reviewers to determine which met the inclusion and exclusion criteria.
2.2 Inclusion/exclusion criteria
In order to include a study the following criteria had to be met: patients with NAP with maximum cumulative (lifetime) antipsychotic exposure of 1 week and no antipsychotic use in the 30 days prior to the study; a matched control group of subjects without psychosis; participants were at least 16 years of age; FG was measured, or a 2HG conducted, after an overnight fast; the paper was published in English; and the data had to be either in the article or accessible from the author upon request. We defined NAP to include schizophrenia, schizophreniform disorder, delusional disorder, brief psychosis, and psychotic disorder not otherwise specified. A diagnosis of schizoaffective disorder was an exclusion criterion, unless separate data for NAP only was available. We chose to study NAP rather than schizophrenia and not including schizoaffective disorder because of evidence from studies of newly diagnosed patients that there is substantial diagnostic stability within this group of disorders, with patients often shifting to a diagnosis of schizophrenia on ten-year follow-up (Bromet et al., 2011; Castro-Fornieles et al., 2011; Schwartz et al., 2000).
2.3 Data Analysis
One author (AG) recorded the data from the studies, which was independently verified by a second (LGB). The statistical analyses were performed in the Stata 13.1 software program. Effect size (ES) estimates (Hedges’ g) were calculated for the outcome variables concentration. Random effects, pooled ES estimates and 95% confidence intervals were calculated using the method of DerSimonian and Laird (1986); p-values were considered statistically significant at the p<0.05 level.
We examined heterogeneity in the ES estimates using chi-square (Cochran, 1950). We also performed sensitivity analyses of FG, INS, 2HG and IR by removing one study at a time and repeating the meta-analysis for each parameter to examine the impact on the ES estimate (Higgins and Green, 2011). Forest plots and funnel plots were examined for FG, INS, 2HG, and IR.
Meta-regression was conducted for the categorical variables of body mass index and smoking, that is, whether or not the groups were matched on these variables; FG, the variable with the most data, was the dependent variable. We also entered family history exclusions as a categorical variable in meta-regression, as in some studies the patient and control groups were matched on the presence/absence of a family history of diabetes. Meta-regression was also conducted for body mass index, using the effect estimate for these in each study as the independent variable and ES for FG as the dependent variable.
3. Results
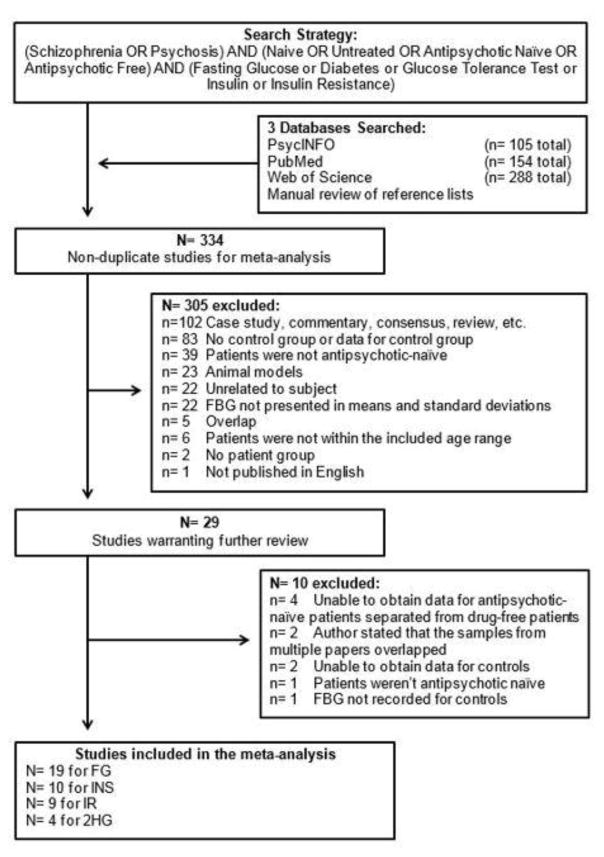
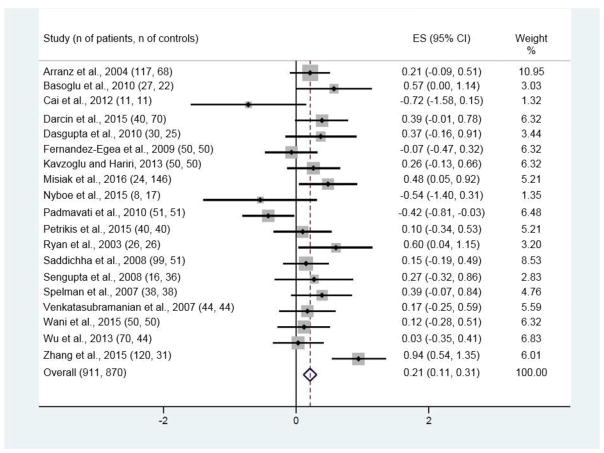
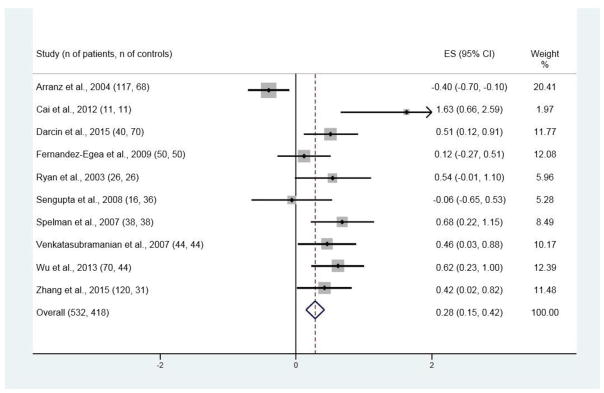
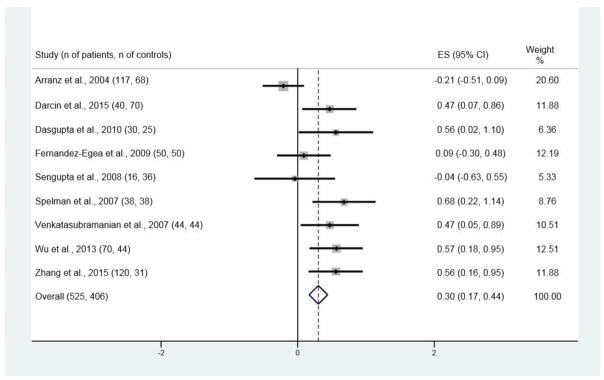
Of the 334 publications originally found with the search strategy, 19 met the inclusion criteria and presented data on FG (Figure 1). These nineteen studies (see Figure 2 for references) had 911 patients with NAP and 870 control subjects; the age of the control subjects was 0.8 years greater than that of patients, which with the large sample sizes was statistically significant (respective ages (SD) for patients and control subjects were 28.7 (7.3) and 29.5 (6.0) years; t = −2.63, p <.01). There were 10 studies with data on INS (see Figure 4 for references), 9 for IR (see Figure 5 for references), and 4 for 2HG (see Figure 3 for references).
Figure 1.
Selection of studies for meta-analysis.
Figure 2.
Forest plot for fasting glucose (FG)
Figure 4.
Forest plot for insulin concentrations (INS)
Figure 5.
Forest plot for insulin resistance (IR)
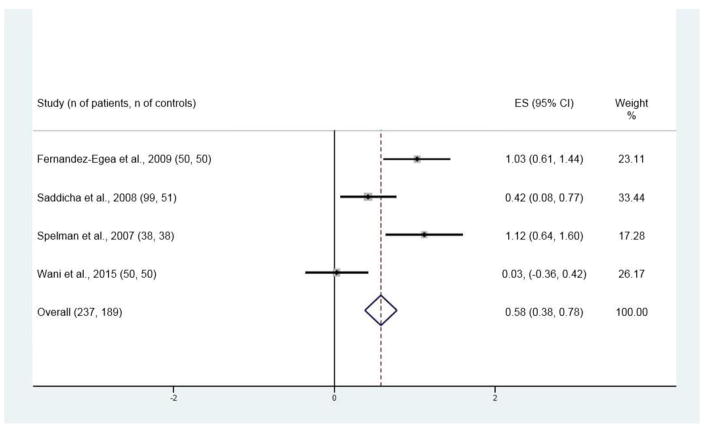
Figure 3.
Forest plot for two-hour glucose values in the two-hour glucose tolerance test (2HG)
Significant differences between patients and control subjects were found for all four variables, with ES estimates of 0.21 [95% CI 0.11, 0.31; p < 0.001; Figure 2] for FG, 0.28 [95% CI 0.15, 0.42; p < 0.001; Figure 3] for INS, 0.30 [95% CI 0.17, 0.44; p < 0.001; Figure 4] for IR, and 0.58 [95% CI 0.38, 0.78; p < 0.001; Figure 5] for 2HG.
Heterogeneity was significant with all studies included in the meta-analysis for FG [I-squared = 55.1%, p = 0.002]. After removal of the most divergent study (Zhang et al., 2015), the heterogeneity was no longer significant for FG [I-squared = 36.5%, p = 0.062] and the ES remained significant [ES = 0.16; p = 0.002]. Heterogeneity was also significant with all studies included in the meta-analysis for 2HG [I-squared = 83.2%, p < 0.001]. In a sensitivity analysis, heterogeneity remained significant after removal of each individual study.
Heterogeneity was significant with all studies included in the meta-analysis for INS [I-squared = 76.5%, p < 0.001]. After removal of the most divergent study (Arranz et al., 2004), heterogeneity was no longer significant [I-squared = 39.6%, p = 0.103] and the ES remained significant [ES = 0.46; p < 0.001].
Heterogeneity was significant with all studies included in the meta-analysis for IR [I-squared = 63.5%, p = 0.005]. After removal of the most divergent study (Arranz et al., 2004), the heterogeneity was no longer significant for INS [I-squared = 9.1%, p = 0.359] and the ES remained significant [ES = 0.44; p < 0.001].
In meta-regression, age was not a significant predictor of ES (p<0.5). No other independent variable we examined (body mass index, cortisol, family history exclusions, and smoking) was a significant predictor of FG in meta-regression (data not shown). There did not appear to be a significant publication bias on funnel plots (Figures 6, 7, 8, and 9 in supplementary materials).
4. Discussion
In a meta-analysis, we found significant differences between antipsychotic-naive patients with NAP and control subjects in FG, INS, and IR. These ES estimates were small, ranging from 0.21 to 0.30. However, when given an oral glucose tolerance test, patients exhibited abnormal glucose tolerance with an ES of 0.58, which is a “medium” effect size. In meta-regression, age was not a significant predictor of ES although patients were on average 0.83 years younger than control subjects. As a consequence, the greater glucose-related abnormalities cannot be attributed to confounding by greater age in the patients. Body mass index, cortisol, family history exclusions, and smoking were also not significant predictors of ES in meta-regression. There did not appear to be a significant publication bias on funnel plots.
There are limitations to our analysis. The number of studies for IR and 2HG was small, nine and four, respectively, although the number of patients and control subjects were 525 and 406, and 237 and 189, respectively. Many of the studies also had limited matching. It would be desirable for any future studies to match on age, sex, body mass index or waist-hip ratio, smoking, and other substance use. The FG studies included patients of Western European, Indian, and East Asian ethnicity, suggesting this is a generalizable effect, but the 2HG studies did not include examination of East Asian patients, raising the question of generalizability of this effect. Only one paper provided information on glucose for schizophrenia and other diagnoses within NAP (Sengupta et al., 2008); unfortunately the number of subjects in each of those groups was small, making any conclusion uncertain.
Another limitation was the heterogeneity that remained after removal of the study of 2HG with the most divergent results. The small number of studies of this variable made it difficult to be confident about the meaning of this heterogeneity. The study of 2HG with the most complete matching (Fernandez-Egea et al., 2009) had ES estimate of 1.03, and in an examination subject from the same project, no relationship was found between 2HG and several other potentially confounding variables (Kirkpatrick et al., 2012).
Our results are consistent with family studies of the relationship between diabetes and NAP (Fernandez-Egea et al., 2008a, 2008b; Mukherjee et al., 1989; Van Welie et al., 2013) as well as studies of glucose intolerance that predate the antipsychotic era (Fernandez-Egea et al., 2013). They are also consistent with the other findings of physiological abnormalities in the periphery in people with NAP, including increased inflammation (Kirkpatrick et al., 2014; Miller et al., 2011) and increases in some lymphocyte populations and in oxidative stress (Flatow et al., 2013; Miller et al., 2013). In one of the studies, a number of potentially confounding variables did not appear to cause the abnormal 2HG results: age, ethnicity, sex, smoking, SES, morning cortisol concentrations, BMI (or waist-hip ratio), measures of diet (blood concentrations of vitamin B12, folate, and homocysteine) and aerobic conditioning, or duration of untreated psychosis (Kirkpatrick et al., 2012).
NAP and type II diabetes appear to share environmental risk factors, including low birth weight and prenatal famine (Cannon et al., 2002; Li et al., 2010; Lumey et al., 2015; St. Clair et al., 2005; Susser and Lin, 1992; Thurner et al., 2013; Whincup et al., 2008). They may also share some genes of risk (e.g., Kajio et al., 2014; Lawford et al., 2016; Liu et al., 2013; Padmanabhan et al., 2016). The schizophrenia gene of risk DISC1 has been shown to influence insulin secretion (Jurczyk et al., 2016).
It is not clear from the studies included in our meta-analysis whether there are clinical correlates of impaired glucose tolerance. A study of antipsychotic-naïve patients with NAP found greater intolerance in patients without primary negative symptoms (Kirkpatrick et al., 2009). The subjects in that study were included in the study of Fernandez-Egea et al. (2009).
Our results do not contradict the strong evidence that certain antipsychotics increase the risk of diabetes (American Diabetes Association et al., 2004; Holt and Peveler, 2006; Newcomer, 2004). However, our results suggest that in addition, people with NAP as a group have an increased risk of diabetes that is apparent in early adulthood and prior to antipsychotic use. As a group, at the time of first clinical contact for psychosis they have a slight increase in FG, which most of them maintain in the normal range, despite a small increase in IR, by secreting additional INS. When faced with a physiological challenge such as a glucose tolerance test or antipsychotics, they are no longer able to maintain a normal glucose concentration.
Supplementary Material
Footnotes
Contributors
Ms. Greenhalgh conducted the literature search, reviewed articles, aided in the first draft of the article, and performed the analysis using the Stata software. Dr. Gonzalez-Blanco helped design the study. Drs. Garcia-Rizo, Fernandez-Egea, and Bernardo Arroyo consulted on data interpretation. Dr. Miller consulted on statistical analysis and choice of articles. Dr. Kirkpatrick designed the study, aided in the first draft of the article, helped in the literature review, and had oversight of all of the steps. All of the authors shared in final approval of the manuscript.
Disclosures
Dr. Kirkpatrick receives licensing royalties and travel support from ProPhase for use of the Brief Negative Symptom Scale (BNSS) by for-profit groups; these fees are donated the Brain and Behavior Research Foundation. He has also received fees and travel support from ProPhase for teaching the BNSS, consulting fees and travel support from Genentech/Roche, and consulting fees to anonymized pharmaceutical companies through Decision Resources, Inc. and to an investment capital company through Guideposts. He also receives fees from Walsh Medical Media for editorial services, and received fees for editorial services from Physicians Postgraduate Press, Inc.
Dr. González-Blanco was supported by a grant from the Spanish Foundation of Psychiatry and Mental Health. She has received speaker fees and travel expenses for attending conferences from Janssen, Otsuka, Pfizer and Lundbeck.
Ms. Greenhalgh has no conflicts of interest to disclose.
Dr. Garcia-Rizo has received honoraria/travel support from Janssen-Cilag, Otsuka and Ferrer.
Dr. Fernandez-Egea has received unrestricted research funding from Genus Pharmaceuticals, and consultancy fees from Roche/Genentech. He is partially supported by a Young Investigator Award (NARSAD).
Dr. Miller receives grant support from the National Institute of Mental Health (K23MH098014), the American Psychiatric Association (Kempf Fund Award) and Georgia Regents University; research support from the National Institutes of Health Clinical Loan Repayment Program; an honorarium from Psychiatric Times; and speaker fees for lectures from the University of Nevada-Reno.
Dr. Bernardo receives grant research support from ABBiotics, Adamed, Amgen, Eli Lilly, Ferrer, Forum Pharmaceuticals, Gedeon, Lundbeck, Otsuka, Roche, Generalitat Catalunya Spanish Ministry of Health, the Spanish Ministry of Science and Education, the Spanish Ministry of Economy and Competiveness, Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), the Government of Catalonia, Secretaria d’Universitats i Recerca del Departamentd’Economia i Coneixement, Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), and the 7th Framework Program of the European Union. He receives consulting fees from ABBiotics, Almirall, Boheringer, Ferrer, Gedeon, Janssen- Cilag, Lundbeck, Otsuka, Pfizer, and Roche. He receives speaker fees or is on the advisory board for Adamed, Ferrer, Forum Pharmaceuticals, Hersill, Janssen-Cilag, Lundbeck, Otsuka, Pfizer, Inc, Servier.
The funding institutions did not participate in drafting the article or have final approval.
Role of the Funding Source
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67(9):923–930. doi: 10.1001/archgenpsychiatry.2010.100. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association and American Psychiatric Association. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- Arranz B, Rosel P, Ramirez N, et al. Insulin resistance and increased leptin concentrations in noncompliant schizophrenia patients but not in antipsychotic-naive first-episode schizophrenia patients. J Clin Psychiatry. 2004;65(10):1335–1342. doi: 10.4088/jcp.v65n1007. [DOI] [PubMed] [Google Scholar]
- Basoglu C, Oner O, Gunes C, et al. Plasma orexin A, ghrelin, cholecystokinin, visfatin, leptin and agouti-related protein levels during 6-week olanzapine treatment in first-episode male patients with psychosis. Int Clin Psychopharmacol. 2010;25(3):165–171. doi: 10.1097/YIC.0b013e3283377850. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, Chang SW. Diagnostic shifts during the decade following first admission for psychosis. Am J Psychiatry. 2011;168(11):1186–94. doi: 10.1176/appi.ajp.2011.11010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HL, Li HD, Yan XZ, et al. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J Proteome Res. 2012;11(8):4338–4350. doi: 10.1021/pr300459d. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Castro-Fornieles J, Baeza I, de la Serna E, Gonzalez-Pinto A, Parellada M, Graell M, Moreno D, Otero S, Arango C. Two-year diagnostic stability in early-onset first-episode psychosis. J Child Psychol Psychiatry. 2011;52(10):1089–98. doi: 10.1111/j.1469-7610.2011.02443.x. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The comparison of percentages in matched samples. Biometrika. 1950;37(3–4):256–266. [PubMed] [Google Scholar]
- Darcin EA, Cavus YS, Dilbaz N, Kaya H, Dogan E. Metabolic syndrome in drug-naïve and drug-free patients with schizophrenia and in their siblings. Schizophr Res. 2015;166(1–3):201–206. doi: 10.1016/j.schres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Dasgupta A, Singh OP, Rout JK, Saha T, Mandal S. Insulin resistance and metabolic profile in antipsychotic naive schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1202–1207. doi: 10.1016/j.pnpbp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Donner T, et al. Metabolic profile of antipsychotic-naive individuals with non-affective psychosis. Br J Psychiatry. 2009;194(5):434–438. doi: 10.1192/bjp.bp.108.052605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Parellada E, et al. Glucose abnormalities in the siblings of people with schizophrenia. Schizophr Res. 2008a;103(1–3):110–113. doi: 10.1016/j.schres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Garcia-Rizo C, Zimbron J, Kirkpatrick B. Diabetes or prediabetes in newly diagnosed patients with nonaffective psychosis? A historical and contemporary view. Schizophr Bull. 2013;39(2):266–267. doi: 10.1093/schbul/sbs134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Miller B, Bernardo M, Donner T, Kirkpatrick B. Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res. 2008b;98(1–3):302–306. doi: 10.1016/j.schres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74(6):400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011;2011 [Google Scholar]
- Holt RI, Peveler RC. Association between antipsychotic drugs and diabetes. Diabetes Obes Metab. 2006;8(2):125–135. doi: 10.1111/j.1463-1326.2005.00495.x. [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Nowosielska A, Przewozniak N, Aryee KE, DiIorio P, Blodgett D, et al. Beyond the brain: disrupted in schizophrenia 1 regulates pancreatic β-cell function via glycogen synthase kinase-3β. FASEB J. 2016;30(2):983–93. doi: 10.1096/fj.15-279810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajio Y, Kondo K, Saito T, Iwayama Y, Aleksic B, Yamada K, et al. Genetic association study between the detected risk variants based upon type II diabetes GWAS and psychotic disorders in the Japanese population. J Hum Genet. 2014;59(1):54–56. doi: 10.1038/jhg.2013.116. [DOI] [PubMed] [Google Scholar]
- Kavzoglu SO, Hariri AG. Intracellular Adhesion Molecule (ICAM-1), Vascular Cell Adhesion Molecule (VCAM-1) and E-selectin levels in first episode schizophrenic patients. Bull of Clin Psychopharm. 2013;23(3):205–214. [Google Scholar]
- Kirkpatrick B, Fernandez-Egea E, Garcia-Rizo C, Bernardo M. Differences in glucose tolerance between deficit and nondeficit schizophrenia. Schizophr Res. 2009;107(2–3):122–127. doi: 10.1016/j.schres.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller B, Garcia-Rizo C, Fernandez-Egea E. Schizophrenia: a systemic disorder. Clin Schizophr Relat Psychoses. 2014;8(2):73–79. doi: 10.3371/CSRP.KIMI.031513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Miller BJ, Garcia-Rizo C, Fernandez-Egea E, Bernardo M. Is abnormal glucose tolerance in antipsychotic-naive patients with nonaffective psychosis confounded by poor health habits? Schizophr Bull. 2012;38(2):280–284. doi: 10.1093/schbul/sbq058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawford BR, Barnes M, Morris CP, Noble EP, Nyst P, Heslop K, et al. Dopamine 2 Receptor Genes Are Associated with Raised Blood Glucose in Schizophrenia. Can J Psychiatry. 2016;61(5):291–297. doi: 10.1177/0706743716644765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Li Z, Zhang M, Deng Y, Yi Z, Shi T. Exploring the pathogenetic association between schizophrenia and type 2diabetes mellitus diseases based on pathway analysis. BMC Med Genomics. 2013;6(Suppl 1):S17. doi: 10.1186/1755-8794-6-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumey LH, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):784–794. doi: 10.1016/S2213-8587(15)00279-X. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223–230. [PubMed] [Google Scholar]
- Miller BJ, Gassama B, Sebastian D, Buckley P, Mellor A. Meta-analysis of lymphocytes in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2013;73(10):993–999. doi: 10.1016/j.biopsych.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiak B, Laczmanski L, Sloka NK, Szmida E, Piotrowski P, Loska O, et al. Metabolic dysregulation in first-episode schizophrenia patients with respect to genetic variation in one-carbon metabolism. Psychiatry Res. 2016;238:60–67. doi: 10.1016/j.psychres.2016.01.077. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. 1989;1:495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic risk during antipsychotic treatment. Clin Ther. 2004;26(12):1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Nyboe L, Vestergaard CH, Moeller MK, Lund H, Videbech P. Metabolic syndrome and aerobic fitness in patients with first-episode schizophrenia, including a 1-year follow-up. Schizophr Res. 2015;168(1–2):381–7. doi: 10.1016/j.schres.2015.07.053. [DOI] [PubMed] [Google Scholar]
- Padmavati R, McCreadie RG, Tirupati S. Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr Res. 2010;121(1–3):199–202. doi: 10.1016/j.schres.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Petrikis P, Tigas S, Tzallas AT, Papadopoulos I, Skapinakis P, Mavreas V. Parameters of glucose and lipid metabolism at the fasted state in drug-naïve first-episode patients with psychosis: Evidence for insulin resistance. Psychiatry Res. 2015;229(3):901–904. doi: 10.1016/j.psychres.2015.07.041. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. Am J Psychiatry. 2003;160(2):284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Saddichha S, Manjunatha N, Ameen S, Akhtar S. Diabetes and schizophrenia – effect of disease or drug? Results from a randomized, double-blind, controlled prospective study in first-episode schizophrenia. Acta Psychiatr Scand. 2008;117(5):342–347. doi: 10.1111/j.1600-0447.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, Lavelle J, Bromet EJ. Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry. 2000;57(6):593–600. doi: 10.1001/archpsyc.57.6.593. [DOI] [PubMed] [Google Scholar]
- Sengupta S, Parrilla-Escobar MA, Klink R, Fathalli F, Ying Kin N, Stip E, et al. Are metabolic indices different between drug-naive first-episode psychosis patients and healthy controls? Schizophr Res. 2008;102(1–3):329–336. doi: 10.1016/j.schres.2008.02.013. [DOI] [PubMed] [Google Scholar]
- Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabet Med. 2007;24(5):481–485. doi: 10.1111/j.1464-5491.2007.02092.x. [DOI] [PubMed] [Google Scholar]
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA. 2005;294(5):557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Susser ES, Lin SP. Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944–1945. Arch Gen Psychiatry. 1992;49(12):983–988. doi: 10.1001/archpsyc.1992.01820120071010. [DOI] [PubMed] [Google Scholar]
- Thurner S, Klimek P, Szell M, Duftschmid G, Endel G, Kautzky-Willer A, Kasper DC. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc Natl Acad Sci. 2013;110(12):4703–4707. doi: 10.1073/pnas.1215626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Welie H, Derks EM, Verweij KH, De Valk HW, Kahn RS, Cahn W. The prevalence of diabetes mellitus is increased in relatives of patients with a non-affective psychotic disorder. Schizophr Res. 2013;143(2–3):354–357. doi: 10.1016/j.schres.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian G, Chittiprol S, Neelakantachar N, et al. Insulin and insulin-like growth factor-1 abnormalities in antipsychotic-naive schizophrenia. Am J Psychiatry. 2007;164(10):1557–1560. doi: 10.1176/appi.ajp.2007.07020233. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Forsen T, Osmond C, Barker DJ, Eriksson JG. Association of schizophrenia with low maternal body mass index, small size at birth, and thinness during childhood. Arch Gen Psychiatry. 2001;58(1):48–52. doi: 10.1001/archpsyc.58.1.48. [DOI] [PubMed] [Google Scholar]
- Wani RA, Dar MA, Margoob MA, Rather YH, Haq I, Shah MS. Diabetes mellitus and impaired glucose tolerance in patients with schizophrenia, before and after antipsychotic treatment. J Neurosci Rural Pract. 2015;6(1):17–22. doi: 10.4103/0976-3147.143182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg SM, Jenkins EA, Marazita ML, Maher BS. Minor physical anomalies in schizophrenia: a meta-analysis. Schizophr Res. 2007;89(1–3):72–85. doi: 10.1016/j.schres.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300(24):2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- Wu X, Huang Z, Wu R, et al. The comparison of glycometabolism parameters and lipid profiles between drug-naive, first-episode schizophrenia patients and healthy controls. Schizophr Res. 2013;150(1):157–162. doi: 10.1016/j.schres.2013.07.051. [DOI] [PubMed] [Google Scholar]
- Xu T, Chan RC, Compton MT. Minor physical anomalies in patients with schizophrenia, unaffected first-degree relatives, and healthy controls: a meta-analysis. PLoS One. 2011;6(9):e24129. doi: 10.1371/journal.pone.0024129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Chen DC, Tan YL, An HM, Zunta-Soares GB, Huang XF, Soares JC. Glucose disturbances in first-episode drug-naïve schizophrenia: Relationship to psychopathology. Psychoneuroendocrinology. 2015;62:376–80. doi: 10.1016/j.psyneuen.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Guan SL, Wang ZQ, You Y, Sun SL, Hui L, et al. No association between the type 2 diabetes mellitus susceptibility gene, SLC30A8 and schizophrenia in a Chinese population. Hum Psychopharmacol. 2012;27:392–396. doi: 10.1002/hup.2239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.